?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Effects of lavender essential oil nanoemulsion (LEON) on the growth, body composition, haematology and immunity of Oncorhynchus mykiss was investigated. A total of 276 healthy fishes were distributed into 12 cages to four treatments in triplicate. The fishe were fed a diet consisting of 0, 100, 150 and 200 ml/kg of LEON for 60 days. Results showed that WG% and SGR (%/day) significantly were increased at the different levels of LEON (p < .05). The highest of WG (%) and SGR were found in 150 ml/kg of LEON (252.16%) followed by 100 and 200 ml/kg. In contrast, FCR was decreased with increasing of dietary supplemental of LEON. The haematology parameters such as RBC, haemoglobin , haematocrit and MCH were significantly increased in 200 ml/kg of LEON compared to the control. ACH50, SOD, lysozyme activity, IgM and total Ig were significantly higher in 200 ml/kg of LEON treatment compared to the control (p < .05). LEON treatments also had a significant influence on body composition like protein and lipid content. Overall, our results interestingly demonstrated that the effect of supplemental dietary LEON especially at the level of 150 and 200 ml/kg had a positive and significant effect on some growth and immunity of rainbow trout.
Introduction
Fish is known as a valuable part of a healthy diet (Adams and Standridge Citation2006). There are enormous differences among countries in the aquaculture growth rate and development. The total aquaculture production of Iran has been 25,800 tons in 1994 which was approximately 8% of the total fisheries production (Kalbassi et al. Citation2013). Improvement of the aquaculture economy depends on biology progresses, nutrition and the environmental management of the production cycle. Many products search for improvement of fish growth performance promoters and antibiotics agrochemicals (Santos et al. Citation2009). The pharmaceutical properties of aromatic plants are partially attributed to essential oils (Edris Citation2007). Essential oils are volatile, natural and complex compounds which are characterized by a strong odour and formed by aromatic plants as secondary metabolites. The biological effects of essential oils are due to the synergism of all molecules or reflection of the main molecules at the highest levels according to the gas chromatographic analysis (Bakkali et al. Citation2008). Essential oils are obtained by various methods such as expression, fermentation or extraction, but the steam distillation method is commercially the most used method. Essential oils basically comprise two classes of compounds: Terpenes and phenylpropenes. Terpenes are subdivided based on the 5 – carbon isoprene unit (building block) into mono, sesqui and diterpenes where the numbers of isoprene units are 2, 3 and 4, respectively, while the phenylpropenes consist of 6 – carbon aromatic ring with a 3 – carbon side chain (C6–C3 compounds) (Clegg et al. Citation1980; Cooke et al. Citation1998). Lavandula officinalis is belonged to Labiatae (Lamiaceae) family and is known as lavender. It carries glycoside, saponins and essential oils. It is a very important drug and has a wide usage such as painkiller, antiseptic, sedative (in asthma and epilepsy), expectorant, relieving the urinary tract infection, healing eczema wounds, strengthening nerves and heart. Zuzarte et al. (Citation2013) reported that lavender essential oil carries different compounds such as camphor, fenchone, borneol, terpineol and cineole.
Rainbow trout (Oncorhynchus mykiss) is the most cultivated cold-water fish in the aquaculture industry of Iran. Due to intensive culture practices for increased production, disease management continues to pose a serious threat to the aquaculture industry. The use of immunostimulants in aqua-feed is considered as a modern and favourable alternative for antibiotics and vaccines as a prophylactic measure in the intensive aquaculture systems.
Immunostimulants also have the ability to increase resistance to microbial infections and stressors like handling, transportation, grading and poor water quality in cultivated fish (Raa Citation2000). Nanoemulsions consist of fine oil-in-water dispersions and have droplets with the size ranges of 100–600 nm (Sarker Citation2005). Nanoemulsion has a higher physical stability period, antibacterial effects and fine particle size that prevent them from joining and flocculation. They also require a low concentration of surfactant. Based on past research, a few research has been done about the effect of EON on fish performance. But so far, no research has been done about the effects of LEON on O. mykiss, which is one of the innovations of this research work. Therefore, this study aimed to determine the effect of different levels of LEON on growth performance, body composition, haematology, and immunity status of O. mykiss.
Material and methods
Rainbow trout were brought from Shirabad, Khanbebin City, Golestan Province, Iran and stocked in cages (80 × 120 cm). For this experiment, 276 healthy fishes (average weight of 10.00 ± 0.2 g) were randomly distributed into 12 cages assigned to 4 treatments repeated in triplicate (23 fishes in each repetition). Physical and chemical characteristics of experimental place were measured, which were included: soluble oxygen (7.97 mg/l), saturated oxygen content percentage in water (81.7), temperature (12°C), EC (395 µs/cm), total soluble solids (189.6 mg/l), salinity (0.19%) and pH = 8.07.
Preparation of LEON
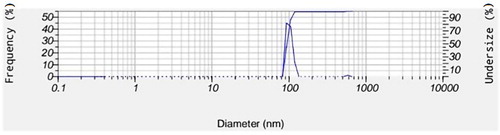
The LEO was prepared from Barij Essence Company (Iran) with 100% purity grade (). Nanoemulsion was prepared with minor changes based on the method of Ebrahimi et al. (Citation2013) and Ebrahimi and Salmanpour (Citation2014). For this purpose, the mixture of lecithin and tween 80 were added to LEO. Also, 2 ml of ethanol was added to the mixture, to obtain a homogenous solution. The prepared solution was kept on the magnetic mixer to be roughly mixed. Deionized water also was simultaneously added to the organic phase of the mixture. The percentage of lecithin, tween 80, essential oil (EO) and water were obtained in a total volume of 10 ml. Prepared samples were kept on the mixer. Then nanoemulsion was placed in an ultrasonic bath according to the specified time to obtain a homogenous solution. The organic solution was separated using rotary at 40°C after the preparation of nanoemulsion. Then after 10 min, the final volume of the water phase was checked and the final volume was set in 10 ml. The particle size for nanoemulsion of lavender is given in .
Table 1. Profile of lavander essential oil (LEO).
Experimental diets
To prepare diet, special commercial concentrate food of O. mykiss was purchased from Faradaneh company (). The nanoemulsion of lavender with the levels of 100, 150 and 200 ml/kg were sprayed uniformly over food surfaces. The basic food was not containing LEO. For the control treatment, only basic nanoemulsion (lecithin and tween 80) without LEO was added to basic diet for the same test conditions. Bovine gelatin was used to protect foods and preventing the release of nanoemulsion of EO to water (Ramsden et al. Citation2009). For this purpose, 50 ml from water solution with 10% bovine gelatin was sprayed on the 400 g of food. Then food containing LEON was dried in the normal room temperature (to prevent evaporation of EO). The prepared diet was weighed, packaged and encoded for the treatment groups and using the zipper nylon bags, then all diets were stored in the refrigerator (Kondoor et al. Citation2013). Experimental treatments were fed for two times in a day, at 08:00 and 15:00, at 3% rate of body weight for 60 days.
Table 2. Diet approximate composition (%).
Growth performance
At the end of the experiment, fish were starved for 24 h to let the stomach be empty and then, the calculation growth performances such as final weight, weight gain percentage (WG%), final length, SGR, FCR, survival rate (SR) and fish net production according to the following relationships (Teimouri et al. Citation2013)
Weight gain percentage = 100 [(Mean final body weight – Mean initial body weight)/ Mean initial body weight],
Specific growth rate = 100[(Ln final weight – Ln initial weight)/ Experiment days],
Feed conservation ratio = Amount of eaten food (g)/ increased body weight,
Survival rate = 100 × (fish number at the beginning of the period- fish number at the end of the period),
Fish Net Production = [(Mean final weight (g)/ mean initial weight (g)] × number of remained fish at the end of the period.
Sample collection and blood analysis
Six fish from each cage were randomly selected. Blood samples were collected using 2 cc syringes from the caudal vein. 0.5 cc of blood was impregnated with anticoagulant (heparin) to determine hematology parameters such as RBC, WBC, Hct, MCV, MCHC, Neutrophils, Lymphocytes, Monocytes and Eosinophils. 1.5 cc of blood remainder was centrifuged at 5000 rpm for 10 min to conduct immunity parameters such as serum alternative complement activity (ACH50), lysozyme activity, superoxide dismutase (SOD), immunoglobulin M (IgM), total immunoglobulin.
Haematology parameters
Haematological tests were measured in blood samples containing heparin anticoagulant. The blood factors including RBC, WBC, Hct, MCV, MCHC, Neutrophils, Lymphocytes, Monocytes and Eosinophils which were measured according to the Feldman et al. (Citation2000).
Immunity parameters
Alternative pathway total haemolytic complement assay
Pathway activity of serum complement was measured based on the RBC haemolysis of rat and with the help of Waley and North (Citation1997), Boesen et al. (Citation1999) and Amar et al. (Citation2000). The curve was drawn for calculation of pathway activity of complement using a Log–Log Graph. The volume of serum which causes 50% haemolysis is the phrase of sample complement activity.where k is the amount of serum (mm), 0.5 is the constant number.
Lysozyme activity of serum
Lysozyme activity of serum was determined according to the Sahu et al. (Citation2006). For this purpose, 15 µl of plasma was added to plates to form of ELISA. Then, 150 µl suspension of Micrococcus lysodeikticus bacteria was added in 0.02 molar buffered sodium citrate (about 0.02 mg/l with pH = 5.5). In the end, optical absorption was read at 450 nm with the help of ELISA reader (Bio-Tek model- made of USA). At 1 h, after storage at room temperature, optical absorption was measured again. Lysozyme lyophilized egg whites (Sigma) was also used to draw a standard curve.
Superoxide dismutase (SOD)
Superoxide dismutase of serum through spectrophotometry and Ferricytochrome C method was measured using zeaxanthin/xanthine oxidase as the source of superoxide radicals. Reaction mixture was included 50 mM buffered potassium phosphate (with pH = 7.8), 0.1 mM EDTA, 0.1 ml xanthine, 0.013 ml cytochrome C and 0.024 ml xanthine oxidase. An activity unit was defined as the amount of required enzyme to produce a 50% inhibition of ferricytochrome C. Then reduction was measured at 550 nm (McCord and Fridovich Citation1969). Enzyme activity was reported according to the unite per ml.
Serum immunoglobulin M (IgM)
Immunoglobulin was measured using the immunoturbidimetric method (Eurolyser model).
Total serum immunoglobulin
Total serum immunoglobulin was measured using Siwicki et al. (Citation1994) method. The basis of the work was based on the colorimetric method (Bradford Citation1976), which absorption of 250-G Blue Brilliant Coomassie solution (as paintable matter) when binding to serum proteins in serum proteins in 595 nm to calculate the number of used proteins. In this method, a standard solution from 0, 250, 500, 1000, 1500 and 2000 µg of Bovine Serum Albumin was used.
Body composition
To measure the composition of the body, six fishes were randomly selected from each treatment after 60 days. After removing the intestines and viscera and separating the head and fins, it has become a fillet and then their fleshy tissue was minced. The resulting mixture was kept at −20°C in the freezer before the analysis. Water content was determined by placing the whole fish in a pre-weighed aluminium foil tray for drying in an electric oven at 65–80°C till constant weight. Ash content was estimated by burning 500 mg of sample in a pre-weighed heat resistant China clay crucible placed in a Muffle furnace for 7 h at 500°C and reweighed after cooling. Fat content was estimated by dry extraction following the method of Bligh and Dyer (Citation1959) and Salam and Davies (Citation1994). Powdered dry tissue (3 mg) was mixed into 10 ml solution of chloroform and methanol (in the ratio 1:2) and stirred with a glass rod. The resultant mixture was left overnight and then centrifuged. After centrifugation, the clear supernatant was removed carefully into washed, dried and pre-weighed small bottles. These bottles were then put in an oven at 40–50°C to evaporate the solvent leaving the lipid fraction. Total protein in dry mass was calculated by the difference method from the mass of other main constituents like ash, lipid and water (Caulton and Bursell Citation1977; Dawson and Grimm Citation1980; Salam and Davies Citation1994; Dempson et al. Citation2004).
Data analysis
Data analysis was performed using SAS software (Version 9.3). Mean comparison analysis of treatments also was done by Duncan test at a 95% confidence level.
Results
Effects of dietary LEON on growth and feed utilization
The data revealed that dietary supplemental level of 200 ml/kg LEON significantly affected on the FW, and the final length of rainbow trout (p < .05). The FW of O. mykiss under treatments 1 and 2 were increased but there were no significant differences in these treatments with control. According to the results, the highest amount of WG (%) was obtained in treatment 2 with 150 ml/kg (252.16%) followed by treatments 1 and 3.
The SGR was significantly higher in the 150 ml/kg of LEON treatment compared to the control and other treatments (p < .05), but no obvious difference was found among the 100 and 200 ml/kg LEON (p > .05). Mean comparison of FCR indicated a significant difference among experimental treatments (p < .05). Hence, the FCR was decreased with increasing of LEON. The highest and lowest FCR were found in the control and 200 ml/kg of LEON. Fishes net production were 61.39, 63.39 and 57.58 g in 1 kg in the dietary supplemental level of 100 (Treatment 1), 150 (treatment 2) and 200 ml/kg (treatment 3) of LEON respectively. The differences in treatments of 1 and 2 were significant in comparison with control only. There was no significant difference in survival rate (SR) among all dietary supplemental levels of LEON (p > .05), although survival rate was increased in treatments 1, 2 and 3 ( and ).
Table 3. Growth and feed performance (Mean ± SD) of O. mykiss fed with LEON after 60 days.
Table 4. Haematological parameters (Mean ± SD) of fish fed with LEON after 60 days.
Non-specific immune response
As shown in , immunity parameters like ACH50, lysozyme activity, SOD, IgM and total immunoglobulin content were significantly higher in fish fed at a level of 200 ml/kg of LEON compared to the control (p < .05). In this experiment, the immune response of rainbow trout gradually increased with increasing LEON levels up to 200 ml/kg.
Table 5. Immune response of O. mykiss fed with LEON after 60 days.
ACH50 of fish fed with treatments of 150 mg/kg LEON also was increased as compared to control treatment, but their differences were not significant (p > .05). Whereas, lysozyme activity of the fish fed with 100 and 150 of LEON were significantly increased as compared to control (p < .05). During the study period, the content of SOD, IgM and total immunoglobulin was not affected by the level of 100 ml/kg of LEON supplementation compared to the control ().
Effects of the dietary LEON on the body composition of O. mykiss
The body composition of fish has been shown in . The dietary supplemental levels of LEON had a significant effect on crude protein and crude lipid content of O. mykiss (p < .05). Protein of fish fed by 200 ml/kg of LEON (20.62%) was higher than control (18.50%) flowed by 150 ml/kg (20.17%). According to the results, crud lipid of fish fed with 150 ml/kg of LEON (9.05%) was higher than other treatments and control. Although, it did not show a significant difference with the treatment of 200 ml/kg of LEON. In the present study, moisture and crud ash of body composition were not affected using different levels of LEON (p > .05).
Table 6. Mean body composition (Mean ± SD) of O. mykiss fed with LEON after 60 days.
Discussion
Medicinal plants could be used as stimulants of growth and immunity in aquaculture (Kirubakaran et al. Citation2010). In recent years, more studies confirmed the positive effects of herbal medicines such as Quercus brantii (Rashidian et al. Citation2018), Rhodomyrtus tomentosa (Na-Phatthalung et al. Citation2018), Stachys lavandulifolia (Moghanlou et al. Citation2018), Coriandrum sativum (Farsani et al. Citation2019), Aloe barbadensis (Mehrabi et al. Citation2019), Pandanus tectorius (Awad et al. Citation2019) and Thymus vulgaris (Zargar et al. Citation2019) on the growth of rainbow trout, physiology and immunity parameters.
L. officinalis is a plant medicine of family Lamiaceae and has long been used as antibacterial, antifungal and antidepressant (Nikfarjam et al. Citation2017). The EO of L. officinalis contains monoterpenes (1–3%). The most important components are linalyl acetate (30–55%), linalool (20–35%), beta-ocimene, cineol, camphor, sesquiterpene, caryophyllene oxide, tannin, rosmarinic acid derivatives, coumarine and flavonoids (Denner Citation2009). The results of this experiment showed that the dietary supplemental levels of LEON positively affected the growth and feed utilization, haematological and immunological parameters of rainbow trout.
In our experiment, supplementation of LEON at the level of 150 and 200 ml/kg increased FW and WG (%) after feeding the fish for 60 days. In contrast, FCR decreased significantly. Similarly, many studies have been reported the potential effect of plant extracts on growth performance. Overall, the positive and significant response of some growth parameters to different levels of LEON, probably because of the presence of some components such as Cineole, Camphor, Limonene and Linalool which have high values in the studied lavender profile. In fact, these compounds may reduce synthesis of the reactive oxygen species (ROS) in cellular liver. Farsani et al. (Citation2019) reported that using 2% of Coriander seed extract (C. sativum) significantly increased values of SGR, final weight (FW) and condition factor (CF) in O. mykiss. Similarly, fish fed from the diets of 2% and 8% S. lavandulifolia extract had the highest weight, SGRs and average daily growth (Moghanlou et al. Citation2018).
Some medicine compounds cause appetite and pancreatic stimulation of digestive enzymes secretion and enhance the digestion (Nobakht et al. Citation2013). Herbs medicine and their derivatives such as natural production could control digestive activities and limit colonization of pathogenic and nonpathogenic species of bacteria in fish guts. This may lead to a greater efficiency in the utilization of food, resulting in enhanced growth performance and improved feed efficiency (Reverter et al. Citation2014).
Crude protein and lipid content of body composition were increased, but parameters of moisture and ash percentage were not affected by dietary supplemental levels of LEON.
Evaluation of haematological parameters is a tool to facilitate fish health management (Chen et al. Citation2004) that can help in the diagnosis of anaemia, poisoning, infectious diseases and food deficiency in aquatic animals (Fazio et al. Citation2003). In this study, RBC, Hct, Hb and MCH in 200 ml/kg of LEON were increased, which indicates the positive effect of higher level of LEON. In this regard, dietary supplemental level of LEON with 200 mg/kg of Echinacea purpurea leaf extract showed a significant increase effect on haematological indices such as RBC and Hb compared with control treatment after 60 days of feeding trial (Akbary and Kakoolaki Citation2019). Adel et al. (Citation2015) reported that haematocrit and haemoglobin values were increased in all the groups of fry Caspian white fish (Rutilus frisii kutum) fed with peppermint (Mentha piperita) diet. Prasad and Priyanka (Citation2011) described that the increase of RBC in Pangasianodon hypophthalmus fed with different levels of fruit extract (Garcinia gummi-gutta), which was related to iron. The blood haemoglobin increase indicates the superiority of the respiratory state of O. mykiss that fed with LEON treatment because the rising rate of haemoglobin causes an increase in the transfer of respiratory gases.
Also WBC, MCV, MCHC, neutrophils, monocytes and eosinophils were not affected by different LEON treatments. Blood index of fish can be affected by some factors such as species, size, age, physiological status, environmental condition and diet (Brunt and Austin Citation2005). Improvements in the parameters of fish health are affected by medicinal plants, which depend on their numerous bioactive compounds (Stauth Citation2007).
Medicinal plants have been demonstrated as the immune stimulants and growth promoters with minor effects on animals (Abdel- Tawwab Citation2016). In the present work, all the immunity parameters such as ACH50, lysozyme activity, superoxide dismutase, IgM and total immunoglobulin were increased with an increase of LEON levels. The highest immunity parameters were observed in fish fed with dietary supplemental level 200 ml/kg supplementation. This indicates an increase of antioxidant activity of LEON to prevent free radicals such as H2O2, singlet oxygen, superoxide, hydroxyl, etc. In fact, antioxidant compounds are combined with free radicals and thus able to prevent oxidation of these compounds. It causes the improvement of immune system of fish body and finally prevent cellular damage (Kohen and Nyska Citation2002). Most plant EO in having phenolic compounds in their structure are similar with each other (Mohajerfar et al. Citation2012). Phenolic compounds due to having a hydroxy factor effectively acts as a hydrogen agent. Therefore, it acts as an effective antioxidant in the cellular mechanisms of animals. Of course, probably the application of various levels of LEON due to their small size in the present experiment (90–100 nm) and long-term physical stability (without any apparent coagulation and sediment or their two-phase) causes more absorption of antioxidants and their efficiency (antimicrobial materials). Schiavone et al. (Citation2007) stated that phenolic compounds are important antioxidants activity responsible compounds. The main activity of phenolic compounds is the improvement of oxidative stability in the body.
In case of increase of enzyme activity of lysozyme under different level of LEON similar results was reported by Fadeifard et al. (Citation2018) in feeding rainbow trout with essential oils of ginger (Zingiber officinale), black seed (Nigella sativa) and cone flower (Echinacea angustifolia) for 21 days with 1% led to significant enhancement in serum lysozyme. Furthermore, the inclusion of 1% Quercetin and 3% N. sativa oil in a rainbow trout diet led to a significant increase in lysozyme (Awad et al. Citation2013).
IgM, the primary antibody of fish, is a major component of the teleost humoral immune system, and it is used to identify and neutralize foreign objects (Ngugi et al. Citation2015). In the current study, the result showed that IgM levels were increased in specimens fed with 150 and 200 ml/kg levels of LEON compared to 100 ml/kg and control treatments. Sivaram et al. (Citation2004) reported increased Ig levels in Epinephelus tauvina (greasy groupers) after feeding them on an herbal diet containing purified active components of Ocimum sanctum (holy basil), Withania somnifera (Indian ginseng) and Myristica fragrans (nutmeg). Similar to our results, Yousefi et al. (Citation2020) reported that dietary lavender (Lavandula angustifolia) extract supplementation significantly improved serum lysozyme, ACH50, and total Ig of common carp after 70 days feeding period.
Immunoglobulin is a natural antibodies component and it is completely regulated and produced in the absence of foreign antigen elicitors, as well as immediately provides immediate protection against unpromising factors. Changes in blood serum immunoglobulin have been reported in most studies (Nayak et al. Citation2007). In this research, diets enriched with LEON at 200 ml/kg enhanced the total immunoglobulin. Hoseini and Yousefi (Citation2019) showed that thyme (T. vulgaris) extract significantly improved the serum total protein, total Ig, lysozyme and ACH50 and mitigated the adverse effects of oxytetracycline on these parameters in rainbow trout. In contrast, Farsani et al. (Citation2019) reported that supplementation of diet with C. sativum extract in rainbow trout had no significant effects on levels of IgM and total immunoglobulin.
Superoxide dismutase enzyme is the first defensive level against oxidative stresses from superoxide radicals (McCord and Fridovich Citation1969). In our study, fish fed with the 200 ml/kg of LEON had the highest SOD level and other immunity parameters. Previous study showed that the oral administration of rosemary leaf powder (Yousefi et al. Citation2019) and artemisia (Artemisia annua) extract (Sarhadi et al. Citation2020) improved SOD activity in common carp. In addition, Rafieepour et al. (Citation2018) showed that different concentrations of oregano extract (Origanum vulgare L.) significantly increased serum SOD activity in rainbow trout. It has been showed that flavonoid and phenolic compounds in herbs and their compounds are responsible for their high antioxidant capacity (Reyes-Cerpa et al. Citation2018). Overall, in the present study, the enhancement of some parameters such as growth, haematological, immunity and body composition of O. mykiss fed with LEON may be due to the efficacy of linalool chemotype of the lavender as a stress-reducing agent (Souza et al. Citation2019).
Conclusion
The current study suggests that dietary supplemental level of LEON, especially 150 and 200 ml/kg, could be considered a good food additive to improve the growth performance and immune status of rainbow trout. The results of the current study activity confirm the potential of LEON as the growth promoter and stimulatory agent of the immune system. The benefits of different levels of LEON on the fish might be due to lower stress that boosts fish immune and health, which characterized by improved growth performance. Therefore, due to a few number of studies on the effect of nanomedicine on fish performance especially LEON, we have added the suggestion in the conclusion to the use of higher supplemental dietary levels of LEON in future studies to obtain more precise results.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Abdel- Tawwab M. 2016. Feed supplementation to freshwater fish: experimental approaches. Berlin: LAP Lambert Academic.
- Adams SM, Standridge JB. 2006. What should we eat? Evidence from observational studies. South Med J. 99(7):744–748.
- Adel M, Amiri AA, Zorriehzahra J, Nematolahi A, Esteban MÁ. 2015. Effects of dietary peppermint (Mentha piperita) on growth performance, chemical body composition and hematological and immune parameters of fry Caspian white fish [Rutilus frisii (kutum)]. Fish Shellfish Immun. 45(2):841–847.
- Akbary P, Kakoolaki S. 2019. Growth, hematological, innate immune responses of Mugil cephalus fed with Echinacea purpurea-supplemented diet against Photobacterium damselae infections. Int J Environ Sci Technol. 16(1):325–334.
- Amar EC, Kiron V, Satoh S, Okamoto N, Watanabe T. 2000. Effects of dietary β- carotene on the immune response of rainbow trout (Oncorhynchus mykiss). Fisheries Sci J. 66:1068–1075.
- Awad E, Austin D, Lyndon AR. 2013. Effect of black seed oil (Nigella sativa) and nettle extract (Quercetin) on enhancement of immunity in rainbow trout [Oncorhynchus mykiss (Walbaum, 1972)]. Aquac J. 388:193–197.
- Awad E, Austin D, Lyndon A, Awaad A. 2019. Possible effect of hala extract (Pandanus tectorius) on immune status, anti-tumour and resistance to Yersinia ruckeri infection in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immun. 87:620–626.
- Bakkali F, Averbeck S, Averbeck D, Idaomar M. 2008. Biological effects of essential oils- a review. Food Chem Toxicol. 46(2):446–475.
- Bligh EG, Dyer WJ. 1959. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 37:911–917.
- Boesen HT, Pedersen K, Larsen JL, Koch C, Ellis AE. 1999. Vibrio anguillarum resistance to Rainbow trout (Oncorhynchus mykiss) serum: role of O- antigen structure of lipopolysaccharide. Infect Immun J. 67(1):294–301.
- Bradford MM. 1976. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein dye binding. Anal Biochem. 72:248–254.
- Brunt J, Austin B. 2005. Use of a probiotic to control lactococcosis and streptococosis in rainbow trout, (Oncorhynchus mykiss). J Fish Dis. 28:693–670.
- Caulton MS, Bursell E. 1977. The relationship between condition and body composition in young Tilapia rendalli (Boulenger). J Fish Boil. 11:143–150.
- Chen CY, Wooster GA, Bowser PR. 2004. Comparative blood chemistry and histopathology of tilapia infected with Vibrio vulnificus or Streptococcus iniae or exposed to carbon tetrachloride, gentamicin, or copper sulfate. Aquac. 239(1–4):421–443.
- Clegg RJ, Middleton B, Bell GD, White DA. 1980. Inhibition of hepatic cholesterol synthesis and S- 3- hydroxyl- 3- methylglutaryl- CoA reductase by mono and bicyclic monoterpenes administered in vivo. Biochem Pharmacol J. 29:2125–2127.
- Cooke CJ, Nanjee MN, Dewey P, Cooper JA, Miller GJ, Miller NE. 1998. Plant monoterpenes do not raise plasma high- density- lipoprotein concentrations in humans. Am J Clin Nutr. 68:1042–1045.
- Dawson AS, Grimm AS. 1980. Quantitative seasonal changes in the protein, lipid and energy contents of carass, ovaries and liver of adult female (Pleuronectes platena L.). J Fish Biol. 16(5):493–504.
- Dempson JB, Schwarz CJ, Shears M, Furey G. 2004. Comparative proximate body composition of Atlantic salmon with emphasis on parr from fluvial and lacustrine habitats. J Fish Biol. 64:1257–1271.
- Denner SS. 2009. Lavandula angustifolia miller: English lavender. Holistic J Nurs Pract. 23(1):57–64.
- Ebrahimi P, Ebrahim-Magham B, Pourmorad F, Honary S. 2013. Ferulic acid lecithin-based nano-emulsions prepared by using spontaneous emulsification process. Iran J Chem Chem Eng. 32:17–25.
- Ebrahimi P, Salmanpour S. 2014. Topical quercetin nanoemulsions: optimization of preparation using chemometric approaches. Pharm Chem J. 48:402–407.
- Edris AE. 2007. Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: a review. Phytother Res J. 21(4):308–323.
- Fadeifard F, Raissy M, Jafarian M, Boroujeni HR, Rahimi M, Faghani M. 2018. Effects of black seed (Nigella sativa), ginger (Zingiber officinale) and cone flower (Echinacea angustifolia) on the immune system of rainbow trout (Oncorhynchus mykiss). Arq Bras Med Vet Zoo. 70(1):199–204.
- Farsani MN, Hoseinifar SH, Rashidian G, Farsani HG, Ashouri G, Van Doan H. 2019. Dietary effects of Coriandrum sativum extract on growth performance, physiological and innate immune responses and resistance of rainbow trout (Oncorhynchus mykiss) against Yersinia ruckeri. Fish Shellfish Immun. 91:233–240.
- Fazio F, Marafioti S, Arfuso F, Piccione G, Faggio C. 2003. Comparative study of the biochemical and hematological parameters of four wild Tyrrhenian fish species. Vet Med. 58(11):576–558.
- Feldman BF, Zinkl JG, Jian NC. 2000. Schalm’s veterinary hematology. 5th Edition. Lippincott Williams and Wilkins publication. . Canada. 1120–1124.
- Hoseini SM, Yousefi M. 2019. Beneficial effects of thyme (Thymus vulgaris) extract on oxytetracycline- induced stress response, immunosuppression, oxidative stress and enzymatic changes in rainbow trout (Oncorhynchus mykiss). Aquac Nutr. 25:298–309.
- Kalbassi MR, Abdollahzadeh E, Salari-Joo H. 2013. A review on aquaculture development in Iran. J Ecopersia. 1(2):159–178.
- Kirubakaran CJW, Alexander CP, Michael RD. 2010. Enhancement of non- specific immune responses and disease resistance on oral administration of Nyctanthes arbortristis seed extract in Oreochromis mossambicus (Peters). Aquac Res. 41:1630–1639.
- Kohen R, Nyska A. 2002. Oxidation of biological systems: oxidative stress and antioxidants. Toxicol Pathol. 30:620–630.
- Kondoor A, Sajadi MM, Sorinezhad A, Daryaie A, Gh M, Khademi F. 2013. Add effect of el catenin supplementary to diet on growth indices and survival of fish of Sparidentex hasta. J Aquat Ecol. 3:35–45.
- McCord JM, Fridovich I. 1969. Superoxide dismutase: an enzymic function for erythrocuprein (Hemocuprein). J Biol Chem. 244:6049–6055.
- Mehrabi Z, Firouzbakhsh F, Rahimi-Mianji G, Paknejad H. 2019. Immune stimulatory effect of Aloe vera (Aloe barbadensis) on non- specific immune response, immune gene expression, and experimental challenge with Saprolegnia parasitica in rainbow trout (Oncorhynchus mykiss). J Aquac. 503:330–338.
- Moghanlou KS, Isfahani EN, Dorafshan S, Tukmechi A, Aramli MS. 2018. Effects of dietary supplementation with Stachys lavandulifolia Vahl extract on growth performance, hemato- biochemical and innate immunity parameters of rainbow trout (Oncorhynchus mykiss). Anim Feed Sci Tech. 237:98–105.
- Mohajerfar T, Hoseinzadeh A, Akhondzadeh basti A, Khanjari A, Misaghi A, Gandomi Nasrabadi H. 2012. Determination of minimum inhibitory concentration (MIC) of Zataria multiflora Bioss. Essential oils and lysozim on Listeria monocytogenes. J Med Plants. 11:70–78.
- Na-Phatthalung P, Teles M, Voravuthikunchai SP, Tort L, Fierro-Castro C. 2018. Immunomodulation effects of Rhodomyrtus tomentosa leaf extract and its derivative compound, Rhodomyrtone, on head kidney macrophages of rainbow trout (Oncorhynchus mykiss). Fish Physiol Biochem. 44(2):543–555.
- Nayak S, Swain P, Mukherjee S. 2007. Effect of dietary supplementation of probiotic and vitamin C on the immune response of Indian major carp, Labeo rohita (Ham.). Fish Shellfish Immun. 23:892–896.
- Ngugi CC, Oyoo-Okoth E, Mugo-Bundi J, Orina PS, Chemoiwa EJ, Aloo PA. 2015. Effects of dietary administration of stinging nettle (Urtica dioica) on the growth performance, biochemical, hematological and immunological parameters in juvenile and adult Victoria Labeo (Labeo victorianus) challenged with Aeromonas hydrophila. Fish Shellfish Immun. 44:533–541.
- Nikfarjam M, Rakhshan R, Ghaderi H. 2017. Comparison of effect of Lavandula officinalis and venlafaxine in treating depression: a double blind clinical trial. J Clin Diagn Res. 11(7):KC01.
- Nobakht A, Rahimzadeh MR, Safa Mehr AR. 2013. Study of various rates of mixture of medicinal herbs of nettle, bee balm and thyme on function, quality of quarcass and biochemical parameters and white blood cells of broiler chicken. Iran J Med Arom Plants. 29(1):224–215.
- Prasad G, Priyanka GL. 2011. Effect of fruit rind extract of Garcinia gummi- gutta on hematology and plasma biochemistry of catfish (Pangasianodon hypophthalmus). Asian J Biochem. 6(3):240–251.
- Raa J. 2000. The use of immunestimulants in fish and shellfish feeds. In: L.E. Cruz-Suarez, D. Ricque-Marie, M. Tapia-Salazar, M.A.Y. Olvera-Novoa, R. Civera-Cerecedo, editors. Avances en Nutriciόn Acuίcόla V. Memorias del V Simposium International de Nutriciόn Acuίcόla. Merida (Yucatan); p. 47–56.
- Rafieepour A, Hajirezaee S, Rahimi R. 2018. Dietary oregano extract (Origanum vulgare L.) enhances the antioxidant defense in rainbow trout, Oncorhynchus mykiss against toxicity induced by organ phosphorus pesticide, diazinon. Toxin Rev. 1–11. https://doi.org/10.1080/15569543.2018.1550092.
- Ramsden SR, Smith TJ, Shaw BJ, Handy RD. 2009. Dietary exposure to titanium dioxide nanoparticles in rainbow trout, (Oncorhynchus mykiss): no effect on growth, but subtle biochemical disturbances in the brain. Ecotoxicol. 18:939–951.
- Rashidian G, Bahrami Gorji S, Farsani MN, Prokić MD, Faggio C. 2018. The oak (Quercus brantii) acorn as a growth promoter for rainbow trout (Oncorhynchus mykiss): growth performance, body composition, liver enzymes activity and blood biochemical parameters. Nat Prod Res. 1–11. https://doi.org/10.1080/14786419.2018.1538994.
- Reverter M, Bontemps N, Lecchini D, Banaigs B, Sasal P. 2014. Use of plant extracts in fish aquaculture as an alternative to chemotherapy: current status and future perspectives. J Aquac. 433:50–61.
- Reyes-Cerpa S, Vallejos-Vidal E, Gonzalez- Bown MJ, Morales Reyes J, Pérez-Stuardo D, Vargas D, Imarai M, Cifuentes V, Spencer E, Sandino AM, Reyes- López FE. 2018. Effect of yeast (Xanthophyllomyces dendrorhous) and plant (Saint John’s wort, lemon balm, and rosemary) extract based functional diets on antioxidant and immune status of Atlantic salmon (Salmo salar) subjected to crowding stress. Fish Shellfish Immun. 74:250–259.
- Sahu S, Das BK, Pradhan J, Mohapatra BC, Mishra BK, Sarangi N. 2006. Effect of Magnifera indica kernel as a feed additive on immunity and resistance to Aeromonas hydrophila in Labeo rohita fingerlings. Fish Shellfish Immun. 23:109–118.
- Salam A, Davies PMC. 1994. Body composition of Northern pike (Esox lucius L.) in relation to body size and condition factor. J Fish Res. 19:193–204.
- Santos EL, Ludke MC, Lima MR. 2009. Extratos vegetais cmo additivos em rações para peixes. Rev Electron Comun Inf Inov Saude. 6:789–800.
- Sarhadi I, Alizadeh E, Ahmadifar E, Adineh H, Dawood MA. 2020. Skin mucosal, serum immunity and antioxidant capacity of common carp (Cyprinus carpio) fed artemisia (Artemisia annua). Ann Anim Sci. doi:10.2478/aoas-2020-0011.
- Sarker DK. 2005. Engineering of nanoemulsion for drug delivery. Curr Drug Deliv. 2:297–310.
- Schiavone A, Righi F, Quarantelli A, Bruni R, Serventi P, Fusari A. 2007. Use of Silybum marianum fruit extract in broiler chicken nutrition: influence on performance and meat quality. J Anim Physiol Anim Nutr (Berl). 91:256–262.
- Sivaram V, Babu MM, Immanuel G, Murugadass S, Citarasu T, Marian M. 2004. Growth and immune response of juvenile greasy groupers (Epinephelus tauvina) fed with herbal antibacterial active principle supplemented diets against Vibrio harveyi infections. J Aquac. 237:9–20.
- Siwicki AK, Anderson DP, Rumsey GL. 1994. Dietary intake of immunostimulants by rainbow trout affects non-specific immunity and protection against furunculosis. Vet Immunol Immunopathol. 41:125–139.
- Souza C, Baldissera M, Baldisserotto B, Heinzmann B, Martos-Sitcha JA, Mancera JM. 2019. Essential oils as stress- reducing agents for fish aquaculture: a review. Front Physiol. 10:785. doi:10.3389/fphys.2019.00785.
- Stauth D. 2007. Studies force new view on biology of flavonoid. Eugene: Oregon State University. http://www.eurekalert.org/pub_releases/2007-03/0su-sfn030507.php.
- Teimouri M, Amir Kolaie AS, Yeganeh S. 2013. The effects of Spirulina platensis meal as a feed supplement on growth performance and pigmentation of rainbow trout (Oncorhynchus mykiss). J Aquac. 14(19):96–399.
- Waley K, North J. 1997. Haemolytic assays for whole complement activity and individual components. In: A.W. Dodds, R.B. Sim, editors. Complement: a practical approach, Vol. 1. Oxford: Oxford University Press; p. 19–47.
- Yousefi M, Hoseini SM, Vatnikov YA, Kulikov EV, Drukovsky SG. 2019. Rosemary leaf powder improved growth performance, immune and antioxidant parameters, and crowding stress responses in common carp (Cyprinus carpio) fingerlings. J Aquac. 505:473–480.
- Yousefi M, Shabunin SV, Vatnikov YA, Kulikov EV, Adineh H, Hamidi MK, Hoseini SM. 2020. Effects of lavender (Lavandula angustifolia) extract inclusion in diet on growth performance, innate immunity, immune-related gene expression, and stress response of common carp, Cyprinus carpio. J Aquac. 515:734588.
- Zargar A, Rahimi-Afzal Z, Soltani E, Taheri Mirghaed A, Ebrahimzadeh Mousavi HA, Soltani M, Yuosefi P. 2019. Growth performance, immune response and disease resistance of rainbow trout (Oncorhynchus mykiss) fed Thymus vulgaris essential oils. Aquac Res J. 50(11):3097–3106.
- Zuzarte M, Goncalves MJ, Cavaleiro C, Cruz MT, Benzarti A, Marongiud B, Maxia A, Piras A, Salgueiroa L. 2013. Antifungal and anti-inflamma1tory potential of Lavandula stoechas and Thymus herba-barona essential oils. Ind Crop and Prod. 44:97–103.