?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Haemonchus contortus (H. contortus) is one of the most pathogenic gastrointestinal nematodes of sheep and goats. The present study was aimed to investigate the impact of H.contortus natural infection in sheep and goats. The haematological, biochemical, immunological and oxidative stress markers were estimated. Blood samples were collected from clinically infected animals for analysis. Abomasum examination was performed after slaughter the animals to confirm the presence of H.contortus adult worm. Parasitologically, H.contortus worms were detected and counted in the abomasum of infected animals. Haematological findings revealed that naturally infected sheep and goats exhibited microcytic hypochromic anaemia, leukopenia, lymphopenia and neutropenia with eosinophilia. H.contortus infection-induced hypoproteinemia, hypoalbuminemia, and hypoglobulinemia. Also, it markedly lowered the serum IgG&IgM and elevated serum IgE levels. Serum concentration of superoxide dismutase (SOD) was reduced, whereas lipid peroxidation (MDA) levels were elevated. However, serum levels of iron (Fe), zinc (Zn) and copper (Cu) were markedly lowered and the total iron-binding capacity (TIBC) was elevated. Finally, this work clears that H.contortus infection in sheep and goats induced alterations in haematological, biochemical and immune marker, with stimulation of oxidative stress, these results may be good and helpful for detect and diagnose of H.contortus infection in sheep and goats.
Introduction
Small ruminants are of considerable economic importance through providing us with meat, milk, skin and wool. Health disorders in small ruminants represent the major problems on sheep and goat production (Bandyopadhyay Citation1999; Lateef et al. Citation2005). Gastrointestinal nematodes of farmed livestock cause severe economic losses in pasture-based grazing systems (Brunsdon Citation1988). Haemonchus contortus (H. contortus), a nematode parasite of the ruminants’ abomasa, which is a main cause of production losses and ill-health in sheep and goats. It is one of the greatest pathogenic nematodes, known as ‘barber’s pole worm or red stomach worm or wire worm’ of small ruminants, which inhabits the abomasum (Roberts and Janovy Citation2005). Also, it is a bloodsucking gastric parasite of ruminants, may induce a life-threatening problem in a severe infection (Silverman and Campbell Citation1959; Urquhart et al. Citation1996), these parasites are more prevalent in the warm temperate and tropical countries (Sissay et al. Citation2007; Qamar et al. Citation2009). Moreover, due to the expensive treatment cost and control measures, it causes high morbidity, mortality and economic loss (Getachew et al. Citation2007; Qamar and Maqbool Citation2012). The acute and emaciated form of disease is commonly seen in young animals, while adult animals are more resistant to infestation (Onyenwe et al. Citation2005). The common clinical signs are ascites, weight loss, anaemia, sub-mandibular edema and death (Abakar et al. Citation2004; Kelkele et al. Citation2012; Tehrani et al. Citation2012). Infection with H.contortus caused a significant reduction in the erythrocyte counts, haemoglobin concentrations and PCV and significant increased WBC and eosinophil, also produce significant reduction in total protein, calcium and iron (Abakar et al. Citation2004). The aim of the present work was to investigate the haematological, biochemical, immunological and oxidative stress marker that may be helpful for diagnosis and controlling strategy of H.contortus infection.
Materials and methods
Sample collection
A total of 170 abomasal samples (90 sheep and 80 goats) were collected from slaughtered sheep and goats of different ages at different abattoirs in Sharkia Province, Egypt, which located in the northern part of the country during the period between September 2016 to August 2017. To collect the samples we visited the abattoirs one time weakly/month during the study period. Blood samples were collected randomly from healthy and clinically infected sheep and goats (All clinically infected animals in this work characterized by pale mucous membrane, submandibular edema [bottle jaw] and weakness) before slaughter, under aseptic condition by vein puncture, 0.5 ml of blood was collected with anticoagulant (EDTA 10%), and another 5 ml was drawn into a screw-capped sterile test tube without anticoagulant slowly to avoid haemolysis for serum separation. Blood samples were labelled with an identification number. Handling of the animals, sample collection and preparation were conducted and approved by a research ethics committee of Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt. All efforts were done to decrease the animal suffering.
Parasitological investigation
The abomasum was collected from all examined animals, ligated at both ends, labelled and immediately taken to the laboratory of Parasitology Department, Zagazig University for examinations. It was open along its greater curvature, their contents and mucosa were washed in 2 litres of water thoroughly mix, then transferred a total of 200 ml in washing jar and washed until all the faecal matter was removed. The water was added to make the volume 50 ml, then small volume was poured in petri dishes and counted the number of adult H.contortus present in the sample. The count was multiplied by 10 to arrive the total worm burden, the degree of infection was determined by the total number of worms as follows: light infection (1–400 worms), moderate infection (400–1500 worms) and heavy infection (more than 1500 worms) (Hansen and Perry Citation1994).
Haematological analysis
Blood samples were collected in vials containing EDTA to evaluate the red blood cells (RBC) count, haemoglobin (Hb) concentration, packed cell volume (PCV) and white blood cells (WBC) count through automatic cell counter (Hospitex Hemascreen 18-Italy). Blood films were prepared, fixed with 95% methyl alcohol and stained with Giemsa for differential leukocyte count (DLC) according to (Feldman et al. Citation2000). MCV and MCHC were calculated according to the following formula.
Biochemical analysis
Serum samples were prepared and stored in clean vials at −20C until used. The serum was used for determining the levels of total proteins and albumin according to Doumas et al. (Citation1981) and Drupt (Citation1974) respectively. Globulin level was calculated by subtracting the determined albumin concentration from total protein concentration (Doumas and Biggs Citation1972). Serum iron (Fe) and total iron binding capacity (TIBC) were determined by automatic analyser. Serum concentrations of copper (Cu) and zinc (Zn) were carried out with a flame Atomic Absorption Spectrometry (USA) at the Central Lab, Faculty of Veterinary Medicine, Zagazig University, according to the method described by Brown et al. (Citation1986) and Brown and Taylor (Citation1995) at the Central Lab, Faculty of Veterinary Medicine, Zagazig University. In order to prevent contamination from glassware, plastic materials were used during the measurements of trace elements.
Immunoglobulins, antioxidant and oxidative stress markers
Serum levels of immunoglobulin G (IgG), immunoglobulin M (IgM) and immunoglobulin E (IgE) were determined by the commercial IgG, IgM and IgE ELISA kits provided by Genorise Scientific, USA (Cat. No. GR116031, GR116032&GR116036) respectively, for sheep, while goats (GR116034, GR116035 &GR116037) respectively. Superoxide dismutase (SOD) activity and lipid peroxidation (MDA) were determined in the serum according to the method described by Nishikimi et al. (Citation1972), and Ohkhawa et al. (Citation1979) respectively.
Statistical analysis
The data were analysed statistically by using SPSS-15 for windows at the 5% level of probability.
Results
Clinical signs
Selected healthy sheep and goats were screened and are free from any endo- and ecto-parasites, while naturally infected sheep and goats appeared emaciated, and some had diarrhoea and ascites.
Parasitological findings
Examination of abomasum samples (90 sheep and 80 goats), the results revealed that, 39 (43.3%) of the examined sheep and 22 (27.5%) of goats harboured adult H.contortus. Small differences in H.contortus worm burdens may cause significant differences in their pathological effect; the degree of infection was recorded as shown in .
Table 1. Parasitological investigation of sheep and goats abomasums.
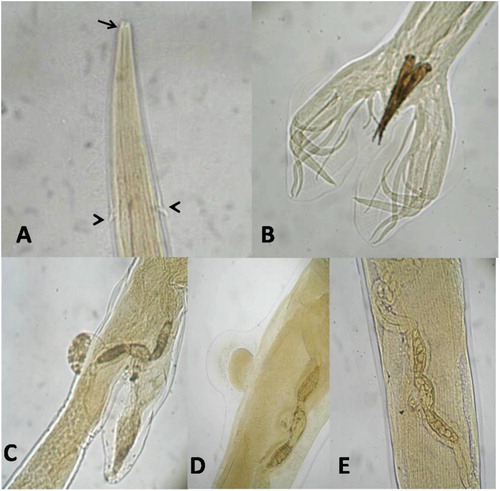
Macroscopically, H.contortus worms were easily seen even amongst the debris in opened abomasum, it is a bright red colour. The female has white ovaries and uteri were twisted spirally around the red blood-filled intestine (barber’s pole or wire appearance). The male was measured 15–20 mm, while the female 24–30 mm in length. Microscopically, the anterior end showed a small buccal capsule containing small lancet and a pair of wedge-shaped cervical papillae. The male bursa was well developed and consisted of two lateral lobes, it was supported by a characteristic Y-shape dorsal ray, and the spicules were long, equal, brownish in color, and end with barbed tips. The female vulva was covered with flap (Linguiform or knobbed) or not (smooth) ().
Figure 1. Shows microscopic examination of H.contortus. (A) Anterior end showing small buccal capsule (arrow) and cervical papillae (arrow head), (B) Male bursa with 2 lateral lobes, Y-shape dorsal ray and the spicules, (C, D&E) Female vulva liguliform, knobbed and smooth form respectively. (A 100x & B,C, D, E 40x).
Haematological finding
Sheep and goats naturally infected with H.contortus (light, moderate and heavy infection) showed a significant decrease (P <0.05) in the RBCs count, Hb concentration, PCV%, MCV and MCHC %, which revealed microcytic hypochromic anaemia compared to healthy control animals. Total leukocyte, lymphocyte and neutrophil count significantly decreased (P <0.05) in infected sheep and goats (light, medium and heavy), while eosinophil count showed a significant increase (P <0.05) in the infected animals compared to control. Basophil and monocyte count showed non-significant changes in infected sheep and goats compared to control ( and ).
Table 2. The effect of H.contortus light, moderate and heavy infection in sheep on haematological parameters.
Table 3. The effect of H.contortus light, moderate and heavy infection in goat on haematological parameters.
Biochemical finding
The serum biochemical parameters recorded in control healthy and naturally infected sheep and goats with H.contortus (light, moderate and heavy infection) are shown in and . Serum total protein and albumin levels significantly decreased (P <0.05) in infected sheep and goats compared to control healthy animals, while serum globulin level was significantly decreased (P <0.05) in heavy infection sheep and goats only in comparison to control. The serum IgG level showed non-significant changes in infected sheep and goats (light, moderate and heavy infection), while the IgM level was significantly decreased (P <0.05) with a significant increase (P <0.05) in the IgE level in infected sheep and goats (light, moderate and heavy) compared to healthy control animals.
Table 4. The effect of H.contortus light, moderate and heavy infection in sheep on biochemical parameters.
Table 5. The effect of H.contortus light, moderate and heavy infestation in goat on biochemical parameters.
Antioxidant/oxidative stress markers
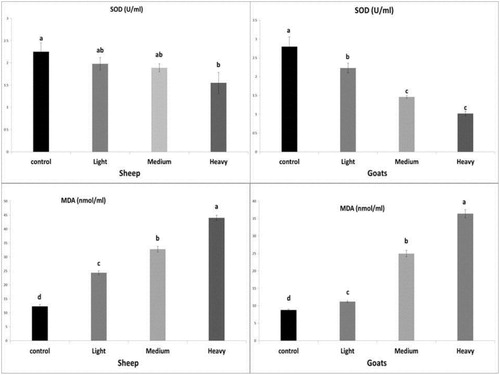
The serum SOD level showed non-significant change in infected sheep (light and moderate infection) but significantly decreased (P <0.05) in heavy infection, while infected goats (light, moderate and heavy) showed a significant decrease (P <0.05) compared to control. Serum lipid peroxidation (MDA) level revealed a significant increase (P <0.05) in infected sheep and goats (light, moderate and heavy infection) in comparison to healthy control animals, these results were shown in .
Serum minerals
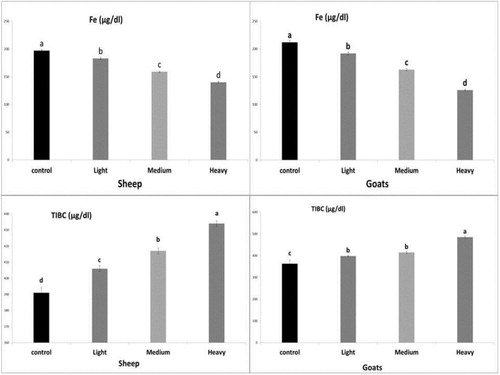
Infected sheep and goats (light, moderate and heavy infection) showed a significant decrease (P <0.05) in serum Fe concentration and a significant increase (P <0.05) in TIBC compared to healthy control animals, these results were shown in .
Figure 3. Effect of natural infection of H.contortus in sheep and goats on serum Fe and TIBC levels. The values shown are means ± SE (n = 5/group). Bars bearing different letters significantly differ at p ≤ 0.05.
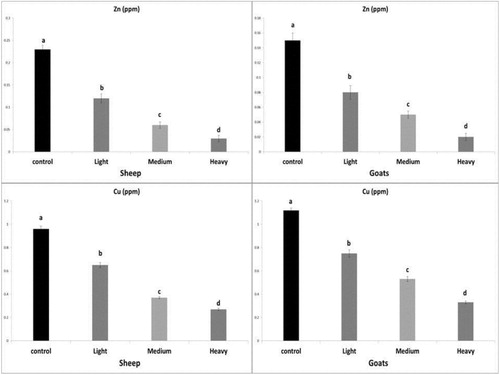
The serum Zn and Cu levels showed a significant decrease (P <0.05) in the infected sheep and goats (light, moderate and heavy infection) in comparison to control, these results were shown in .
Discussion
Gut parasites induce a reduction in the digestibility of appetite and food as well as diversion of nutrients from production sites toward the repair of tissue-injury, which leads to alterations in the haematological, biochemical and antioxidant markers (Kyriazakis and Houdijk Citation2006; Cardia et al. Citation2011). The present work revealed a marked reduction in RBC count, Hb, PCV%, MCV and MCHC%, which confirmed the results of Misra et al. (Citation1996). Our results revealed microcytic hypochromic anaemia in infected sheep and goats that may be attributed to the injuries of abomasa caused by the H.contortus leading to haemorrhage and chronic blood loss (Coles Citation1986), these findings similar to that recorded by Abdel (Citation1992) and Kumar et al. (Citation2013). The present data showed reduction in the total leukocyte, lymphocyte and neutrophil counts, that may be attributed to increased sequestration of white blood cells to abomasum where is the worm localized, or maybe due to the worm release substance stimulates the migration of neutrophil and lymphocyte cells from circulation or indicates immunodepression. Ortolani et al. (Citation2013) recorded reduction in WBC, neutrophil and lymphocyte count in sheep experimentally infected with haemonchosis. Also, there is a significant elevation in eosinophil count in the infected sheep and goats, that it is the main leukocyte increased in the parasitic infestation to antagonize the action of histamine hormone which released to initiate inflammation and immune response during parasitism (Feldman et al. Citation2000).
Sheep and goats naturally infected with H. contortus revealed a marked reduction in total protein, albumin and globulins, which attributed to decrease food intake, loss of protein in the gut during infection with haemonchosis, that is responsible for protein losing enteropathy (Soulsby Citation1982). A reduction of plasma protein concentrations (Wallace et al. Citation1996) was due to blood loss, haemorrhagic gastritis and leakage of proteins to gastric lumen caused disruption of intercellular unions and increased gut permeability (Baker et al. Citation2003). Also, heavy infection with H.contortus produces abomasum haemorrhage that leads to loss of albumin from the blood vessels (Urquhart et al. Citation1996; Taylor et al. Citation2007; Bowman Citation2009). Moreover, serum globulin level in infected animals increases due to elevated synthesis of gamma globulin that is the main component of humoral immune response (Tarazona et al. Citation1982), in the present work, serum globulin levels significantly reduced in heavy infected sheep and goats, which may be attributed to exhaustion and release humoral antibodies from circulation to overcome the infection in their sites (abomasum).
Regarding to the humoral immune response in the body, H.contortus infection significantly reduced the serum immunoglobulin (IgG&IgM) in heavy infected sheep and goats, which may be attributed to the presence of histamine and leukotriene in the abomasal mucus that associated with parasitic infection to facilitate the translocation of plasma proteins, including humoral antibodies into the lumen of the abomasum (Miller Citation1996), consequently the levels of IgG and IgM reduced in the circulation and released into abomasum to protect it against H.contortus and lower motility of its larvae. These findings are in agreement with Martínez-Valladares et al. (Citation2005) and Muñoz-Guzmán et al. (Citation2006). Moreover, there was a marked elevation in the serum IgE level in infected sheep and goats, that may be due to natural infection with haemonchosis that elevate the production of specific antibodies IgE that associated with worm fertility control, which is characteristic of helminthic infection, it was resulted from the response of a Th2-type, and it has been related to gastrointestinal nematode resistance in sheep and goats (De la Chevrotière et al. Citation2012; Pernthaner et al. Citation2005).
Parasitic infection in sheep as Trichostrongylidae, Fasciola, and Eimeria spp. induced elevation in the production of free radicals and induction of oxidative stress that resulted in the increased lipid peroxidation production (Dede et al. Citation2000). In the present study, haemonchosis in sheep and goats (light, moderate and heavy infection) produced an elevation in the MDA levels, which attributed to increase the formation of reactive oxygen species (ROS) that resulted from oxidative stress (Nanev et al. Citation2011), also the results revealed depletion in the antioxidant state of the infected animals which represented by lowering in the serum SOD level, which attributed to exhaustion of the antioxidant enzyme in scavenging the free radicals and/or it was inhibited due to higher production of H2O2 by enzymatic oxidation of the superoxide anion radical (Halliwell and Gutteridge Citation1999). Furthermore, H.contortus infection in sheep and goats lowered the serum level of Zn which may be attributed to depletion of Zn in the immune response, it is may be due to the role of Zn in the response of protective Th1 cells (Lightbody et al. Citation2001). Zinc is essential for intestinal immunity, against gastrointestinal nematodes depends on the zinc nutrition and status (Hughes and Kelly Citation2006). Serum Zn concentrations reduced in many diseases associated with anorexia (Kozat et al. Citation2007) and is associated with increased the adult parasite burdens (Shi et al. Citation1995). Our result in accordance with Awad et al. (Citation2016). Serum Cu concentration was also reduced in this work, which attributed to the infection interfere with intestinal absorption that may change gastrointestinal PH (Mulcahy et al. Citation2004). Our data were in accordance with Nanev et al. (Citation2012).
Conclusion
Haemonchus contortus is one of the important bloodsucking parasite which produce anaemia and hypoproteinaemia that is the major feature of H.contortus pathogenesis in sheep and goats, which may be fatal to young animals. The present study describes the clinical signs of haemonchosis and their correlation with clinicopathological findings and parasitological examination for diagnosis of haemonchosis infection. Sheep and goats similarly response to natural H.contortus infection.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Abakar AD, Alamin AE, Osman AY. 2004. Haematological and biochemical changes following Concurrent Infections with Coccidia and Haemonchus contortus in Desert Lambs. J Animal Vet Adv. 3:643–647.
- Abdel ATS. 1992. Haematological and biochemical studies on the efficiency of synthetic drugs against gastrointestinal nematode parasites in sheep. Aus Vet Med. 42:197–203.
- Awad AH, Ali AM, Hadree DH. 2016. Some haematological and biochemical parameters assessments in sheep infection by Haemonchus contortus. Tikrit J Pure Sci. 21(1):11–14.
- Baker RL, Nagad S, Rodriguez-Zas SL, Southey BR, Audho JO. 2003. Resistance and resilience to gastro-intestinal nematode parasites and relationships with productivity of Red Massai, Dorper and Red Massai X Dorper crossbred lambs in the sub-humid tropics. Anim. Sci. 76:119–136.
- Bandyopadhyay B. 1999. Gastrointestinal parasitic infections of sheep and goats at Salboni, West Bengal. J Vet Parasitol. 13:79–80.
- Bowman D. 2009. Georgis Parasitology for veterinarians, 9th ed. Ithaca, New York: Saunders, an imprint of Elsevier Inc. 161.
- Brown A, Halls JD, Taylor A. 1986. Atomic spectrometry update-clinical materials, foods and beverages. J Anal At Spectrom. 1:21–35.
- Brown AA, Taylor A. 1995. Applications of a slotted quartz tube and a flame atomic absorption spectrophotometer to the analysis of biological samples. Analyst. 110:579–582.
- Brunsdon RV. 1988. The economic impact of nematode infection in sheep: implications for future research and control. In: Heath ACG, editor. The Economic Importance of Parasites of Livestock in New Zealand. Vol. 1. New Zealand Society for Parasitology Miscellaneous Publication; p. 4–16.
- Cardia DFF, Rocha-Oliveira RA, Tsunemi MH, Amarante AFT. 2011. Immune response and performance of growing Santa Ines lambs to artificial Trichostrongylus colubriformis infections. Vet Parasitol. 182(2-4):248–258.
- Coles EH. 1986. Veterinary clinical Pathology, 4th ed. Philadelphia: W. Saunders Company.
- Dede S, Deger Y, Deger S, Alkan M. 2000. Determination of the status of lipid peroxidation and antioxidants in sheep infected with certain endoparasites (Fasciola spp.,trichostronglidae spp., Eimeria spp.). Acta Parasit Turc. 24:190–193.
- De la Chevrotière C, Bambou JC, Arquet R, Jacquiet P, Mandonnet N. 2012. Genetic analysis of the potential role of IgA and IgE responses against Haemonchus contortus in parasite resistance of Creole goats. Vet Parasitol. 186(3-4):337–343.
- Doumas BT, Bay so DD, Carter RJ, Peters T, Schaffer R. 1981. Determination of serum total protein. Clin Chem. 27:1642–1650.
- Doumas BT, Biggs HG. 1972. Determination of serum globulin. In: Cooper, editor. Standard Methods of Cilnical Chemistry. Vol. 7. New York: Academic Press; p. 358–362.
- Drupt F. 1974. Colorimetric determination of albumin. Pharm Biol. 9:777–779.
- Feldman BF, Zinki JG, Jain VC. 2000. Schalm, s Veterinary hematology, 5th Ed. Philadilphia: Lippincott Williams and Wilkins.
- Getachew T, Dorchies P, Jacquiet P. 2007. Trends and challenges in the effective and sustainable control of Haemonchus contortus infection in sheep- review. Parasite. 14(1):3–14.
- Halliwell B, Gutteridge JMC. 1999. Free radicals in Biology and Medicine. 3rd ed. Oxford: Oxford University Press; p. 1–25.
- Hansen J, Perry B. 1994. The Epidemiology, diagnosis and control of Helminth parasites of ruminants, 2nd ed. Nairobi: ILRAD.
- Hughes S, Kelly P. 2006. Interaction of malnutrition and immune impairment, with specific reference to immunity against parasites. Parasite Immunol. 28:577–588.
- Kelkele FA, Tolossa YH, Kassa GM. 2012. Experimental infection of Ethiopian highland sheep by different infective doses of Haemonchus contortus (L3): haematological and parasitological parameters, serum protein concentrations and clinical responses. Ethiopian Vet J. 16(1):41–57.
- Kozat S, Göz Y, Yörük BH. 2007. Some trace elements and vitamins A, C, and E levels in ewes infected with gastrointestinal parasites. Yyü Vet Fak Derg. 18(2):9–12.
- Kumar R, Ranjan S, Vishnu P G, Negi M, Senapati P K. 2013. Haematological and biochemical changes in black Bengal goats infected with Haemonchus contortus. Indian J Small Rumminant. 19:172–174.
- Kyriazakis I, Houdijk J. 2006. Immunonutrition: nutritional control of parasites. Small Ruminant Res. 62:79–82.
- Lateef M, Iqbal Z, Jabbar A, Khan MN, Akhtar MS. 2005. Epidemiology of trichostrongylid nematode infections in sheep under traditional husbandry system in Pakistan. Int J Agricul Biol. 7:596–600.
- Lightbody J, Stevenson L, Jackson F, Don-aldson K, Jones D. 2001. Comparaive aspects of plasma antioxidant status in sheep and goats and the influence of experimental abomasal nematode infection. J Comp Pathol. 124:192–199.
- Martínez-Valladares M, Vara-Del Río MP, Cruz-Rojo MA, Rojo-Vázquez FA. 2005. Genetic resistance to Teladorsagia circumcincta: IgA and parameters at slaughter in Churra sheep. Parasite Immunol. 27(6):213–218.
- Miller HR. 1996. Prospects for the immunological control of ruminant gastrointestinal nematodes: natural immunity, can it be harnessed. Int J Parasitol. 26(8–9):801–811.
- Misra SC, Panda DN, Prida S. 1996. Haemotological and Histological alterations of immature paramphistomiasis in lambs. Indian Vet J. 73:1274–1276.
- Mulcahy GO, Neill S, Donnelly S, Dalton JP. 2004. Helminths at mucosal barriers-interaction with the immune system. Adv Drug Deliv Rev. 56:853–868.
- Muñoz-Guzmán MA, Cuéllar-Ordaz JA, Valdivia-Anda AG, Buendía-Jiménez JA, Alba-Hurtado F. 2006. Correlation of parasitological and immunological parameters in sheep with high and low resistance to haemonchosis. Can J Anim Sci. 86(3):363–371.
- Nanev V, Gabrashanska M, Hrusanov D, Mihaylov M, Vladov I. 2011. Oxidative stress status in lambs experimentally infected with Haemonchus contortus (Nematoda). Conference: Scientific Conference with International Participation Tradition and Modernity in Veterinary Medicine.
- Nanev V, Gabrashanska M, Vladov I, Yordanova I, Ermakov V, Tjutikov S. 2012. Alterations in trace elements associated with haemonchosis in lambs. Project: Geochemical ecology of organisms in terms of background areas and biogeochemical province.
- Nishikimi M, Rao NA, Yagi K. 1972. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun. 46:849–854.
- Ohkhawa H, Ohsini N, Yagi K. 1979. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 95:351–358.
- Onyenwe IW, Onwe C, Onyeabor A, Onunkwo JI. 2005. Abattoir-Based study of the Susceptibility of Two natural infected Breeds of goat to Haemonchus contortus in Nsukka Area of Enugu state, Nigeria. Animal Res Int. 2(2):342–345.
- Ortolani EL, do RêgoLeal ML, Minervino AHH, Aires AR, Coop RL, Jackson F, Suttle NF. 2013. Effects of parasitism on cellular immune response in sheep experimentally infected with Haemonchus contortus. Vet Parasitol. 196(1-2):230–234.
- Pernthaner A, Shaw RJ, McNeill MM, Morrison L, Hein WR. 2005. Total and nematode-specific IgE responses in intestinal lymph of genetically resistant and susceptible sheep during infection with Trichostrongylus colubriformis. Vet Immunol Immunopathol. 104(1-2):69–80.
- Qamar MF, Maqbool A. 2012. Biochemical studies and serodiagnosis of haemonchosis in sheep and goats. J Animal Plant Sci. 22(1):32–38.
- Qamar MF, Maqbool A, Khan MS, Ahmad N, Muneer MA. 2009. Epidemiology of haemonchosis in sheep and goats under different managemental conditions. Veterinary World. 2(11):413–417.
- Roberts LS, Janovy J. 2005. Phylum Nematoda: form, Function, and Classification. In: Gerald D. Schmidt, Larry S. Roberts, editor. Foundations of Parasitology. 7th ed. New York: McGraw-Hill; p. 367–396.
- Shi RN, Scott ME, Koski KG, Boulay M, Stevenson MM. 1995. Energy restriction and severe zinc deficiency influence growth, survival and reproduction of Heligmosomoides polygyrus (Nematoda) during primary and challenge infections in mice. Parasitology. 110:599–609.
- Silverman PH, Campbell JA. 1959. Studies on parasitic worms of sheep in Scotland. Parasitology. 49:23–38.
- Sissay MM, Uggla A, Waller PJ. 2007. Epidemiology and seasonal dynamics of gastrointestinal nematode infections of sheep in a semi-arid region of eastern Ethiopia. Vet Parasitol. 143(3-4):311–321.
- Soulsby EJL. 1982. Helminths, arthropods and protozoa of domesticated animals, 7th edn. The English Language Book Society and Bailliere, Tindall. Taylor M A, Coop R L, Wall R L. 2007. Veterinary Parasitology. Third Edn. Blackwell Publishing.
- Tarazona JM, Sanz-Pastor A, Babin-M-del M, Dominguez T, Parra I, Sanz P-A, Del-Mar-Babin M, Sanz P-A. 1982. Caprine Trichostrongylidosis II clinical studies of field infections. Analesdel-Instituto-Nacional-de-Investigaciones Agrarias; Ganadera, Spain 14: 111–124.
- Taylor MA, Coop RL, Wall RL. 2007. Veterinary Parasitology. 3rd ed. Blackwell Publishing.
- Tehrani A, Javanbakht J, Jani M, Sasani F, Solati A, Rajabian M, Khadivar F, Akbari H, Mohammadian M. 2012. Histopathological study of Haemonchus contortus in Herrik sheep abomasum. J Bacteriol Parasitol. 3(5):1000144–1000149.
- Urquhart GM, Armour J, Duncan JL, Dunn AM, Jennings FW. 1996. Veterinary Parasitology, 2nd ed. Oxford: Blackwell.
- Wallace DS, Bairden K, Duncan JL, Fishwick G, Gill M. 1996. Influence of soybean meal supplementation on the resistance of Scottish Blackface lambs to haemonchosis. Res Vet Sci. 60:138–143.