ABSTRACT
The experiment was performed to determine the effect of 20% dietary DL-Methionine (Met) in excess of modern breeder requirement level compared to Tilmicosin (Pulmotil AC ®) on the humoral immune response and histopathological parameters of male broilers challenged with Mycoplasma gallisepticum (MG). Birds of Treatments 1–4 remained unchallenged while those of Treatments 5–8 were challenged with MG at 10 days of age. Diets with excess Met were formulated for birds of Treatments 3, 4, 7 and 8. Broilers of Treatments 2, 3, 6 and 7 were administered Pulmotil AC® in drinking water. The 20% excess dietary Met significantly reduced tracheal deciliation, preserved the integrity of tracheal mucosal layer and significantly decreased goblet cell degeneration in MG-challenged birds. Furthermore, MG-challenged broilers administered 20% excess Met manifested higher ELISA titers against MG and a significantly higher antibody response to p33 (MG-cytadhesin putative gene) and p66 (MG-hemagglutinin protein A (pMGA)) in comparison to the other treatments. The 20% excess Met thereby maintained the health of the respiratory system and embodied an increased protection against MG-putative gene and a greater humoral response to MG challenge in broilers.
Introduction
Globally, Mycoplasma gallisepticum outbreaks remain a persistent obstacle resulting in considerable economic losses in poultry production and high morbidity and mortality of flock as it predisposes birds to severe secondary infections. The frequent antigenic or phenotypic variation of major surface antigens allows the MG microorganism to modulate its surface proteins in order to evade the host lymphatic response (Levisohn et al. Citation2000, Bencina, Citation2002; Noormohammadi, Citation2007; Ramadan, Citation2018). In addition, MG has high frequency phase variability; a mechanism for coping with rapid varying environments without the need of random mutation (Glew et al., Citation2000; Pflaum et al., Citation2016).
Substantial effort has been made to identify MG antigens, especially those with cytadhesin or hemagglutinin properties, which may be of vital pathogenesis process of, and immune response to, MG infection. As integral membrane proteins, adhesins possess regions exposed on the surface of the cell that adhere to receptor sites on host epithelial cells, granting colonization and infection; thereby they are perceived as crucial virulence factors (Ramadan, Citation2018).
According to several research studies, proteins or lipoproteins of MG with molecular weights of 60–75 kDa have been deduced as immunodominant adhesins or hemagglutinins (Boguslavsky et al., Citation2000; Kleven, Citation2008; Ley, Citation2008; Markham, Citation2012; Hassan et al., Citation2014; Ramadan, Citation2018).
In regard to histopathology, thickening of mucosal membranes is highlighted in MG-infected tissues (trachea, chorioallantoic membrane, sinus membrane, lateral borders of lungs, air sac) due to the penetration of mononuclear cells and the hyperplasia of mucosal glands (Islam et al., Citation2011; Wijesurendra et al., Citation2015; Limsatanun, Citation2017). In tracheal tissues, destruction and loss of cilia are indicated in MG-infected birds, along with swollen epithelial cells (Ramadan, Citation2018). Commonly, lymphoid hyperplasia is observed in submucosa (Haley, Citation2017). Ciliostasis and the cytadherance of MG to host's villi are apparent (Ley, Citation2008; Majumder, Citation2014). Lung inflammation caused by MG is characterized with pneumonic regions, alterations in the lympho-follicles and granulomatous lesions (Ley, Citation2008). Kerato-conjunctivitis in MG-infected layers is outlined by epithelial hyperplasia, sub-epithelial edema, drastic cellular infiltration, and stroma of the central fibrovascular connective tissue that lead to thickening of the eyelids (Kawashima et al., Citation2010). In salpingitis cases, thickening of the oviductal mucosa and lymphoplasmacytic infiltration are notable (Nunoya et al., Citation1997). Proliferation of lymphocytes and plasma cells following germinal centres is also indicated in the lamina propia, specifically in the sub-epithelial region with a consequence of irregular elevations of the hyperplastic epithelial layer (Nunoya et al., Citation1997). Encephalitis caused by MG has been examined from moderate to severe forms comprising fibrinoid vasculitis, lymphocytic cuffing of vessels, parenchymal necrosis, and meningitis (Ley, Citation2008).
Methionine (Met) supplementation in broiler diets ensures proper metabolic and immune functioning. Beside being an essential component of tissue proteins, Met participates in transmethylation and transsulfuration pathways and reduces reactive oxygen species (ROS) level in tissues. Moreover, and as Met is a sulfur-bearing amino acid, it constitutes a vital component of innate, humoral and cell-mediated immunity (Lai et al., Citation2018). Swain and Johri (Citation2000) indicated that cellular immune response reflected by leucocyte migration inhibition increased significantly (P < 0·05) in 21 day old broilers when broiler diets were supplemented with various concentrations of methionine demonstrating enhanced immunity. Histological studies of broilers fed Met deficient rations reported congestion in cortex and medulla of thymic lobule with loosely arranged and significantly reduced quantity of lymphocytes in the medulla in methionine deficient formulated diet (Wu et al., Citation2012a). Decline in lymphocytes was also observed in lymphoid follicles with thinner cortices and wider medullae in the bursa of Fabricius (Wu et al., Citation2012b). The histological structure of spleen was disarranged and its lymphocytes were significantly decreased in the white and red pulp. Vacuolated mitochondria of lymphocytes and greater apoptotic lymphocytes were detected in the spleens of broiler on methionine deficient ration (Wu et al., Citation2012a; Wu et al., Citation2012b).
Met in excess of modern breeder requirement has proven successful as immunopotentiator against Mycoplasma gallisepticum (MG) infection in broilers as concluded from the work of Ramadan (Citation2018). This study revealed that the treatment of 20% excess Met has significantly increased IgG titers, bursal indices and hematocrit percentage in MG-challenged Ross 308 broilers. In addition, the percentage of birds with severe tracheitis caused by MG-infection was significantly reduced from 40 to 10% at 17–35 days of age.
This study was performed to investigate the role of 20% excess dietary methionine above the modern broiler recommended level in the protection of Ross 308 broilers against mycoplasma gallisepticum-challenge treated with or without Pulmotil AC® ; by assessing: (1) the quantitative and qualitative increase in humoral response in targeting MG putative genes and (2)preserving tissue integrity of the respiratory system.
Materials and methods
Birds, housing and treatments
The trial was initiated after receiving the approval from the Institutional Animal Care and Use Committee (IACUC) of the American University of Beirut (AUB). A total of 1200 day-old male broilers chicks of Ross 308 strain originating from the same breeding flock and hatchery were raised on the floor in two adjacent environmentally-controlled poultry houses of similar design and dimensions at the Agricultural Research and Education Center (AREC) of the American University of Beirut, Lebanon. On the same day of purchasing the experimental birds, day-old chicks were tested, using culture of trachea and airsac specimens in Frey's medium, to confirm they were MG free.
Each house included 12 floor pens accommodating 50 broilers per pen using wood shavings as litter materials, one large manual circular feeder and an automatic hanging bell shaped drinker, a continuous lighting programme was provided. The pen's dimensions were 1.8 m × 2.75 m and -birds in each house were served by a separate attendant. The house temperature was adjusted, through a temperature control panel, as per the recommendations of the Ross 308 broiler management handbook 2018 starting with 30◦C for the first two days and then gradually decreased until it reached 20◦C at day 27. The temperature was then stabilized at 20◦C until the end of the experiment. The trial was composed of eight treatments with three replicates per treatment. The treatments were allocated as per .
Table 1. Treatments arrangement to evaluate the immunopotentiating effect of 20% excess DL-Methionine on Mycoplasma gallisepticum infected broilers with a comparison to an antimicrobial administration.
Challenge
Birds within the first house were challenged each with a field F strain of Mycoplasma gallisepticum suspension of 8.3 × 106 CFU/ml Frey's broth. The challenge strain was administered intrathoracically and intratracheally with 0.2 ml and 50 ul of the Mycoplasma gallisepticum suspension, respectively at 10 days of age. In the second house, birds remained unchallenged.
Antibiotic
Challenged and unchallenged birds were administered Tilmicosin active-based antibiotic Pulmotil AC ® in drinking water during the first three days of age and from 18–20 days of age as recommended by the manufacturer.
Diets
Starter, grower and finisher corn/soybean meal-based diets were formulated for groups 1, 2, 5 and 6 to contain the recommended level of Methionine to meet the modern breed requirement suggested by the Ross 308 Broiler company (Aviagen). Diets containing 20% additional Methionine above the recommended level for the modern Ross 308 Broiler requirement () were formulated for groups 3, 4, 7 and 8. Feed and water were offered ad libitum.
Pathological parameters
Histopathology. At 35 days of age, three birds from each pen, representing the average pen weight, were selected to perform the histopathological studies. The experimental and control birds were euthanized and tracheas were removed and preserved in 10% formalin. Tracheas were then subjected to cross-sectioning of 4 μm, and stained with Hematoxylin and Eosin (H&E). The microscopic observations included tracheal deciliation, mucosal hypertrophy (at x400) goblet cell degeneration, and heterophil infiltration (at x1000) in three tracheal cuts/bird within three microscopic fields/cut, located at four, eight, and twelve clock positions. A score of (+) was assigned for each of the following tracheal tissue alterations, namely, deciliation, mucosal hypertrophy, goblet cell degeneration, and heterophil infiltration, while a score of (-) was donated to the absence of tracheal malformation. Images were taken for all recorded microscopic lesions and documented.
Serological analysis.
ELISA titers against Mycoplasma gallisepticum. At 10, 17 and 35 days of age, blood was collected from 5 birds/pen (15 birds/treatment) chosen at random. Seroconversion to MG was determined using Enzyme-linked immunosorbent assay (ELISA). ELISA calculations were obtained via (IDEXX) equations (Ramadan Citation2018).
Humoral immunity to p33 (MG-cyadhesin) and p66 (pMGA-Hemagglutinin). Two serum samples were taken from each pen (six sera samples/treatment) to evaluate the humoral immunity to p33 (MG-cyadhesin) and p66 (pMGA-Hemagglutinin) using western immunoblotting technique. The Sodium Dodecyl Sulfate- Polyacrylamide Gel Electrophoresis (SDS- PAGE) (Bio-Rad Lab. 200 Alfred Nobel Dr., Hercules, CA, USA) was performed using polyacrylamide gel and gel lanes were each loaded with a 25 l volume reflecting that each well contained 15 μg/μl of the purified MG peptides and SDS-PAGE ran for 60 min at 45 V (or 60 mA). The following steps were performed for western-immunoblotting: The polyacrylamide gel was transferred into a nitrocellulose membrane (NCM) for blotting. The NCM was then stained with Ponceau S, and individual's lanes were cut and followed with TBS wash. Blocking of the active site was achieved via 5% gelatin-TBS soak for 1 h with continuous shaking. The NCM lanes were then washed with TTBS. Sera samples were diluted with 1% gelatin-TTBS to 1:250 and incubated for 1 h at 37°C allowing MG-specific antibodies to bind. NCM lanes were washed with TTBS. The NCM lanes were immersed in a sheep anti-chicken IgG (H + L) Peroxidase Conjugate (Sigma, St. Louis, MO, USA) 1:1000 diluted solution in 1% gelatin-TTBS and were incubated at 37°C for 30 min. The NCM lanes were washed with TTBS and further washed with TBS. The NCM lanes were then soaked in DAB peroxidase Substrate solution (Sigma, St. Louis, MO, USA) and were shaken at 37°C. Image scanning of the NCM lanes was obtained for image and band analysis. Optical densities of the obtained bands were determined on five randomly selected areas (3×2 square pixels) using Photoshop and averaged.
Statistical analyses
The trial included an arrangement of eight treatments replicated three times (pens) with 50 birds per replicate in a completely randomized design. Data were analysed using One Way Analysis of Variance (ANOVA) followed by Tukey's test for mean separation (P < 0.05, SPSS v.24, 2018).
Results
Tracheal tissue integrity
Histopathology results of Ross 308 broilers at 35 days of age are illustrated in while microscopic slide photographs of tracheal histopathology of male Ross 308 broilers are shown in . With the exception of Treatment 8 (20% excess Met), MG-challenged groups showed numerically higher frequencies of microscopic lesions among birds in comparison to those of unchallenged groups. According to the statistical results shown in , the 20% excess dietary methionine has shown the lowest percentage value of deciliation in birds challenged with MG compared to other treatments. The percentage of deciliation in birds challenged with MG and supplemented with excess Met (Treatment 8) was also significantly lower than that of MG challenged untreated birds (Treatment 5) and numerically lower than that of MG-challenged birds treated with tilmicosin (Pulmotil AC ®) (Treatment 6).
Figure 1. A microscope slide photograph of healthy trachea in ‘A’ (200x magnification) and ‘B’ (400x magnification) demonstrating normal ciliation (arrow), presence of intact goblet cells (dashed arrow), and integrity of mucosal layer (dotted arrow) in tracheas versus photograph ‘C’ and ‘D’ (both 400x magnification) deciliation and degeneration of goblet cells (dashed oval), and thickening of mucosal layer in tracheas of male Ross 308 broilers (curved arrow). (H & E stain).
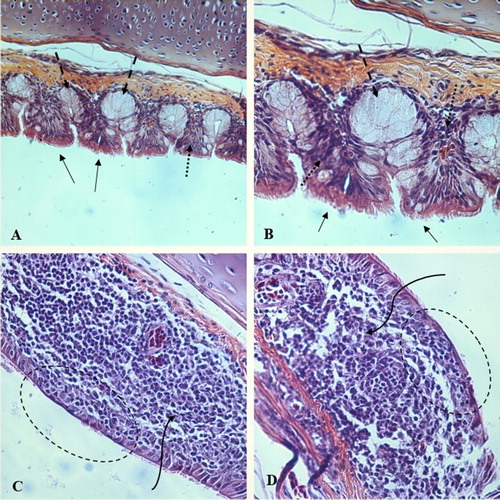
Table 2. The mean1 percentages (%) of negative tracheal histopathology parameters of male Ross 308 broilers at 35 days of age of different designated treatments.
Regarding tracheal mucosal thickening, all treatments resulted in significantly lower values than the untreated MG-challenged group. Note that birds in MG-unchallenged pens administered excess Met have shown the lowest percentage of tracheal mucosal thickening.
The integrity of tracheal tissues in birds supplemented with 20% dietary methionine was further reflected in goblet cell persecution. MG-challenged birds with excess Met (Group 8) demonstrated no goblet cell degeneration and were not significantly different than pens unchallenged with MG. However, the MG-challenged untreated pens with adequate methionine levels (group 5) illustrated the significantly highest percentage of goblet cell degeneration. Birds treated with Tilmicosin whether MG-challenged or not were not significantly different than birds treated with Tilmicosin plus excess Met in regards to goblet cell atrophy.
Protective humoral immunity
Western-immunoblotting analysis showed that broilers challenged with M. gallisepticum whether remained untreated or supplemented with excess methionine responded to specific proteins with high band intensity. However, differences were observed in the immune profile of broilers depending on their age at onset of challenge and on the time of blood collection post-challenge. Prior to MG-challenge, broilers demonstrated no antibody response to p33 and p66, i.e. MG-cytadhesin gene and MG- hemagglutinin protein A (pMGA) (). When challenge was administered at 10 days of age and immunoblotting assessment was carried out 7 days post challenge (17 days of age) no antibodies to p33 and p66 appeared (, and ). However when MG-infection was prolonged to a later age with 25 days post-challenge; antibodies to p33 and p66 were detected (, and ). For the cytadhesin gene (33KD); unchallenged groups (1–4) showed no antibody response while MG-challenged untreated pens (Treatment 5) were not significantly different in terms of band intensity compared to all MG- unchallenged treatments (Treatments 1–4). Similarly, MG-challenged untreated pens were not significantly different from MG-challenged pens administered tilmicosin solely or in combination with excess methionine. However, broilers in pens challenged with MG and administered 20% excess dietary Met (Treatment 8) have shown significantly enhanced band intensity, and consequently higher antibody levels, for p33 compared to unchallenged treatments and MG-challenged treatments administered tilmicsosin.
Figure 2. Western immunoblotting of 10 day old male broilers MG unchallenged sera: L = molecular marker; 1 = positive MG serum, 10-day old sera; MG-unchallenged (lanes 2–5).
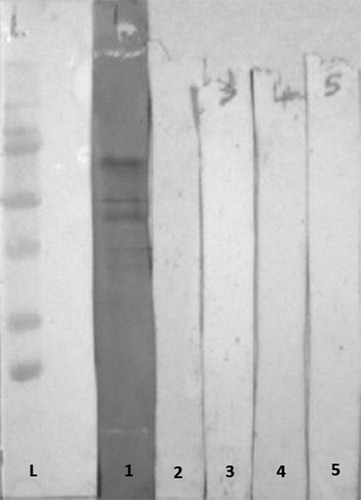
Figure 3. Western immunoblotting of 17 day old MG unchallenged male broilers sera: L = molecular marker; 1 = positive MG serum, MG-unchallenged pens no Pulmotil and adequate Methionine (lanes 2–7); MG-unchallenged pens treated with Pulmotil (lanes 8–13); MG-unchallenged pens treated with Pulmotil + 20% Methionine (lanes 14–19), MG-unchallenged pens treated with 20% Methionine, no pulmotil (lanes 20-23).
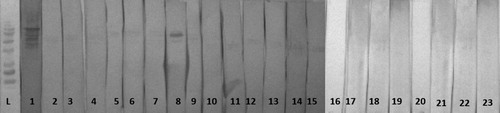
Figure 4. Western immunoblotting of 17 day old MG challenged male broilers sera: L = molecular marker; 1 = positive MG serum, MG-challenged pens no Pulmotil and adequate Methionine treated (lanes 2–7); MG-challenged pens treated with Pulmotil (lanes 8–13); MG-challenged pens treated with Pulmotil + 20% Methionine (lanes 14–19), MG-unchallenged pens treated with 20% Methionine, no pulmotil (lanes 20–23).
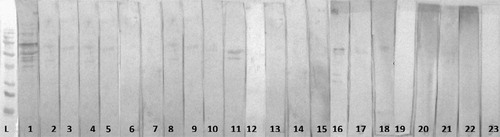
Figure 5. Western immunoblotting of 35 day old MG challenged male broilers sera: L = molecular marker; 1 = positive MG serum, MG-challenged pens treated with Pulmotil (lanes 2–9); MG-challenged pens treated with Pulmotil + 20% Methionine (lanes 6–13).
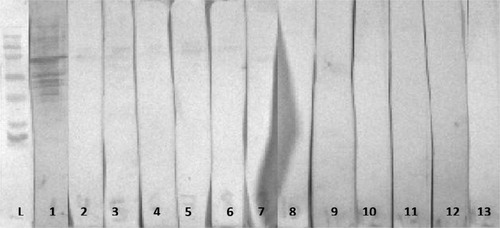
Figure 6. Western immunoblotting of 35 day old MG challenged male broilers sera: L = molecular marker; 1 = positive MG serum, MG-challenged untreated pens (lanes 2–5), MG-challenged + 20% Methionine treated pens (lanes 6–9).
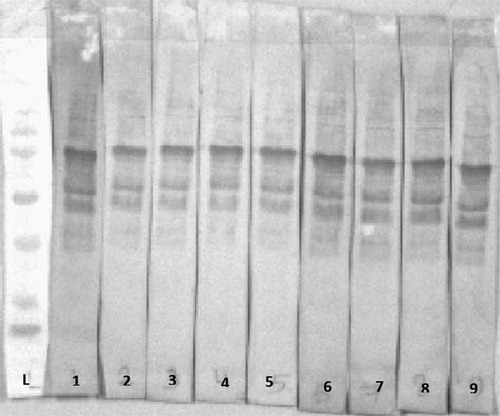
Table 3. Band density1 of Mycoplasma gallisepticum cytadhesin gene and hemagglutinin gene (pMGA) at 33 and 66 kilodalton (KD), respectively of male Ross 308 broilers at 17 and 35 days of age.
As for the pMGA gene (p66), unchallenged groups did not exhibit any humoral response at 35 days of age. The MG-challenged untreated group has demonstrated a significant difference compared to all unchallenged treatments and MG-challenged treatments administered tilmicosin solely or with the addition of excess methionine. Yet, MG-challenged pens with excess Met have illustrated significantly enhanced band intensity to p66 in comparison to all treatments.
On the account of an evident numerically increasing trend in band density for both p33 and p66 for MG-challenged pens administered excess methionine, it can be concluded that the 20% excess dietary methionine is increasing the protection level against MG-infection. The results at 35 days of age of treatments 5 and 8 stress the importance of protein p33 and p66 observed as bands in the immunoblots ().
ELISA results revealed in our previous data (Ramadan Citation2018; ) that the significantly greatest titer to MG was illustrated in the challenged excess methionine treated pens (Treatment 8), apparent at 35 days of age (P < 0.05) along a low CV value of 14.84% (Ramadan Citation2018). Male Ross 308 broilers demonstrated IgG titers against MG on 35 days of age only in Treatment 5 (MG-challenged untreated group) and in Treatment 8 (MG-challenged excess Met group). No detectable MG titers were recorded for all the birds at 10 and 17 days of age (<400).
Table 4. IgG ELISA titers1 of Mycoplasma gallisepticum of male Ross 308 broilers at 35 days of age.
Discussion
In all the observed tracheal histopathological parameters, the MG-challenged untreated group illustrated the highest percentages of tracheal atrophy with significantly increased deciliation, mucosal thickening layer and deterioration of goblet cells. On the other hand, an apparent trend is illustrated from histopathological result with 20% excess dietary methionine revealing significant reduced deciliation, increased coherence of mucosal layer and integrity of goblet cells. This is true for birds unchallenged and challenged with MG, treatments 4 and 8, respectively. This indicates that the 20% excess dietary methionine treatment has a significant role in preserving the integrity of tracheal tissues in broilers, specifically of birds challenged with Mycoplasma gallisepticum. Literature sustaining these findings is abundant; and similar results were obtained in several studies that emphasized the role of excess dietary methionine on the preservation of broiler tissue integrity and resistance to various enteric and respiratory diseases of poultry (Dahiya et al., Citation2007; Rubin et al., Citation2007; Martinez et al., Citation2017; Lai et al., Citation2018)
MG gene families of hemagglutinin protein A (pMGA) and phase variable putative adhesion protein A (PvpA) are acknowledged for encoding major surface proteins accompanying attributes of pathogenicity, antigenic, and immune escape. MG cells express pMGA as an abundant surface lipoprotein (Hu et al. Citation2015). Therefore, the increase in the antibody level against this specific protein plays a vital role as a protective element against the attachment of MG colonies to the host cell.
The pMGA on the M. gallisepticum cells enables the host to further recognize and produce antibodies that are specific to the target antigen. It consequently protects effectively against M. gallisepticum cells. That was proven by one of several studies that showed the effectiveness and specificity of anti-pMGA in hindering the metabolism of MG cells whereby they used two pMGA-specific monoclonal antibodies (MAbs), (66 and 86 KDa) with M. gallisepticum cells in liquid culture. The metabolism rates of cultures of Mycoplasma synoviae were not affected by the inclusion of MAb 66 within the growth medium, ruling out a nonspecific growth inhibition; this assures the specificity of the pMGA. As for the detection of their effect in masking the metabolism, the MAb 66 caused a reduction in the growth and metabolic activity of cells compared to the control culture lacking Mab (Markham et al., Citation1998). By increasing the immune specific response to pMGA, the methionine would be playing a major role in prohibiting the colonization of the host respiratory tissue with Mycoplasma gallisepticum as revealed in this study. The enhanced humoral immunity could be mainly explained by the fact that methionine, a sulfur-containing amino acid, is essential for the formation Immunoglobulins that contain disulfide bonds between their light and heavy chains (Tsiagbe et al., Citation1987). In addition, and since methionine is essential for protein synthesis in cells, literature illustrates the role of excess dietary methionine in augmenting the immune response through enhancing T cell proliferation, development of Thymus and Bursa tissues, and production of antibodies (Swain and Johri, Citation2000; Jankowski et al., Citation2014).
The same applies for the cytadhesin protein PvpA (p33) gene that controls the attachment and adhesion to the host cells, as a primordial step in the infection process, and attributes the characteristics of pathogenicity, antigenicity, and immune escape to MG. Therefore, increasing the production of specific- PvpA antibodies by the host provides protection against MG infection as reported in the literature (Ley, Citation2008; Szczepanek et al., Citation2010; Cizelj et al., Citation2011; Tan et al., Citation2015; Xu et al., Citation2015). When the dietary methionine level in this study was increased, the specific immune response to MG via targeting the PvpA and pMGA proteins was enhanced; this was reflected by the preservation of the integrity of the tracheal tissue of MG-challenged broilers as shown in the histopathological study section.
The immunoblotting profile at 35 days of age illustrates a direct proportionality in results to the ELISA titers and M. gallisepticum IgG response. The intensity of western-immunoblotting bands was observed to be significantly the highest at p33 and p66, 25 days post-challenge for MG-challenged broilers supplemented with 20% excess Met. This further reflects and confirms the immunoprotective performance of excess Met treatment in the presence of MG-infection. It is worth noting that Pulmotil AC ® treatment (Groups 6 and 7) cleared MG infection in challenged groups (5–8); which was reflected by the subsequent absence of antibody response to MG proteins, as revealed by ELISA, in comparison to those untreated with tilmicosin (Groups 5 and 8). These results offer an additional proof of the superiority of tilmicosin in treating MG infection in poultry (Charleston et al., Citation1998; Abd El-Ghany, Citation2009; Mavromati, Citation2011)
Conclusion
The excess methionine treatment has demonstrated a significant role in preserving the integrity of tracheal tissues in broilers, specifically of birds challenged with Mycoplasma gallisepticum. Excess dietary methionine resulted in lower values compared to other treatments regarding observed deciliation, and this can be reflected in the immunoblotting results demonstrating methionine's protective ability to bind to hemagglutinin and adhesin protein. The integrity of tracheal tissues was further reflected in goblet cell persecution and the coherence of the tracheal mucosal layer.
It is recommended to use 20% excess dietary Met in broiler farms with high exposure risk to MG. Methionine offers a source of sulfur, and hence as observed in the previous study, excess methionine has demonstrated greater immunity, enhancing specifically humoral immune response. In addition, Pulmotil AC ® treatment alone was sufficient to reduce significantly humoral response to MG proteins as a result of bacterial clearance from the respiratory tissue of MG-challenged broilers. The combination of 20% excess dietary Met and Pulmotil AC ® treatment forms a beneficial strategy to treat broilers with MG infection.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Abd El-Ghany W. 2009. The In-vitro and In-vivo Evaluation of Tiamulin and Tilmicosin for the treatment of Mycoplasma gallisepticum infected broiler Chickens. Int J Poult Sci. 8(12):1189–1198. doi: 10.3923/ijps.2009.1189.1198
- Bencina D. 2002. Haemagglutinins of pathogenic avian mycoplasmas. Avian Pathol. 31:535–547. doi: 10.1080/0307945021000024526
- Boguslavsky S , Menaker D , Lysnyansky I , Liu T , Levisohn S , Rosengarten R , García M , Yogev D. 2000. Molecular characterization of the Mycoplasma gallisepticum pvpA gene which encodes a putative variable cytadhesin protein. Infect Immun. 68:3956–3964. doi: 10.1128/IAI.68.7.3956-3964.2000
- Charleston B , Gate JJ , Aitken IA , Reeve-Johnson L. 1998. Assessment of the efficacy of tilmicosin as a treatment for Mycoplasma gallisepticum infections in chickens. Avian Pathol. 27(2):190–195. doi: 10.1080/03079459808419322
- Cizelj I , Bercic RL , Dusanic D , Narat M , Kos J , Dovc P , Bencina D. 2011. Mycoplasma gallisepticum and Mycoplasma synoviae express a cysteine protease CysP, which can cleave chicken IgG into Fab and Fc. Microbiology. 157:362–372. doi: 10.1099/mic.0.045641-0
- Dahiya JP , Hoehler D , Van Kessel AG , Drew MD. 2007. Effect of different dietary methionine sources on intestinal microbial populations in broiler chickens. Poultry Science 86(11):2358–2366. doi: 10.3382/ps.2007-00133
- Glew MD , Browning GB , Markham PF , Walker ID. 2000. pMGA phenotypic variation in Mycoplasma gallisepticum Occurs In Vivo and Is mediated by Trinucleotide Repeat Length variation. Immunity. 68:6027–6033. doi: 10.1128/IAI.68.10.6027-6033.2000
- Haley PJ. 2017. The lymphoid system: a review of species differences. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5406590/ .
- Hassan S , Mukhtar N , Ur S , Asghar A. 2014. Molecular Epidemiology of Mycoplasma gallisepticum in different Types of Chickens. Int J Agric Biol; Faisalabad. 16(1):165–170.
- Hu F , Zhao C , Bi D , Tian W , Chen J , Sun J , Peng X. 2015, November 9. Mycoplasma gallisepticum (HS strain) surface lipoprotein pMGA interacts with host apolipoprotein A-I during infection in chicken. https://link.springer.com/article/10.1007/s00253-015-7117-9 .
- Islam A , Aslam A , Chaudhry ZI , Ahmed MUD , Rehman HU , Saeed K , Ahmad I. 2011. Pathology of Mycoplasma gallisepticum in Naturally infected broilers and its Diagnosis through PCR. Int J Agric Biol. 13:835–837.
- Jankowski J , Kubińska M , Zduńczyk Z. 2014. Nutritional and immunomodulatory function of methionine in poultry diets – a review. Ann Anim Sci. 14(1):17–31. doi: 10.2478/aoas-2013-0081
- Kawashima M , Kawakita T , Higa K , Satake Y , Omoto M , Tsubota K , Shimazaki J. 2010, December 15. Subepithelial corneal fibrosis partially due to epithelial-mesenchymal transition of ocular surface epithelium. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3002964/ .
- Kleven SH. 2008. Mycoplasmosis. In: L. Dufour-Zavala , D. E. Swayne , J. R. Glisson , J. E. Pearson , W. M. Reed , M. W. Jackwood , P. R. Woolcock , editors. A laboratory manual for the isolation, identification and characterization of avian pathogens. 5th. Athens, GA : American Association of Avian Pathologists; p. 59–64.
- Lai A , Dong G , Song D , Yang T , Zhang X. 2018. Responses to dietary levels of methionine in broilers medicated or vaccinate against coccidian under Eimeria tenella challenged condition. BMC Vet Res. 14:140–150. doi: 10.1186/s12917-018-1470-8
- Levisohn S , Kleven SH. 2000. Avian mycoplasmosis (Mycoplasma gallisepticum) - oie.int. Retrieved from https://www.oie.int/doc/ged/D9309.PDF .
- Ley D. 2008. Mycoplasmomsis. Mycopalsma gallispeticum infection. In: Saif Y.M. , Barnes H.J. , Glisson J.R. , Fadly A.M. , McDougald L.R. , Swayne D.E. , editors. Diseases of poultry. Twelfth Edition. Blackwell Publishing; p. 807–845.
- Limsatanun A. 2017. Development of inactivated mycoplasma gallisepticum vaccine in chickens [Thesis Ph.D.]. Chulalongkorn University. Faculty of Veterinary Science.
- Majumder S. 2014. Role of Mycoplasma gallisepticum and host airway epithelial cell interaction in inflammation. [Doctoral dissertations]. (651).
- Markham PF. 2012. A novel transposon construct expressing PhoA with potential for studying protein expression and translocation in Mycoplasma gallisepticum . BMC Microbiol. 12:138. doi: 10.1186/1471-2180-12-138
- Markham PF , Glew MD , Browning GF , Whithear KG , Walker ID. 1998. Expression of two members of the pMGA gene family of Mycoplasma gallisepticum oscillates and is influenced by pMGA-specific antibodies. Infect Immun. 66(6):2845–2853.
- Martínez Y , Li X , Liu G , Bin P , Yan W , Más D , Valdivié M , Andy Hu C , Ren W , Yin Y. 2017. The role of methionine on metabolism, oxidative stress, and diseases. Amino Acids. 49(12):2091–2098. doi: 10.1007/s00726-017-2494-2
- Mavromati J , Mavromati E , Gjeta Z. 2011. Effect of macrolide antibiotics on mycoplasma control and production efficiency in broilers. Biotechnol Anim Husbandry. 27:721–731. doi: 10.2298/BAH1103721M
- Noormohammadi AH. 2007. Role of phenotypic diversity in pathogenesis of avian mycoplasmosis. Avian Pathol. 36:439–444. doi: 10.1080/03079450701687078
- Nunoya T , Kanai K , Yagihashi T , Hoshi S , Shibuya K , Tajima M. 1997. Natural case of salpingitis apparently caused by Mycoplasma gallisepticum in chickens. Avian Pathol. 26:391–398. doi: 10.1080/03079459708419221
- Pflaum K , Tulman ER , Beaudet J , Liao X , Geary SJ. 2016. Global changes in Mycoplasma gallisepticum phase-variable lipoprotein gene vlhA expression during in vivo infection of the natural chicken host. Infect Immun. 84(1):351–355. doi: 10.1128/IAI.01092-15
- Ramadan NM. 2018. DL-Methionine: an Immunopotentiator, In: Mycoplasma gallisepticum Challenged Broilers Treated With Pulmotil Ac®. Master’s Thesis. Department of Animal and Veterinary Sciences; Faculty of Agricultural and Food Sciences. American University of Beirut. Jafet Library. Published May 11th, 2018.
- Rubin LL , Canal CW , Ribeiro ALM , Kessler A , Silva I , Trevizan L , Viola T , Raber M , Gonçalves TA , Krás R. 2007. Effects of methionine and arginine dietary levels on the immunity of broiler chickens submitted to immunological stimuli. Brazilian J Poult Sci. 9(4):241–247. doi: 10.1590/S1516-635X2007000400006
- Swain BK , Johri TS. 2000. Effect of supplemental methionine, choline and their combinations on the performance and immune response of broilers. Br Poult Sci. 41:83–88. doi: 10.1080/00071660086457
- Szczepanek SM , Jr FS , Schumacher VL , Liao X , Padula M , Djordjevic SP , Geary SJ. 2010. Identification of lipoprotein MslA as a Neoteric virulence Factor of Mycoplasma gallisepticum . Infect Immun. 78(8):3475–3483. doi: 10.1128/IAI.00154-10
- Tan L , Hu M , Yu S , Wang X , Lu F , Liu F , Qiu X , Song C , Sun Y , Ding C. 2015. Characterization of the chaperonin GroEL in Mycoplasma gallisepticum . Arch Microbiol. 197:235–244. doi: 10.1007/s00203-014-1047-2
- Tsiagbe VK , Cook ME , Harper AE , Sunde ML. 1987. Enhanced immune responses in broiler chicks fed methionine supplemented diets. Poult Sci. 66:1147–1154. doi: 10.3382/ps.0661147
- Wijesurendra KA , Tivendale KA , Bacci B , Noormohammadi AH , Browning GF , Markham PF. 2015. Development of a Mycoplasma gallisepticum infection model in turkeys. Avian Pathol. 44:35–42. doi: 10.1080/03079457.2014.992390
- Wu BY , Cui HM , Peng X , Fang J , Cui W , Liu XD. 2012a. Effect of Methionine deficiency on the thymus and the subsets and proliferation of peripheral blood T-cell, and serum IL-2 contents in broilers. J Integrative Agriculture. 11:1009–1019. doi: 10.1016/S2095-3119(12)60093-8
- Wu BY , Cui HM , Peng X , Fang J , Cui W , Liu XD. 2012b. Pathology of spleen in chickens fed on a diet deficient in Methionine. Health. 4:32–38. doi: 10.4236/health.2012.41007
- Xu J , Teng D , Jiang F , Zhang Y , El-Ashram SA , Wang H , Sun Z , He J , Shen J , Wu W , Li J. 2015. Mycoplasma gallisepticum MGA_0676 is a membrane-associated cytotoxic nuclease with a staphylococcal nuclease region essential for nuclear translocation and apoptosis induction in chicken cells. Appl Microbiol Biotechnol. 99:1859–1871. doi: 10.1007/s00253-014-6185-6
