ABSTRACT
This study comprehensively explored muscle fibre characteristics and expression patterns of myosin heavy chain (MyHC) family in skeletal muscles of Large White and Mashen pigs exhibiting differences in muscle fibre development in postnatal muscle growth. Muscle fibre density and diameter were analysed using haematoxylin and eosin staining. The expression patterns of MyHCI, MyHCIIa, MyHCIIx and MyHCIIb were detected by quantitative real-time polymerase chain reaction. The muscle fibre diameter increased and density decreased gradually with aging. In individuals of the same age, the muscle fibre diameter was significantly lower and density was significantly higher in Mashen than in Large White pigs. The MyHCI expression increased, whereas that of MyHCIIa first increased and then decreased. The expression of MyHCI and MyHCIIa was significantly higher in Mashen. The expression of MyHCIIx and MyHCIIb decreased with increasing age; their expression was significantly lower in Mashen pigs. Muscle fibre diameter was negatively correlated with MyHCI expression and positively correlated with MyHCIIx and MyHCIIb expression (except the expression of MyHCI in psoas major). Muscle fibre diameter, muscle fibre density, and MyHCs expression follow certain patterns in skeletal muscles. These results provide valuable information for understanding the molecular mechanism responsible for pig growth performance and meat quality.
Introduction
Recently, increased emphasis has been placed on improving meat quality (England et al. Citation2013; Listrat et al. Citation2016). Many physical and biochemical factors such as breed, genotype, sex, age, nutrition, and slaughter conditions have been identified to affect meat quality. Particularly, it is widely accepted that skeletal muscle fibre characteristics are one of the important factors influencing meat quality by affecting muscle tenderness, intramuscular fat content, meat succulence, and other properties (Gil et al. Citation2003; Klosowska and Fiedler Citation2003; Choi et al. Citation2007; Joo et al. Citation2013).
Porcine muscle fibres are classified into four types: type I, type IIa, type IIx, and type IIb (Schiaffino and Reggiani Citation1994; Wimmers et al. Citation2008; Lee et al. Citation2010). Type I fibres (oxidative fibres) have greater oxidative capacity to support sustained muscle contractions, whereas type IIb (glycolytic fibres) fibres are predominantly glycolytic fibres that utilize the rapid conversion of glycogen for short bursts of energy. The IIa and IIx types of fibres are intermediate to type I and IIb (Chang et al. Citation2003). The expression of myosin heavy chain (MyHC) genes is commonly used to indicate the fibre types in pig muscles because it is more precise and reliable than traditional methodologies (Gunawan et al. Citation2007; Choi and Kim Citation2009; Park et al. Citation2009). Among the breeds Berkshire, Duroc, Landrace, Meishan, Yorkshire, and LYD (Landrace × Yorkshire sows × Duroc as terminal sires) crossbreed, Meishan pigs have the lowest percentage of type IIA fibres and the highest percentage of type IIB fibres, whereas LYD has the highest proportion of type IIA fibres and the lowest of type IIB fibres (Kim et al. Citation2017). The fibre diameter in Duroc pigs is significantly larger than that in Laiwu pigs, and the proportion of oxidative fibres in longissimus dorsi is greater, while the proportion of glycolytic fibres is lower in Laiwu pigs than in Duroc (Hu et al. Citation2008). Until now, there have been no reports on the developmental regularity of muscle fibre diameter, muscle fibre density, and the composition of muscle fibres in pig skeletal muscles. Furthermore, the correlations between muscle fibre diameter, density, and fibre type composition remain unclear. Therefore, a comprehensive study on the developmental patterns of skeletal muscle fibre diameter, density, and composition can provide a theoretical basis for studies on the mechanism of improving pork meat quality.
Mashen pig is an important indigenous breed in northern China. Compared with the Western commercial breed Large White pig, Mashen pig exhibits higher adaptability, better meat quality, slower growth, and lower feed conversion rate (Zhang et al. Citation2001; Yang et al. Citation2005; Zhao et al. Citation2015). In the present study, Mashen and Large White pigs were used as experimental animals to explore the muscle fibre characteristics, the developmental expression patterns, and the effect of MyHCs expression on the diameter and density of muscle fibres. These results could provide a basis for elucidating the growth and development of skeletal muscles and improvement of meat quality.
Materials and methods
Animals
All animal procedures were conducted per the Code of Ethics of the World Medical Association (Declaration of Helsinki) for animal experiments (http://ec.europa.eu/environment/chemicals/lab_animals/legislation_en.htm). The experiment was approved by the Animal Ethics Committee of Shanxi Agricultural University (Shanxi, China). A total of 80 male pigs (40 Mashen pigs, 40 Large White pigs) that were castrated when weaned and under the same feeding management conditions were selected from the Datong Pig Farm (Shanxi, China). The pigs were slaughtered at 60, 90, 120, 150, and 180 d of age, and 16 pigs (8 Mashen, 8 Large White) were selected at each stage. Electric shock was used to stun the pigs, which were then exsanguinated.
Histological structure of muscle fibres
Within 45 min post mortem, M. longissimus thoracis, biceps femoris, and psoas major samples were taken and immediately placed in 4% paraformaldehyde solution. M. longissimus thoracis was taken from the height of the last thoracic vertebra. Biceps femoris was taken from the muscle median transverse section. Psoas major was taken from the muscle transverse section. After 24 h of immobilization, the samples were stored at 70% alcohol. First, gradient alcohol dehydration. Second, clearing. Then put the organization in 60°C wax pot for 2.5 h, the embedding was completed when liquid paraffin completely frozen. After embedding, the wax was modified and sections were made. Sections with a thickness of 5 μm were subjected to haematoxylin and eosin (HE) staining. The microscopic images were captured at 100× magnification (BX51, Olympus Corporation, Japan BX51). Muscle fibre diameter was measured on approximately 100 fibres based on the measurement method of the elliptical long-short axis, that is, the distance between the longest two points on the cross-sectional area of the muscle fibre was first obtained as the long axis, then the midpoint was taken as the short axis perpendicular to the long axis, and then the geometric mean of the two axes was obtained as the diameter of each muscle fibre. Muscle fibre density was calculated by dividing the average number of muscle fibres in 10 randomly selected microscope fields by the field area (0.59 mm2), which was then converted to the number of muscle fibre roots per square millimetre.
The expression patterns of MyHCs mRNA
M. longissimus thoracis, biceps femoris, and psoas major samples of Mashen and Large White pigs were taken shortly after exsanguination, immediately frozen in liquid nitrogen, and stored at −80°C for subsequent use. The expression patterns of MyHCs (MyHCI, MyHCIIa, MyHCIIx, and MyHCIIb) were detected by quantitative real-time polymerase chain reaction (qPCR). The primers used for amplification of MyHCs were obtained from Hu et al. (Citation2008). 18S rRNA was used as the internal control. All primers used in this study were synthesized by Sangon Biotech Co. (China). Details of primers are shown in . Total RNA was extracted using Invitrogen Ambion TRIzol LS Reagent (Life Technologies, USA). The cDNA was synthesized by reverse transcription from 500 ng total RNA using the PrimeScript RT Reagent Kit with gDNA Eraser (Takara, China) according to the manufacturer’s instructions. qPCR was performed using a SYBR® PrimeScriptTM RT–PCR Kit (Takara, China) in conjunction with an ABI-7500 real-time PCR system (Applied Biosystems, USA). The reaction conditions were as follows: pre-denaturation at 95°C for 30 s; 45 cycles at 95°C for 30 s and 60°C for 34 s; and one cycle at 95°C for 30 s, 60°C for 1 min, and 95°C for 30 s. To ensure robustness, each sample was analysed in triplicate. The relative expressions were quantified using the 2−ΔΔCt method (Ramakers et al. Citation2003).
Table 1. Primer sequences of MyHCs and 18S rRNA used in qPCR.
Statistical analyses
Data were analysed by one-way ANOVA in SPSS 22.0 software (IBM Corp., USA), and significant difference was considered at P < 0.05. Data were expressed as means ± standard error. Pearson correlation coefficients were used to evaluate the relationships between the diameter and density of muscle fibres and the expression of MyHCs.
Results
The diameter and density of muscle fibres in Mashen and Large White pigs
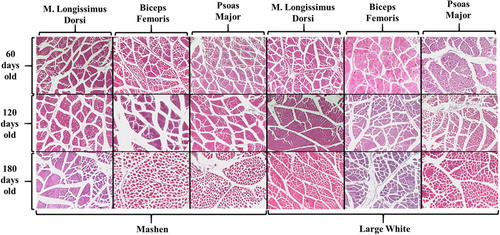
The histological structures of muscle fibres at different developmental stages of Mashen and Large White pigs are shown in . After HE staining, the cytoplasm appeared red under the microscope, the cross section was round or polygonal, the nucleus was round and stained blue-violet, and several nuclei were attached to the cell surface. The diameter and density of muscle fibres were significantly different at different developmental stages of Mashen and Large White pigs (). As the age of pigs increased, the diameter of muscle fibres increased significantly. In contrast, muscle fibre density decreased significantly with age of pigs. The diameter and density of muscle fibres was significantly different in different skeletal muscles.
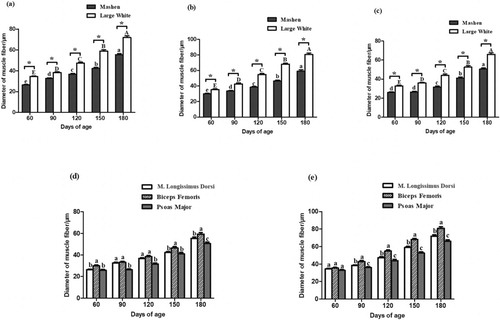
As shown in , the developmental growth patterns of the muscle fibre diameter followed a similar trend in the three skeletal muscles based on the analysis of the muscle fibre diameter – the diameter increased gradually with aging of the animals (except for the psoas major in Mashen pigs at 60 and 90 d of age), reaching the highest diameter at 180 d of age. The muscle fibre diameter in Mashen pigs was significantly lower than that in Large White pigs of the same age, and there were significant differences in fibre diameter of the three skeletal muscles (except for Large White pigs at 60 d of age) (P < 0.05). In animals of the same age, the fibre diameter of the femoral biceps was significantly larger than that of the m. longissimus thoracis and psoas major (In addition to 90 and 120 days old Mashen pigs and 60 days old Large White pigs), showing a great growth potential. The smallest fibre diameter was observed in the psoas major, indicating late development and growth potential. The density of muscle fibres followed an opposite pattern: the density of muscle fibres decreased gradually with increasing age of the animals. M. longissimus thoracis had the largest density of muscle fibres at 60, 90 of Mashen pig and 60,120 days of age in Large White pig. The density of muscle fibres in Psoas Major was the largest at 120, 150, 180 days of Mashen pig and 90,150,180 days of Large White pig. Biceps Femoris has a significantly lower muscle fibre density than the other two muscle tissues at almost stages (except the 60 days old Large White pig). The specific results are shown in supplement .
Figure 2. Muscle fibre diameter of the m. longissimus thoracis (a), biceps femoris (b), and psoas major (c) in Mashen and Large White pigs at different developmental stages. Comparison of muscle fibre diameters of the three skeletal muscles in Mashen pigs (d) and Large White pigs (e). (a–c): In different stages of Mashen pig, different lowercase letters indicate significant difference, while the same lowercase letters indicate no significant difference; In different stages of Large White pig, different capital letters indicate significant difference, while the same capital letters indicate no significant difference. * indicates that there are significant differences between Mashen and Large White pigs at the same stage. (d,e): In different tissues of the same age, different lowercase letters mean significant difference, while the same lowercase letters mean no significant difference.
The developmental expression patterns of MyHCs in Mashen and Large White pigs
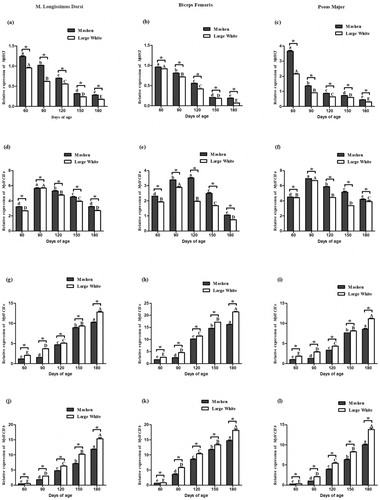
The expression of MyHCs in Mashen and Large White pigs is shown in . The expression of MYHCI decreased significantly in the m. longissimus thoracis with increasing animal age of the two breeds, reaching the lowest levels in 180-day-old animals ((a)). The expression of MyHCI in Mashen pigs was significantly higher than that in Large White pigs (P < 0.05). The expression of MyHCI followed a similar pattern in the three skeletal muscles ((b,c)). The expression of MyHCIIa in skeletal muscles of the two breeds increased initially and then decreased as the animals got older ((d–f)). The expression of MyHCIIa in Mashen pigs was significantly higher than that in Large White pigs. The expression of MyHCIIx showed a rising trend as the age of both Mashen and Large White pigs increased. Its expression in the skeletal muscles was significantly different between the two breeds (P < 0.05) ((g–i)), it was significantly lower in Mashen pigs than in Large White pigs of the same age. The expression of MyHCIIb in the skeletal muscle of both breeds increased significantly with age ((j–l)), and its expression was significantly lower in Mashen pigs than in Large White pigs of the same age.
Figure 3. The developmental expression patterns of MyHCs in Mashen and Large White pigs at different developmental stages. The developmental expression patterns of MyHCI (a), MyHCIIa (d), MyHCIIx (g) and MyHCIIb (j) in m. longissimus thoracis. The developmental expression patterns of MyHCI (b), MyHCIIa (e), MyHCIIx (h) and MyHCIIb (k) in biceps femoris. The developmental expression patterns of MyHCI (c), MyHCIIa (f), MyHCIIx (i) and MyHCIIb (l) in psoas major. Note: In different stages of Mashen pig, different lowercase letters indicate significant difference, while the same lowercase letters indicate no significant difference; In different stages of Large White pig, different capital letters indicate significant difference, while the same capital letters indicate no significant difference. * indicates that there are significant differences between Mashen and Large White pigs at the same stage.
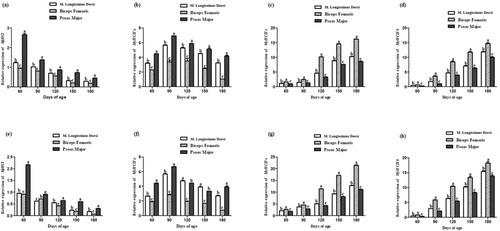
The expression of MyHCI was the highest in the psoas major and the lowest in the femoral biceps (except 90 days old Mashen pig) in both Mashen and Large White pigs (). The expression of MyHCIIa in the psoas major was higher than that in the other two skeletal muscles (except 120 and 150 days old Large White pig). The expressions of MyHCIIx and MyHCIIb were the highest in the femoral biceps and the lowest in the psoas major.
Figure 4. The expression patterns of MyHCs in different skeletal muscles. The expression patterns of MyHCI (a), MyHCIIa (b), MyHCIIx (c) and MyHCIIb (d) in Mashen pigs; (e-h) The expression patterns of MyHCI (e), MyHCIIa (f), MyHCIIx (g) and MyHCIIb (h) in Large White pigs. Note: In different tissues of the same age, different lowercase letters mean significant difference, while the same lowercase letters mean no significant difference.
Correlation analysis
The correlation analysis () revealed that the diameter of muscle fibres was negatively correlated with the expression of MyHCI, but significantly positively correlated with the expression of MyHCIIx and MyHCIIb (except the expression of MyHCI in psoas major). There was a positive correlation between muscle fibre density and the expression of MyHCI and a significant negative correlation between muscle fibre density and the expression of MyHCIIx and MyHCIIb (except the expression of MyHCI in psoas major).
Table 2. Correlation analysis between the diameter and density of muscle fibres and the expression of MyHCs.
Discussion
In oxidative fibres (MyHCI), the content of myoglobin and phospholipids is higher, the content of glycogen and the activity of ATPase are lower, the diameter of muscle fibres is smaller, and the capacity for oxygen metabolism is stronger. The characteristics of glycolytic fibres (MyHCIIb) are opposite to those of the oxidative fibres, whereas the metabolic activity and contraction properties of MyHCIIa and MyHCIIx are between those of the glycolytic and oxidative fibres. The flesh colour, marbling score, and fat content of the muscle would be higher, the muscle would be tender, the fibre diameter would be thinner, and the water retention of the muscle would be higher in muscles with higher proportion of oxidative fibres (Larzul et al. Citation1997; Maltin et al. Citation1997; Immonen et al. Citation2000). Fast-growing Large White pigs had muscles with a higher expression of MyHCIIb compared with Mashen pigs, which indicated that Large White pigs may had higher percentage glycolytic fibres than Mashen pigs. These results were consistent with previous studies (Larzul et al. Citation1997; Zhang et al. Citation2013). In addition, as the animals grew older, the diameter of muscle fibres gradually increased. It was found that the muscle fibre diameter was positively correlated with the expression of MyHCI but negatively correlated with the expression of MyHCIIx and MyHCIIb. The diameter of oxidative fibres was smaller than that of glycolytic fibres. Therefore, the increase in muscle fibre diameter may be caused by the change in muscle fibre composition, that is, the increase in the number of glycolytic muscle fibres and the decrease in the number of oxidative muscle fibre.
Katsumata et al. examined the expression patterns of MyHCs in different muscles of 1-, 12-, 26-, 45-, and 75-day-old pigs and discovered that the expression of MyHCIIb in the longissimus dorsi and biceps femoris rapidly and considerably increased from the late foetal stage to the early postnatal stage in association with the development of type IIb fibres, at least in the longissimus dorsi (Katsumata et al. Citation2017). Yang et al., who studied the longissimus dorsi in 1-day-, 1-week-, 2-week-, 1-month, 2-month, and 6-month-old Large White pigs, found that the expression of MyHCI decreased, the expression of MyHCIIb increased gradually, the expression of MyHCIIa increased and then decreased from 1 d to 2 weeks of age, and the expression of MyHCIIx did not change significantly during the development of the longissimus dorsi (Yang et al. Citation2005). Wu et al. reported a decrease in the percentage of oxidative red fibres and an increase in the percentage of glycolytic white fibres in the growing period, which contributed to the increasing glycolytic capacity and maximum contraction speed of the longissimus dorsi, but the decreasing oxidation capacity during different developmental stages (63, 98, 161 days old) (Wu et al. Citation2015). In the current experiment, we found that the expression of MyHCI showed a downward trend as the age of pigs from the two breads increased from 60 to 180 days, while the MyHCIIb increased in number. These results indicated that the percentage of oxidative fibres showed a downward trend and the glycolytic fibres showed an increased trend with the increase of the age in pig, thus corroborating the above studies.
As an excellent local breed in China, Laiwu pig has fine meat characteristics, such as tender and juicy meat and rich fragrance, among others. In this breed, the proportion of oxidative fibres in longissimus dorsi is higher and the proportion of glycolytic fibre is lower than they are in Duroc, indicating a lower glycolytic but higher oxidative metabolism of the longissimus dorsi in Laiwu pigs as compared with Duroc (Hu et al. Citation2008). Consequently, potentially more lipids are used as an energy substrate, resulting in higher intramuscular fat content, tenderness, and water-holding capability of the longissimus dorsi in Laiwu (Hu et al. Citation2008). The Meishan pigs exhibit lower growth rate, poorer feed efficiency, and lower lean meat content than the conventional Western pig breeds (Bidanel et al. Citation1990; White et al. Citation1995), but the sensory quality of their meat is superior (Touraille et al. Citation1989; Suzuki et al. Citation1991). Lefaucheur et al. compared the mRNA expression and protein levels of four MyHCs in the longissimus dorsi and rhomboids in the Large White and Meishan pigs and found that the expression of MyHCIIb was significantly lower in Meishan pigs than in Large White pigs, which may be caused by higher meat quality in Meishan pigs (Lefaucheur et al. Citation1997, Citation2004). Comparisons between wild (Large White, Duroc, Landrace) and domestic pigs (Meishan, Erhualian) suggested that intensive selection for lean muscle growth in modern pigs induced a shift in muscle metabolism toward a more glycolytic and less oxidative fibre type (Rahelic and Puac Citation1981; Weiler et al. Citation1995). Subsequently, the ability of glucose catabolism was improved, while the ability of oxidative metabolism was weakened, which was related to the lean meat rate, feed conversion rate, and growth rate of pigs (Tanabe et al. Citation2001; Muller et al. Citation2002; Lefaucheur et al. Citation2004). The present experimental results were consistent with previous studies. The percentage of oxidative fibres was higher and that of glycolytic fibres was lower in Mashen pigs than in Large White pigs, which was consistent with the slow growth rate, low feed utilization rate, and low lean meat rate of Mashen pigs. This indicated that the muscle of Mashen pigs exhibits a high ability to use fat as a source of energy.
Conclusion
As the age of Mashen and Large White pigs increased, the diameter of muscle fibres increased and their density decreased gradually, the expression of MyHCI increased, the expression of MyHCIIa decreased after an initial increase, and the expression of MyHCIIx and MyHCIIb decreased. The diameter of muscle fibres was negatively correlated with the expression of MyHCI and positively correlated with the expression of MyHCIIx and MyHCIIb (except the expression of MyHCI in psoas major). These data provide valuable information for understanding the developmental expression patterns of MyHCs and the correlation between muscle fibre characteristics and MyHCs expression in pig skeletal muscles.
TAAR_1756823_supplement_Table
Download MS Word (17.6 KB)Acknowledgments
Thanks to all the staff of the Datong pig farm. This work was supported by the Program for Sanjin Scholar (grant numbers 2016, 2017), the Fund for Shanxi 1331 Project (grant number 2017), the Foundation of Science and Technology Innovation Team of Shanxi Province (grant number 201705D131028-19), the Science and Technology Key Project of Shanxi Province (grant number 201803D221022-1), the National Natural Science Foundation of China (grant number 31872336) and the Program for the Top Young Academic Leaders of Higher Learning Institutions of Shanxi.
Disclosure statement
No potential conflict of interest was reported by the author(s).
ORCID
Guoqing Cao http://orcid.org/0000-0002-1313-8469
Additional information
Funding
References
- Bidanel JP, Caritez JC, Legault C. 1990. Ten years of experiments with Chinese pigs in France. 1. Breed evaluation. Pig News Info. 11:345–348.
- Chang KC, da Costa N, Blackley R, et al. 2003. Relationships of myosin heavy chain fibre types to meat quality traits in traditional and modern pigs. Meat Sci. 64:93–103. doi: 10.1016/S0309-1740(02)00208-5
- Choi YM, Kim BC. 2009. Muscle fiber characteristics, myofibrillar protein isoforms, and meat quality. Livest Sci. 122:105–118. doi: 10.1016/j.livsci.2008.08.015
- Choi YM, Ryu YC, Kim BC. 2007. Influence of myosin heavy- and light chain isoforms on early postmortem glycolytic rate and pork quality. Meat Sci. 76:281–288. doi: 10.1016/j.meatsci.2006.11.009
- England EM, Scheffler TL, Kasten SC, Matarneh SK, Gerrard DE. 2013. Exploring the unknowns involved in the transformation of muscle to meat. Meat Sci. 95:837–843. doi: 10.1016/j.meatsci.2013.04.031
- Gil M, Oliver MA, Gispert M, et al. 2003. The relationship between pig genetics, myosin heavy chain I, biochemical traits and quality of M. longissimus thoracis. Meat Sci. 65:1063–1070. doi: 10.1016/S0309-1740(02)00324-8
- Gunawan AM, Park SK, Pleitner JM, Feliciano L, Grant AL, Gerrard DE. 2007. Contractile protein content reflects myosin heavy-chain isoform gene expression. J Anim Sci. 85:1247–1256. doi: 10.2527/jas.2006-511
- Hu H, Wang J, Zhu R, Guo J, Wu Y. 2008. Effect of myosin heavy chain composition of muscles on meat quality in Laiwu pigs and Duroc. Sci China C Life Sci. 51:127–132. doi: 10.1007/s11427-008-0016-x
- Immonen K, Ruusunen M, Hissa K, Puolanne E. 2000. Bovine muscle glycogen concentration in relation to finishing diet, slaughter and ultimate pH. Meat Sci. 55:25–31. doi: 10.1016/S0309-1740(99)00121-7
- Joo ST, Kim GD, Hwang YH, Ryu YC. 2013. Control of fresh meat quality through manipulation of muscle fiber characteristics. Meat Sci. 95:828–836. doi: 10.1016/j.meatsci.2013.04.044
- Katsumata M, Yamaguchi T, Ishida A, Ashihara A. 2017. Changes in muscle fiber type and expression of mRNA of myosin heavy chain isoforms in porcine muscle during pre- and postnatal development. Anim Sci J. 88:364–371. doi: 10.1111/asj.12641
- Kim JM, Lee SH, Ryu YC. 2017. Comparisons of meat quality and muscle fibre characteristics on multiple pig breeds and sexes using principal component analysis. Anim Prod Sci. 58:2091–2099. doi: 10.1071/AN16223
- Klosowska D, Fiedler I. 2003. Muscle fibre types in pigs of different genotypes in relation to meat quality. Anim Sci Pap Rep. 21:49–60.
- Larzul C, Lefaucheur L, Ecolan P, et al. 1997. Phenotypic and genetic parameters for longissimus muscle fiber characteristics in relation to growth, carcass, and meat quality traits in large white pigs. J Anim Sci. 75:3126–3137. doi: 10.2527/1997.75123126x
- Lee SH, Joo ST, Ryu YC. 2010. Skeletal muscle fiber type and myofibrillar proteins in relation to meat quality. Meat Sci. 86:166–170. doi: 10.1016/j.meatsci.2010.04.040
- Lefaucheur L, Hoffman R, Okamura C, et al. 1997. Transitory expression of alpha cardiac myosin heavy chain in a subpopulation of secondary generation muscle fibers in the pig. Dev Dyn. 210:106–116. doi: 10.1002/(SICI)1097-0177(199710)210:2<106::AID-AJA4>3.0.CO;2-K
- Lefaucheur L, Milan D, Ecolan P, Le Callennec C. 2004. Myosin heavy chain composition of different skeletal muscles in Large White and Meishan pigs. J Anim Sci. 82:1931–1941. doi: 10.2527/2004.8271931x
- Listrat A, Lebret B, Louveau I, et al. 2016. How muscle structure and composition influence meat and flesh quality. Sci World J. 2016:1–14. doi: 10.1155/2016/3182746
- Maltin CA, Warkup CC, Matthews KR, Grant CM, Porter AD, Delday MI. 1997. Pig muscle fibre characteristics as a source of variation in eating quality. Meat Sci. 47:237–248. doi: 10.1016/S0309-1740(97)00055-7
- Muller E, Rutten M, Moser G, Reiner G, Bartenschlager H, Geldermann H. 2002. Fibre structure and metabolites in M. longissimus dorsi of wild Boar, Pietrain and Meishan pigs as well as their crossbred generations. J Anim Breed Genet. 119:125–137. doi: 10.1046/j.1439-0388.2002.00328.x
- Park SK, Gunawan AM, Scheffler TL, Grant AL, Gerrard DE. 2009. Myosin heavy chain isoform content and energy metabolism can be uncoupled in pig skeletal muscle. J Anim Sci. 87:522–531. doi: 10.2527/jas.2008-1269
- Rahelic S, Puac S. 1981. Fibre types in longissimus dorsi from wild and highly selected pig breeds. Meat Sci. 5:439–450. doi: 10.1016/0309-1740(81)90042-5
- Ramakers C, Ruijter JM, Deprez RH, Moorman AF. 2003. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci Lett. 339:62–66. doi: 10.1016/S0304-3940(02)01423-4
- Schiaffino S, Reggiani C. 1994. Myosin isoforms in mammalian skeletal muscle. J Appl Physiol (1985). 77:493–501. doi: 10.1152/jappl.1994.77.2.493
- Suzuki A, Kojima N, Ikeuchi Y, et al. 1991. Carcass composition and meat quality of Chinese purebred and European x Chinese crossbred pigs. Meat Sci. 29:31–41. doi: 10.1016/0309-1740(91)90021-H
- Tanabe R, Murakami T, Kawahara T, et al. 2001. Composition of myosin heavy chain isoforms in relation to meat texture in Duroc, Landrace and Meishan pigs. J Anim Sci. 72:230–237.
- Touraille C, Monin G, Legault C. 1989. Eating quality of meat from European x Chinese crossbred pigs. Meat Sci. 25:177–186. doi: 10.1016/0309-1740(89)90070-3
- Weiler U, Appell HJ, Kremser M, Hofacker S, Claus R. 1995. Consequences of selection on muscle composition. A comparative study on gracilis muscle in wild and domestic pigs. Anat Histol Embryol. 24:77–80. doi: 10.1111/j.1439-0264.1995.tb00013.x
- White BR, Lan YH, McKeith FK, Novakofski J, Wheeler MB, McLaren DG. 1995. Growth and body composition of Meishan and Yorkshire barrows and gilts. J Anim Sci. 73:738–749. doi: 10.2527/1995.733738x
- Wimmers K, Ngu NT, Jennen DG, et al. 2008. Relationship between myosin heavy chain isoform expression and muscling in several diverse pig breeds. J Anim Sci. 86:795–803. doi: 10.2527/jas.2006-521
- Wu F, Zuo JJ, Yu QP, et al. 2015. Effect of skeletal muscle fibers on porcine meat quality at different stages of growth. Genet Mol Res. 14:7873–7882. doi: 10.4238/2015.July.14.13
- Yang WP, Cao GQ, Shi JZ, Liu JH, Zhou ZX. 2005. Study on the finishing ability of different cross combination in pig. Chinese J Anim Sci. 41:48–49.
- Zhang JG, Wang X, Du MH, Zhou ZX. 2001. Species diversity and the way to protect Ma Shen Zhu. J Shanxi Agric Univ. 21:188–191.
- Zhang SH, Zhu L, Wu ZH, et al. 2013. Effect of muscle-fiber type on glycogenin-1 gene expression and its relationship with the glycolytic potential and pH of pork. Genet Mol Res. 12:3383–3390. doi: 10.4238/2013.September.4.4
- Zhao YY, Gao PF, Li W, et al. 2015. Study on the developmental expression of Lbx1 Gene in longissimus dorsi of Mashen and large white pigs. Ital J Anim Sci. 14:109–113.