ABSTRACT
This study was carried out to compare the analgesic effects and haematobiochemical changes produced by the administration of lidocaine, detomidine and lidocaine–detomidine combination in the epidural space of goats. All goats were assigned to all of the three different epidural treatments using a balanced crossover design with two-weeks washout period. Nociception was investigated by pinprick; times to the onset and duration of anti-nociception in the perineal region were demonstrated. Incoordination, ataxia and analgesic effects were carefully observed and recorded according to the scoring system. The blood samples for haematological and biochemical changes were collected at 0, 30, 60, 90 and 120 min post-treatment. Our results showed that detomidine treatment produced a highly significant (P < 0.01) and (P < 0.0001) earlier onset time than lidocaine and lidocaine–detomidine combination, respectively. Detomidine treatment had a significantly longer duration of analgesia than lidocaine (P < 0.01) and lidocaine–detomidine combination (P < 0.0001) treatments. Moreover, all treatments showed no effect on haematological or biochemical parameters, with no obvious systemic effects. In conclusion, administering a detomidine provided a longer duration of analgesia and a useful level of systemic sedation in goats.
1. Introduction
Regurgitation, ruminal tympany and excessive salivation are the main complications of general anaesthesia in the digestive system of small ruminants which needs to be considered (Hall et al. Citation2001). Thus, local anaesthesia in standing position is preferred in surgical interventions in ruminants (Skarda Citation2007). Epidural injection is commonly used in small ruminant surgeries (Skarda Citation2007), obstetrical operations and vaginal and uterine prolapses (Hanie Citation2006; Wachida and Kisani Citation2011) as well. Epidural injection is a simple and effective technique used to manage pain during surgeries involving the perineal regions without producing digestive system complications. Lidocaine, a local anaesthetic drug, is the most common drug used for epidural injection. It acts by modifying the fast voltage-gated sodium channels at the neuronal cell membrane leads to the blocking of signal conduction (Catterall Citation2002). Therefore, this action is not specific to the sensory tracts, but it blocks motor and sympathetic fibres causing hind limb weakness and occasionally recumbency (Hall et al. Citation2001; Skarda Citation2007).
One of the drawbacks of local analgesic drugs is a short duration of action which is not appropriate in long-duration surgical operations. Consequently, anaethetics re-administration is essential to conduct surgical or obstetrical procedures that require long-time (Hall et al. Citation2001; Skarda Citation2007). However, larger volumes produce an increased risk of motor paralysis to the hind limbs (Moulvi et al. Citation2011a). Therefore, local anaesthetics can be used in combination with other analgesic or anaesthetic agents such as xylazine or ketamine, respectively (Mpanduji et al. Citation2000; Dehkordi et al. Citation2012; Re et al. Citation2016; Shokry and Elkasapy Citation2018). Alpha-2 adrenergic agonist medications such as detomidine and medetomidine are commonly used to induce longer and adequate analgesia. These medications act by stimulating their specific receptors in the dorsal horn of the spinal cord (Singh et al. Citation2009). Recently, the administration of medetomidine at different doses has been reported to induce analgesic effect, sedation and immobilization in various animal species (Buck et al. Citation2017). Detomidine is used for horses and other animals to provide sedation, analgesia and muscle relaxation to facilitate examination and surgical procedures (Lawrence et al. Citation1997). Moreover, opioids and alpha-2 adrenergic agonists can be used alone or in combination with lidocaine for sufficient analgesia (Atiba et al. Citation2015).
Ruminants have remarkable alpha-2 adrenergic receptors, which make them sensitive to the sedative effects of their agonists. Several researchers have been considered medetomidine and detomidine as sedative and analgesic agents (Lemke Citation2004; Singh et al. Citation2009). Hence, the administration of medetomidine in epidural space of buffalo can produce the whole analgesia of the tail, perineum, inguinal region and upper parts of the hind limbs (Singh et al. Citation2005). In addition, some adverse effects of anaesthetic and/or analgesic agents on haematological and biochemical parameters were previously reported; intramuscular injection of detomidine in combination with atropine decreased the packed cell volume (PCV) in goats (Dilipkumar et al. Citation1997), and similar results were reported in horses (Wagner et al. Citation1991). The effect of detomidine infusion on total serum protein (TSP) was also demonstrated in horses (Daunt et al. Citation1993). However, the haematobiochemical changes after epidural injection of detomidine or combination with lidocaine in goats are still not fully covered.
The aim of this study was to compare the analgesic effects and haematobiochemical changes produced by the administration of detomidine or lidocaine–detomidine combination with that produced by lidocaine administration in the epidural space in goats.
2. Materials and methods
This study was conducted according to the Animal Ethics Committee of the South Valley University for Veterinary Research, Qena, Egypt. Five clinically healthy goats of 12.20 ± 1.70 months-old (±SD) and 25.20 ± 7.90 kg body weight (±SD) from the College of Veterinary Medicine Animal Farm, South Valley University were used. All goats were maintained under uniform feeding and management conditions during the whole period of the study. Each animal fasted from food for 24 h and water was withheld 12 h before the experiment.
2.1. Experimental design
Three different treatments were applied to all animals by a balanced crossover design; all goats received injection with two-weeks washout period. For the epidural injection, the lumbosacral area was clipped and scrubbed with povidone iodine (The Nile Co. for Pharmaceuticals and Chemical Industries). Local infiltration with 1 mL 2% lidocaine (Sigma-Tec pharmaceutical Indust.) was performed to prevent animal movement during injection. Sterile 18- gauge, 8 cm-long spinal needle (Braun Melsungen Co., Germany) was inserted into the epidural space at the interspace between the last lumbar and first sacral vertebrae after penetrating the ligamentum flavum. Then, the needle was inserted at an angle of about 45° to the skin surface and guided anteriorly and ventrally to locate the site appropriately. Accurate siting of the needle into the extradural space was confirmed by the loss of resistance to the injection and absence of any fluid or blood on aspiration.
The first group (A) was given 2% lidocaine hydrochloride 1 mL/7 kg (2.86 mg/kg body weight), the second group (B) was given 20 µg/kg detomidine (Zoetis) epidurally and the third group (C) was given a combination of lidocaine and detomidine (1.43 mg/kg of lidocaine incombination with 10 µg/kg of detomidine). The volume was standardized with sterile saline solution up 3 mL.
Time from injection to loss of sensation was judged as the time of onset and the time from loss of sensation till reappearance of the response was judged as the duration of action. Scoring system of incoordination of hind limbs, posture, response to pinprick and desensitized area are shown in and were scored at 0, 5, 10, 15, 30, 45, 60 and 90 mins. For animals where the analgesia lasted more than 90 mins, further evaluation was performed every 30 mins until recovery. Analgesia was outlined as a response to pinprick as manifested by an avoidance response to pricking the surface of the skin. Pinprick test using an 18-gauge needle was applied first in the perineal area and then shifted cranially toward the thoracic region until a response was detected (). The observer evaluating analgesia was unaware of the different treatments. Heart rate (HR) and respiration rate (RR) were measured by thoracic auscultation using a stethoscope, and rectal temperature (RT) was monitored using a digital thermometer at the same time points.
Table 1. Incoordination, posture and pin prick test scoring between groups.
2.2. Haematobiochemical parameters
Blood samples (5 mL; 2 mL-sample for haematological analysis and 3 mL-sample for biochemical analysis) were obtained from jugular vein at 0, 30, 60, 90 and 120 min intervals. All blood samples were collected with a 17-gauge needle on a 5-mL syringe. Samples for haematology were placed immediately in 5000 μL plastic tubes containing lithium heparin, whereas samples for clinical chemistry were placed immediately in 5000 μL plastic plasma separator tubes with lithium heparin and promptly centrifuged. Haematology and serum chemistry results were determined by using goat’s software on automated haematology analyzer A (Hitachi 747–200, Hoffman–La Roche, and Basel).
2.3. Statistical analyses
All results were expressed as means ± standard deviation. Data were analysed statistically using one- or two-way(s) ANOVA with Tukey Comparison Test as a post-test using the computer statistics Prism 6.0 package (GraphPad Software, Inc.). Mean ± SD was used. P-values less than 0.05 were considered statistically significant. *P < 0.05, **P < 0.01, *** P < 0.001 and ****P < 0.0001.
3. Results
3.1. Analgesic effects of epidurally administered lidocaine, detomidine and lidocaine–detomidine combination
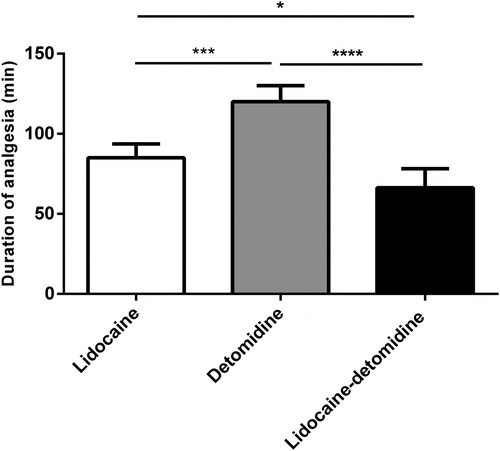
Detomidine treatment showed a significant (P < 0.01) and (P < 0.0001) earlier onset of analgesia (5.00 ± 1.26 min) than lidocaine (9.50 ± 0.44 min) and lidocaine–detomidine combination (13.00 ± 1.89 min), respectively (). Additionally, detomidine treatment demonstrated a significant (P < 0.001) increase in the duration of analgesia (120.00 ± 8.94 min) than lidocaine (85.00 ± 7.70 min) and lidocaine–detomidine combination (66.25 ± 10.60 min) treatments. At the same time, lidocaine treatment produced a significant (P < 0.05) increase in the duration of analgesia than lidocaine–detomidine combination treatment (). After lidocaine treatment, incoordination of hind limb started early (8–10 min) and scored 3, however hind limb incoordination in detomidine treatment was scored 1. All animals did not show any signs of incoordination or recumbancy in lidocaine–detomidine treatment group.
Figure 1. Onset of analgesia of epidurally administered lidocaine, detomidine, and lidocaine– detomidine combination in goats. Columns: relative frequency plus SD (n = 5). **P < 0.01, ***P < 0.001 and ****P < 0.0001 (one-way ANOVA followed by post-hoc Tukey’s test).
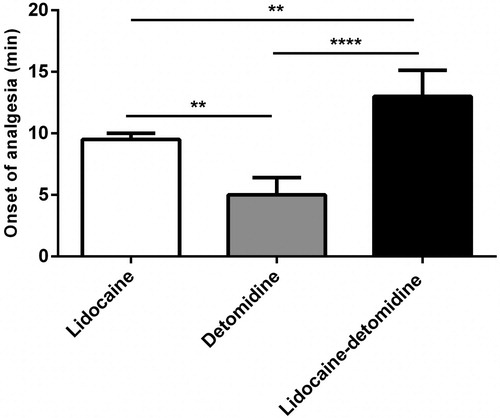
Figure 2. Duration of analgesia of epidurally administered lidocaine, detomidine, and lidocaine– detomidine combination in goats. Columns: relative frequency plus SD (n = 5). *P < 0.05, ***P < 0.001 and ****P < 0.0001 (one-way ANOVA followed by post-hoc Tukey’s test).
The response of animals to pinprick varied clearly between the three different groups. In lidocaine treatment, the response to pinprick started late, and a moderate to very weak response was noticed starting from 10 min, only one animal showed complete abolished response throughout the period of observation. A moderate to very weak response to pinprick was noticed starting from 5 min in detomidine treatment, and was completely abolished up to 90 min. In lidocaine–detomidine combination, a moderate response to pinprick was noticed starting from 15 min up to 30 min. In detomidine treatment, the total scores for pinprick were significantly lower than that in goats given lidocaine alone or in combination with detomidine. Interestingly, animals in detomidine treatment, exhibited a wide range area of loss of analgesia including tail, perineum region, upper parts of the hind limb and flanks than the other two groups which exhibited analgesic effect mainly in tail and perineum region. No significant change in the mean of RT was noticed in all groups, and variable depression effects on the mean of RR and HR were demonstrated in groups detomidine and lidocaine–detomidine treatment. However, lidocaine treatment showed an increase in the mean of RR and HR.
3.2. Effects of epidurally administered lidocaine, detomidine and lidocaine–detomidine combination on haematological parameters, kidney functions markers and liver enzymes activity
No differences were observed for the values of all haematological parameters except for white blood cells (WBCs) between the three groups (). For differential leukocytic count, detomidine, lidocaine or lidocaine–detomidine combination treatment did not affect the differential leukocytic counts in all time points. A significant increase in monocytes % was recorded at 30, 60, 90 and 120 min intervals, respectively, in comparison to those at 0 min in lidocaine treatment. While, in lidocaine–detomidine treatment, a significant increase in monocytes % at 60 min in comparison to that at 0 min time point was recorded ().
Table 2. Effects of epidurally administered lidocaine (A), detomidine (B) and lidocaine– detomidine combination (C) on haematology parameters in goats.
Table 3. Effects of epidurally administered lidocaine (A), detomidine (B) and lidocaine– detomidine combination (C) on differential leukocytic count in goats.
All treatments had no significant effect on both kidney function markers; blood urea nitrogen concentration (BUN) and creatinine concentration in all time points. For liver enzymes activity, all treatments had no effect on alanine aminotransferase enzyme activity (ALT) in all-time points, and the aspartate aminotransferase enzyme activity (AST) was significantly increased at 30-min time point after lidocaine treatment and at 60-min time point after detomidine treatment. There was a significant difference between the values of AST at 0-time point of lidocaine alone or in combination with detomidine ().
Table 4. Effects of epidurally administered lidocaine (A), detomidine (B) and lidocaine– detomidine combination (C) on liver enzymes activity and kidney functions markers in goat.
4. Discussion and conclusions
The present study compared the anti-nociceptive, haematological and biochemical responses of three different epidurally administered local anaesthetics; lidocaine, detomidine and lidocaine–detomidine combination in goats. More specifically, this study clearly demonstrated that, detomidine epidural administration in lumbosacral space could be useful clinically to provide rapid onset, prolonged and safe epidural analgesia which is required for long-duration surgical and obstetrical operations in goats. In recent literatures, several research groups evaluated the analgesic effects of different anaesthetic drugs and their combinations with variable results. The mechanism of action of the local anaesthetics is not specific to the sensory tracts and therefore, undesired effects such as motor paralysis are common side effects (Skarda Citation2007). Lidocaine is most commonly used as a local anaesthetic drug in small ruminants to induce epidural anaesthesia (Khajuria et al. Citation2014). Because of its side effects and short duration of action, alternative analgesia using different effective and safe anaesthetic drugs is necessary.
In our study, detomidine epidural injection produces adequate and rapid onset analgesia with minimum adverse effects. Moreover, epidural injection of detomidine in goats has a prolonged duration of analgesia in comparison with lidocaine and lidocaine–detomidine combination treatments. Prolonged analgesia of detomidine treatment (120.00 ± 8.94 min) is clinically useful in long-duration surgical and/or obstetrical operations. The prolonged analgesic effect of detomidine treatment is supported by the previous study which stated that epidural administration of detomidine has a prolonged analgesic effect in equine (Fischer et al. Citation2009; Lopes et al. Citation2016). We suggested that the lower volume of spreading for epidural detomidine is likely correlated to the prolonged duration of pain perception. Furthermore, the vasodilatation effect of epidurally injected lidocaine decreased the duration of analgesia due to sympathetic blockade produced (Gomez de Segura et al. Citation2000). In our study, the reduced/short duration of action in lidocaine and lidocaine–detomidine combination treatments is inconsistent. Our results showed that severe recumbency occurred after epidural administration of lidocaine treatment but slight in detomidine treatment, suggesting minimal adverse effects of detomidine. Recumbency and ataxia following epidural administration of lidocaine are critical adverse effects that may occur due to blocking of both sensory and motor nerve fibres (Day and Skarda Citation1991), and may adversely affect surgical and obstetrical operations that need an animal in a standing position. Moreover, the rapid onset of detomidine treatment is advantageous to save the time required to start the surgical or obstetrical interference which positively enhances the success of such operations. However, the combination of lidocaine and detomidine was less effective, these results were likely because of incompatibility of the two drugs together (Becker Citation2011). Detomidine and lidocaine–detomidine combination treatments had variable effects on HR and RR while lidocaine treatment induced HR and RR increase. This increase in HR and RR possibly due to sympathetic nerve block and vasodilatation induced by lidocaine as demonstrated before (Gomez de Segura et al. Citation2000).
Limited information is available about the measurements of haematobiochemical parameters following epidural administration of lidocaine, detomidine and their combination in animals, however, other recent studies reported similar findings after epidural administration of lignocaine-xylazine combination in cow calves (Moulvi et al. Citation2011b). In our study, detomidine treatment is likely to be more appropriate that it did not alter the differential leukocytic counts during its administration epidurally.
Furthermore, we investigated and compared the effects of the three different epidural treatments on liver and kidney function parameters. The present findings showed no systemic effect of all treatments on kidney functions all over the time of analgesia. However, a previous study reported an increase in BUN due to an increase in hepatic urea production from amino acid degradation result (Eichner et al. Citation1979) and after injection of medetomidine (Hugar et al. Citation1998). These effects of epidural injection on BUN reported by other research groups are likely due to the transient preventive effect of the drugs on the renal blood flow or as a result of prerenal azotaemia, which in turn might have caused such rise in BUN (Kinjavdekar et al. Citation2006). The increase in the AST levels as one of the liver enzymes at different time point intervals in lidocaine and detomidine may occur due to changes in cell membrane permeability, which may affect these enzymes to leak to or from the cells with intact membranes (Koichev et al. Citation1988).
In conclusion, detomidine treatment produced a safe and adequate prolonged analgesic effect with a rapid onset and extended area of anti-nociception in comparison with lidocaine and lidocaine treatment combination treatments. Detomidine treatment did not affect haematological parameters, BUN, creatinine levels, nor the ALT. However, the effect on AST was transient. Detomidine would be clinically useful as an appropriate local analgesic for epidural injection in goats.
Acknowledgements
Authors are grateful to the director and staff of the College of Veterinary Medicine Animal Farm, Qena for providing the necessary facilities for conducting this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
ORCID
Mohammed Zayed http://orcid.org/0000-0002-3361-0943
References
- Atiba A, Ghazy A, Gomaa N, Kamal T, Shukry M. 2015. Evaluation of analgesic effect of caudal epidural tramadol, tramadol-lidocaine, and lidocaine in water buffalo calves (Bubalus bubalis). Vet Med Int. 2015:1–6. doi: 10.1155/2015/575101
- Becker DE. 2011. Adverse drug interactions. Anesth Prog. 58(1):31–41. doi: 10.2344/0003-3006-58.1.31
- Buck RK, Meyer LR, Stegmann GF, Kästner SB, Kummrow M, Gerlach C, Fosgate GT, Zeiler GE. 2017. Propofol-medetomidine-ketamine total intravenous anaesthesia in thiafentanil-medetomidine-immobilized impala (Aepyceros melampus). Vet Anaesth Analg. 44(1):138–143. doi: 10.1111/vaa.12402
- Catterall WA. 2002. Molecular mechanisms of gating and drug block of sodium channels. sodium channels and neuronal hyperexcitability. Novartis Found Symp. 241:206–218.
- Daunt DA, Dunlop CI, Chapman PL, Shafer SL, Ruskoaho H, Vakkuri O, Hodgson DS, Tyler LM, Maze M. 1993. Cardiopulmonary and behavioral responses to computer-driven infusion of detomidine in standing horses. Am J Vet Res. 54(12):2075–2082.
- Day TK, Skarda RT. 1991. The pharmacology of local anesthetics. Veterinary Clinics of North America: Equine Practice. 7:489–500.
- Dehkordi SH, Bigham-Sadegh A, Gerami R. 2012. Evaluation of anti-nociceptive effect of epidural tramadol, tramadol-lidocaine and lidocaine in goats. Vet Anaesth Analg. 39(1):106–110. doi: 10.1111/j.1467-2995.2011.00655.x
- Dilipkumar D, Sharma AK, Gupta OP. 1997. Studies on haematological and biochemical changes induced during alpha adrenoreceptor agonist sedation in goats. Indian Vet J. 74:496–498.
- Eichner RD, Prior RL, Kvasnicka WG. 1979. Xylazine-induced hyperglycemia in beef cattle. Am J Vet Res. 40(1):127–129.
- Fischer BL, Ludders JW, Asakawa M, Fortier LA, Fubini SL, Nixon AJ, Radcliffe RM, Erb HN. 2009. A comparison of epidural buprenorphine plus detomidine with morphine plus detomidine in horses undergoing bilateral stifle arthroscopy. Vet Anaesth Analg. 36(1):67–76. doi: 10.1111/j.1467-2995.2008.00422.x
- Gomez de Segura IA, Vazquez I, De Miguel E. 2000. Antinociceptive and motor-blocking action of epidurally administered IQB-9302 and bupivacaine in the dog. Reg Anesth Pain Med. 25(5):522–528. doi: 10.1097/00115550-200009000-00015
- Hall LW, Clark KW, Trim CM. 2001. Veterinary anaesthesia. London: W.B. Saunder’s Co.
- Hanie EA. 2006. Prolapse of the vaginal and uterus: textbook of large animal clinical procedures for veterinary technicians. Mosby: Elsevier. 218–221.
- Hugar B, Gupta OP, Singh GR. 1998. A note on the effects of medetomidine with and without ketamine in goats. Indian Vet Med J. 22:139–140.
- Khajuria A, Fazili M, Shah RA, Khan A, Bhat H, Ganai N. 2014. Comparison of two doses of ropivacaine hydrochloride for lumbosacral epidural anaesthesia in goats undergoing laparoscopy assisted embryo transfer. Int Sch Res Notices. 2014:1–8. doi: 10.1155/2014/937018
- Kinjavdekar P, Aithal HP, Amarpal Pawde A, Pratap K, Singh GR. 2006. Potential effect of romifidine with lidocaine administration in goats. Small Rumin Res. 64(3):293–304. doi: 10.1016/j.smallrumres.2005.04.029
- Koichev K, Golemanov D, Houbenov H, Aminkov B. 1988. Experimental study on the effect of ‘domosedan’ in sheep and cattle. Vet Anaesth Analg. 15(1):114–126.
- Lawrence CJ, Prinzen FW, de Lange S. 1997. Hemodynamic and coronary vascular effects of dexmedetomidine in the anesthetized goat. Acta Anaesthesiol Scand. 41(7):830–836. doi: 10.1111/j.1399-6576.1997.tb04796.x
- Lemke KA. 2004. Perioperative use of selective alpha-2 agonists and antagonists in small animals. Can Vet J. 45(6):475–480.
- Lopes C, Luna SP, Rosa AC, Quarterone C, Crosignani N, Taylor PM, Pantoja JC, Puoli JN. 2016. Antinociceptive effects of methadone combined with detomidine or acepromazine in horses. Equine Vet J. 48(5):613–618. doi: 10.1111/evj.12483
- Moulvi BA, Kalim MO, Athar H, Singh M, Baba MA. 2011a. Comparative efficacy of lignocaine alone and in combination with ketamine as epidural anaesthesia in cow calves. Vet World. 4(8):364–367. doi: 10.5455/vetworld.2011.364-367
- Moulvi BA, Parrah JD, Kalim MO, Athar H, Dedmari F. 2011b. Haemato-biochemical response to lignocaine alone or in combination with xylazine for epidural analgesia in cow calves. J Adv Vet Res. 1:17–20.
- Mpanduji DG, Bittegeko SB, Mgasa MN, Batamuzi EK. 2000. Analgesic, behavioural and cardiopulmonary effects of epidurally injected medetomidine (Domitor) in goats. J Vet Med A Physiol Pathol Clin Med. 47(2):65–72. doi: 10.1046/j.1439-0442.2000.00254.x
- Re M, Canfrán S, Largo C, de Segura IA. G. 2016. Effect of lidocaine–ketamine infusions combined with morphine or fentanyl in sevoflurane-anesthetized pigs. J Am Assoc Lab Anim Sci. 55(3):317–320.
- Shokry MM, Elkasapy AH. 2018. Epidural anesthesia in Egyptian water buffalo (Bubalus bubalis): a comparison of lidocaine, xylazine and a combination of lidocaine and xylazine. Vet Anaesth Analg. 45(1):707–710. doi: 10.1016/j.vaa.2018.03.013
- Singh V, Amarpal, Kinjavdekar P, Aithal HP, Pratap K. 2005. Medetomidine with ketamine and bupivacaine for epidural analgesia in buffaloes. Vet Res Commun. 29(1):1–18. doi: 10.1023/B:VERC.0000046736.78612.f7
- Singh V, Amarpal, Kinjavdekar P, Aithal HP. 2009. Effect of bupivacaine on epidural analgesia produced by xylazine or medetomidine in buffaloes (Bubalus bubalis). Vet Anaesth Analg. 36(1):77–85. doi: 10.1111/j.1467-2995.2008.00429.x
- Skarda RT. 2007. Local and regional anesthetics and analgesic techniques: ruminants and swine. In: Tranquilli WJ, Thurmon JC, Grimm KA, editors. Lumb & Jones’ veterinary anesthesia and analgesia. 4th ed. Ames, IA: Blackwell; p. 731–746.
- Wachida N, Kisani AI. 2011. Uterine prolapse in a doe goat: a case report. J Anim Vet Adv. 3(3):135–137.
- Wagner AE, Muir WW, Hinchcliff KW. 1991. Cardiovascular effects of xylazine and detomidine in horses. Am J Vet Res. 52(5):651–657.
