ABSTRACT
Pomegranate pulp (PP), an antioxidant source, is a major by-product of pomegranate processing industry. Four-hundred and forty d-old broilers were randomly allocated to eight treatments consisting of five replicates. The birds were divided into one of 2×4 factorial experiments, including two controlled housing temperatures (21°C for 24 h/d thermoneutral, TN; or 37°C for 8 h/d heat stress, HS) and four PP levels(0, 40, 70, and 100 gram PP/Kg diet). Significant interactions were observed in body weight gain, FCR and production broiler index between PP and HS at 42 d. Heat stress decreased feed intake compare to control. The interaction between HS and PP significantly affects the concentration of uric acid, malondialdehyde, the enzyme activity of Glutathione peroxidase (GPx) and total antioxidant capacity (TAC; P < 0.05). The HS-birds had lower plasma protein, the enzyme activities of superoxide dismutase(SOD) compared to TN-birds. The abdominal fat and liver percentage were significantly affected by the interactions between HS and PP(P < 0.05). The PP diets reduced the plasma concentration of cholesterol and LDL compared to the control. It is concluded that HS shows reduced performance, total protein and antioxidant status of broilers, while PP at the levels higher than 7%, improves the performance, blood cholesterol, and antioxidant status of HS-birds.
Introduction
Heat stress is a major environmental stressor threatening the poultry industry worldwide. It adversely affects the growth rate, feed efficiency, and the mortality rate of poultry, which consequently results in huge economic losses (Lara and Rostagno Citation2013; Sahin et al. Citation2013, Citation2016; Hosseini-Vashan et al. Citation2015). Heat stress begins some undesirable processes in physiological, immune, and antioxidant system in birds (Sahin et al. Citation2013, Citation2016; Hosseini-Vashan et al. Citation2015), which negatively influences the growth performances. High environmental temperature increases the oxidation process in the plasma, liver, and heart of chickens and produces a great concentration of free radicals (Halliwell and Gutteridge Citation1989; Lin et al. Citation2006; Mujahid et al. Citation2006). In the normal condition, free radicals were trapped by the body antioxidant defence system, while in the abnormal condition, especially with higher environmental stressors, the production of free radicals was amplified and the oxidative reaction was heightened (Wang et al. Citation2008). Then, the body antioxidant defence system cannot remove the undesirable free radicals. To prevent the undesirable effects of free radicals, supplementation of natural or synthetic antioxidant to poultry diets is necessary (Hosseini-Vashan et al. Citation2012, Citation2015).
Usage of agricultural by-products is one way to decrease the cost of ratio in poultry production and cut back environmental waste. Many agricultural by-products have a nutritional value to meet the requirements of poultry and they can be substituted with cereals in poultry diets to reduce the cost of poultry products. Pomegranate (Punica granatum L.; Punicaceae) is a fruit that is produced in different countries (Khan Citation2009). The annual production of pomegranate and pomegranate by-products is over 1,100,000 and 140,000 t/year in Iran, respectively (Amarnameh Ministry of Agriculture-Jahad Citation2016). Today, several products were produced from pomegranate such as pomegranate seed, juice, pulp, and peel (Shabtay et al. Citation2008). Prakash and Prakash (Citation2011) reported that the main components of pomegranate seeds were classified into eight groups including 1-Hydroxy-benzoic acids such as ellagic acid; 3,3 di-O-methyl ellagic acid; 3, 3, 4 tri-O-ellagic acid; 2-Conjugated fatty acids such as punicic acid (9 cis, 11 trans, 13 cis Octadecatrienoic acid); 3-Unconjugated fatty acids: linoleate, oleate, palmitate, stearate; 4-Esterols: stigmasterol, sitosterol, decosterol, testosterone, cholesterol; 5-Tocopherols: gamma-tocopherol; 6-Triterpenes: ursolic acid, oleanolic acid; 7-Isoflavones: daidzein (DZ) and genistein (GE); 8-Phenyl aliphatic Glycosides or lignin. The pomegranate pulp is a nutritional by-product that has important levels of metabolizable energy and crude protein (Persia et al. Citation2003).
The chemical composition of pomegranate seed pulp, reported by many researchers, was 9–12% crude protein, 6–10% crude fat, 30–35% crude fibre, and metabolisable energy 2400–2500 kcal/kg (Hosseini-Vashan and Ghaznavi Citation2016; Khosravi et al. Citation2015; Taher-Maddah et al. Citation2012; Mirzaei-Aghsaghali et al. Citation2011; Abbasi et al. Citation2008). The pomegranate seed pulp has also a valuable concentration of polyphenolic components involved in ellagic acid, punicalagin, and punicalin (Abbasi et al. Citation2008). The pomegranate seed pulp had no significant effect on broiler performance, lipid profile including, cholesterol, triglyceride, and LDL; however, the concentrations of HDL were increased when birds were fed pomegranate pulp (Hosseini et al. Citation2014; Luo et al. Citation2018). The antioxidant properties of pomegranate pulp and peel were reported higher than those of green tea (Louba Citation2007). Pomegranate pulp at the levels up to 15% in layer diets increased the production traits, egg quality indices, and blood cholesterol. However, the antioxidant indices, such as plasma malondialdehyde and total antioxidant capacity, did not alter by the inclusion of pomegranate pulp to layer diets (Saki et al. Citation2014). Therefore, the present study aimed to assess the antioxidant and immune system status, plasma lipid, abdominal fat, and growth performance of broilers exposed to heat stress and fed diets supplemented with pomegranate seed pulp (Punica granatum, L.).
Materials and methods
Chemical analysis
The chemical compositions of pomegranate seed pulp (PP) involved dry matter (DM), crude protein (CP, Kjeldahl N×6.25), ether extract (EE), crude fibre (CF), ash, calcium, and phosphorus were analysed (three replicates) according to AOAC (Citation2005) (). To the measurement of the phenolic components, the PP samples were extracted in methanol–water (80:20 vol/vol, 20 mL/g of PP flour) by shivering at room temperature for 3 h. The PP extracts were centrifuged (10 min, 3000×g), and supernatants were collected and kept in the dark at 4°C until analysis. The method of Makkar et al. (Citation1993) was used to determine the concentration of total phenolic compounds and tannins in PP.
Birds, diets, and experimental design
A total of 440 one-day-old male Ross broiler chicks were used in accordance with animal welfare regulations at the Veterinary Control and Research and Animal Care Committee of Birjand University, Iran. The birds were divided into one of 2×4 factorially experiments including two controlled housing temperatures (22°C for 24 h/d; thermoneutral, TN or 37°C for 8 h/d; heat stress, HS) and four levels of PP included 0, 40, 70, and 100 g of PP per kilogram diet. Each treatment had five replicates with 11 birds each. The chicks were reared in two controlled chambers under the same temperature, moisture, vaccination, and ventilation throughout 1–24 days. The initial room temperature (1 d old) was set at approximately 33 ± 1°C, thereafter; the daily temperature was gradually (2.5°C per week) reduced to normal management practice to 21°C by 25 days of age. In order to adjust the room temperature, a thermostat was installed. At 25 days of age, the TN chamber was maintained at 21°C with the relative humidity of 55% during 25–42 days. While in the HS chamber, birds were kept at 21°C for 14 h and exposed to 2 h of 21–37°C, 6 h of 37 ± 1°C, 2 h of 37–21°C (). The relative humidity of 55% for 6 h/day was also applied in HS (10.00 am–16.00 pm; Heat stress, HS groups). Birds were fed a mash starter diet until 10 d of age and a mash grower diet from 11 to 24 d and a mash finisher diet from 25 to 42 days of age (). Feed and water were provided ad libitum.
Figure 1. The experimental pattern of heat stress used for broilers (25–42 d). A constant of 21°C was applied for thermoneutral (TN) chamber and, whereas daily heat stress (21–37°C) was applied for heat-stressed chambers.
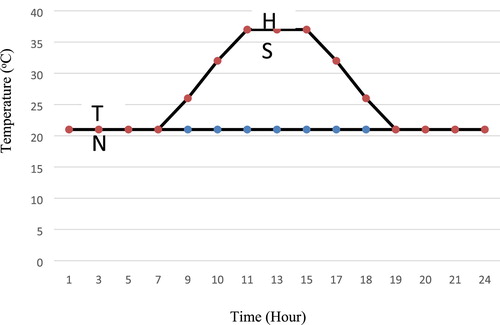
Table 1. The chemical composition, phenolic compounds of pomegranate seed pulp.
Growth performance measurements
The growth performances, such as feed intake (FI) and body weight, were weekly recorded for each replicate (pen) and also documented at 10, 24, and 42 d after fasting for 4 h. The feed conversion ratio (FCR; g feed intake: g weight gain) and production broiler index (PBI; 100*[(body weight*livability)/ (FCR* rearing period (day))]) were computed (Marcu et al. Citation2013).
Blood biochemical indices
To study the blood biochemical indices, eight birds (two/replicate) from each treatment were randomly selected and the blood was sampled from the left wing venipuncture after a 5 h feed deprivation at 42 d. The blood samples were collected in heparinized and non-heparinized tubes, then blood samples were centrifuged at 2500 g for 15 min to obtain plasma and serum, which were kept at −20°C in Eppendorf test tubes until analysis. The buffy coat was carefully eliminated and then sedimented cells were washed three times by resuspending in isotonic phosphate-buffered saline, followed by the recentrifugation and elimination of supernatant fluid and buffy coats. To prepare erythrocyte haemolysates, erythrocytes were washed and lysed with ten volumes of ice-cold distilled water, then stored at −70°C for later analysis (Perai et al. Citation2015).
The serum concentrations of cholesterol, triglyceride, low density lipoprotein (LDL), high density lipoprotein (HDL), total protein, and uric acid were measured by the Gesan chem autoanalyzer instrument (Gesan Chem 200 autoanalyzer, Italy) with ParsAzmon kits (Pars Azmon Ltd Co; Tehran Iran). The enzyme activities of aspartate aminotransferase (AST) and lactate dehydrogenase (LDH) were also measured by the Gesan chem autoanalyzer and using ParsAzmon kits.
Antioxidant status
The enzyme erythrocyte activity of glutathione peroxidase (GPx) and superoxide dismutase (SOD) were quantified in erythrocyte haemolysates using assay kits (Ransel and Ransod test kits, Randox Laboratories Ltd, UK). Another indictor used to evaluate antioxidant status is total antioxidant capacity (TAC). There are several methods to assess the TAC. The ferric-reducing ability of plasma (FRAP) directly measures antioxidants (TAC) with a reduction potential below the reduction potential of the Fe3+/Fe2+ couple (Benzie and Strain Citation1996; Halvorsen et al. Citation2002). Briefly, either sample or standards were blended with FRAP reagent and incubated at 37°C for 30 min. Then the absorbance was read at 593 nm and plotted the standard data on the curve using different concentrations (100–1100 μmol/L) of FeSO4–7H2O. The results were compared and calculated from the standard curve and expressed as mmol Fe2+/L.
The plasma lipid peroxidation was evaluated as thiobarbituric acid-reactive substance (TBARS) by the method described by Yoshioka et al. (Citation1979). Briefly, 0.5 mL plasma samples or standards were blended with 1.0 mL of 0.67% TBA and 2.5 mL of 20% trichloroacetic acid (TCA), and then heated in a boiling water bath (100°C) for 30 min. Four mL of n-butanol was added to the mixtures, after cooling in a water bath (2oC) for 10 min. The mixtures was then blended and vortexed for 2 min and centrifuged at 2500 rpm for 15 min. Following this, the supernatant n-butanol layer was separated and absorbance was read at 535 nm. The serial dilution curves were plotted based on a series of standard solutions (1, 1, 3, 3-tetramethoxypropane). The results were compared to the standard curve and the values were expressed as nmol/mL.
Immune system
In order to evaluate immune system especially humoral immune response of broilers, two strategies were made: (i) live Newcastle disease vaccine (La Sota strain) was administrated via drinking water at 8, 18, and 28 days of age, and (ii) The sheep red blood cells (SRBCs) were injected into the vein of broilers at 18 and 35 d. The sera were collected and the antibody titres against SRBCs (Nelson et al. Citation1995) and NDV (using haemagglutination inhibition test) were evaluated at 24 and 42 d. At 42 d, 1.0 mL of whole blood was gathered in heparinized capillary tubes, the blood smear was organized using May-Grunwald-Geimsa stain and then heterophil and the lymphocytes were counted to a total number of 100 cells/slide (Gross and Siegel Citation1983).
Carcass characteristics measurements
To study carcass characteristics, at 42 d of age, two birds from each replicate were randomly selected with a body weight close to the group average and weighed individually after a 4 h fasting. Broilers were scarified by cervical dislocation and abdominal fat, bursa of Fabricius, and spleen were weighed individually. The organ weights were stated as a percentage of live body weight.
Statistical analysis
In order to evaluate the normality, the data were evaluated using Shapiro–Wilk test, stem and leaf plots and normal probability plots using SPSS statistical software. The data were analysed as a completely randomized design with factorially 2×4 arrangements using the GLM procedure of SAS (SAS Institute Inc.). The differences in means of treatment were detected using Tukey’s test. Statements of significance were based on P ≤ 0.05.
Results
Chemical composition and bioactive components
The data related to the chemical composition and phenolic components of pomegranate seed pulp (PP) are shown in . The PP had 91.42%, 11.87%, 9.69%, and 29.97% dry matter, crude protein, ether extract, and crude fibre, respectively. The ME value of PP was 2320 kcal/kg; however, this value is very variable with alteration of the CF or EE in PP. The phenolic components of PP are also displayed in . The total phenolic and total tannins of PP were 3.68% and 2.89%, respectively. The amount of hydrolyzable tannins in PP containing gallic acid, tanic acid, ellagic acid, punicallin, punicallagin A, and punicallagin B was 0.42, 0.21, 1.53, 0.1, 0.1, and 0.39, respectively.
Table 2. Composition of starter (1–10 d), grower (11–24 d) and finisher (25–42 d) diets.
Growth performance measurements
As can be seen in , there were no significantly differences among performance traits (feed intake, body weight, and FCR) of birds reared in each of two chambers before applying heat stress. The pomegranate pulp at the levels of 7% and 10% increased the body weight gain as compared to control at 24 d (P < 0.05). Significant interactions were observed in body weight gain, FCR, and PBI at 42 d between heat stress and pomegranate pulp (P < 0.05). The birds’ fed diets contained 4% and 10% PP at TN had higher body weight gain and PBI as compared to birds’ fed diets contained 0% and 4% PP at HS condition. The lower FCR was observed in birds fed 10% PP at TN or HS as compared to heat-stressed control group. Heat stress significantly decreased feed intake compared to TN condition (P < 0.05).
Table 3. Effects of pomegranate pulp on growth production parameters of broilers reared in heat-stressed conditions.
Blood biochemical indices
As depicted in , there were no interactions between PP and HS for serum concentration of cholesterol, triglyceride, HDL, LDL, total protein, and the enzyme activity of LDH and AST. The heat stress also had no significant effects on the blood concentration of cholesterol, triglyceride, LDL, and HDL. The heat stress broilers compared to TN significantly reduced the concentration of total protein. A significant interaction was observed in the serum concentration of uric acid between heat stress and pomegranate pulp (P < 0.05). The birds were fed 4% PP at HS had higher concentration of uric acid compared to other treatments. The PP diets significantly reduced the blood concentration of cholesterol and LDL compared to control diets (P < 0.05). The concentration of HDL, triglyceride, and total protein was not influenced by the levels of PP diets.
Table 4. Effects of pomegranate pulp on some blood parameters of broilers reared in heat-stressed conditions.
The plasma enzyme activity of lactate dehydrogenase was not altered by alterations of temperature or PP levels (). The high ambient temperature significantly increased the enzyme activity of aspartate aminotransferase (P < 0.05).
Antioxidant status
As shown in , significant interactions were observed in the enzyme activities of GPx, and indices of TAC and TBARS between HS and PP (P < 0.05). The enzyme activities of SOD were significantly lower in HS broilers than TN (P < 0.05). Supplementation of 10% PP significantly increased the enzyme activity of SOD throughout the HS period (P < 0.05). The birds fed 10% PP at TN had higher enzyme activity of GPx and total antioxidant capacity (TAC) compared to HS birds and the control group. The lower TBARS values were observed in TN birds compared to HS birds or HS birds fed PP ().
Table 5. Antioxidant status of broilers fed pomegranate pomace reared under thermoneutral or heat-stressed conditions.
Immune system
The data related to titre antibody production against NDV, and titres of total, IgM, and IgG antibodies against SRBCs, and the ratio of heterophil/lymphocyte (H/L) are shown in . There were no significant interactions in immune indices between HS and PP diets. Heat stress significantly reduced the titres of total and IgM antibodies for the secondary response to SRBCs and antibody production against NDV. The heterophil: lymphocyte ratio (H/L) was increased when birds were exposed to HS. The PP diets increased the titres of total and IgM antibodies for the primary and secondary response to SRBCs and antibody production against NDV. The H/L ratio was significantly reduced by decreasing PP levels in diets.
Table 6. Effects of pomegranate pomace on growth production parameters of broilers reared in heat-stressed conditions.
Carcass characteristics
The relative weight of breast, thigh, bursa of fabricius, and spleen were not affected by the interaction between HS and PP (). The TN control group had higher relative weight of abdominal fat and liver as compared to HS control (P < 0.05). Heat stress also did not affect the relative weight of breast, thigh, burs of fabricius, and the spleen of broilers (). The relative weight of bursa and spleen significantly increased when birds’ fed diets contained PP (P < 0.05).
Table 7. Effects of pomegranate pulp (PP) on carcass relative weights (percentages of live weight) of broilers reared in heat-stressed conditions (42 d).
Discussion
The results of the present study indicated that PP had valuable nutrient especially for protein and fat. There are many reports that indicate the chemical compositions of PP, especially for CP and EE, are valuable for animal and poultry (Mirzaei-Aghsaghali et al. Citation2011; Taher-Maddah et al. Citation2012; Khosravi et al. Citation2015; Emami et al. Citation2015, Hosseini-Vashan and Ghaznavi Citation2016). The previous studies have been described that some indices, such as the growing condition, variety and method of processing, can affect the chemical composition of PP (Taher-Maddah et al. Citation2012). Due to the development of oil-extracting factories, the oil percentage of PP is very different and reduced. The metabolisable energy of PP is related to the percentage of EE and CF. The ME value of PP was 2320 kcal/kg; however, this value is very variable with alteration of CF or EE in PP (Hosseini-Vashan and Ghaznavi Citation2016).
The similar data related to total phenolic, total tannins, and hydrolyzable tannins in PP were reported by Emami et al. (Citation2015). However, there are great variations in the percentage of phenolic components of different varieties of PP. In addition, some of other factors, such as growing region, climate, maturity, and cultivar type, also affect the phenolic compounds of PP (Holcroft et al. Citation1998; Heshi et al. Citation2001; Ozkan Citation2002). The phenolic component had antioxidant and antimicrobial activities that could affect the broiler performance and immune response (Mirzaei-Aghsaghali et al. Citation2011; Taher-Maddah et al. Citation2012). Recently, Emami et al. (Citation2015) found that PP increased the total phenolic content and total antioxidant capacity in the plasma, liver and muscle of goat kids.
Luo et al. (Citation2018), Hosseini-Vashan and Ghaznavi (Citation2016) and Geraret et al. (Citation1996) found that HS reduced the body weight and FCR. Chronic heat stress is reduced FI, body weight, and FCR (Mashaly et al. Citation2004; Hosseini-Vashan et al. Citation2012; Habibian et al. Citation2014; Akhavan-Salamat and Ghasemi Citation2016). The weakened of broiler performance in the HS could be attributed to a greater spending of energy for a physiological adaptation to a change in environmental temperature (Renaudeau et al. Citation2012). The lower feed intake and appetite at high ambient temperatures may be due to a crucial defence mechanism to reduce the heat increment and body temperature. HS has a negative effect on illume and cecal microflora, and decreases digestion, absorption, and retention of nutrients (Feng et al., Citation2012), consequential to body weight, feed intake, and PBI. The greater tolerance to HS may also be associated with the genetic make-up of the birds and the reduced size and secretion of the thyroid gland (Abidin and Khatoon Citation2013).
To our knowledge, there is no study that evaluated the effect of PP supplementation on broiler growth performance at high ambient temperature. The addition of PP to broiler diet had no effect on body weight, feed intake and FCR (Hosseini et al. Citation2014). Similar to the present study, supplementation of PP with multi-enzyme has significantly improved the laying hen production traits including feed intake, FCR, egg production and egg quality (Rabet et al. Citation2012). Hamady et al. (Citation2015) reported that pomegranate peel extract improved the broiler performance and carcass efficiency. The phenolic compounds, especially tannins and Ellagic acid, are antioxidant components of PP that can relieve HS' undesirable effects on the performance of birds. The PP had valuable bioactive compounds, such as punicic acid, that may have valuable effects on the performance parameters of broilers.
The AST is usually used as an indicator of hurt to the skeletal, cardiac muscles and to the liver. It was also used as a screening test for hepatocellular damage in heat stress (Hoffmann and Solter Citation2008). The enhanced activities of AST suggest that the HS may have experimented with liver injury. The HS is also developed lipid peroxidation that it may be the cause of liver damage (Abidin and Khatoon Citation2013). The similar findings were reported by previous researchers that enhancing the chronic or cyclic heat stress causes an increase in the activities of AST and ALT in broilers (Habibian et al. Citation2014; Hamady et al. Citation2015; Hosseini-Vashan et al. Citation2015; Luo et al. Citation2018). The HS had no effect the plasma enzyme activity of Lactate dehydrogenase (LDH). LDH is a marker of heart trauma or myocardial injury (Martins et al. Citation1996). The pomegranate pulp had no significant effects on the enzyme activity of AST and LDH. There is no research related to the rearing of broilers fed by PP in HS condition.
High ambient temperature reduced the synthesis of protein, essential amino acids and increased catabolism of protein in the body (Gous Citation2005). Similar to this study, a lower concentration of total protein in heat-stressed broilers was reported (Luo et al. Citation2018). HS instigates the plasma concentration of uric acid. The higher body temperature leads to increased maintenance energy requirements (Hurwitz et al. Citation1980) to keep body temperature around the normal range. Since the lower feed consumption and higher energy requirements are the probable reasons for the mobilization of amino acids for gluconeogenesis (Rezaei and Hajati Citation2010). In the case of poultry, the mobilization of the amino acids leads to increased plasma uric acid as a consequence of the increased liver gluconeogenesis pathway in HS to provide energy demands (Sylvia and Mader Citation1998). The highest plasma concentrations of uric acid in HS birds compared to TN were in agreement with Sun et al. (Citation2015). Pomegranate pulp decreased plasma uric acid concentration of heat-stressed birds. This can be reduced by the mobilization of uric acid in heat-stressed birds.
A higher concentration of cholesterol and triglyceride were reported in birds reared at high ambient temperature (Luo et al. Citation2018). The concentration of cholesterol and LDL decreased when the levels of pomegranate pulp gradually increased. The similar findings were reported by Hosseini-Vashan et al. (Citation2015), and Saki et al. (Citation2014) that the PP reduce blood LDL, HDL and triglyceride of laying hens; however, the serum cholesterol concentrations were increased with the upper levels of pomegranate pulp. In contrast, Gursu et al. (Citation2004) found higher concentrations of cholesterol, triglycerides, and HDL in heat-stressed birds. The antioxidant components in PP have a lowering effect on blood lipids (Emami et al. Citation2015); therefore, the supplementation of PP to the diet may have reduced serum lipid profiles especially for cholesterol and LDL.
Malondialdehyde (MDA) is an index that has been used to evaluate the oxidative damage to membranes and the peroxidation of lipids in the animal and poultry body (Lykkesfeldt Citation2007; Ibrahim et al. Citation2008). This finding is in agreement with Emami et al. (Citation2015) and Devatkal and Naveena (Citation2010) who reported that the PP reduced the MDA concentration of meat. The polyphenols and tannins, especially punicalagin, have a great inhibitory effect on lipid peroxidation and radical scavenging activities (Kulkarni et al. Citation2004; Sreelatha and Padma Citation2009). On the other hand, the heat stress stimulates the peroxidation of lipids in the body and increases the production of free radicals and damages the muscle. Therefore, the pomegranate pulp could be used in broiler production, especially in heat-stressed birds, to improve their quality.
Pomegranate pulp had an improvement influence on humoral antibody response against SRBCs and the relative weight of bursa of fabricius. Polyphenols can improve immune responses in domestic animals, which seems to be through three mechanisms. Firstly, these compounds can enhance the proliferation of beneficial bacteria, while reducing the abundance of pathogenic ones through their bactericidal and bacteriostatic activities, thus indirectly improving host immunity and health (Gessner et al. Citation2017). Secondly, these molecules possess antioxidant capacity and can counteract the free radical generated during the immune responses and heat stress, thereby improving the animal’s immune system (Lipiński et al. Citation2016). Polyphenols are also able to positively regulate the production of cytokines, HSPs, and transcription factors (Lipiński et al. Citation2016). This study suggested that the PP supplementation relieved all of these changes by regulating the enzymatic and nonenzymatic antioxidant systems as well as by modulating the expression of heat shock genes in the immune organs. The stress condition, duration and the severity of heat stress, the bioavailability of phenolic compounds, the breed, and the age of the birds can affect the immune response of birds to dietary polyphenol supplementation (Nelson et al. Citation1995; Renaudeau et al. Citation2012).
The pomegranate pulp has a lowering effect on abdominal fat which could be the result of lower absorption of dietary fat and/or de novo lipogenesis. This effect increases the heat loss capacity of broilers during heat-stressed conditions (Renaudeau et al. Citation2012). Furthermore, the inclusion of PP further reduced the abdominal fat in the HS broilers suggests the delipidating effect of the pomegranate pulp. The PP has an anti-adipogenic effect and exerts this action by inhibiting fat accumulation processes and promoting lipolytic and oxidative pathways (Emami et al. Citation2015). This result is in accordance with the results of Eid et al. (Citation2003), who found that a mixture of catechins extracted from green tea reduced the percentage of abdominal fat in glucocorticoid-treated broilers. Finally, according to this finding, Starčević et al. (Citation2015) demonstrated that the supplementation of tannic acid to broiler diets markedly increased fat percentage in breast and thigh muscles.
Conclusion
HS had detrimental effects on the growth performance, immunity, and antioxidant status of broiler chickens. Supplementation of PP to HS broiler, in particular 10%, reduces the adverse effects of HS on immunity, and antioxidant status and improved blood lipid profiles. It suggests that PP added to heat-stressed-broiler diet more than 7%.
Disclosure statement
No potential conflict of interest was reported by the authors.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Abbasi H , Rezaei K , Rashida L. 2008. Extraction of essential oils from the seeds of pomegranate using organic solvents and supercritical CO2. J Am Oil Chem Soc. 85:83–89. doi: 10.1007/s11746-007-1158-x
- Abidin Z , Khatoon A. 2013. Heat stress in poultry and the beneficial effects of ascorbic acid (vitamin C) supplementation during periods of heat stress. World’s Poult Sci J. 69:135–152. doi: 10.1017/S0043933913000123
- Akhavan-Salamat H , Ghasemi HA. 2016. Alleviation of chronic heat stress in broilers by dietary supplementation of betaine and turmeric rhizome powder: dynamics of performance, leukocyte profile, humoral immunity, and antioxidant status. Trop Anim Health Prod. 48(1):181–188. doi: 10.1007/s11250-015-0941-1
- Amarnameh Ministry of Agriculture-Jahad . 2016. Annual production of agricultural in Iran. Tehran : Ministry of Agriculture-Jahad publisher.
- AOAC . 2005. Official methods of analysis, 18th ed. Washington, DC : Association of Official Analytical Chemists.
- Benzie IFF , Strain JJ. 1996. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal Biochem. 239(1):70–76. doi: 10.1006/abio.1996.0292
- Devatkal SK , Naveena B. 2010. Effect of salt, kinnow and pomegranate fruit by-product powders on color and oxidative stability of raw ground goat meat during refrigerated storage. Meat Sci. 85:306–311. doi: 10.1016/j.meatsci.2010.01.019
- Eid Y , Ohtsuka A , Hayashi K. 2003. Tea polyphenols reduce glucocorticoid-induced growth inhibition and oxidative stress in broiler chickens. Br Poult Sci. 44:127–132. doi: 10.1080/0007166031000085427
- Emami A , Ganjkhanlou M , Nasri MF , Zali A , Rashidi L. 2015. Pomegranate seed pulp as a novel replacement of dietary cereal grains for kids. Small Rum Res. 123:238–245. doi: 10.1016/j.smallrumres.2014.12.001
- Feng Y , Yang XJ , Wang YB , Li WL , Liu Y , Yin RQ , Yao JH. 2012. Effects of immune stress on performance parameters, intestinal enzyme activity and mRNA expression of intestinal transporters in broiler chicken. Asian Australas J Anim Sci. 25:701–707. doi: 10.5713/ajas.2011.11377
- Geraret PA , Padilha JCF , Guillauimn S. 1996. Metabolic and endocrine changes by chronic heat exposure in broiler chickens: growth performance, body composition and energy retention. Br J Nutr. 75:195–204.
- Gessner D , Ringseis R , Eder K. 2017. Potential of plant polyphenols to combat oxidative stress and inflammatory processes in farm animals. J Anim Physiol Anim Nutr. 101(4):605–628. doi: 10.1111/jpn.12579
- Gous RM. 2005. Nutritional interventions in alleviating the efficacy of high temperature in broiler production. World’s Poult Sci J. 48:175–180.
- Gross WB , Siegel HS. 1983. Evaluation of the heterophil/lymphocyte ratio as a measure of stress in chickens. Avian Dis. 27(4):972–979. doi: 10.2307/1590198
- Gursu MF , Onderci M , Gulcu F , Sahin K. 2004. Effects of vitamin C and folic acid supplementation on serum paraoxonase activity and metabolites induced by heat stress in vivo. Nutr Res. 24:157–164. doi: 10.1016/j.nutres.2003.11.008
- Habibian M , Ghazi S , Moeini MM , Abdolmohammadi A. 2014. Effect s of dietary selenium and vitamin E on immune response and biological blood parameters of broilers reared under thermoneutral or heat stress conditions. Int J Biometeorol. 58:741–752. doi: 10.1007/s00484-013-0654-y
- Halliwell B , Gutteridge JMC. 1989. Free radicals in biology and medicine, 2nd ed. Oxford : Clarendon.
- Halvorsen BL , Holte K , Myhrstad MC , Barikmo I , Hvattum E , Remberg SF , Wold AN , Haffner K , Andersen LF. 2002. A systematic screening of total antioxidants in dietary plants. J Nutr. 132(3):461–471. doi: 10.1093/jn/132.3.461
- Hamady GAA , Abdel-Moneim MA , El-Chaghaby GA , Abd-El-Ghany Z M , Hassanin MS. 2015. Effect of pomegranate peel extract as natural growth promoter on the productive performance and intestinal microbiota of broiler chickens. Afr J Agr Sci Tech. 3(12):514–519.
- Heshi A , Garande V , Wagh A , Katore H. 2001. Effect of pre-harvest sprays of chemicals on the quality of pomegranate fruit (Punica granatum L.). Cv. G-137. Agr Sci Digest. 21(1):25–27.
- Hoffmann WE , Solter PF. 2008. Diagnostic enzymology of domestic animals. In: Kaneko JJ , Harvey JW , Bruss M.L. , editor. Clinical biochemistry of domestic animals. Massachusetts : Academic Press; p. 351–378.
- Holcroft DM , Gil MI , Kader AA. 1998. Effect of carbon dioxide on anthocyanins, phenylalanine ammonia lyase and glucosyltransferase in the arils of stored pomegranates. J Am Soc Hortic Sci. 123(1):136–140. doi: 10.21273/JASHS.123.1.136
- Hosseini-Vashan SJ , Ghaznavi T. 2016. Determination metabolizable energy content and nutritive value of pomegranate peel and pulp, using adult cockerels. J Anim Prod. 18(3):513–524.
- Hosseini-Vashan S , Golian A , Yaghobfar A. 2015. Growth, immune, antioxidant, and bone responses of heat stress-exposed broilers fed diets supplemented with tomato pomace. Inter J Biometerol. 60:1183–1192. doi: 10.1007/s00484-015-1112-9
- Hosseini-Vashan S , Golian A , Yaghobfar A , Zarban A , Afzali N , Esmaeilinasab P. 2012. Antioxidant status, immune system, blood metabolites and carcass characteristic of broiler chickens fed turmeric rhizome powder under heat stress. Afr J Biotechnol. 11(94):16118–16125. doi: 10.5897/AJB12.1986
- Hosseini SM , Amoli M , Modaresi SJ. 2014. Effect of different pomegranate seed pulp levels on performance traits and some blood parameters of broilers. Anim Prod Res. 3(2):29–38.
- Hurwitz S , Weiselberg M , Eisner M , Bartov I , Riesenfeld G , Sharvit M , Niv A , Bornstein S. 1980. The energy requirements and performance of growing chickens and turkeys as affected by environmental temperature. Poult Sci. 59:2209–2299.
- Ibrahim WH , Habib HM , Chow CK , Bruckner GG. 2008. Isoflavone-rich soy isolate reduces lipid peroxidation in mouse liver. Int J Vitam Nutr Res. 78:217–222. doi: 10.1024/0300-9831.78.45.217
- Khan SA. 2009. The role of pomegranate (Punica granatum L.) in colon cancer. Pak J Pharm Sci. 22:346–348.
- Khosravi F , Fathi Nasri MH , Farhangfar H , Modaresi SJ. 2015. Nutritive value and polyphenol content of pomegranate seed pulp ensiled with different tannin-inactivating agents. Anim Feed Sci Tech. 207:262–266. doi: 10.1016/j.anifeedsci.2015.06.004
- Kulkarni AP , Aradhya SM , Divakar S. 2004. Isolation and identification of a radical scavenging antioxidant – punicalagin from pithand carpellary membrane of pomegranate fruit. Food Chem. 87:551–557. doi: 10.1016/j.foodchem.2004.01.006
- Lara JJ , Rostagno MH. 2013. Impact of heat stress on poultry production. Anim. 3:356–369. doi: 10.3390/ani3020356
- Lin H , Decuypere E , Buyse J. 2006. Acute heat stress induces oxidative stress in broiler chickens. Comp Biochem Physiol A Mol Integr Physiol. 144(1):11–17. doi: 10.1016/j.cbpa.2006.01.032
- Lipiński K , Mazur M , Antoszkiewicz Z , Purwin C. 2016. Polyphenols in monogastric nutrition-a review. Ann Anim Sci. 17(1):41–58. doi: 10.1515/aoas-2016-0042
- Louba BN. 2007. What are the medical properties of pomegranates? J Chin Clin Med. 2:530–538.
- Luo J , Song J , Liu L , Xue B , Tian G , Yang Y. 2018. Effect of epigallocatechin gallate on growth performance and serum biochemical metabolites in heat-stressed broilers. Poul Sci. 97(2):599–606. doi: 10.3382/ps/pex353
- Lykkesfeldt J. 2007. Malondialdehyde as biomarker of oxidative damageto lipids caused by smoking. Clin Chim Acta. 380:50–58. doi: 10.1016/j.cca.2007.01.028
- Makkar HP , Blümmel M , Borowy NK , Becker K. 1993. Gravimetric determination of tannins and their correlations with chemical and protein precipitation methods. J Sci Food Agr. 61(2):161–165. doi: 10.1002/jsfa.2740610205
- Marcu A , Vacaru-Opriş I , Dumitrescu G , Ciochina LP , Marcu A , Nicula M , Peţ I , Dronca D , Kelciov B. 2013. The influence of genetics on economic efficiency of broiler chickens growth. Anim Sci Biotech. 46(2):339–346.
- Martins JT , Li DJ , Baskin LBJ , Keffer JH. 1996. Comparison of cardiac troponin I and lactate dehydrogenase isoenzymes for the late diagnosis of myocardial injury. Am J Clin Path. 106(6):705–708. doi: 10.1093/ajcp/106.6.705
- Mashaly MM , Hendricks GL , Kalama MA , Gehad AE , Abbas AO , Patterson PH. 2004. Effect of heat stress on production parameters and immune responses of commercial laying hens. Poult Sci. 83:889–894. doi: 10.1093/ps/83.6.889
- Mirzaei-Aghsaghali A , Maheri-Sis N , Mansouri H , Razeghi ME , Mirza-Aghazadeh A , Cheraghi H , Aghajanzadeh-Golshani A. 2011. Evaluating potential nutritive value of pomegranate processing by-products for ruminants using in vitro gas production technique. J Agr Biol Sci. 6(6):45–51.
- Mujahid A , Sato K , Akiba Y , Toyomizu M. 2006. Acute heat stress stimulates mitochondrial superoxide production in broiler skeletal muscle, possibly via downregulation of uncoupling protein content. Poult Sci. 85:1259–1265. doi: 10.1093/ps/85.7.1259
- Nelson N , Lakshmanan N , Lamont S. 1995. Sheep red blood cell and Brucella abortus antibody responses in chickens selected for multitrait immunocompetence. Poult Sci. 74:1603–1609. doi: 10.3382/ps.0741603
- Ozkan M. 2002. Degradation of anthocyanins in sour cherry and pomegranate juices by hydrogen peroxide in the presence of added ascorbic acid. Food Chem. 78(4):499–504. doi: 10.1016/S0308-8146(02)00165-6
- Perai AH , Kermanshahi H , Nassiri Moghaddam H , Zarban A. 2015. Effects of chromium and chromium+ vitamin C combination on metabolic, oxidative, and fear responses of broilers transported under summer conditions. Inter J Biometeorol. 59:453–462. doi: 10.1007/s00484-014-0860-2
- Persia ME , Parsons CM , Schang M , Azcona J. 2003. Nutritional evaluation of dried tomato seeds. Poult Sci. 82(1):141–146. doi: 10.1093/ps/82.1.141
- Prakash CVS , Prakash I. 2011. Bioactive chemical constituents from pomegranate (Punica granatum) juice, seed and peel- A Review. Inter J Res Chem Environ. 1:1–18.
- Rabet M , Saki AA , Zamani P , Vatankhah AM , Mirzaei S. 2012. Performance traits of laying hens response to pomegranate pulp and multi-enzyme. The Conference of Science and Applied in Using of Agricultural, Urbans and Industries in Ration of Animal, Poultry and Aquatic, Iran, Tabriz, University of Tabriz, 19.
- Renaudeau D , Collin A , Yahav S , De Basilio V , Gourdine J , Collier RJ. 2012. Adaptation to hot climate and strategies to alleviate heat stress in livestock production. Animal. 6:707–728. doi: 10.1017/S1751731111002448
- Rezaei M , Hajati H. 2010. Effect of diet dilution at early age on performance, carcass characteristics and blood parameters of broiler chicks. Ital J Anim Sci. 9(1):e19–100. doi: 10.4081/ijas.2010.e19
- Sahin K , Orhan C , Smith MO , Sahin N. 2013. Molecular targets of dietary phytochemicals for alleviation of heat stress in poultry. World’s Poult Sci J. 69:113–124. doi: 10.1017/S004393391300010X
- Sahin K , Orhan C , Tuzcu M , Sahin N , Hayirli A , Bilgili S , Kucuk O. 2016. Lycopene activates antioxidant enzymes and nuclear transcription factor systems in heat-stressed broilers. Poult Sci. 95:1088–1095. doi: 10.3382/ps/pew012
- Saki AA , Rabet M , Zamani P , Yosefi Y. 2014. The effect of different levels of pomegranate seed pulp with multi-enzyme on performance, egg quality and serum antioxidant in laying hens. Iran J Appl Anim Sci. 4(4):803–808.
- Shabtay A , Eitam H , Tadmor Y , Orlov A , Meir A , Weinberg P , Weinberg ZG , Chen Y , Brosh A , Izhaki I. 2008. Nutritive and antioxidative potential of fresh and stored pomegranate industrial byproduct as a novel beef cattle feed. J Agr Food Chem. 56(21):10063–10070. doi: 10.1021/jf8016095
- Sreelatha S , Padma P. 2009. Antioxidant activity and total phenolic con-tent of Moringa oleifera leaves in two stages of maturity. Plant Food Hum Nutr. 64:303–311. doi: 10.1007/s11130-009-0141-0
- Starčević K , Krstulović L , Brozić D , Maurić M , Stojević Z , Mikulec Ž , Bajić M , Mašek T. 2015. Production performance, meat composition and oxidative susceptibility in broiler chicken fed with different phenolic compounds. J Sci Food Agr. 95:1172–1178. doi: 10.1002/jsfa.6805
- Sun X , Zhang H , Sheikhahmadi A , Wang Y , Jiao H , Lin H , Song Z. 2015. Effects of heat stress on the gene expression of nutrient transporters in the jejunum of broiler chickens (Gallus gallus domesticus). Inter J Biometeorol. 59(2):127–135. doi: 10.1007/s00484-014-0829-1
- Sylvia S , Mader W. 1998. Biology, 6th edn. New York : McGraw-Hill. p. 185.
- Taher-Maddah M , Maheri-Sis N , Salamatdoustnobar R , Ahmadzadeh A. 2012. Estimating fermentation characteristics and nutritive value of ensiled and dried pomegranate seeds for ruminants using in vitro gas production technique. Open Vet J. 2:40–45.
- Wang H , Gao XD , Zhou GC , Cai L , Yao WB. 2008. In vitro and in vivo antioxidant activity of aqueous extract from Choerospondias axillaris fruit. Food Chem. 106:888–895. doi: 10.1016/j.foodchem.2007.05.068
- Yoshioka T , Kawada K , Shimada T , Mori M. 1979. Lipid peroxidation in maternal and cord blood and protective mechanism against activated-oxygen toxicity in the blood. Am J Obstet Gynec. 135:372–376. doi: 10.1016/0002-9378(79)90708-7
