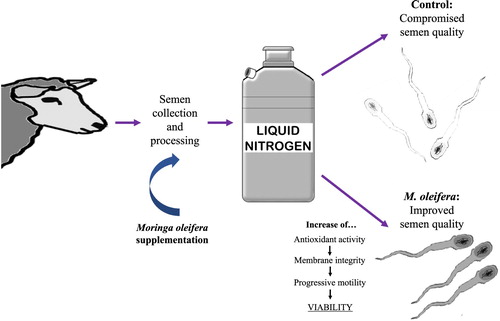
ABSTRACT
Semen cryopreservation damages sperm due to oxidative stress. This study evaluated the antioxidant capacity of Moringa oleifera seed extract in cryopreserved ram semen and the impact of the extract on sperm characteristics. Semen from eight hair rams (four rams per sampling) was allocated into four groups, according to their treatment prior to cryopreservation: Control (no extract), 0.5 (M0.5), 5.0 (M5.0), and 10.0 (M10) mg/mL of M. oleifera extract. The antioxidant activity (ferric reducing antioxidant power, FRAP) and the spermatic characteristics (sperm viability; progressive motility; fast motility; slow motility; acrosome damage; membrane damage; and mitochondrial activity) were assessed post-thawing. Variables were evaluated with analysis of variance followed by Tukey test. While no significant differences were detected in acrosomal damage, mitochondrial activity, fast-, or slow motility, the antioxidant activity was higher (P < 0.05) in M0.5 and M5 treatments. Viability and progressive motility increased in the M0.5 group (P < 0.05), whereas sperm membrane damage was lower (P < 0.05) in the same treatment. In conclusion, supplementation of ram semen with M. oleifera seed extract enhances antioxidant activity, sperm membrane integrity, viability, and progressive motility after thawing. This suggests that M. oleifera extract could be used as an antioxidant to improve the outcome of semen cryopreservation.
It is widely known that semen cryopreservation induces sublethal damage to sperm, deteriorating spermatic characteristics, which is largely attributed to oxidative stress.
There are scarce studies regarding the use of plant extracts as a replacement for conventional antioxidants to conserve sperm viability in cryopreserved ram semen.
Addition of Moringa oleifera seed extract to a concentration of 0.5 and 5.0 mg/mL prior to ram semen freezing increased antioxidant activity after cryopreservation.
M. oleifera seed extract at 0.5 mg/mL decreased post-thawing damage to the sperm membrane, increasing both viability and progressive motility.
M. oleifera seed extract could be potentially used as a replacement for conventional antioxidants added to maintain sperm viability in cryopreserved ram semen.
Highlights
1. Introduction
Semen cryopreservation, commercialization, and storage are widely used tools in livestock industry that promote genetic improvement. However, semen freezing induces sublethal damage to sperm, causing deterioration of cellular characteristics. This is attributed to thermal shock, ice crystal formation, osmotic changes, membrane lipids and proteins rearrangement, and high oxidation levels (Bucak et al. Citation2010). Oxidative stress occurs when there is an imbalance in the production of reactive oxygen species (ROS), in which ROS generation is greater than the biological ability to detoxify their reactive intermediates or easily repair the resulting damage (Agarwal and Said Citation2003). This condition triggers toxic effects due to the production of peroxides and free radicals that damage cell components, including proteins, lipids, and DNA (Schafer and Buettner Citation2001). Thus, it is necessary to limit sperm damage generated by oxidative stress induced during cryopreservation.
The addition of antioxidants, such as alpha-tocopherol (Benhenia et al. Citation2016), Trolox (Sicherle et al. Citation2011), and taurine (Baghshahi et al. Citation2014) to ovine semen cryopreservation diluents has shown, to some extent, protective capacity against the adverse effects of ROS. This supplementation increases motility, viability, and post-thawing fertility (Sicherle et al. Citation2011; Baghshahi et al. Citation2014; Benhenia et al. Citation2016). Likewise, plant extracts with antioxidant properties have been recently studied in order to improve the quality of cryopreserved semen. These extracts contain widely known antioxidant phytochemicals including carotenoids, polyphenols, and flavonoids (Allai et al. Citation2016). In this regard, tissues from the Moringa oleifera tree have been demonstrated to possess high antioxidant activity due to the presence of ascorbic acid, flavonoids, polyphenolics, and carotenes (Jayawardana et al. Citation2015). These components decrease both free radical levels and the risk of cell death (Wang et al. Citation2017). However, research suggesting the potential use of vegetal extracts to replace conventional antioxidants and preserve sperm viability is still scarce (Berkovich et al. Citation2013). Moreover, exploration of the impact of M. oleifera extracts addition on the antioxidant status of cryopreserved ram semen and spermatic characteristics has not yet been reported. Therefore, because of all the aforementioned, the aims of the present study were to evaluate the effect of M. oleifera seed extract supplementation on the antioxidant capacity of frozen-thawed ram semen and identify the influence of different concentrations of this vegetal extract on sperm characteristics in cryopreserved ram semen.
2. Materials and methods
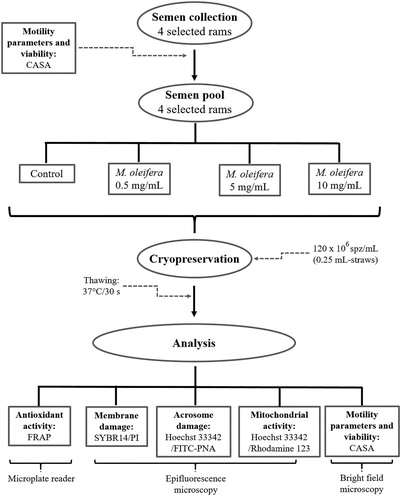
A schematic summary of the methods used in this work is shown in .
Figure 1. Scheme summarizing the methodology used to assess the effects of Moringa oleifera seed extract supplementation on the antioxidant activity and spermatic characteristics of cryopreserved ram semen. M. oleifera: Moringa oleifera, mg/mL: milligrams per millilitre, spz/mL: spermatozoa per millilitre, mL: millilitre, °C: Celsius degrees, s: seconds, CASA: computer assisted semen analysis, PI: propidium iodide, FITC-PNA: fluorescein isothiocyanate-coupled peanut agglutinin, FRAP: ferric reducing antioxidant power.
2.1. Animals and semen collection
The authors assert that all procedures contributing to this work complied with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals in Mexico (NOM-051-ZOO-1995: Humanitarian care of animals during mobilization; NOM-033-ZOO-1995: Humanitarian slaughter of domestic and wild animals; and the institutional regulation of the Bioethics Committee of the Universidad Autónoma de Ciudad Juárez).
The present investigation was conducted at the Universidad Autónoma de Ciudad Juárez, Ciudad Juárez, Chihuahua, México (31° 44′ 22″ N, 106° 29′ 13″ W) during the breeding season (autumn and winter). Eight healthy hair breed rams (Katahdin, Black Belly, Pelibuey), two to four years old, kept in confinement with natural light and ad libitum access to forage and water, supplemented with 500 g/head/day of ground corn were employed for this study. Males were kept under similar management conditions during the entire study. Four out of the eight rams available were randomly selected and used per sampling session, which was carried out twice a week using the artificial vagina collection technique. The four samples were pooled to eliminate individual differences. 10 μL of the sample were placed in a standard 20 μm count chamber (Leja Camera, Leja Products, The Netherlands) to be evaluated by computer assisted semen analysis (CASA) system (AndroVision, Minitube, Germany) under a vertical microscope (AxioScope.A1, Zeiss, Germany). The minimum levels required to freeze were established as it follows: Volume of the ejaculate, ≥0.5 mL (Banday et al. Citation2017); progressive motility, ≥80%; and concentration, 3 × 109 spermatozoa/mL (Câmara et al. Citation2011). shows the CASA system settings used to evaluate ram sperm.
Table 1. Settings of the CASA system* used to evaluate ram spermatozoa.
2.2. Production and characterization of Moringa oleifera seed extract
Seeds of M. oleifera were collected in the state of Sonora, in Northwestern Mexico. Mature pods were manually collected in November 2017 and transported in plastic bags at room temperature. Seeds were obtained and oven-dried at 60°C for 8 h. Then, these structures were stored in plastic bags in a dark room. All samples were ground and passed through a mesh sieve (60 meshes). The extract of M. oleifera was obtained from lyophilized defatted seeds processed following the method described by Núñez-Gastélum et al. (Citation2019) with some modifications. Specifically, 100 mL of 80% (v/v) methanol were added to 10 mg of sample. The mixture was then sonicated at 40 kHz for 30 min in the dark. The extract was centrifuged at 1600 × g for 30 min at 4°C and the supernatant was recovered by filtration. The residue was re-extracted under the same conditions. Finally, supernatants were combined, rota-evaporated (Buchi R-3, Flawil, Switzerland), lyophilized (FreeZone 4.5, Labconco, Kansas City, MO), and stored at −80°C until use. To characterize the extracts, the total polyphenolics and flavonoids content was determined following the procedure described by Núñez-Gastélum et al. (Citation2018) by using the method of Folin–Ciocalteu and complexation with aluminum chloride, respectively.
2.3. Semen processing and treatments
A diluent based on Tris (Two Step, Continental, Delavan, WI) was used with 6% (v/v) glycerol and 10% (v/v) egg yolk. Diluted samples were assigned into four treatments: Control, which included conventional antibiotic (55-, 275-, 330-, and 165 μg/mL of a mixture tylosin, gentamicin, spectinomycin, and lincomycin, respectively), while the three remaining treatments contained no antibiotic but extract of M. oleifera seed at final concentrations (in the straw) of 0.5 (M0.5), 5.0 (M5), and 10.0 (M10) mg/mL. Semen concentration was adjusted to 120 × 106 motile sperm/mL. Samples were manually aspirated into 0.25 mL straws (IMV Technologies, France) at room temperature, sealed with polyvinyl alcohol powder, and immediately cooled to 5°C at a rate of −0.2°C/min. Once semen was refrigerated and equilibrated at 5°C for one hour, straws were placed in an automatic freezer (Cryobath CL-8800, CryoLogic, Australia) previously stabilized at 5°C. Semen was frozen from 5 to −120°C at a rate of −12.5°C/min and then stored in a cryogenic container at −196°C until further analysis.
2.4. Evaluation of antioxidant activity in post-thawed semen
2.4.1. Sample preparation
For seminal plasma recovery it was followed the procedure described by Gallardo (Citation2007) with some modifications. Briefly, straws were thawed 30 days after freezing at 37°C for 30 s in a water bath and centrifuged at 800 × g for 10 min at 4°C. The supernatant (seminal plasma and diluent) was removed and stored at −80°C until use.
2.4.2. Ferric reducing antioxidant power test
An acetate buffer was prepared for the ferric reducing antioxidant power (FRAP) assay. For that purpose, 1.3 g of sodium acetate (CH₃COONa) and 8 mL of glacial acetic acid were mixed with 300 mL of distilled water. The pH was adjusted to 3.6 and brought to a final volume of 500 mL. The reagent TPTZ was prepared with 31.2 mg of 2,4,6-Tri (2-pyridyl)-s-triazine (TPTZ) and 33 μL of 1.21 M hydrochloric acid, adjusted to 10 mL with distilled water, and stored in the absence of light. The FRAP reagent was prepared using 1 mL of the TPTZ reagent and 1 mL of 20 mM FeCl2. Afterwards, this mixture was adjusted to 10 mL with acetate buffer and then incubated at 37°C for 30 min. Immediately after FRAP reagent incubation, the sample (seminal plasma and diluent) was thawed at room temperature and 24 μL were placed per microplate well. 180 μL of the FRAP reagent were added to each well for subsequent reading at 595 nm in a microplate spectrophotometer (xMark, BioRad, Hercules, CA) at 37°C. The results were expressed as millimoles of Trolox equivalents (mmol TE) upon comparison with a Trolox standard curve (Núñez-Gastélum et al. Citation2018).
2.5. Evaluation of sperm characteristics in post-thawed semen
2.5.1. Viability and motility
Semen samples were thawed as previously explained and evaluated with the CASA system for the viability, progressive motility, fast motility, and slow motility variables.
2.5.2. Sperm membrane damage
SYBR14/PI staining (15407/0001, Minitube, Germany) was used for sperm membrane damage evaluation. Propidium iodide only penetrates permeated membranes. Therefore, damaged sperm nuclei were stained red. 50 μL of semen and 2.5 μL of dye were mixed and incubated at 37°C during 15 min protected from light. A 10 μL-drop of this preparation was placed on a slide and covered with a coverslip. The sample was then analyzed with the membrane integrity module of the CASA system under an epifluorescence microscope (200X; AxioScope.A1, Zeiss, Germany). The fluorescence filter (AHF Analysentechnik, Germany) characteristics were: 481–501 nm and 560–589 nm excitation; 503 and 589 nm beam splitters (BS); 512–550 nm and 600–660 nm emission.
2.5.3. Acrosome damage
A double fluorescence stain with Hoechst 33342/FITC-PNA (15407/0011, Minitube) was used to determine the grade of acrosome damage. 50 μL of thawed semen and 27 μL of dye were mixed and incubated for 20 min at 37°C in the dark. 10 μL of this mixture were loaded on a slide and covered with glass. This preparation was evaluated with the acrosome integrity module of the CASA system with an epifluorescence microscope (200X). The fluorescence filter (AHF Analysentechnik) characteristics were: 392–414 nm and 481–501 nm excitation; 419 and 503 nm BS; 440–470 nm and 512–550 nm emission. Heads of all spermatozoa showed blue colouration. Meanwhile, damaged acrosomes were stained green.
2.5.4. Mitochondrial activity
A double fluorescent stain was performed with Hoechst 33342/Rhodamine 123 (15407/0012, Minitube) to evaluate mitochondrial function. 50 μL of thawed semen and 4 μL of dye were mixed and incubated for 20 min at 38°C protected from light. The sample was then centrifuged at 800 × g for 4 min and the supernatant was removed. 50 μL of BTS diluent (13525/0001, Minitube) was added and incubated for 5 min at 38°C. A 10 μL-drop was placed on a slide, covered with glass, and analyzed with the mitochondrial activity module of the CASA system with a microscope of epifluorescence (200X). The fluorescence filter (AHF Analysentechnik) characteristics were: 392–414 nm and 481–501 nm excitation; 419 and 503 nm BS; 440–470 nm and 512–550 nm emission. Heads of all spermatozoa were stained blue due to the action of Hoechst 33342, whereas the intermediate part of the spermatozoa with functional mitochondria showed green colour due to the Rhodamine 123 stain.
2.6. Statistical analysis
Six replicates for each test were carried out. The experimental unit was each of the six mixtures formed by the ejaculate of the four rams used in each collection. Data analysis was performed with the statistical package SAS (SAS, 2009/STAT v. 9.3, SAS Institute, Cary, NC). The variables were assessed with one-way ANOVA and compared with a Tukey test. A P < 0.05 was considered as significant. Percent values of viability, progressive motility, fast motility, slow motility, sperm membrane damage, acrosomal damage, and mitochondrial activity were transformed to arcsine before analysis, although all data were expressed as non-transformed means ± SEM.
3. Results
Data of the total polyphenolic and flavonoid composition of the M. oleifera seed extract supplemented prior to ram semen freezing are shown in . Upon such treatment ram semen was cryopreserved. Subsequently, both antioxidant capacity and spermatic characteristics were evaluated, rendering the following findings:
Table 2. Total polyphenolics and flavonoids in Moringa oleifera seed extract.
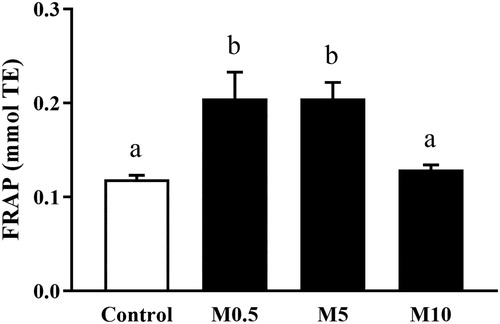
First, it was observed that M0.5 (0.205 ± 0.028 mmol TE) and M5 (0.205 ± 0.017 mmol TE) groups had similar results (P > 0.05) in relation to the antioxidant activity in seminal plasma, as indicated in . Nevertheless, either M0.5 or M5 treatments showed significantly higher antioxidant activity (P < 0.05) when compared to M10 (0.129 ± 0.005 mmol TE) and Control groups (0.119 ± 0.004 mmol TE).
Figure 2. Antioxidant activity in ram semen following cryopreservation. FRAP: ferric reducing antioxidant power, mmol TE: millimoles of Trolox equivalents, Control: control group, M0.5: 0.5 mg/mL of Moringa oleifera seed extract, M5: 5 mg/mL of M. oleifera seed extract, M10: 10 mg/mL of M. oleifera seed extract. Data are presented as mean ± SEM. a,b Different literals across bars indicate significant differences (P < 0.05).
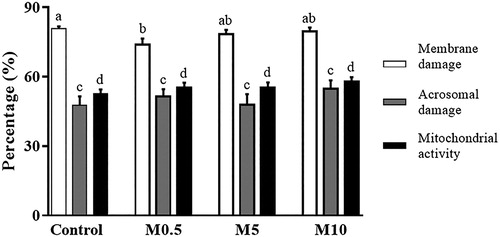
Concerning integrity and activity of spermatic structures, the percentage of sperm membrane damage was significantly lower (P < 0.05) in M0.5 (73.88 ± 2.64%) when compared to the Control group (80.75 ± 1.06%). However, the membrane damage rate of the M0.5 group did not differ (P > 0.05) from that of M5 (78.38 ± 1.91%) and M10 (79.50 ± 1.79%) treatments (). On the other hand, the results of both acrosomal damage and mitochondrial activity were similar (P > 0.05) among Control (47.63 ± 3.80% and 52.75 ± 1.73%, respectively), M0.5 (51.38 ± 3.2% and 55.38 ± 2.05%), M5 (47.88 ± 4.50% and 55.38 ± 2.16%), and M10 (54.87 ± 3.50% and 58.00 ± 1.84%) treatments, as shown in .
Figure 3. Sperm membrane damage, acrosome damage, and mitochondrial activity in ram semen following cryopreservation. Control: control group, M0.5: 0.5 mg/mL of Moringa oleifera seed extract, M5: 5 mg/mL of M. oleifera seed extract, M10: 10 mg/mL of M. oleifera seed extract. Data are presented as mean ± SEM. a,b Different literals across bars of the same colour indicate significant differences (P < 0.05).
In relation to cryopreserved semen quality parameters (drawn from motility analysis), the percentage of both post-thaw sperm viability and progressive motility were higher (P < 0.05) in M0.5 in comparison to the Control treatment (). Nevertheless, M0.5 resulted similar to M5 and M10 groups in terms of viability and progressive motility (P > 0.05). In contrast to sperm viability and progressive motility, there were no significant differences among all treatments regarding the variables of fast- and slow motility in post-thawed semen (P > 0.05).
Table 3. Quality of cryopreserved ram semen supplemented with Moringa oleifera extract at 0.5, 5.0, and 10.0 mg per mL.
4. Discussion
The polyphenolic and flavonoid content of M. oleifera seed extracts used in the present study is in the range reported for defatted seed extracts in other studies (Singh et al. Citation2013; Nascimiento et al. Citation2017; de la Mora-López et al. Citation2018). This reflects the antioxidant capacity of the extract since it is widely known that both phenolic and flavonoid compounds possess high antioxidant activity (Covas et al. Citation2010; Castaldo et al. Citation2019; Olszowy Citation2019).
Although other studies have reported a protective effect of the addition of antioxidants, such as hypotaurine, on acrosome integrity and mitochondrial activity (Bucak et al. Citation2013), this investigation did not find any significant effect of the M. oleifera extract on either the acrosomal status or mitochondrial activity in cryopreserved ram sperm. In agreement, Câmara et al. (Citation2011) also found no differences neither in the percentage of ram sperm with intact acrosome nor in mitochondrial membrane potential in samples added with antioxidants. However, these authors mention that there might be latent damage, in which the lesion degree manifests during post-thaw incubation, reducing sperm longevity in the female genital tract after insemination. Similarly, Silva et al. (Citation2011) also found no difference in the results of fluorescent tests of acrosome integrity and mitochondrial membrane potential when they added superoxide dismutase or reduced glutathione to cryopreserved semen. However, ultrastructural analysis by transmission electron microscopy showed that the addition of these antioxidants helped to conserve acrosome and mitochondria structure (Silva et al. Citation2011). This might suggest that in the present study, although an effect of the seed extract of M. oleifera on the variables of acrosomal integrity and mitochondrial activity was not found, there could be a protective effect that was reflected in the increase in progressive motility. Nevertheless, clarification of these possibilities deserves further exploration.
In the present study, it was observed that a low concentration of M. oleifera seed extract (0.5 mg/mL) protected the sperm membrane, apparently due to the antioxidant properties of this vegetal extract. It can be inferred that conservation of sperm membrane structure was due to an antioxidant function given the significantly higher levels of antioxidant activity detected in semen after the addition of M. oleifera in this investigation. As mentioned before, this plant contains compounds encompassing polyphenols, flavonoids, vitamin C, vitamin E, β-carotene, zinc, selenium, vitamin A, riboflavin, nicotinic acid, folic acid, pyridoxine, cryptochlorogenic acid, isoquercetin, and astragalin. All these substances have strong antioxidant potential (Moyo et al. Citation2012; Vongsak et al. Citation2013; Jayawardana et al. Citation2015). In this way, it could be suggested that the higher antioxidant capacity detected here upon M. oleifera extract addition likely diminished formation of ROS in sperm plasma membrane. This is supported by the fact that antioxidant compounds decrease spermatic lipid peroxidation that results from the attack to the lipid matrix by ROS (Agarwal et al. Citation2008; Bucak et al. Citation2010; Allai et al. Citation2016). However, the physiology underlying sperm protection by the extract of M. oleifera is not completely clear since the positive effects may be a consequence of combined actions of its components (Cai et al. Citation2002). Therefore, future research is guaranteed to shed light on the exact basis of the mechanism of action that protected the sperm membrane, as well as the identification of the involved compound (or combination of substances). On the other hand, it is conceivable that the higher sperm membrane integrity observed in this work after supplementation of the vegetal extract in turn improved both viability and progressive motility of sperm. Accordingly, it is widely known that maintenance of spermatic plasma membrane is required for both vitality and motility, which are landmarks for male fertility throughout species, as indicated by Fraser et al. (Citation2001), Ahmad et al. (Citation2015), Reis et al. (Citation2016). Thus, conservation of plasma membrane structure, as a consequence of increased antioxidant capacity upon M. oleifera extract addition here, also appears as a requirement for sperm viability and progressive motility in ram semen.
In conclusion, supplementation of the diluent used to freeze ram semen with M. oleifera seed extract increased antioxidant activity, viability, and progressive motility, while sperm membrane damage was decreased. These results, arisen from in vitro analysis, suggest that M. oleifera extracts could be an option of antioxidant additive for ram semen freezing (). Future studies should be conducted to better understand the mechanism of action, as well as to identify the specific active components in M. oleifera extracts involved in the improvement of antioxidant capacity and spermatic characteristics during ram semen cryopreservation.
Disclosure statement
No potential conflict of interest was reported by the author(s).
ORCID
José Maria Carrera-Chávez http://orcid.org/0000-0002-6899-9485
Edson Eduardo Jiménez-Aguilar http://orcid.org/0000-0003-0847-529X
Theisy Patricia Acosta-Pérez http://orcid.org/0000-0002-5768-5050
José Alberto Núñez-Gastélum http://orcid.org/0000-0002-7098-4485
Andrés Quezada-Casasola http://orcid.org/0000-0003-0758-9762
Angélica María Escárcega-Ávila http://orcid.org/0000-0002-4066-0586
Mateo Fabián Itza-Ortiz http://orcid.org/0000-0003-0313-586X
Ernesto Orozco-Lucero http://orcid.org/0000-0003-3238-2341
Additional information
Funding
References
- Agarwal A, Makker K, Sharma R. 2008. Clinical relevance of oxidative stress in male factor infertility: an update. Am J Reprod Immunol. 59:2–11. https://onlinelibrary.wiley.com/doi/epdf/10.1111/j.1600-0897.2007.00559.x.
- Agarwal A, Said TM. 2003. Role of sperm chromatin abnormalities and DNA damage in male infertility. Hum Reprod Update. 9:331–345. https://doi.org/10.1093/humupd/dmg027.
- Ahmad M, Ahmad N, Riaz A, Anzar M. 2015. Sperm survival kinetics in different types of bull semen: progressive motility, plasma membrane integrity, acrosomal status and reactive oxygen species generation. Reprod Fertil Dev. 27:784–793. https://doi.org/10.1071/RD13400.
- Allai L, Druart X, Öztürk M, BenMoula A, Nasser B, El Amiri B. 2016. Protective effects of Opuntia ficus-indica extract on ram sperm quality, lipid peroxidation and DNA fragmentation during liquid storage. Anim Reprod Sci. 175:1–9. https://doi.org/10.1016/j.anireprosci.2016.09.013.
- Baghshahi H, Riasi A, Mahdavi AH, Shirazi A. 2014. Antioxidant effects of clove bud (Syzygium aromaticum) extract used with different extenders on ram spermatozoa during cryopreservation. Cryobiology. 69:482–487. https://doi.org/10.1016/j.cryobiol.2014.10.009.
- Banday MN, Lone FA, Rasool F, Rashid M, Shikari A. 2017. Use of antioxidants reduce lipid peroxidation and improve quality of crossbred ram sperm during its cryopreservation. Cryobiology. 74:25–30. https://doi.org/10.1016/j.cryobiol.2016.12.008.
- Benhenia K, Lamara A, Fatmi S, Iguer-Ouada M. 2016. Effect of cyclodextrins, cholesterol and vitamin E and their complexation on cryopreserved epididymal ram semen. Small Rum Res. 141:29–35. https://doi.org/10.1016/j.smallrumres.2016.06.009.
- Berkovich L, Earon G, Ron I, Rimmon A, Vexler A, Lev-Ari S. 2013. Moringa oleifera aqueous leaf extract down-regulates nuclear factor-kappa B and increases cytotoxic effect of chemotherapy in pancreatic cancer cells. BMC Complement Altern Med. 13:212–219. https://doi.org/10.1186/1472-6882-13-212.
- Bucak MN, Keskin N, Taşpınar M, Çoyan K, Başpinar N, Cenariu MC, Bilgili A, Öztürk C, Kurşunlu AN. 2013. Raffinose and hypotaurine improve the post-thawed Merino ram sperm parameters. Cryobiology. 67:34–39. https://doi.org/10.1016/j.cryobiol.2013.04.007.
- Bucak MN, Tuncer PB, Sarıözkan S, Başpinar N, Taşpinar M, Çoyan K, Bilgili A, Akalın PP, Büyükleblebici S, Aydos S, et al. 2010. Effects of antioxidants on post-thawed bovine sperm and oxidative stress parameters: antioxidants protect DNA integrity against cryodamage. Cryobiology. 61:248–253. https://doi.org/10.1016/j.cryobiol.2010.09.001.
- Cai Y-J, Ma L-P, Hou L-F, Zhou B, Yang L, Liu Z-L. 2002. Antioxidant effects of green tea polyphenols on free radical initiated peroxidation of rat liver microsomes. Chem Phys Lipids. 120:109–117. https://doi.org/10.1016/S0009-3084(02)00110-X. doi: 10.1016/S0009-3084(02)00110-X
- Câmara DR, Silva SV, Almeida FC, Nunes JF, Guerra MMP. 2011. Effects of antioxidants and duration of pre-freezing equilibration on frozen-thawed ram semen. Theriogenology. 76:342–350. https://doi.org/10.1016/j.theriogenology.2011.02.013.
- Castaldo L, Narváez A, Izzo L, Graziani G, Gaspari A, Minno GD, Ritieni A. 2019. Red wine consumption and cardiovascular health. Molecules. 24:E3626. https://doi.org/10.3390/molecules24193626.
- Covas MI, Gambert P, Fitó M, de la Torre R. 2010. Wine and oxidative stress: up-to-date evidence of the effects of moderate wine consumption on oxidative damage in humans. Atherosclerosis. 208:297–304. https://doi.org/10.1016/j.atherosclerosis.2009.06.031.
- de la Mora-López GS, López-Cervantes J, Gutiérrez-Dorado R, Cuevas-Rodríguez EO, Milán-Carrillo J, Sánchez-Machado DI, Reyes-Moreno C. 2018. Effect of optimal germination conditions on antioxidant activity, phenolic content and fatty acids and amino acids profiles of Moringa oleifera seeds. Rev Mex Ing Quím. 17:547–560. http://www.rmiq.org/ojs311/index.php/rmiq/article/view/59/43. doi: 10.24275/uam/izt/dcbi/revmexingquim/2018v17n2/Servin
- Fraser L, Gorszczaruk K, Strzezek J. 2001. Relationship between motility and membrane integrity of boar spermatozoa in media varying in osmolarity. Reprod Dom Anim. 36:325–329. https://doi.org/10.1046/j.1439-0531.2001.00310.x.
- Gallardo JM. 2007. Evaluation of antioxidant system in normal semen. Rev Invest Clin. 59:42–47. http://www.scielo.org.mx/pdf/ric/v59n1/v59n1a6.pdf.
- Jayawardana BC, Liyanage R, Lalantha N, Iddamalgoda S, Weththasinghe P. 2015. Antioxidant and antimicrobial activity of drumstick (Moringa oleifera) leaves in herbal chicken sausages. LWT – Food Sci Technol. 64:1204–1208. https://doi.org/10.1016/j.lwt.2015.07.028.
- Moyo B, Oyedemi S, Masika PJ, Muchenje V. 2012. Polyphenolic content and antioxidant properties of Moringa oleifera leaf extracts and enzymatic activity of liver from goats supplemented with Moringa oleifera leaves/sunflower seed cake. Meat Sci. 91:441–447. https://doi.org/10.1016/j.meatsci.2012.02.029.
- Nascimiento OK, Pereira RI, Maria AI. 2017. Total phenolic and antioxidant capacity of flower, leaf and seed of Moringa oleifera. Int J Food Nutr Res. 1:1–6. https://escipub.com/Articles/IJFNR/IJFNR-2017-03-1501.
- Núñez-Gastélum JA, Hernández-Rivas R, Rodrigo-García J, de la Rosa LA, Alvarez-Parrilla E, Díaz-Sánchez ÁG, Muñoz-Bernal OA, Cota-Ruíz K, Martínez-Martínez A. 2018. Polyphenolics content, antioxidant and antimicrobial activities of Ibervillea sonorae root. Biotecnia. 20:23–27. http://biotecnia.unison.mx/index.php/biotecnia/article/view/702.
- Núñez-Gastélum JA, Rodríguez-Núñez JR, de la Rosa LA, Díaz-Sánchez AG, Alvarez-Parrilla E, Martínez-Martínez A, Villa-Lerma G. 2019. Screening of the physical and structural properties of chitosan-polycaprolactone films added with Moringa oleifera leaf extract. Rev Mex Ing Quím. 18:99–105. https://doi.org/10.24275/uam/izt/dcbi/revmexingquim/2019v18n1/Nunez.
- Olszowy M. 2019. What is responsible for antioxidant properties of polyphenolic compounds from plants? Plant Physiol Biochem. 144:135–143. https://doi.org/10.1016/j.plaphy.2019.09.039.
- Reis LSLS, Ramos AA, Camargos AS, Oba E. 2016. Integrity of the plasma membrane, the acrosomal membrane, and the mitochondrial membrane potential of sperm in Nelore bulls from puberty to sexual maturity. Arq Bras Med Vet Zootec. 68:620–628. https://doi.org/10.1590/1678-4162-8748.
- Schafer FQ, Buettner GR. 2001. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 30:1191–1212. https://doi.org/10.1016/S0891-5849(01)00480-4. doi: 10.1016/S0891-5849(01)00480-4
- Sicherle CC, Maia MS, Bicudo SD, Rodello L, Azevedo HC. 2011. Lipid peroxidation and generation of hydrogen peroxide in frozen-thawed ram semen supplemented with catalase or Trolox. Small Rum Res. 95:144–149. https://doi.org/10.1016/j.smallrumres.2010.10.011.
- Silva SV, Soares AT, Batista AM, Almeida FC, Nunes JF, Peixoto CA, Guerra MMP. 2011. In vitro and in vivo evaluation of ram sperm frozen in Tris egg-yolk and supplemented with superoxide dismutase and reduced glutathione. Reprod Dom Anim. 46:874–881. https://doi.org/10.1111/j.1439-0531.2011.01758.x.
- Singh RSG, Negi PS, Radha C. 2013. Phenolic composition, antioxidant and antimicrobial activities of free and bound phenolic extracts of Moringa oleifera seed flour. J Funct Foods. 5:1883–1891. https://doi.org/10.1016/j.jff.2013.09.009.
- Vongsak B, Sithisarn P, Gritsanapan W. 2013. Bioactive contents and free radical scavenging activity of Moringa oleifera leaf extract under different storage conditions. Ind Crops Prod. 49:419–421. https://doi.org/10.1016/j.indcrop.2013.05.018.
- Wang Y, Gao Y, Ding H, Liu S, Gui J, Liu D. 2017. Subcritical extraction of flavonoids from Moringa oleifera leaf and evaluation of antioxidant activity. Food Chem. 218:152–158. https://doi.org/10.1016/j.foodchem.2016.09.058.