 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The study investigated, for fixed time artificially inseminated (AI) Menz ewes in field trials, the reproductive performance of prostaglandin-based treatments simultaneously to the standard ‘P4+eCG’ protocol. A total of 483 Menz ewes were assigned to either the ‘P4+eCG’ protocol, using progesterone impregnated intravaginal sponges in combination with equine chorionic gonadotropin (eCG) injection at sponge withdrawal, or the ‘PGFs’ treatment where sheep received a single injection of prostaglandin or ‘PGF7’ and ‘PGF11’ where ewes were synchronized with 2 injections of prostaglandin 7 or 11 days apart, respectively. AI was implemented with fresh semen at 55 ± 1 h after the end of the hormonal treatment. Conception rate (CR; 60.87 ± 4.2) was highest for PGF11 ewes (P < 0.05); PGFs ewes had the lowest CR (34.07 ± 4.1). Other factors did not affect variation in CR (P > 0.05). A higher proportion of ewes in the P4+eCG group yielded twins and triplets compared to the 3 prostaglandin-based protocols (P < 0.01). Nevertheless, a higher (P = 0.02) proportion (17.11 ± 4.3) of PGF11 ewes yielded twins by comparison to their PGF7 counterparts (2.50 ± 2.5). PGF-based protocol with 2 injections 11 days apart, preceded by a careful selection of non-pregnant ewes for cervical fixed-time AI, is a feasible reproductive management for sheep breeding programmes in Ethiopia.
Introduction
Community-based breeding programmes (CBBPs) for sheep and goats have been adopted in Ethiopia as the nationwide main breeding approach for small ruminants and genetic progress in growth traits and reproduction has been evidenced together with other socio-economic benefits (Haile et al. Citation2019). For breeding programmes to be effective, delivery systems of the improved sires should be put in place and artificial insemination (AI) is universally represented as being the most common process through which the products of the primary breeding activities are out and upscaled. In this regard and with reference to the context of CBBPs in Ethiopia, Mueller et al. (Citation2019) have identified AI, in complementarity of natural mating, as one strategy to further increase genetic progress and dissemination relying on a more intense use of sires. Fixed-time AI using fresh, cooled semen is the most widely used method in sheep (Palacín et al. Citation2012). Such a method requires synchronization of the estrus and ovulations in recipient females which are inseminated without prior estrous detection. Compared to other domestic species, AI in sheep has several technical and organizational limitations which were reviewed by Santolaria et al. (Citation2011). One important factor which may condition the insemination outcome in sheep is the synchronization treatment type and its duration (Baril et al. Citation1996; Maurel et al. Citation2003). The most popular treatment uses intravaginal devices containing synthetic progestogens for 12–14 days (whether synchronization is implemented outside or during the natural breeding season) coupled with an intramuscular injection of eCG at the end of treatment. In spite of the advantages of such treatments in inducing high estrus response in and out of the breeding season, there are many drawbacks like disruptions in LH secretions (Leyva et al. Citation1998; Viñoles et al. Citation2001), alterations in the preovulatory ovarian events (Berlinguer et al. Citation2007; Vilariño et al. Citation2011), disability of sperm motility (Manes et al. Citation2016) and production of eCG antibodies with repeated applications of the treatment on the same animals (Hervé et al. Citation2004) even if animals are treated once a year. Furthermore, intravaginal hormone-releasing devices have a high cost, are not registered products in many parts of the world and even when registered, their availability remains very restricted (Flores-Najera et al. Citation2010). In inter-tropical and equatorial Africa, seasonality of reproduction in sheep is not pronounced because of the very limited changes in photoperiod and non-pregnant female sheep tend to express continuous cyclic ovarian activity and oestrus behaviour. Such a physiological status is advantageous allowing a larger spectrum of synchronization options for field applications. Indeed, and as reported by Mekuriaw et al. (Citation2015) and Rekik et al. (Citation2016), prostaglandin and GnRH (Gonadotropin-Releasing Hormone) analogues were effective in inducing satisfactory levels of estrous synchrony and reproductive performance after natural mating. In comparison with progestagen intravaginal devices, such protocols are easy to use because they involve intramuscular injections only, and because they are not invasive as well. Data from our team in Ethiopia evaluates the cost of the progestogen + eCG protocol at US$ 8.5 which is a 6.5-fold increase of the protocol based on 2 injections of a natural prostaglandin (M. Rekik, personal communication). To our knowledge, there are no published results on the effectiveness of sheep AI in Ethiopia and current study aimed, under on-farm field conditions of the Ethiopian Highlands, at comparing the reproductive performance of different prostaglandin-based protocols after fixed-time AI of Menz ewes participating in the breed CBBP.
Materials and methods
Study area
The study was conducted in different privately-owned flocks’ members of the Menz breed CBBP (n = 8) and belonging to two regions, Debre Birhan and Menz. The climate of the two locations has a bi-modal rainfall pattern, where the main rainy season is from June to September. Debre Birhan is a woreda (administrative sub-division) in central Ethiopia (120 km North of Addis Ababa at 2840 m above sea level), located in the Semien Shewa Zone of the Amhara Region. Menz area is located in North Shewa administrative zone of Amhara regional state at north east of Addis Ababa. It is the homeland for Menz sheep and is found in the subalpine and cold highland agro-ecological zones of Ethiopia (Gizaw et al. Citation2007). Mixed crop-livestock dominated by sheep-barley is the principal production system of both areas. Menz sheep is small in body size (the smallest of the 14 Ethiopian breeds described by Gizaw et al. (Citation2007)), has a very low twinning rate, usually less than 3% and is well adapted to the harsh climate of Ethiopian highlands, feed scarcity, and shows resistance to parasite infestation (Haile et al. Citation2002; Gizaw et al. Citation2008). This breed is mainly reared for income generation from the sale of lambs, coarse wool, manure and presents various socio-cultural benefits (Gizaw et al. Citation2008; Getachew et al. Citation2010).
Experimental animals
In this trial, a total of 483 non-pregnant and non-suckling Menz ewes were selected. Prior to synchronization, non-pregnancy was verified using CBBP database records and ultrasonography (B-Ultrasonic Diagnostic 23500/1000 Minitub GmbH; Tiefenbach, Germany). Ewe’s parity ranged from 1 to 4 and body condition score (BCS) was assessed using the method of Russel et al. (Citation1969). The experimental animals were housed in the night and allowed to graze during the day on natural pastures daily for 6–7 h. Starting 20 days before AI and the subsequent 2 weeks, all experimental animals were fed quality hay ad libitum and received a mixed commercial concentrate (200 g head/day). They had free access to fresh water twice a day. The experimental animals were drenched against internal parasites (Albendazole 300 mg at 1 bolus/30 kg body weight, Ashish life Science Pvt. Ltd., India), and were vaccinated against Ovine Pasteurellosis, Peste des Petits Ruminants (PPR) and Sheep and Goat Pox (National Veterinary Institute, Debre Zeit, Ethiopia). The manuscript does not contain clinical studies or patient data. There are no ethical concerns to be reported and animals were handled in the presence of their owners by adhering to local animal practices and handling rules.
Experimental procedures
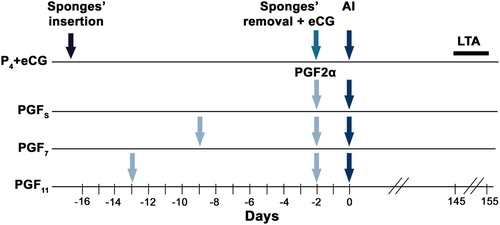
Ewes were assigned randomly to four groups synchronized with different hormonal protocols. Ewes belonging to the same flock were randomly assigned to only 2 protocols to ensure enough females are allocated per treatment for statistical purposes. The first group (P4+eCG, n = 121) received the standard protocol using intravaginal polyurethane sponges impregnated with 30 mg fluorogestone (Syncro-part®; CEVA laboratories, Libourne, France) that were placed in the vagina for 14 days. All ewes were checked twice daily (morning and evening) to ensure that sponges remained in place during the treatment period. At sponge withdrawal, each ewe received an i.m. injection of 300 I.U. of equine chorionic gonadotropin (eCG) (Syncro-part PMSG®; CEVA laboratories, Libourne, France). Ewes in the second group (PGFS, n = 135) received a singular i.m. of 5 mg of the PGF2α analogue dinoprost (1 ml Enzaprost®; CEVA Laboratories, Libourne, France). Ewes in the two other groups (PGF7 and PGF11, n = 89 and n = 138, respectively) received each 2 i.m. of 5 mg of the PGF2α analogue dinoprost (1 ml Enzaprost®; CEVA laboratories, Libourne, France) administered 7 and 11 days apart. The schedule of implementation of the 4 protocols was arranged so that fixed-time AI is carried out the same day for all sheep ().
Figure 1. Schematic representation of the experimental design. P4+ECG group: ewes synchronized with the standard protocol using intravaginal polyurethane sponges and 1 injection of ECG. PGFS group: ewes synchronized with 1 injection of dinoprost. PGF7 and PGF11 groups: ewes synchronized with two injections of dinoprost given 7 and 11 days apart respectively, starting 13 days before the timed artificial insemination (Day 0). AI: day of artificial insemination. LTA: Lambing to AI between 145 and 155 days.
Artificial insemination
Semen from 8 adult Menz rams, 4 per location (selected on the basis of their estimated breeding value and checked for normal breeding soundness) was collected using an artificial vagina. Ejaculates that were retained for AI’s had a threshold concentration of 2.5 × 109 sperm ml-1, determined by spectrophotometry (Accucell®, Instruments de Médecine Vétérinaire, IMV Paris, France) and an individual motility of at least 3.5 on a scale of 5 using a contrast-phase microscope. Immediately after collection, the semen was diluted with a commercial sheep-specific diluent (OVIXcell®, Instruments de Médecine Vétérinaire, IMV Paris, France) and ewes were inseminated cervically with 0.2 ml of diluted semen containing in average 400 × 106 sperm. Cervical artificial insemination AI was performed by two well-trained technicians using a speculum equipped with a light source and an insemination gun (Instruments de Médecine Vétérinaire, IMV Paris, France) as described by Evans and Maxwell (Citation1987). The 2 inseminators randomly inseminated ewes in the 8 flocks irrespectively of the synchronization treatment. Ewes in all treatment groups were inseminated at 55 ± 1 h after the end of the hormonal treatment (Fierro et al. Citation2017).
Data collection and statistical analyses
Conception rate (CR) was attributed the value of 1 if lambing date ∈ [145, 155 days after AI] and 0 if the ewe did not lamb or the lambing date >155. When lambing occurred, the number of lambs born was also recorded. For each protocol, lambing rate (LR), litter Size (LS) and ewes with multiple bearings (MB) were calculated according to the formulas:
For statistical purposes, multiple LS used to calculate MB was considered as a binomial variable: 0 for single-born lambs and 1 for multiple-born lambs (2 and 3 in the case of this study) and since CR and MB follow a binomial distribution, an analysis of variance for categorical data was performed and a general linear model (GLM) procedures using the logit model of R-Studio® open source (R version 3.4.3; 2017-11-30), was performed. The following model was used:where Fijklm is the outcome of the insemination (CR or MB = 0/1); u = Overall mean; Loci is the location, i = Menz area or Debre Birhan; BCSj is the body condition score, j = ≤2, [2, 3] or [3, 4]; Protk is the used hormonal protocol of oestrus synchronization, k = P4+ECG, PGFS, PGF7 or PGF11; Pl is the parity of the ewe, l = from 1 to 4; eijklm is the residual error. For factors showing significant effect, a chi-square test was used to compare different classes (biostat TGV, https://biostatgv.sentiweb.fr/?module=tests/chideux) for CR and MB. ANOVA (IBM SPSS Statistics® ver. 23.0) was conducted to compare the effect of factors of variation on LR and LS and a Tukey test was conducted for levels of LS comparisons between protocols (IBM SPSS Statistics® ver. 23.0).
Results
Conception rate to AI
For the 483 ewes included in the experiment, 225 ewes conceived in response to AI giving an overall CR of 46.58 ± 2.3. Only the protocol affected significantly the CR and the highest value was recorded for PGF11 (60.87 ± 4.2). The two by two chi square comparison showed that ewes treated with PGF11 protocol had significant higher CR than animals synchronized with P4+eCG, PGFS and PGF7 (p < 0.05). CR tended to be higher for animals receiving the P4+eCG treatment compared to PGFS (P = 0.06) ().
Table 1. Differences in conception rate (CR) and litter size (LS) according to the different parameters.
Litter size and multiple bearings
For the 225 ewes which conceived, the data for litter size was only available for 217 ewes. For the remaining 8 ewes which conceived to AI, data on the number of lambs born was not recorded. The average number of lambs at lambing was 1.19 ± 0.4 and the frequencies of ewes bearing single, double and triple LS were 82.95 ± 2.6, 15.21 ± 2.4 and 1.84 ± 0.9, respectively. Statistical analysis showed that LS was higher in Debre Birhan area (1.26 ± 0.5 vs 1.13 ± 0.4) (P = 0.037). The effect of the synchronization protocol on the LS was also significant (P < 0.001) (). The Tukey test showed that ewes in P4+eCG had higher LS (P < 0.01) when compared to the 3 other protocols.
In the same way, the proportion of ewes having multiple litters tended to be statistically higher in Debre Birhan area (22.68 ± 4.3 vs 12.50 ± 3.0). The effect of the synchronization protocol on the proportion of animals bearing multiple litters just reached significance (p = 0.05) (). The two by two chi square comparison showed that more ewes in P4+eCG yielded multiple litters (P < 0.01) when compared to the 3 other protocols. Percentages of animals having multiple LS was significantly higher when treated with PGF11 protocol than PGF7 ewes (P = 0.02) and tended to be higher than for sheep in PGFS treatment (P = 0.09).
Lambing rate
The overall LR following oestrous synchronization and artificial insemination was 53.42%. ANOVA showed that synchronization protocols had a significant effect on lambing rate and the highest percentages were recorded for P4+eCG and PGF11 protocols (65.29 and 64.49, respectively) ().
Table 2 Effect of synchronization protocols on lambing rate in Menz sheep
Discussion
Compared to the positive control (P4+eCG), PGF7 and PGFS, the longest interval between prostaglandin injections (PGF11) improved CR at fixed-time AI. Such a finding is very important in tropical and inter-tropical countries such as Ethiopia and East Africa where sheep are non-seasonal breeders and the use of prostaglandins for estrus synchronization might be applied throughout the year for a cost-effective management of reproduction (Contreras-Solís et al. Citation2009). The lower CR for the standard P4+eCG protocol is backed by previous findings (Rekik et al. Citation2016) in which lambing rate of nearly 50% only was obtained after natural mating of synchronized Menz ewes. Such a low result was explained by the same authors as being associated to the drawbacks of progestogen-based protocols for the control of reproduction in sheep (Lopez-Sebastian et al. Citation2007).
Beyond the practical significance of these results, some features of the reproductive performance in terms of both conception rate and litter size are important to discuss.
The estrous response is the physiological response to injections of the prostaglandin-analogue at different moments during the luteal phase in randomly cycling ewes. When longer intervals between prostaglandin injections are applied, they result in longer periods of higher progesterone levels prior to mating, higher oestradiol plasma levels around the onset of estrus (Fierro et al. Citation2016) and a higher fertilization rate (Fairnie et al. Citation1977). This has been strongly evidenced in the recent work of Fierro et al. (Citation2017) who concluded that long-term prostaglandin-based protocols with intervals between 12 and 16 days between the 2 injections enhance conception of ewes at cervical AI with fresh semen. For short intervals (7 days in this study), there is an altered progesterone profile during the luteal period preceding luteolysis which may depress the preovulatory events and the success of AI (Fierro et al. Citation2017). As anticipated, the least conception rate was obtained with the single injection of PGF2α. With only one single injection, ewes which are in the early luteal phase (days 1–4) and those which have already initiated luteolysis are not responsive to the treatment and are not synchronized (Pope and Cárdenas Citation2004). Conception rates in this study using the PGFs protocol are backed by the findings of Mekuriaw et al. (Citation2015) who obtained lambing rates varying between 25 and 55% only when Menz ewes are naturally mated after being synchronized with only one single injection of different analogues and doses of prostaglandin. There is a very high variability in the response of ewes when synchronized with only one single injection of prostaglandin and this is a major drawback to the purpose for which AI is used as a delivery method of improved genetics to the largest possible proportion of females in the flocks.
The variation factors that were captured in this study, namely location, BCS and parity did not affect the results of conception rate. For parity, different results are found in the literature and Palacín et al. (Citation2012) reported that the likelihood of pregnancy decreased in ewes with more than five previous parturitions by a factor of 0.87, 0.79 and 0.66 for the 6th, 7th and ≥8 parturitions, respectively. It may be that in the case of our study, the ewes had between 1 and 4 previous parturitions and this is the range of parturitions when fertility of the female sheep is at its peak. Although not statistically significant, conception rate was lower for ewes with a BCS less than 2. The relationship between BCS and the outcome of reproduction is very well documented, and our results are very similar to those by Bru et al. (Citation1995) who recorded the lowest pregnancy rate (32.7%) for Rasa Aragonesa ewes with a BCS < 2. In this trial, the inexistence of differences in CR for classes of BCS may be attributed to the size of the samples and to the hardiness of the breed which is able to preserve satisfactory reproductive performance even under poor management conditions causing depressed body condition.
Menz sheep is a non-prolific breed and average figures of litter size are very often inferior to 1.1. The proportion of ewes bearing multiple litters (2 and 3) in the P4+eCG treatment group was anticipated because of the effect of eCG, even at lower doses, in increasing ovulation rate. Which was not anticipated is the increase of the proportion of ewes yielding twins in the PGF11 group (13/76) corresponding to an average litter size of 1.17. Such an increase was reflected in ewes bearing twins only and no triplet births were recorded. Although, Menz is a breed which thrives in a very harsh environment of the Ethiopian Highlands, the observed increase in litter size in the PGF11 compared to the PGF7 group, from a production point of view, cannot be considered as an undesirable increase of prolificacy and the extra lambs born can be easily managed by farmers. Physiologically, the effect of prostaglandin analogues on ovulation rate and prolificacy is controversial, with reports showing no change, a decrease, and an increase in ovulation rate (Fierro et al. Citation2013). Combining litter size and the number of inseminated ewes under what we called lambing rate, puts the P4+eCG and the PGF11 treatment groups in a similar rank significantly exceeding the reproductive outcome in both PGFs and PGF7 treatments.
Conclusions
The findings of this study indicate that the use of a prostaglandin-based protocol composed of 2 injections 11 days apart, preceded by a careful selection of non-pregnant ewes (BCS > 2) using ultrasonography for cervical fixed-time AI with fresh semen, is a feasible reproductive management option for the dissemination of genetic gain in the framework of CBBPs in Ethiopia.
Acknowledgement
The authors acknowledge the contribution of the staff at Debre Birhan (Amhara Regional Agricultural Research Institute) for their technical support. They are also indebted to the engagement of the Menz CBBP farmers for contributing with their sheep and labour to the experimental work.
Disclosure statement
The authors declare no conflict of interest that would prejudice the impartiality of this scientific work.
Additional information
Funding
References
- Baril G, Remy B, Leboeuf B, Beckers JF, Saumande J. 1996. Synchronization of estrus in goats: the relationship between eCG binding in plasma time of occurrence of estrus and fertility following artificial insemination. Theriogenology. 45:1553–1559. doi: 10.1016/0093-691X(96)00123-9
- Berlinguer F, Gonzalez-Bulnes A, Succu S, Leoni G, Mossa F, Bebbere D, Ariznavarreta C, Treguerres JAF, Veiga-Lopez A, Naitana S. 2007. Effects of progestagens on follicular growth and oocyte developmental competence in FSH-treated ewes. Domest. Anim. Endocrinol. 32:303–314. doi: 10.1016/j.domaniend.2006.04.007
- Bru C, Fantova E, Sevilla E, Quintin FJ, Alabart JL. 1995. Resultados de insemiancion artificial de las ovejas Rasa aragonea de las ganaderías de Carne Aragón, S.C.L. Influencia de la condición corporal, Proceedings of the XX Jornadas Científicas de la Sociedad Española de Ovinotecnia y Caprinotecnia, Madrid (España).
- Contreras-Solís I, Vásquez B, Díaz T, Letelier C, López Sebastian A, González Bulnes A. 2009. Efficiency of estrous synchronization in tropical sheep by combining short-interval Cloprostenol-based protocols and “male effect”. Theriogenology. 71:1018–1025. doi: 10.1016/j.theriogenology.2008.11.004
- Evans G, Maxwell WMC. 1987. Insemination. In: Evans G, Maxwell WMC, editors. Salamon's artificial insemination of sheep and goats. Editorial Butterworths 2nd ed. Sydney, Australia: Publisher; p. 194 pp.
- Fairnie IJ, Wales RG, Gherardi PB. 1977. Time of ovulation, fertilisation rate, and blastocyst formation in ewes following treatment with a prostaglandin analogue (ICI 80996). Theriogenology. 8:183. doi: 10.1016/0093-691X(77)90145-5
- Fierro S, Gil J, Viñoles C, Olivera-Muzante J. 2013. The use of prostaglandins in controlling estrous cycle of the ewe: a review. Theriogenology. 79:399–408. doi: 10.1016/j.theriogenology.2012.10.022
- Fierro S, Viñoles C, Olivera-Muzante J. 2016. Concentrations of steroid hormones, estrous, ovarian and reproductive responses in sheep estrous synchronized with different prostaglandin-based protocols. Anim. Reprod. Sci. 167:74–82. doi: 10.1016/j.anireprosci.2016.02.009
- Fierro S, Viñoles C, Olivera-Muzante J. 2017. Long term prostaglandin based-protocols improve the reproductive performance after timed artificial insemination in sheep. Theriogenology. 90:109–113. doi: 10.1016/j.theriogenology.2016.11.031
- Flores-Najera MJ, Meza-Herrera CA, Echavarrıa FG, Villagomez E, Iniguez L, Salinas H, Gonzalez-Bulnes A. 2010. Influence of nutritional and socio-sexual cues upon reproductive efficiency of goats exposed to the male effect under extensive conditions. Anim. Prod. Sci. 50:897–901. doi: 10.1071/AN10030
- Getachew T, Haile A, Tibbo M, Sharma AK, Sölkner J, Wurzinger M. 2010. Herd management and breeding practices of sheep owners in a mixed crop-livestock and a pastoral system of Ethiopia. Afr. J. Agric. Res. 5:685–691.
- Gizaw S, Komen H, Hanotte O, Van Arendonk JAM. 2008. Indigenous sheep resources of Ethiopia: types, production systems. Anim. Genet. Resour. Inf. 43:25–39. doi: 10.1017/S1014233900002704
- Gizaw S, Van Arendonk JAM, Komen H, Windig JJ, Hanotte O. 2007. Population structure, genetic variation and morphological diversity in indigenous sheep of Ethiopia. Anim. Genet. 38:621–628. doi: 10.1111/j.1365-2052.2007.01659.x
- Haile A, Gizaw S, Getachew T, Mueller JP, Amer P, Rekik M, Rischkowsky B. 2019. Community-based breeding programmes are a viable solution for Ethiopian small ruminant genetic improvement but require public and private investments. J. Anim. Breed. Genet. 136:319–328. doi: 10.1111/jbg.12401
- Haile A, Tembely S, Anindo D, Mukasa-Mugerwa E, Rege JE, Yami A, Baker RL. 2002. Effects of breed and dietary protein supplementation on the responses to gastrointestinal nematode infections in Ethiopian sheep. Small Rumin. Res. 44:247–261. doi: 10.1016/S0921-4488(02)00080-9
- Hervé V, Roy F, Bertin J, Guillou F, Maurel MC. 2004. Anti-equine chorionic gonadotropin (eCG) antibodies generated in goats treated with eCG for the induction of ovulation modulate the luteinizing hormone and follicle-stimulating hormone bioactivities of eCG differently. Endocrinology. 145:294–303. doi: 10.1210/en.2003-0595
- Leyva V, Buckrell BC, Walton JS. 1998. Regulation of follicular activity and ovulation in ewes by exogenous progestogen. Theriogenology. 50:395–416. doi: 10.1016/S0093-691X(98)00148-4
- Lopez-Sebastian A, Gonzalez-Bulnes A, Carrizosa JA, Urrutia B, Diaz-Delfa C, Santiago-Moreno J, Gomez-Brunet A. 2007. New estrus synchronization and artificial insemination protocol for goats based on male exposure, progesterone and cloprostenol during the non-breeding season. Theriogenology. 68:1081–1087. doi: 10.1016/j.theriogenology.2007.08.003
- Manes J, Rios G, Fiorentino MA, Ungerfel R. 2016. Vaginal mucus from ewes treated with progestogen sponges affects quality of ram spermatozoa. Theriogenology. 85:856–861. doi: 10.1016/j.theriogenology.2015.10.033
- Maurel MC, Roy F, Hervé V, Bertin J, Vaiman D, Cribiu E, Manfredi E, Bouvier F, Lantier I, Boue P, Guillou F. 2003. Réponse immunitaire à la eCG utilisée dans le traitement de l’induction d’ovulation chez la chèvre et la brebis. Gynécologie Obs. Fertil. 31:766–769. doi: 10.1016/S1297-9589(03)00214-5
- Mekuriaw Z, Assefa H, Tegegne A, Muluneh D. 2015. Estrus response and fertility of Menz and crossbred ewes to single prostaglandin injection protocol. Trop. Anim. Health Prod. 48:53–57. doi: 10.1007/s11250-015-0919-z
- Mueller JP, Haile A, Getachew T, Rekik M, Rischkowsky B. 2019. Genetic progress and economic benefit of community-based breeding programs for sheep out- and upscaling options in Ethiopia. Small Rumin. Res. 177:124–132. doi: 10.1016/j.smallrumres.2019.06.025
- Palacín I, Yániz JL, Fantova E, Blasco ME, Quintín-Casorrán FJ, Sevilla-Mur E, Santolaria P. 2012. Factors affecting fertility after cervical insemination with cooled semen in meat sheep. Anim. Reprod. Sci. 132:139–144. doi: 10.1016/j.anireprosci.2012.05.005
- Pope WF, Cárdenas H. 2004. Sensitivity of sheep to exogenous prostaglandin F2α early in the estrous cycle. Small Rumin. Res. 55:245–248. doi: 10.1016/j.smallrumres.2004.01.004
- Rekik M, Haile A, Abebe A, Muluneh D, Goshme S, Ben Salem I, Hilali ME, Lassoued N, Chanyalew Y, Rischkowsky B. 2016. GnRH and prostaglandin-based synchronization protocols as alternatives to progestogen-based treatments in sheep. Reprod. Domest. Anim. 51:924–929. doi: 10.1111/rda.12761
- Russel AJF, Doney JM, Jun RG. 1969. Subjective assessment of body fat in live sheep. J. Agric. Sci. 72:451–454. doi: 10.1017/S0021859600024874
- Santolaria P, Palacin I, Yaniz J. 2011. Management factors affecting fertility in sheep, artificial insemination in farm animals, Dr. Milad Manafi (Ed.), ISBN: 978-953-307-312-5, InTech, 2. Available from: http://www.intechopen.com/books/artificial-insemination-in-farm-animals/management-factors-affectingfertility-in-sheep.
- Vilariño M, Rubianes E, Menchaca A. 2011. Re-use of intravaginal progesterone devices associated with the short-term protocol for timed artificial insemination in goats. Theriogenology. 75:1195–1200. doi: 10.1016/j.theriogenology.2010.11.030
- Viñoles C, Forsberg M, Banchero G, Rubianes E. 2001. Effect of long-term and short-term progestagen treatment on follicular development and pregnancy rate in cyclic ewes. Theriogenology. 55:993–1004. doi: 10.1016/S0093-691X(01)00460-5
