ABSTRACT
The objective of this study was to evaluate the serum protein pattern and selected acute phase proteins in dairy cows with chronic diarrhoea associated with seropositivity to Mycobacterium avium subsp. paratuberculosis (MAP). Forty-four dairy cows with chronic diarrhoea and MAP-seropositive and 19 clinically healthy MAP-seronegative cows were included in the study. The concentrations of total protein (TP), protein fractions and selected acute phase proteins – serum amyloid A (SAA), haptoglobin (Hp) and C-reactive protein (CRP) were measured in blood serum. In cows with diarrhoea the mean values of TP, albumin and the albumin/globulin ratio were significantly lower (P < 0.001), the relative concentrations of α1-, β1- and γ-globulins were significantly (P < 0.001) and α2- and β2-globulins were non-significantly higher. The electrophoretic pattern of serum proteins showed β-γ bridging in 32 from 44 diseased cows. The concentrations of SAA and Hp were non-significantly higher and CRP significantly lower (P < 0.001) in cows with diarrhoea. Presented results indicate a marked effect of chronic diarrhoea in MAP-seropositive cows on the protein metabolism suggesting possible diagnostic significance of some biomarkers from the protein profile in the evaluation of the severity of the disease and changes caused by this protein-losing enteropathy.
1. Introduction
Chronic diarrhoea associated with malabsorption is less frequently documented in older or adult cattle than in calves, but represents a great health problem on the affected farms causing high economic losses due to decreased milk production, higher risk of early culling, as well as decreased slaughter value (Tiwari et al. Citation2007; Smith et al. Citation2009; Vázquez et al. Citation2012; Wittek Citation2016). Besides nutritional factors, there are several pathogens that may cause chronic diarrhoea in adult cattle. Paratuberculosis, or Johne’s disease, is one of the most important intestinal chronic progressive granulomatous infections of ruminants with major economic impact (Groenendaal and Galligan Citation2003; Pillars et al. Citation2009). According to the results presented by Chi et al. (Citation2002), the economic losses in dairy herds caused by paratuberculosis may be higher than those for other diseases, including bovine viral diarrhoea, enzootic bovine leukosis and neosporosis. The disease is caused by Mycobacterium avium subspecies paratuberculosis (MAP) through oral infection from forage, water, milk or environment contaminated with faeces containing bacteria shed by the infected animals (Chiodini et al. Citation1984; Sweeney et al. Citation1992). The infection develops into granulomatous lymphadenitis, thickening and oedema of the intestinal mucosa resulting in intermittent treatment-resistant diarrhoea (Sweeney Citation1996; Clarke Citation1997). Consequently, the intestinal absorption of nutrients decreases resulting in malabsorption associated with protein-losing enteropathy, and in decreased concentrations of blood proteins in the advanced stages of the disease (Bendixen Citation1978; Tiwari et al. Citation2006). On the other hand, the infection with MAP is accompanied by immune and inflammatory reactions of the body, manifested also by the activation of macrophages, release of tumor necrosis factor alpha (TNF-α) and other cytokines, as well as by increased production of acute phase proteins (Buza et al. Citation2003; Coussens Citation2004). However, the mechanism of these reactions is not yet completely understood. Similarly, little is known about the effect of chronic diarrhoea in cows seropositive for MAP-antibodies (MAP-Ab) on the distribution of serum proteins. Therefore, the study aimed at evaluating the protein profile and assessing the changes of electrophoretic pattern of serum proteins and the concentrations of some acute phase proteins in dairy cows suffering from chronic diarrhoea and seropositive for MAP-antibodies.
2. Material and methods
2.1. Animals and blood sample collection
Blood samples from forty-four dairy cows affected with chronic diarrhoea persisting for more than two weeks were included in this study. The sampled animals were of a low land black-spotted breed, Slovak spotted breed and their crossbreeds at the age of 3.5–8 years, and were from four conventional dairy farms with similar feeding and management regimes, and in the previous period with occurrence of clinical signs and laboratory confirmation of Mycobacterium avium subspecies paratuberculosis (MAP) infection in the herds. The evaluated diseased cows were positive for MAP-antibodies. All of these animals despite normal appetite showed obvious clinical signs of the disease, characterized by persistent diarrhoea, reduced milk yield, loss of body weight, and general muscle wasting. The body condition score (BCS) in all of these cows was lower than 2.5 (Ferguson et al. Citation1994). Out of these sick and MAP-positive animals, nineteen clinically healthy and MAP-negative cows without any signs of diseases and in good general condition and BCS >3.0 were selected as control animals.
Blood samples were taken from these animals by private veterinarians on the farms from v. jugularis into serum gel separator tubes without any additives or anticoagulants (Meus, Piove di Sacco, Italy). After transportation to the laboratory of the Clinic of Ruminants of the University of Veterinary Medicine and Pharmacy in Košice (Slovak Republic), all blood samples were immediately centrifuged at 3000 g for 20 min. The harvested serum was dispensed into plastic tubes, and stored at −20°C until it was analysed.
2.2. Laboratory analyses
Immunoenzymatic test for specific detection of anti-Mycobacterium avium subsp. paratuberculosis antibodies in ruminant serum was used for specific detection of anti-MAP antibodies in blood serum (PrioCHECK Ruminats MAP Ab Serum Plate Kit, Prionics Lelystad, Lelystad, The Netherlands). To assess the changes in the protein profile, serum samples were analysed for the concentration of total protein, main protein fractions, and selected specific inflammatory proteins. The total protein (TP, g/l) were assessed using an automated biochemical analyser Alizé (Lisabio, Poully en Auxois, France) according to the biuret method with commercially available diagnostic kits (Randox, Crumlin, United Kingdom). The serum protein fractions were separated by zone electrophoresis on an agarose gel using an automated electrophoresis system Hydrasys (Sebia Corporate, Lisses, Evry Cedex, France) with commercial diagnostic kits Hydragel 7 Proteine (Sebia Corporate, Lisses, Evry Cedex, France) according to the procedure described by the manufacturer. The densitometry scanning system Epson Perfection V700 (Epson America Inc., CA, USA) was used to scan the electrophoretic gels based on the method of light transmission and conversion into an optical density curve. The gel images were visualized using the computer software Phoresis version 5.50 (Sebia Corporate, Lisses, Evry Cedex, France). The following protein fractions were identified: albumin, α1- and α2-globulins, β1- and β2-globulins, and γ-globulins. Each protein fraction was expressed as relative amount (%) according to the obtained optical density. Consequently, their absolute concentrations (g/l) were calculated from the total serum protein concentrations. The ratios of albumin to globulins (A/G) were calculated also.
Among inflammatory acute phase proteins, the serum concentrations of serum amyloid A (SAA, μg/ml), haptoglobin (Hp, mg/ml) and C-reactive protein (CRP, μg/ml) were evaluated. SAA was assessed by sandwich enzyme-linked immunosorbent assay (ELISA) using commercial multispecies kit (Tridelta Development, Kildare, Ireland). Haptoglobin was determined according to its biochemical activity to bind haemoglobin using commercial colorimetric kit (Tridelta Development, Kildare, Ireland) in microplates. CRP was measured by solid-phase ELISA assay using commercially available test (Life Diagnostics, Inc., West Chester, PA, USA). The absorbancies were read on automatic microplate reader Opsys MR (Dynex Technologies, Chantilly, VA, USA). The results were calculated using the computer software Revelation QuickLink version 4.25 (Dynex Technologies, Chantilly, VA, USA).
2.3. Statistical analyses
The statistical analyses were done with the programme GraphPad Prism V5.02 (GraphPad Software Inc., CA, USA). Descriptive statistical procedures were used to calculate arithmetic means (x) and standard deviations (SD) for each evaluated variable and group of animals. The distribution of data was evaluated by Kolmogorov–Smirnov Test for normality. All parameters showed normal distribution. The significance of differences in values between cows with chronic diarrhoea and healthy animals was examined by unpaired t-test.
3. Results
The data obtained from healthy and diseased cows are presented in –. Representative examples of the electrophoretic pattern of serum proteins in a cow with chronic diarrhoea and in a clinically healthy cow are presented in .
Figure 1. (a,b) Representative electrophoretograms in cows: (a) healthy cow, (b) MAP-positive cow with chronic diarrhoea.
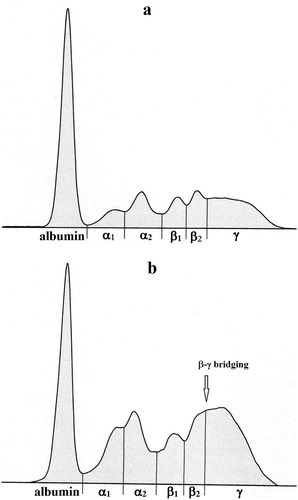
Table 1. Differences in the relative concentrations of serum protein fractions (%) and albumin/globulin ratio (A/G) between cows with chronic diarrhoea and clinically healthy cows (mean ± SD).
Table 2. Differences in the concentrations of total serum proteins (TP, g/l) and absolute values of protein fractions (g/l) between cows with chronic diarrhoea and clinically healthy cows (mean ± SD).
Table 3. Differences in the concentrations of SAA, Hp and CRP between cows with chronic diarrhoea and clinically healthy cows (mean ± SD).
The relative values of albumin were significantly lower in diarrhoeic cows compared with healthy ones (P < 0.001; ). An opposite trend was observed in the relative concentrations of α-globulins. The α1-globulins were significantly (P < 0.001) and the α2-globulins non-significantly higher in sick cows than in healthy animals. Similarly, the diarrhoeic cows were found to have significantly higher relative concentrations of β1-globulins (P < 0.001) and non-significantly higher β2-globulins compared with healthy animals. The relative concentrations of γ-globulins showed a tendency of significantly higher values in cows with chronic diarrhoea (P < 0.001). Similarly, the mean value of A/G ratios was significantly lower in sick cows compared with clinically healthy animals (P < 0.001). Furthermore, while no β-γ bridging was found in clinically healthy cows, the electrophoretic pattern of serum proteins showed β-γ bridging in 32 from 44 cows with chronic diarrhoea ((b)). The serum of cows with chronic diarrhoea had significantly lower concentrations of total protein than clinically healthy animals (P < 0.001; ). Significantly lower values of the absolute concentrations of albumin were found in diarrhoeic cows as well (P < 0.001). While the absolute concentrations of α1-globulins were significantly higher in cows suffering from diarrhoea (P < 0.001), the absolute concentrations of α2-globulins were significantly higher in healthy animals (P < 0.05). No differences in means were observed in the concentrations of β1- and β2-globulins between sick and healthy cows. The absolute concentrations of γ-globulins were significantly higher in cows with diarrhoea (P < 0.01).
The evaluation of the concentrations of SAA and Hp showed differences in means between the analysed groups of cows with a tendency of non-significantly higher mean values in cows with diarrhoea. On the other hand, the mean concentrations of CRP were significantly higher in healthy animals compared with diseased cows (P < 0.001; ).
4. Discussion and conclusion
The concentration of serum proteins reflects the balance between their synthesis, metabolism and loss. Protein-losing enteropathies are characterized by increased loss of proteins into the gastrointestinal tract compared to their synthesis (Braamskamp et al. Citation2010). The results of our study showed in cows with chronic diarrhoea associated with seropositivity to MAP infection lower concentrations of total serum proteins, which was more marked in high MAP-positive animals. Lower total protein concentrations in MAP-positive dairy cows compared with MAP-negative animals were observed also in German Holstein dairy herds and in young bulls with paratuberculosis (Szilágyi et al. Citation1989; Körmendy et al. Citation1990; Donat et al. Citation2014). According to Raizman et al. (Citation2007), hypoproteinemia may be found not only in cows with severe clinical signs of paratuberculosis and high shedder animals, but also in moderate or low shedders, although to a lower extent. Furthermore, they expected that the values of total protein in the blood serum may be reduced prior to the appearance of clinical signs. Seeing that paratuberculosis is accompanied by enteropathy and malabsorption syndrome, the loss of essential metabolites, including proteins, through the gastrointestinal mucosa might lead to decreased total serum protein concentrations observed also in our study (Levitt and Levitt Citation2017). In the early or subclinical stages of the disease, the body tries to compensate the protein loss by increasing protein synthesis or degradation of muscle proteins to assure the synthesis of functional, as well as milk proteins in cows (Donat et al. Citation2014; Copland and DiBaise Citation2017). Furthermore, the serum concentrations of some rapid turnover proteins, including prealbumin, may remain relatively unchanged despite severe hypoproteinemia (Takeda et al. Citation2003). On the other hand, low serum concentrations may be found in case of slower turnover proteins, e.g. albumin, ceruloplasmin, or transferrin (Schmidt et al. Citation1995). Consequently, when the body is not able to sufficiently compensate the loss of blood proteins and maintain milk protein synthesis, the ongoing muscle degradation may result in wasting weight loss in the affected animals (Patterson and Berret Citation1968). In the study conducted by Donat et al. (Citation2014), a decrease in total protein concentrations was observed in relation to the MAP status and increase in bacteria shedding. Similar pattern was seen in our study, when we in MAP-positive dairy cows found lower total protein values compared to clinically healthy animals.
Although protein-losing enteropathies are of non-selective nature characterized by the loss of all kinds of blood proteins independently of the molecular weight, the main focus is on albumin (Dossin and Lavoue Citation2011; Levitt and Levitt Citation2017). Albumin is one of the main slow turnover proteins with a relatively long half-life of about 14–16 days in ruminants, and even slow leaks may increase its clearance from the body and thus reduce its serum concentrations (Prinsen and de Sain-van der Velden Citation2004; Levitt and Levitt Citation2016). The liver is then not able to fully compensate for sustained losses resulting in moderate or severe hypoalbuminemia. Significant and marked decrease of albumin concentrations were also obtained in our study in cows affected by chronic diarrhoea. Similarly, Osterstock et al. (Citation2005) recorded very low albumin values in a bull with protothecal infection which is very similar to paratuberculosis. Protein-losing enteropathy associated with various gastrointestinal disorders was characterized by marked hypoalbuminemia also in dogs due to its excessive loss through the gastrointestinal mucosa (Dossin and Lavoue Citation2011). Furthermore, Craven et al. (Citation2004) and Allenspach et al. (Citation2007) reported that low concentrations of albumin may suggest poor prognosis in dogs with chronic enteritis. Similarly, Mair et al. (Citation1993) found lower albumin values in horses with chronic diarrhoea that died than in those that survived. These findings indicate that the concentrations of albumin may reflect the magnitude of changes and predict adverse clinical outcome. Vincent et al. (Citation2003) suggested that low concentrations of albumin in the blood serum serve as an important prognostic indicator that is associated with increased mortality and morbidity.
The effect of chronic diarrhoea associated with chronic intestinal infection and malabsorption syndrome on the concentrations of further serum proteins is up to now less well documented. Copland and DiBaise (Citation2017) stated that the reduction of serum proteins other than albumin seldom causes clinically significant problems. However, Constable et al. (Citation2017) reported that enteropathies with a loss of proteins are characterized by initial changes in the plasma albumin concentrations, but panhypoproteinemia with changes also in the other protein concentrations ensues as the disease progresses. Seth et al. (Citation2009) identified transthyretin (prealbumin) and retinol-binding protein as significant biomarkers related to MAP infections in cattle. Transthyretin belongs to the group of negative acute phase proteins with tendency to decrease in conditions related to inflammation, tissue injury, malnutrition, changes in protein-energy status (Ingenbleek and Young Citation2002). Thus, it might be an important biomarker to monitor the progression of paratuberculosis and protein wasting in the affected animals (Seth et al. Citation2009). On the other hand, retinol-binding protein plays important roles in the vitamin A based host response to MAP due to the stimulation of monocyte differentiation and inhibition of the multiplication of mycobacteria in macrophages (Crowle and Ross Citation1989). In addition, some other proteins associated with the activation of host immune response were observed in cattle during mycobacterial infections. One of them identified in bovine tuberculosis, as well as paratuberculosis was vitamin D binding protein (vitamin DBP), involved in the transportation of vitamin D, modulation of inflammatory responses and consequently upregulation of cathelicidin that has antimicrobial activity (Yamamoto and Naraparaju Citation1996; Speeckaert et al. Citation2014). Merhan et al. (Citation2017) found an increase in the concentrations of haptoglobin (by 26%) and serum amyloid A (by 37%) in cattle infected with Mycobacterium bovis. Furthermore, they determined a fair prognosis for animals with Hp values between 0.1 and 1.0 g/l. The aforementioned proteins belong to the group of so-called positive acute phase proteins, thus, their increase might be related to the tissue damage caused by the bacteria. Increased, thought insignificant mean Hp and SAA concentrations were also observed in our study in cows seropositive for MAP-antibodies clinically manifested by chronic diarrhoea due to inflammatory changes in the intestinal wall. While the mean concentrations of SAA were approximately 1.7-fold higher in diarrhoeic cows compared with healthy ones, the mean values of Hp were about 2.9-fold higher in diseased animals. These differences in the rate of increase may be related to differences in the reactivity among several acute phase proteins, since Hp is characterized by more prolonged response. Thus, haptoglobin may be preferable in the field to evaluate disease processes, especially the course of chronic diseases (Petersen et al. Citation2004). However, the wider range of measured concentrations and higher values of standard deviations suggest that there are great differences in the reactivity of animals to respond to various inflammatory stimuli. Different disease severity might be another reason for wider range of values, i.e. more severe disease processes are accompanied by higher concentrations of acute phase proteins (Lomborg et al. Citation2008). Elevated concentrations of SAA were found also in patients with inflammatory bowel disease associated with active gut inflammation and in mice exposed to Mycobacterium avium subsp. paratuberculosis manifested with colitis and weight loss (De Beer et al. Citation1982; Singh et al. Citation2007). The majority of acute phase proteins belongs to the α-globulin fraction, thus their increased production caused by granulomatous intestinal inflammatory processes might result in increases of the fraction of α-globulins (both α1- and α2-globulin fraction) observed in our study in cows with chronic diarrhoea. Higher α2-globulin concentrations (probably due to elevated Hp values) were found by Mair et al. (Citation1993) in horses with chronic diarrhoea that died compared with those that survived, which reflect the degree of inflammation. Gurau et al. (Citation2016) observed in children with protein losing enteropathy slightly elevated α1- and α2-globulins reflecting the response of the body to the damage of intestinal mucosa.
A tendency of higher relative values in cows with chronic diarrhoea was observed also in the β1- and β2-globulin fractions. Similarly, higher concentrations of β1-globulins were found in horses with chronic diarrhoea caused by larval cyathostomiasis and in children affected by protein-losing enteropathy (Mair et al. Citation1993; Gurau et al. Citation2016). Some diagnostically important proteins were identified in the β-globulin region, including complement, transferrin, ferritin, as well as β2-microglobulin or β-lipoproteins (Bernabucci et al. Citation2009). These proteins are involved in the regulation of inflammatory processes and, thus, may be attributed to the marked elevation of β-globulins due to inflammation and damage of mucosal surface in cows suffering from chronic diarrhoea. Furthermore, as part of the response to different antigenic stimulation, some immunoglobulins (mainly IgM or IgA) may migrate into the β region or β-γ interzone and produce a beta-gamma fusion with no clear valley between these fractions so that they are indistinguishable (Morris and Johnston Citation2002). Yang et al. (Citation2017) observed in patients with severe malabsorption due to immunoproliferative small intestinal disease high concentrations of IgA in the serum, but with no monoclonal immunoglobulin bands on the protein electrophoretogram. This pattern was observable also in our study in thirty-two from forty-four cows with chronic diarrhoea. Evans and Duncan (Citation2003) stated that the so-called β-γ bridging is pathognomic for chronic liver diseases, especially portal cirrhosis of the liver. Presented results suggest that β-γ fusion may be observed also in cows with chronic diarrhoea associated with seropositivity to MAP-infection, and this finding was not yet described.
C-reactive protein is another protein that belongs to the β-globulin fraction. In ruminants, it is a constitutively synthesized protein, with only a minor increase during disease processes (Eckersall Citation2008). In the present study, the concentrations of CRP were lower in cows with chronic diarrhoea compared with clinically healthy animals. Ostrow et al. (Citation2006) recorded in patients with abnormal enteric protein loss elevated concentrations of CRP, but there was no correlation with protein loss. Higher serum CRP values were found also in dogs with protein-losing enteropathy being not predictive of survival time (Equilino et al. Citation2015). The behaviour of CRP in cattle affected by chronic diarrhoea associated with protein-losing enteropathy was not yet described. One of the reasons for lower concentrations of CRP in cows with chronic diarrhoea may be its excessive loss through the gastrointestinal mucosa, similarly to typically elevate fecal loss of α1-antitrypsin in human patients, as well as in dogs suffering from protein-losing enteropathy (Strygler et al. Citation1990; Murphy et al. Citation2003). However, further studies are needed to evaluate the usefulness of CRP in the differential diagnosis of chronic diarrhoea in cattle associated with various degree of hypoproteinemia.
According to Braamskamp et al. (Citation2010), more severe cases of protein-losing enteropathies may be associated with reduced concentrations of γ-globulins. However, Willard (Citation2015) observed in dogs with protein-losing enteropathy that initially had high globulin concentrations the loss of over half of their blood proteins, but consequently had globulin concentrations in the reference range. Gurau et al. (Citation2016) found in children with protein-losing enteropathy slightly elevated concentrations of γ-globulins, but cases with severe malnutrition may be accompanied by hypogammaglobulinemia due to the loss of immunoglobulins through the gastrointestinal mucosa. Similarly to our results obtained in cows, Bonelli et al. (Citation2017) and Schroeder et al. (Citation2001) found higher values of γ-globulins in goats positive for paratuberculosis, suggesting chronic inflammatory processes and severe infection. The above-mentioned changes in the concentrations of albumin and globulin fractions resulted also in alterations in the A/G ratio. The values recorded in cows with chronic diarrhoea were significantly lower compared with clinically healthy animals. These low A/G values are consistent with protein-losing enteropathy in paratuberculosis infections, caused by excessive loss of albumin or the overproduction of globulins (Osterstock et al. Citation2005).
In conclusion, because the pathogenesis of Mycobacterium avium subsp. paratuberculosis infection in cows and clinical manifestation of chronic diarrhoea with subsequent protein-losing enteropathy has so far lacked knowledge of their impact on the changes of serum protein fractions and acute phase protein values, the results of the presented study represent significant widening of the knowledge in this area of research. They suggest a significant effect of chronic diarrhoea in cows seropositive for paratuberculosis on the protein metabolism characterized by alterations in the electrophoretic pattern of serum proteins, as well as changes in the concentrations of the evaluated acute phase proteins. The values of total serum protein, albumin and A/G ratio were lower in sick cows compared with healthy animals, while the concentrations of α1-, α2-, β1-, β2- and γ-globulins were higher. Differences in the values were obtained also for haptoglobin and serum amyloid A. The results suggest possible diagnostic significance of some biomarkers from the protein profile in the evaluation of the severity of the disease and the magnitude of changes. However, further investigations are needed to establish the diagnostic accuracy of serum protein electrophoresis in the differential diagnosis of chronic diarrhoea in cattle.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Allenspach K, Wieland B, Grone A, Gachen F. 2007. Chronic enteropathies in dogs: evaluation of risk factors for negative outcome. J Vet Intern Med. 21:700–708. doi: 10.1111/j.1939-1676.2007.tb03011.x
- Bendixen PH. 1978. Immunological reactions caused by infection with Mycobacterium paratuberculosis. A review. Nord Vet Med. 30:63–168.
- Bernabucci U, Lacetera N, Danieli PP, Bani P. 2009. Influence of different periods of exposure to hot environment on rumen function and diet digestibility in sheep. Int J Biometeorol. 53:387–395. doi: 10.1007/s00484-009-0223-6
- Bonelli F, Fratini F, Turchl B, Cantile C, Ebani VV, Colombani G, Galiero A, Sgorbini M. 2017. Evaluation of clinical pathology parameters in fecal PCR-positive or PCR-negative goats for Johne’s disease. Trop Anim Health Pro. 49:1489–1493. doi: 10.1007/s11250-017-1351-3
- Braamskamp MJAM, Dolman KM, Tabbers MM. 2010. Protein-losing enteropathy in children. Eur J Pediatr. 169:1179–1185. doi: 10.1007/s00431-010-1235-2
- Buza JJ, Mori Y, Bari AM, Hikono H, Aodon-Geril HH, Hirayama S, Shu Y, Momotani E. 2003. Mycobacterium avium subsp. paratuberculosis infection causes suppression of RANTES, monocyte chemoattractant protein 1, and tumor necrosis alpha expression in peripheral blood of experimentally infected cattle. Infect Immun. 71:7223–7227. doi: 10.1128/IAI.71.12.7223-7227.2003
- Chi J, VanLeeuwen J, Weersink A, Keefe G. 2002. Direct production losses and treatment costs from bovine viral diarrhoea virus, bovine leukosis virus, Mycobacterium avium subspecies paratuberculosis, and Neospora caninum. Prev Vet Med. 55:137–153. doi: 10.1016/S0167-5877(02)00094-6
- Chiodini RJ, Van Kruiningen HJ, Merkal RS. 1984. Ruminant paratuberculosis (Johne’s disease): The current status and future prospects. Cornell Vet. 74:218–262.
- Clarke CJ. 1997. The pathology and pathogenesis of paratuberculosis in ruminants and other species. J Comp Pathol. 116:217–261. doi: 10.1016/S0021-9975(97)80001-1
- Constable PD, Hinchcliff KW, Done SH, Grünsberg W. 2017. Veterinary medicine: A textbook of the diseases of cattle, horses, sheep, pigs, and goats. 11th ed. St. Louis, MO: Elsevier. p. 1090.
- Copland AP, DiBaise JK. 2017. Protein losing enteropathy: diagnosis and management. Pract Gastroenterol, Nutr Issues Gastroenterol, Ser. 162:22–35.
- Coussens PM. 2004. Model for immune responses to Mycobacterium avium subsp. paratuberculosis in cattle. Infect Immun. 72:3089–3096. doi: 10.1128/IAI.72.6.3089-3096.2004
- Craven M, Simpson JW, Ridyard AE, Chandler ML. 2004. Canine inflammatory bowel disease: Retrospective analysis of diagnosis and outcome in 80 cases (1995–2002). J Small Anim Pract. 45:336–342. doi: 10.1111/j.1748-5827.2004.tb00245.x
- Crowle AJ, Ross EJ. 1989. Inhibition of retinoic acid of multiplication of virulent tubercle bacilli in cultured human macrophages. Infect Immun. 57:840–844. doi: 10.1128/IAI.57.3.840-844.1989
- De Beer FC, Mallya RK, Fagan EA, Lanham JG, Hughes GR, Pepys MB. 1982. Serum amyloid-A protein concentration in inflammatory diseases and its relationship to the incidence of reactive systemic amyloidosis. Lancet. 31:231–234. doi: 10.1016/S0140-6736(82)90321-X
- Donat K, Erhardt G, Soschinka A, Brandt HR. 2014. Decreased serum protein associated with Mycobacterium avium subspecies paratuberculosis shedding in German Holstein cows. Vet Rec. 174:408–413. doi: 10.1136/vr.101957
- Dossin O, Lavoue R. 2011. Protein-losing enteropathies in dogs. Vet Clin N Am-Small. 41:399–418. doi: 10.1016/j.cvsm.2011.02.002
- Eckersall PD. 2008. Proteins, proteomics, and the dysproteinemias. In: Kaneko JJ, Harvey JW, Bruss ML, editors. Clinical biochemistry of domestic animals. 6th ed. San Diego, CA: Academic Press; p. 117–155.
- Equilino M, Théodoloz V, Gorgas D, Doherr MG, Heilmann RM, Suchodolski JS, Steiner JM, Burgener IA. 2015. Evaluation of serum biochemical marker concentrations and survival time in dogs with protein-losing enteropathy. J Am Vet Med Assoc. 246:91–99. doi: 10.2460/javma.246.1.91
- Evans EW, Duncan JR. 2003. Proteins, lipids, and carbohydrates. In: Latimer KW, Mahaffey EA, Prasse KW, editors. Duncan & Prasse’s veterinary laboratory medicine clinical pathology. 4th ed. Ames: Blackwell; p. 162–192.
- Ferguson JD, Galligan DT, Thomsen N. 1994. Perincipal descriptors of body condition score in Holstein cows. J Dairy Sci. 77:2695–2703. doi: 10.3168/jds.S0022-0302(94)77212-X
- Groenendaal H, Galligan DT. 2003. Economic consequences of control programs for paratuberculosis in midsize dairy farms in the United States. J Am Vet Med Assoc. 23:1757–1763. doi: 10.2460/javma.2003.223.1757
- Gurau G, Earar K, Coman M, Dinu CA, Busila C, Voicu DC, Macovei LA, Calin AM, Arbune M. 2016. The electrophoretic patterns of serum proteins in children. Rev Chim-Bucharest. 67:190–194.
- Ingenbleek Y, Young VR. 2002. Significance of prealbumin in protein metabolism. Clin Chem Lab Med. 40:1281–1291.
- Körmendy B, Szilágyi M, Suri A, Tuboly S, Nagy G. 1990. Some diagnostic features of the pathogenesis of bovine paratuberculosis (Johne’s disease) and serum biochemical changes after oral reinfection. Zbl Veterinärmed B. 37:229–235.
- Levitt DG, Levitt MD. 2016. Human serum albumin homeostasis: a new look at the roles of synthesis, catabolism, renal and gastrointestinal excretion, and the clinical value of serum albumin measurements. Int J Gen Med. 9:229–255. doi: 10.2147/IJGM.S102819
- Levitt DG, Levitt MD. 2017. Protein losing enteropathy: comprehensive review of the mechanistic association with clinical and subclinical disease states. Clin Exp Gastroenterol. 10:147–168. doi: 10.2147/CEG.S136803
- Lomborg SR, Nielsen LR, Heegaard PMH, Jacobsen S. 2008. Acute phase proteins in cattle after exposure to complex stress. Vet Res Commun. 32:575–582. doi: 10.1007/s11259-008-9057-7
- Mair TS, Cripps PJ, Ricketts SW. 1993. Diagnostic and prognostic value of serum protein electrophoresis in horses with chronic diarrhoea. Equine Vet J. 25:324–326. doi: 10.1111/j.2042-3306.1993.tb02973.x
- Merhan O, Bozukluhan K, Çelebi Ö, Öğün M, Atakişi E, Büyük F. 2017. Levels of acute phase protein and some biochemical parameter in cattle infected with Mycobacterium bovis. Vet Fak Derg. 14:101–105.
- Morris D, Johnston JK. 2002. Alterations in blood proteins. In: Smith BP, editor. Large animal internal medicine. 3rd ed. St. Louis: Mosby; p. 426–433.
- Murphy KF, German AJ, Ruaux CG, Steiner JM, Williams DA, Hall EJ. 2003. Fecal alpha-1 protease inhibitor concentration in dogs with chronic gastrointestinal disease. Vet Clin Pathol. 32:67–72. doi: 10.1111/j.1939-165X.2003.tb00316.x
- Osterstock JB, Mansell JL, Roussel AJ. 2005. Protothecal enteritis as a cause of protein-losing enteropathy in a bull. J Am Vet Med Assoc. 227:1476–1479. doi: 10.2460/javma.2005.227.1476
- Ostrow AM, Freeze H, Rychik J. 2006. Protein-losing enteropathy after fontan operation: investigations into possible pathophysiologic mechanisms. Ann Thorac Surg. 82:695–700. doi: 10.1016/j.athoracsur.2006.02.048
- Patterson DSP, Berret S. 1968. Malabsorption in Johne’s disease of cattle: depressed in vitro amino-acid uptake by isolated intestinal tissue preparations. Vet Rec. 81:51–56.
- Petersen HH, Nielsen JP, Heegaard PMH. 2004. Application of acute phase protein measurements in veterinary clinical chemistry. Vet Res. 35:163–187. doi: 10.1051/vetres:2004002
- Pillars RB, Grooms DL, Wolf CA, Kaneene JB. 2009. Economic evaluation of Johne’s disease control programs implemented on six Michigan dairy farms. Prev Vet Med. 90:223–232. doi: 10.1016/j.prevetmed.2009.04.009
- Prinsen BHCM, de Sain-van der Velden MG. 2004. Albumin turnover: experimental approach and its application in health and renal diseases. Clin Chim Acta. 347:1–14. doi: 10.1016/j.cccn.2004.04.005
- Raizman EA, Wells SJ, Godden SM, Fetrow JP, Oakes MJ. 2007. The association between culling due to clinical Johne’s disease or the detection of Mycobacterium avium subsp. paratuberculosis fecal shedding and the diagnose of clinical or subclinical disease in two dairy herds in Minnesota, USA. Prev Vet Med. 78:166–178. doi: 10.1016/j.prevetmed.2007.02.005
- Schmidt PN, Blirup-Jensen S, Svendsen PJ, Wandall JH. 1995. Characterization and quantification of plasma proteins excreted in faeces from healthy humans. Scand J Clin Lab Invest. 55:35–45. doi: 10.3109/00365519509075376
- Schroeder C, Seeliger F, Gaede W, Westermeier G, Ganter M. 2001. Diagnosis, epidemiology, signs and pathology of paratuberculosis in a goat herd in Germany. Tierartzl Prax. 29:27–34.
- Seth M, Lamont EA, Janagama HK, Widdel A, Vulchanova L, Stabel JR, Waters WR, Palmer MV, Sreevatsan S. 2009. Biomarker discovery in subclinical mycobacterial infections of cattle. PLoS ONE. 4:e5478. doi: 10.1371/journal.pone.0005478
- Singh UP, Singh S, Singh R, Karls RK, Quinn FD, Potter ME, Lillard Jr JW. 2007. Influence of Mycobacterium avium subsp. paratuberculosis on colitis development and specific immune responses during disease. Infect Immun. 75:3722–3728. doi: 10.1128/IAI.01770-06
- Smith RL, Grohn YT, Pradhan AK, Whitlock RH, Van Kessel JS, Smith JM, Wolfgang DR, Schukken YH. 2009. A longitudinal study on the impact of John’s disease status on milk production in individual cows. J Dairy Sci. 92:2653–2661. doi: 10.3168/jds.2008-1832
- Speeckaert MM, Speeckaert R, van Geel N, Delanghe JR. 2014. Vitamin D binding protein: a multifunctional protein of clinical importance. Adv Clin Chem. 63:1–57. doi: 10.1016/B978-0-12-800094-6.00001-7
- Strygler B, Nicar MJ, Santangelo WC, Porter JL, Fordtran JS. 1990. Alpha 1-antitrypsin excretion in stool in normal subjects and in patients with gastrointestinal disorders. Gastroenterology. 99:1380–1387. doi: 10.1016/0016-5085(90)91165-3
- Sweeney RW. 1996. Transmission of paratuberculosis. Vet Clin N Am-Food A. 12:305–312. doi: 10.1016/S0749-0720(15)30408-4
- Sweeney RW, Whitlock RH, Rosenburg AE. 1992. Mycobacterium paratuberculosis isolated from fetuses of infected cows not manifesting signs of disease. Am J Vet Res. 53:477–480.
- Szilágyi M, Koermendy B, Suri A, Tuboly S, Nagy G. 1989. Experimental paratuberculosis (Johne’s disease)-studies on biochemical parameters in cattle. Arch Exp Vet Med. 43:463–470.
- Takeda H, Ishihama K, Fukui T, Fujishima S, Orii T, Nakazawa Y, Kawata S. 2003. Significance of rapid turnover proteins in protein-losing gastroenteropathy. Hepatogastroenterology. 50:1963–1965.
- Tiwari A, Van Leeuwen JA, Dohoo IR, Keefe GP, Haddad JP, Tremblay R, Scott HM, Whiting T. 2007. Production effects of pathogens causing bovine leukosis, bovine viral diarrhea, paratuberculosis, and neosporosis. J Dairy Sci. 90:659–669. doi: 10.3168/jds.S0022-0302(07)71548-5
- Tiwari A, Van Leeuwen JA, Mc Kenna LB, Keefe GP, Barkema HW. 2006. Johne’s disease in Canada part I: clinical symptoms, pathophysiology, diagnosis, and prevalence in dairy herds. Can Vet J. 47:874–882.
- Vázquez P, Garrido JM, Juste RA. 2012. Effects of paratuberculosis on Friesian cattle carcass weight and age at culling. Span J Agric Res. 10:662–670. doi: 10.5424/sjar/2012103-2728
- Vincent JL, Dubois MJ, Navickis RJ, Wilkes MM. 2003. Hypoalbuminemia in acute illness: is there a rationale for intervention? A meta-analysis of cohort studies and controlled trials. Ann Surg. 237:319–334.
- Willard M. 2015. Canine protein losing enteropathies. Isrl J Vet Med. 70:17–20.
- Wittek T. 2016. Overview of malassimilation syndromes in large animals. In: Aiello SE, Moses MA, editors. The Merck veterinary manual. 11th ed. Kenilworth: Merck; p. 353–360.
- Yamamoto N, Naraparaju VR. 1996. Role of vitamin D3-binding in activation of mouse macrophages. J Immunol. 157:1744–1749.
- Yang J, Chen S, Chen L, Ouyang M, Li F. 2017. Chronic diarrhea associated with high serum level of immunoglobulin A and diffuse infiltration of plasma cell in small intestine. Med (Baltimore). 96:5. (e6057).
