ABSTRACT
This study was carried out to compare Day 0 and traditional protocols for in vivo embryo production. Twenty-two crossbred ewes were used as embryo donors and assigned to two groups. Ewes from Traditional protocol were submitted to estrus synchronization with CIDR for 14 days and superovulation treatment was induced 60 h before the withdrawal of CIDR with 240 mg pFSH. Donors from Day 0 protocol were subjected to a short-term protocol of estrus synchronization (6 days) and superovulation treatment was the same to the other group, but it started 84 h after CIDR withdrawal. Embryo recoveries were performed using the transcervical method. All embryo donors (100%) presented estrus regardless of the hormone protocol used. The treatments did not affect the ovarian response, as well as quality and viability of embryos (P > 0.05). Treatments had a small dispersion of estrus, 81.81% (9/11) animals from the Traditional protocol group presented estrus 20 h after CIDR withdrawal and 90.9% (10/11) of the Day 0 protocol group presented estrus 24 h. Regarding embryo production, there was no significant difference between hormonal treatments for the different parameters evaluated (P > 0.05). It can be concluded that the Day 0 protocol was not different from the Traditional protocol in crossbred ewes.
Introduction
The MOET (multiple ovulation and embryo transfer) technique is an important tool as it accelerates and produces greater precision in the animal selection process (Brasil et al. Citation2016). Currently, it is known that the ovarian response to superovulatory treatments is related to the ovarian follicular status at the start of the treatment with follicle-stimulating hormone (FSH).
The Day 0 (Day 0 = day of ovulation) protocol is an effective and easy approach to starting treatment with FSH during the early follicular emergence because it involves a pre-synchronization of estrus and ovulation, therefore, ensuring small follicles emergence. Thus, the Day 0 protocol is initiated simultaneously with a new follicular wave emergence, which gets acceptable superovulatory responses (Menchaca et al. Citation2007). However, the Day 0 protocol efficiency has contradictory results in scarce studies (Menchaca et al. Citation2007; Menchaca et al. Citation2009b; Lehloenya and Greyling Citation2010). In this context, this study aimed to compare the Day 0 and Traditional protocols on the production and quality of embryos from crossbred (Dorper vs. Santa Ines) ewes in semiarid, sub medium region of São Francisco valley.
Material and methods
Animal care
This study was conducted in accordance with the bioethical rules and guidelines applicable to studies involving animals set forth by the Animal Ethics Committee (or the Ethics Committee on the use of animals in research) in Brazil.
Experimental animals
Twenty-two crossbred (Dorper vs. Santa Ines) ewes were used, with 36.0 ± 4.5 months old and weight 41.7 ± 2.3 kg, which came from Division of Animal Production in Bahia State University (UNEB) located in Juazeiro, State of Bahia, at 9°24’42’’S and 40°29’55’’W. The animals were selected after ultrasonography and used as embryo donors. They were not previously treated with hormones. During the day, ewes were managed in an area of Tanzania grass (Panicum maximum cv. Tanzania), and they were housed in a covered pen at night. The animals also had free access to water, pasture and mineral supplement; the diet met the nutritional requirements in accordance with NRC (Citation2007).
Two Dorper rams were used as sires of proven fertility and a Santa Ines ram was used as a teaser. Rams remained in a covered pen during all time and received Napier grass, chopped hay and a mixture of concentrate feed, the diet met the nutritional requirements in accordance with NRC (Citation2007).
Hormonal treatments and mating
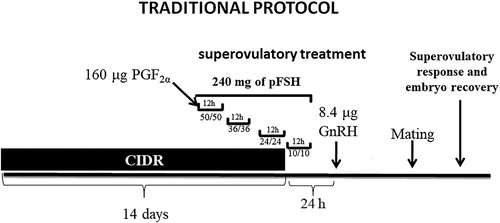
Traditional protocol (n = 11 donors) was composed to estrus synchronization with CIDR (EASI – BREED CIDR®, Pfizer, Brazil) for 14 days. The superovulatory treatment with pFSH (Folltropin V®, Vetrepharm, Canada) has started 60 h before CIDR withdrawal. The donors received 240 mg pFSH via intramuscular divided into eight decreasing doses (50/50; 36/36; 24/24; 10/10 mg), every 12 h. Simultaneous to the first application of pFSH, 160 µg of a PGF2α analogue (cloprostenol) (Ciosin®, Schering – Plough, Brazil) was also administered by intramuscular. In addition, 24 h after CIDR withdrawal, it was administered 8.4 mg of a GnRH analogue (buserelin acetate) (Sincrofort®, Ourofino, Brazil) (). Estrus detection in embryo donors was initiated after 12 h post-CIDR withdraw, with a Santa Inês ram. After confirmation of estrus, females were subjected twice to natural mating. The first was at estrus onset and another 24 h later with Dorper ram.
Figure 1. Traditional protocol schematic, 14 days with CIDR®, applications of PGF2α, superovulatory treatment with 240 mg of pFSH, divided into eight decreasing doses with 12 h intervals, 12 h after CIDR withdrawal, it was administered 8.4 mg of a GnRH.
Embryo donors (n = 11) were allocated to the Day 0 protocol, initially, they were subjected to estrus and ovulation synchronization. This treatment was performed using a short-term protocol (six days) using CIDR. By the intramuscular way, 160 µg cloprostenol was administered at the time of CIDR insertion and 300 IU equine chorionic gonadotropin (eCG) (Novormon®, Intervet / Schering – Plough, Brazil) was administered simultaneously with CIDR withdrawal. The donors received (36 h after CIDR withdrawal) 8.4 mg buserelin acetate (). Estrus behaviour was examined as described for the animals undergoing Traditional protocol, but this examination started 84 h after CIDR withdrawal.
Figure 2. Day 0 protocol schematic, 6 days with CIDR®, applications of PGF2α, superovulatory treatment with 240 mg of pFSH, divided into eight decreasing doses with 12 h intervals, started 84 h after CIDR withdrawal, two times of 8.4 mg of GnRH. *transrectal ultrasound exam.
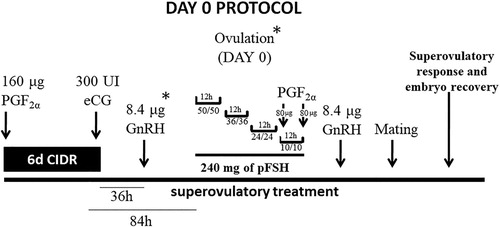
To confirm the exact ovulation time, two transrectal ultrasound exams were performed, one during the administration of GnRH analogue and another at the beginning of the superovulatory treatment with pFSH. Ovulation was confirmed by identification of the largest ovarian follicle in the first ultrasound evaluation 36 h after CIDR withdrawal, and its disappearance following transrectal ultrasound exam. The pFSH was administered as in the Traditional protocol, but it started 84 h after CIDR withdrawal. Two doses of 80 µg cloprostenol were administered simultaneously with the last two pFSH applications. Estrus detection in embryo donors restarted along with the last dose application of pFSH and mating was performed as previously reported. To synchronize the LH peak and ovulation, 8.4 mg buserelin acetate was administered 12 h after the last pFSH application.
Superovulatory response and embryo recovery
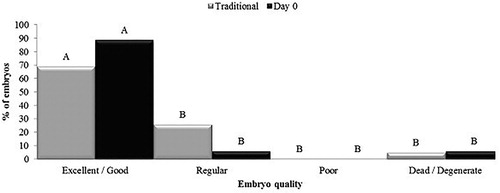
On the fifth post-mating day, all ewes were fasted for feed (24 h) and water (12 h). In addition, ewes were subjected to a laparoscopy respecting the order of estrus onset six days after mating and immediately before embryo collection to verify the ovulatory response. All animals underwent transcervical embryo collection for embryo recoveries. The recovered lavage fluid was poured into plastic Petri dishes (Petri dish 100®, Nutricell, Brazil) and submitted to search and identification of structures. It was used a stereomicroscope (SMZ 645®, Nikon, Japan) using 40–70X magnification for embryo assessment and the development stage and quality following morphological criteria of the International Embryo Transfer Society (Robertson and Nelson Citation2010), using embryo Code I (excellent or good), II (regular), III (poor) and IV (dead or degenerated). Rate of transferable embryos was considered as number of embryos of grade I, II and III / number of embryos collected. Freezability rate was calculated by number of embryos of grade I and II / number of embryos collected.
Statistical analysis
Results were expressed as the mean ± standard error. Analysis of variance was applied for comparison of various parameters between the protocols. Means were compared using Tukey’s test and were considered significantly different when P < 0.05. Data expressed as percentages were subjected to the Fisher and Chi-square test. Data that were not distributed normally were subjected to the Kruskal–Wallis test. ASSISTAT Version 7.6 beta (2013) was used for the calculations.
Results
All embryo donors (100%) presented estrus regardless of the hormone protocol used. Regarding the interval between CIDR withdrawal and estrus onset, the values found for Traditional and Day 0 protocols were 20.73 ± 3.09 and 25.82 ± 1.74 h, respectively (P > 0.05). Treatments had a small dispersion of estrus, 81.81% (9/11) animals from the Traditional protocol group presented estrus 20 h after CIDR withdrawal and 90.9% (10/11) of the Day 0 protocol group presented estrus 24 h.
In relation to ultrasound exams performed in the Day 0 protocol, the first exam indicated that only 9% (1/11) animals exhibited pre-ovulatory follicles (POF) (larger than 6 mm) and the second exam showed small follicles (smaller than 4 mm) in 27% (3/11) animals.
Concerning ovarian response after superovulatory treatment, there was no significant difference between the protocols (). Both superovulatory treatments were efficient (mean ± SEM) with 17.55 ± 3.48 and 14.00 ± 2.63 corpora lutea, respectively. However, in the Traditional protocol, 18.2% (2/11) did not express to superovulatory treatment.
Table 1. Number of recovered structures, total number of embryos, transferable and freezable embryos (mean ± SEM), fertilization, transferable embryos and freezability rates of crossbred (Dorper vs. Santa Ines) ewes according to the hormonal protocol used (Traditional vs. Day 0).
It is necessary to report the persistent presence of POF in two animals of the Traditional protocol (one POF in each animal) and in four of the Day 0 protocol group (13 POF; nine POF in only one animal). In addition, one animal (Traditional protocol) presented three follicular cysts.
Regarding embryo production, there was no significant difference between hormonal treatments for the different parameters evaluated (P > 0.05) (). The hormone protocol did not interfere (P > 0.05) with embryo quality (). In both treatments, embryos were not collected in grade III (), which resulted in equality in rate of transferable embryos and freezability rate ().
Discussion
Percentage of ewes presenting estrus in the Day 0 protocol was higher than reported by Lehloenya and Greyling (Lehloenya and Greyling Citation2010), who also used Day 0 protocol in sheep and achieved 71.4% estrus percentage. However, our data agree with Brasil et al. (Citation2016), who worked with CIDR for 14 days in hair sheep (Santa Ines breed) and also achieved 100% females in estrus. In addition, our results are consistent with Menchaca et al. (Citation2007), who compared the Day 0 and Traditional protocols in sheep and obtained 100% and 95.5% females in estrus after Traditional and Day 0 protocols, respectively.
In this study, the time interval between CIDR withdrawal and estrus onset was earlier than observed by Lehloenya and Greyling (Citation2010), after the application of Day 0 protocol (36.4 ± 0.5 h) or Traditional protocol with (33.0 ± 1.0 h) and without PGF2α (28.6 ± 0.5 h), respectively. This shorter time interval can be associated with GnRH used, because it is an efficient synchronization agent of estrus and ovulation (Menchaca et al. Citation2009a).
Day 0 protocol presented a low follicular recruitment at the onset of superovulation treatment. This was indicated by the low percentage of animals with small follicles (27%) at the time of pFSH administration, observed in the second ultrasound. Menchaca et al. (Citation2007, Citation2009a, Citation2009b) explained that ovulation should be confirmed and before the onset of superovulation treatment. The ovary showed the large follicles absence (>4 mm) probably by the small follicles in the ovary, which promoted a higher follicular recruitment with a homogeneous pool of growing small follicles.
Brasil et al. (Citation2016) suggested that a longer exposure time to progesterone associated with GnRH increased the fertilization rate. According to these authors, the greater fertilization is due to the greater synchronization of ovulation. Thus, the Traditional protocol had fewer females responding to the superovulatory treatment, 81% (9/11). However, the number of embryos produced was not different between the treatments (P > 0.05). It could be assumed that synchronization with longer exposure time to CIDR can be inhibited by the presence of a large follicle that can suppress the ovarian response.
Another very important factor is the timing of the first follicular wave that may vary in different breeds (Brasil et al. Citation2016) and can be influenced by local factors, becoming unpredictable due to individual variation (Menchaca et al. Citation2009a). According to the results obtained in the first ultrasound (when we expected to observe POF in the ovaries), there were only 9% animals exhibiting POF, i.e. an indication that in crossbred Dorper and Santa Ines ewes, reared in semiarid Northeastern Brazil, the predetermined time for the onset of superovulatory treatment occurred early.
Lehloenya and Greyling (Citation2010), also testing the Day 0 protocol, found inferior results of Day 0 protocol when compared to the Traditional protocol, attributing it to the earlier initiation of treatment with pFSH. However, these authors report that eCG was not administered, which is very important for concentration and anticipation of ovulation.
The occurrence of POF and follicular cysts at the time of embryo collection may have been the result of ovarian overstimulation caused by the dose of pFSH (240 mg). Some studies suggest that by reducing the pFSH dose could show the same result of superovulatory treatment (Loiola Filho et al. Citation2015) and maybe without overstimulation. This dose was administered in animals that were at latitude 9°24’4’’S, i.e. where there is no dominance of photoperiod sexual activity and thus, the animals are naturally receptive to breeding.
Simonetti et al. (Citation2008) reported that the presence of anovulatory large follicles together with their greater individual hormonal production can produce high estradiol levels. These high concentrations can affect the uterine environment and thus interfere with oocytes capture by the fimbriae or the transport of female and male gametes through the female genital tract (Menchaca et al. Citation2009a).
Superovulation protocols use high and decreasing doses of pFSH because the follicles are more responsive to pFSH when they are small, as they grow the response decreases (Menchaca et al. Citation2009a). This higher stimulation may be related to a fast and abnormal follicular development, thus impairing the synchronization of oocyte-follicle growth resulting in decreasing embryo quality (Simonetti et al. Citation2008), but in this experiment, there was no prejudice in embryos quality. This is consistent with the pattern of an association between greater ovarian stimulation and a decrease in oocyte or embryo production (Menchaca et al. Citation2009a). Moreover, this decline has been regularly observed in animals treated with high doses of gonadotropin, suggesting that it may be related to excessive follicle stimulation or very high circulating estrogen levels during the early luteal phase.
Conclusion
Both protocols presented satisfactory results in relation to estrus concentration, in vivo embryo production and quality from crossbred Dorper × Santa Ines ewes, wherein the Day 0 protocol did not differ than Traditional protocol. However, the effect of the time of superovulation onset in Day 0 protocol and the pFSH dose require further studies in different sheep breeds.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Brasil OO , Moreira NH , Santos G , Silva BDM , Mariante AS , Ramos AF. 2016. Superovulatory and embryo yielding in sheep using increased exposure time to progesterone associated with a GnRH agonist. Small Rum Res. 136:54–58. doi: 10.1016/j.smallrumres.2016.01.005
- Lehloenya KC , Greyling JPC. 2010. The ovarian response and embryo recovery rate in Boer goat does following different superovulation protocols, during the breeding season. Small Rum Res. 88:38–43. doi: 10.1016/j.smallrumres.2009.11.007
- Loiola Filho JB , Do Monte APO , Souza T , Miranda M , Magalhães LC , Barros CHSC , Silva A , Santos AO , Guimarães A , Costa J , et al. 2015. Effect of pFSH dose reduction on in vivo embryo production in Dorper ewes. Semina: Ci Agrarias. 36(2):4215–4224. doi:10.5433/1679-0359.2015v36n6Supl2p4215.
- Menchaca A , Vilariño M , Crispo M , De Castro T , Rubianes E. 2009a. New approaches to superovulation and embryo transfer in small ruminants. Reprod Fert Develop. 22:113–118. doi: 10.1071/RD09222
- Menchaca A , Vilariño M , Crispo M , Pinczak A , Rubianes E. 2007. Day 0 protocol: superstimulatory treatment initiated in the absence of a large follicle improves ovarian response and embryo yield in goats. Theriogenology. 68:1111–1117. doi: 10.1016/j.theriogenology.2007.07.020
- Menchaca A , Vilariño M , Pinczak A , Kmaid S , Saldaña JM. 2009b. Progesterone treatment, FSH plus eCG, GnRH administration, and Day 0 protocol for MOET programs in sheep. Theriogenology. 72:477–483. doi: 10.1016/j.theriogenology.2009.04.002
- NRC . 2007. Nutrient requirements of small ruminants: sheep, goats, cervids and new world camelids. Washington, DC : Natl. Acad. Press.
- Robertson I , Nelson R. 2010. Certification and identification of embryos. In: Stringfellow DA , Givens MD , editors. Proceedings of guide of International Embryo Transfer Society. 4th ed. Champaign, Illinois, USA : IETS; p. 86–105.
- Simonetti L , Forcada F , Rivera OE , Carou N , Alberio RH , Abecia JA , Palacin I. 2008. Simplified superovulatory treatments in Corriedale ewes. Anim Reprod Sci. 104:227–237. doi: 10.1016/j.anireprosci.2007.01.020