ABSTRACT
The aim of current trial was to investigate the efficacy of a new plant-specific fungus (Piriformospora indica) rich in mannan as a fungal feed additive in chicks. A control diet was formulated and 5 incremental levels (0.1, 0.2, 0.4, 0.6, and 0.8 g/kg of diet) of P. indica and 750 mg/kg of diet yeast culture or 500 mg/kg L. acidophilus were added to the control diet. Different characteristics and responses were measured during experimental period. P. indica inclusion increased BW and significantly decreased FCR (P ≤ 0.01) but had no effect on feed intake. P. indica significantly enhanced blood glucose level and reduced cholesterol, LDL, total protein, albumin and uric acid concentration of plasma (P ≤ 0.01; P ≤ 0.05). Higher villus height and thinner crypt depth were obtained in chicks fed 0.6 g P. indica in comparison with the chicks fed control diet or diets containing Lactobacillus acidophilus or yeast culture. Protein digestibility was enhanced in chicks fed yeast culture or 0.1, 0.2 and 0.4 g of P. indica (P ≥ 0.001). Gizzard pH was increased and ileal pH was decreased and the relative weight of carcass, bursa of Fabricius and thymus were increased by inclusion of P. indica.
Introduction
With the increasing demand for antibiotic free poultry products, prebiotics and probiotics proposed as an alternative to antibiotics and have been increasingly applied to improve day-old chicks by improving their intestinal microbial balance and immune system (Fuller Citation1989, p. 366). The new trials results are encouraging for the improvement of intestinal health and gut development and improve overall efficiency of the broiler chicken especially by using the gastrointestinal growth promoters. Various types of trials in this specific area demonstrated that dietary effects such as ingredient composition, enzymes and other antimicrobials inclusion, intestinal microflora balances, pathogens existence, as well as probiotics and prebiotics administration directly affect the development of the gastrointestinal tract in birds and have been manipulated to achieve increased feed conversion and pathogen reduction in broiler (Apajalahti et al. Citation2004; Banerjee and Ray Citation2017; Carnevali et al. Citation2017). Sohail et al. (Citation2012) defined prebiotics as indigestible feed ingredients that beneficially affect the host by selectively stimulating the proliferation and activity of one or a few bacteria. Mannan oligosaccharides are prebiotics that are polysaccharide-protein complexes derived from yeast. They are known to create favourable conditions for Lactobacillus but they also act as competitive binding sites for pathogens with type 1 fimbriae that recognize D-mannose receptors (Hutsko et al. Citation2016). Solis de los Santos et al. (Citation2007) reported that in turkey fed diets supplemented with a mannan oligosaccharide, the proportion of acidic mucins within goblet cells was increased. Growth promoting activity of prebiotics on beneficial bacteria, such as Lactobacillus and Bifidobacteria, can boost the metabolism of host birds and improve gut efficiency by increasing nutrient absorption (Yokota and Coates Citation1982) and hastening gut development (Palmer and Rolls Citation1983). This bacteria can improves animals and poultry performance by prohibiting pathogens, strengthening intestinal barrier function, and positively modulating the immune system (Ng et al. Citation2009). Many researches have corroborated the potential link between gut health and gut microflora (Thompson and Applegate Citation2006), and it is contemplate to find new candidate of probiotics to increase broiler profitability, gut health and enhance gut associated immunity, for diminishing antibiotic usage in poultry feed and also enhancing our knowledge on the mechanisms behind the beneficial action of probiotics in poultry.
Piriformospora indica (P.indica), is a root endophytic fungus rich in mannan oligosaccharides which has been isolated from rhizosphere soil of plants growing in extreme hot conditions of Thar desert of Rajasthan, India (Varma et al. Citation1999). This fungus is similar to arbuscular mycorrhizal fungi; however, it can be axenically cultivated on a variety of synthetic media (Singh et al. Citation2011). It grows inter- and intracellularly, and forms a symbiosis with the roots of a wide variety of plants and can promote the growth and enhance the activity of secondary metabolite in a range of economical and medicinally important plants (Ghabooli et al. Citation2013, Citation2014; Ghaffari et al. Citation2016). Besides stimulating growth, P. indica increases the uptake and metabolism of nitrogen and phosphate and allows plants to survive under extreme abiotic stress (Sun et al. Citation2010). It also confers resistance to toxins, pathogenic microorganisms, and increases host biomass yield (Waller et al. Citation2008).
A literature review surveys showing that there have been no reports on the effect of plant-specific fungus such as P. indica in poultry and animals. Therefore, it is of interest to investigate the growth promoter effects of P. indica as a mannan oligosaccharide content prebiotic candidate in poultry. Therefore, findings from the present study may open up a new field; possibly identifying new prebiotic targets to increase broiler profitability, gut health and reduce antibiotics usage in poultry feed and also enhance our knowledge on the mechanisms behind the beneficial action of prebiotics in poultry.
Material and methods
The animal experiment was carried out in accordance with the procedure and guidelines approved by the Animal Care Committee of Iranian Council of Animal Care, 1995.
Fungal growth and Piriformospora indica preparation
Piriformospora indica were cultured in accordance with the procedure described by Ghabooli et al. (Citation2013). Briefly, cultures were maintained on a complex agar medium supplemented with 15 g l-1 agar at 28 ± 1°C. For liquid culture of the fungus, discs from the actively growing edges of the petri dish culture were punched out and inoculated in 250-mL Erlenmeyer flasks containing 100 mL complex medium without agar at 28 ± 1°C on a gyratory shaker at 150 rpm for 6–7 d. After completing the growth step of fungus (), culture medium was mixed with wheat bran (100, 200, 400, 600, and 800 mL of medium) and then added to the different diet to produce diets with different levels of P. indica. Each gram of P. Indica contained 180 mg of mannan determined by method described by Jones and Ballou (Citation1969).
Bird management and experimental design
A total of 384 male Ross 308 broilers (1 d old) were obtained from a parent stock farm (40 week) and raised over a 42-d experimental period. The chicks were placed in pens (1 × 1 m2) and kept inside a building where was heated using the 2 heaters to maintain concrete temperatures above 32°C degrees at arrival time. The experiment was conducted as a completely randomized design and involved 8 treatments of 6 replicates and 8 chicks in each pen. A 3-phase (0–10, 11–24 and 25–42 d of age) control diet was formulated and 5 incremental levels (0.1, 0.2, 0.4, 0.6, and 0.8 g/kg of diet) of P. indica and 750 mg/kg of diet yeast culture (XPC, Diamond V co. 2525 60th Ave SW, Cedar Rapids, IA 52404, United States) or 500 mg/kg Lactobacillus acidophilus (Lallemand Animal Nutrition, France) was added to control diet. Control diets are shown in . Before the formulation of diets the protein content of soybean meal and corn were determined precisely according to the AOAC method (AOAC, Citation2003) and calcium and phosphorus content of soybean meal and corn were determined using a spectrophotometer (UNICO 2150, Germany) measuring absorbance at 600 and 340 nm (method 946.06, AOAC International Citation2000) respectively and the values were considered in formulation of the diets. Broilers had ad libitum access to feed and water throughout the experimental period. At the beginning of the trail and at the beginning of each week, total amount of feed needed for 1 wk consumption of birds in each pen (equal to the Ross performance objective) was mixed and fed every morning (0800 h) by placing into the bucket plastic gallon pan feeder. At the end of d 7, the amount of feed consumption of each pen was calculated by total amount of mixed feed minus the residual feed in pan. Automatic bell drinkers with the same polyethylene tanks were used for water as a drinking system for broilers and all chicks received similar water. Chicks were brooded following standard temperature regimens, which gradually decreased from 32 to 24°C, and under a 23:1 light:dark cycle. Body weight was measured weekly and cumulative feed intake was measured at the end of the first week and then on weeks 3 and 7 for each pen for calculating the FCR.
Table 1. Composition and calculated analyses of the basal diets.
Blood characteristics
On d 14 and 42, 4 chicks from each pen were selected and 2-mL blood samples were collected in heparinized and nonheparinized tubes by puncturing the brachial vein. Two samples (nonheparinized tubes) centrifuged at 1734 × g at 0°C for 20 min, and the serum was used for measuring triglyceride, cholesterol, low-density lipoprotein (LDL), and high-density lipoprotein (HDL). Plasma from heparinized tubes was used for measuring the total protein, albumin, gamma-glutamyl transpeptidase (SGGT), alanine aminotransferase (SGPT) and plasma uric acid concentration. The triglyceride, total cholesterol, HDL, and LDL cholesterol content in the samples were analysed with an automatic biochemical analyzer (Hitachi Boehringer Mannheim 717) using colorimetric method. Plasma from heparinized tubes was used for determination of total protein, albumin, gamma-glutamyl transpeptidase (SGGT) and alanine aminotransferase (SGPT) with an automatic biochemical analyzer (Hitachi 717, Boehringer Mannheim, Ingelheim am Rhein, Germany) using an Elitech diagnostic kit (catalog number A.110537). The pooled serum was then analysed for plasma uric acid using commercial reagent kits (Pointe Scientific, Canton, MI).
Intestinal morphology parameters
On days 14 and 42 d of age 2 chicks from each pen were slaughtered in accordance with the procedures allowed by animal care committee of the university and a 2-cm segment of the middle of the jejunum samples were washed in physiological saline solution and fixed in 10% buffered formalin and the fixing solution was changed 3 times in daily intervals for fixation. A single 0.5-cm sample was cut from each ileal section, dehydrated using increasing ethanol concentrations, cleared with xylene and placed into polyfin embedding wax. Tissue sections (2 µm) were cut by microtome (Easy cut 202, Italy), floated onto slides and stained with haematoxylin (Gill number 2; Sigma, St. Louis, MO) and eosin (Sigma Aldrich, Darmstadt, Germany). Villus height and crypt depth were measured on images taken from samples and micrometer using a digital camera equipped with light microscopy (Motic-SMZ-140, Germany). Twenty images from 8 tissue sections of each ileal section were taken and 34 villus heights and crypt depths were measured by imaging software. Measurements for villus lengths were taken from the tip of the villus to the valley between individual villi, and measurements for crypt depth were taken from the valley between individual villi to the basolateral membrane.
Enumeration of cecal bacterial population
On d 35, one gram of the cecal contents of two birds from each pen was pooled and used for serial dilution. Microbial populations were determined by serial dilution (10− 5 to 10− 6) of cecal samples in anaerobic diluents (saline solution 9%) before inoculation onto petri dishes of sterile agar as described by Bryant and Burkey (Citation1953). Salmonella Shigella agar (Liofilchem, Zona Ind.le-Roseto d. Abruzzi-Lot: 111816503, Italy) is used to salmonella culture and McConkey agar (Liofilchem, Zona Ind.le-Roseto d. Abruzzi-Lot: 120316502, Italy) for coliforms and E. coli. Plates were incubated at 37°C and were counted between 24 and 48 h after inoculation. Colony forming units were counted immediately after removal from the incubator (GmbH, D-91126, Germany). Agars used to grow Clostridium perfringens were prepared according to the U.S. Food and Drug Administration (FDA Citation1992). Plates for C. perfringens was incubated anaerobically (73% N:20% CO2:7% H2) at 37°C. E. coli was incubated aerobically at 37°C. Plates were counted between 24 and 48 h after inoculation. Finally, colony forming units were defined as distinct colonies measuring at least 1 mm in diameter.
Digestibility
All feed samples were ground through a 1-mm screen and protein content of samples was determined according to the AOAC method (AOAC, Citation2003). Ileal apparent digestibility of protein was calculated by using the acid insoluble ash (AIA) as an inner marker inner. During d 35–42, birds were randomly chosen, weighed and slaughtered in accordance with the procedures allowed by animal care committee of the university and the digestive tract was removed and the ileum was separated from the Meckel’s diverticulum up to 1 cm proximal to the ileocecal junction and then dried with soft paper; then, the digesta gently was collected from half of the ileum into plastic cups by flushing with distilled water. Then after samples were pooled and frozen until being lyophilized and grounded. Samples were then used to determine DM content by oven drying at 105°C for 24 h. Nitrogen content and acid insoluble ash content of the samples were determined according to the AOAC international (AOAC Citation2003).
Digestive tract traits
On days 14 and 42, 2 chicks from each pen were slaughtered in accordance with the procedures allowed by animal care committee of the university and the weight of carcass, breast, thigh, abdominal fat, liver, spleen, thymus, gizzard, and bursa of Fabricius and the length of the duodenum, jejunum, and ileum were measured. The weight of the empty organs was expressed relative to live BW. The gizzard, duodenum, jejunum, and ileum were cut longitudinally and pH of their contents was measured in triplicate by using a digital pH meter (Testo 205, Testo-Strabe 1, 79853 Lenzkirch, Germany).
Immune measurements
On days 21 and 28 0.1 mL/kg BW of 0.5 cc/100 sheep red blood cell (SRBC) was injected into brachial vein of 2 chicks per each pen. Seven days after each injection, blood was collected in nonheparinized tubes by puncturing the brachial vein. Serum was obtained by centrifuging at 1500 × g for 15 min and stored at −30°C until assayed. Individual serum samples were analysed for antibody responses against SRBC by ELISA technique using commercial kits, and the plates were read at 405 nm on an ELISA reader.
Statistical methods
Data were analysed in a completely randomized design with 8 treatments of 6 replicates and 8 chicks in each pen and the effects (control, yeast culture, L. acidophilus, and P. indica inclusion levels) were estimated using the general linear model procedure of SAS software (SAS Citation2003). Normal distribution of the residual was checked by Shapiro-Wilks test using the UNIVARIATE procedure of SAS software. When the effect was significant, differences between treatment means were tested using Duncan multiple comparison test. For the level of inclusion of P. indica in the diet, linear and quadratic effects were also fitted in the model. Also, orthogonal polynomial contrasts were used to assess the significance of linear and quadratic models to describe the response in the dependent variable to total differences between P. indica with control, yeast culture, or L. acidophilus using the Mixed procedure of SAS for performance. Results are reported as means in tables, and differences among treatments were considered significant at a threshold of P < 0.05. Microbiological concentrations were subject to log10 transformation before analysis.
Results
Growth performance
Influence of P. indica on growth performance of broilers was shown in and . Piriformospora indica had no prominent effect on BW during the 0–14 d of age, but by aging the chicks the effects of P. indica on BW was increased and different levels of P. indica gained significantly much higher than that of control diet (linear, P ≤ 0.01, and quadratic, P ≤ 0.05). The highest BW was observed in birds received 0.2 g of P. indica at 42 d of age. Chicks fed yeast culture or L. acidophilus showed lower BW in comparison with the chicks fed P. indica on d 42. Feed intake was increased by addition of 0.4, 0.6, and 0.8 g of P. indica at 21 d of age; also, similar results were obtained in chicks fed yeast culture or L. acidophilus on d 21, but there were no significant effects of the additives on feed intake at 42 d of age. The FCR was significantly decreased in chicks fed yeast culture and L. acidophilus as well as chicks fed 0.6 and 0.8 g of P. indica during the first week after hatch. But there was no significant effect of additives on FCR at 42 d of age. The highest BW gain and lowest FCR was obtained by inclusion of 0.2 g P. indica in control diet during the experimental period (). Overall, P. indica increased BW without any changes in feed intake () and the results for BW were comparable to yeast culture and L. acidophilus on d 42.
Table 2. Influence of Piriformospora indica, Lactobacillus acidophilus, and yeast culture in diet on growth performance of broilers from 1 to 42 d of age.1
Table 3. Influence of Piriformospora indica, Lactobacillus acidophilus, and yeast culture in diet on growth performance of broilers using orthogonal polynomial contrasts from 1 to 42 d of age.1
Blood characteristics
The effects of P. indica on blood metabolites at 14 and 42 d of ages were shown in and . Except for SGGT and SGPT there were no significant effects of treatment on blood metabolites on d 14. The lowest and highest SGGT concentration in plasma was shown in control and L. acidophilus fed birds on d 14 (linear, P ≤ 0.01, and quadratic, P ≤ 0.05). Piriformospora indica significantly increased SGPT, but contrariwise, yeast culture reduced SGPT on d 14 (linear, P ≤ 0.01, and quadratic, P ≤ 0.06). Results for blood metabolites at 42 d of age showed that P. indica significantly enhanced blood glucose level and reduced cholesterol, LDL, total protein, albumin, and uric acid concentration of plasma (linear, P ≤ 0.01, and quadratic, P ≤ 0.05). A linear increase in plasma glucose and linear reduction of plasma uric acid was achieved by incremental levels of P. indica at 42 d of age. Gamma-glutamyl transpeptidase and alanine aminotransferase of plasma also significantly was changed by treatments. Highest level of SGGT and SGPT at 42 d of age was observed in chicks fed 0.1 and 0.8 g of P. indica, respectively.
Table 4. Influence of Piriformospora indica, Lactobacillus acidophilus, and yeast culture in diet on plasma metabolites of broilers at 14 d of age.1
Table 5. Influence of Piriformospora indica, Lactobacillus acidophilus, and yeast culture in diet on plasma metabolites of broilers at 42 d of age1.
Effect of Piriformospora indica on intestinal morphology parameters
A cross-section of the ileal morphology for chicks fed control diet or diets containing P. indica, L. acidophilus, or yeast culture are shown in . There were prominent differences in villus height and crypt depth among the treatments. Villus height and crypt depth were significantly (P ≥ 0.001) affected by P. indica inclusion in control diet at 14 and 42 d of ages (). Villus height in chicks fed 0.6 g P. indica was greater (linear, P ≤ 0.01, and quadratic, P ≤ 0.01) than the corresponding values in chicks fed control diet or diets containing L. acidophilus or yeast culture. The villus height was 200 and 253 µm higher than that of control diet in chicks fed 0.6 g P. indica at 14 and 42 d of age, respectively. Piriformospora indica inclusion significantly reduced crypt depth (linear, P ≤ 0.01, and quadratic, P ≤ 0.01) in comparison with the control diet and also villus to crypt ratio (linear, P ≤ 0.01, and quadratic, P ≤ 0.01) was significantly increased in chicks fed P. indica at 14 and 42 d of ages.
Figure 2. Ileal morphology of chicks fed different treatments: (a) control, (b) Piriformospora indica, (c) yeast culture, and (d) Lactobacillus acidophilus.
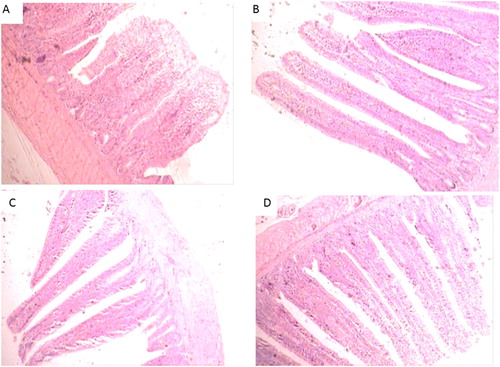
Table 6. Influence of Piriformospora indica, Lactobacillus acidophilus, and yeast culture in diet on ileum morphological characteristics at 14 and 42 d of age and cecal microbial population and apparent ileal digestibility coefficient of protein on d 35.1
Protein digestibility and enumeration of cecal bacterial population
Ileal digestibility of protein was enhanced in chicks fed yeast culture or 0.1, 0.2 and 0.4 g of P. indica (linear, P ≤ 0.007 and quadratic, P ≥ 0.001). The highest and lowest CP digestibility coefficient was obtained in chicks were fed diets containing yeast culture or L. acidophilus, respectively (). The cecal E. coli population was reduced in chicks fed diets containing P. indica or yeast culture (P ≤ 0.05), but L. acidophilus–fed chicks showed the highest levels of E. coli population in their ceca in comparison with the control diet (). Clostridium perfringens and salmonella were not detected in the serial dilution.
Gastrointestinal pH and carcass analysis
Results of carcass analysis are shown in and . The relative weight of carcass significantly (P ≤ 0.01) increased by supplementing of the diets with 0.6 g of P. indica at 14 d of age, but the rest were unchanged under the influence of treatments. The relative length of ileum quadratically declined (P ≤ 0.05) as the proportion of P. indica increased in control diet at 42 d of age. The relative weight of carcass, bursa of Fabricius, and thymus were increased by inclusion of P. indica and yeast culture, especially when P. indica was added at 0.2 g of diet at 42 d of age. The relative weight of liver, spleen, breast, thigh, and abdominal fat remained unchanged by inclusion of P. indica and also yeast culture and L. acidophilus in control diet at 42 d of age. Incorporation of P. indica instead of yeast culture or L. acidophilus in control diet significantly (P ≤ 0.05) increased gizzard pH and decreased jejunum pH, but the reduction of pH in gizzard and jejunum was obtained by inclusion of yeast culture or L. acidophilus (). In the duodenum and ileum, the pH showed no changes by addition of these products.
Table 7. Influence of Piriformospora indica, Lactobacillus acidophilus, and yeast culture in diet on carcass analysis of broilers at 14 d of age.1
Table 8. Influence of Piriformospora indica, Lactobacillus acidophilus, and yeast culture in diet on carcass analysis of broilers at 42 d of age.1
Table 9. Influence of Piriformospora indica, Lactobacillus acidophilus, and yeast culture in diet on antibody response at 28 and 42 d of age and pH of different parts of gastrointestinal at 42 d of age.1
Immune response
Piriformospora indica did not affect primary or secondary antibody response against the SRBC (), but immune related organs weight such as bursa of Fabricius, and thymus were increased by inclusion of P. indica.
Discussion
Prebiotics have a profound effect on overall nutrient utilization, gut health, immunity and growth of broiler chickens. Prebiotics form part of nutritional strategies targeting broiler performance and health and are being broadly investigated for their effects on gut microbiota (Applegate et al. Citation2010). In spite of the success showed by the development of the Lactobacillus probiotic for use in commercial poultry, there is still an indispensable need for commercial probiotics that are shelf stable, cost effective, and feed stable (tolerant to heat pelletization procedures) to increase compliance and widespread use (Latorre et al. Citation2014). The primary aim of this study was to generate new attitude on the effects of plant specific fungus which is rich in mannan as a poultry prebiotics and explore its effects and feasibility in broilers. In agreement with earlier reports discussing the influence of prebiotics on growth performance (Kabir et al. Citation2004), the present study showed that P. indica inclusion has resulted in increased BW and decreased FCR by aging the birds. It is reported (Kabir et al. Citation2004) that better growth performance and feed efficiency of broiler chickens fed prebiotics will be induced by the total effects of prebiotic action including the maintenance of beneficial microbial population improving feed intake and digestion and altering bacterial population and intestinal morphology (Cole et al. Citation1987).
Piriformospora indica addition showed no impressive effect on BW and FCR in starter (0–7 d) and also grower (11–21 d) period but its effects on BW at finisher phase were prominent and birds fed diets supplemented with P. indica gained higher weight than those of birds fed control, L. acidophilus, or yeast culture supplemented diets. This fungus is similar to arbuscular mycorrhizal fungi (Singh et al. Citation2011); It grows inter- and intracellularly and forms a symbiosis with the roots of a wide variety of plants and can promote the growth and enhance the activity of secondary metabolite in a range of economical and medicinally important plants (Ghabooli et al. Citation2013, Citation2014; Ghaffari et al. Citation2016). Overall, the results corroborate studies by Fritts et al. (Citation2000), which all suggest that the effects of supplementing a broiler diet with Bacillus spore can improve growth performance at late stage of rearing period. However, there are some conflicting studies. For example, Knap et al. (Citation2011) reported significant differences in daily gain and FCR with Bacillus subtilis DSM17299 supplementation during the experimental period. In addition, Santoso et al. (Citation1999) reported that supplementation with B. subtilis spore did not affect broiler performance compared with the control treatment. It is speculate that the variation in the results of the aforementioned studies with spore and fungus base pro-and prebiotics can be ascribed to several factors, including the age of the animals, the dose of administration, germination and increase their load in intestine, diet composition, feed form, and interaction with other dietary feed additives (Chesson Citation1994). Our results demonstrated that performance improvement by dietary supplementation with P. indica compared with control is age related, indicating that the tendency for improved BW and FCR may occur as a result of an increase in load of fungus and mannan oligosaccharides in gastrointestinal lumen by growing up the chicks and increase in feed intake and improvement in villi growth, pH reduction and overall nutrient digestibility.
In the current study, protein digestibility enhanced and plasma total protein, uric acid and albumin concentration reduced linearly by incremental levels of P. indica in diets. In broilers, plasma uric acid level is an important indicator for protein digestibility and amino acid utilization and its decreases by increase in protein and amino acid utilization (Donsbough et al. Citation2010). Researchers reported that, besides stimulating growth, biomass and seed production in the host, P. indica increases the uptake and metabolism of nitrogen and phosphate and allows plants to survive under extreme abiotic stress (Sun et al. Citation2010). Furthermore, P. indica enhances nutrient uptake, enables plants to cope with environmental conditions, and survives under abiotic stresses. In this study, the improvement in growth performance in P. indica and yeast culture fed chicks was concomitant with an improved total tract CP digestibility. Only a few studies have examined nutrient digestibility in broilers fed probiotics. It was shown that depending on the probiotic inclusion level in the diet, probiotic intake resulted in an improved apparent digestibility of nitrogen and fat in broilers diets and ileal apparent digestibility of energy, calcium, phosphate, CP, and most amino acids in broilers (Li et al. Citation2008).
Results of current study showed that there is no differences between treatments on blood glucose, cholesterol, triglyceride and LDL concentration on d 14, however at the end of 42 d of age blood glucose was enhanced and cholesterol, triglyceride and LDL content of plasma were reduced in chicks fed diets containing P. indica. Several studies have evaluated the effect of feeding yeast and prebiotics on blood glucose regulation in chickens. Bacterial β-glucosidase contributes to the hydrolysis of glucose monomers from nonstarch polysaccharides (e.g. cellulose, β-glucans), and play an important role in the carbohydrate digestion in small intestine and reduces undigested carbohydrates and, ultimately, increase blood glucose concentration and animal performance and health. However, changes in the glycolytic activity in the intestinal tract did not measured in the current study, but plasma glucose enhancement along with decrease in E. coli population and increase in protein digestion by addition of P. indica maybe resulted from changes in enzymatically activities in gastrointestinal tract. Several studies have proven that live organism reduces serum cholesterol, triglyceride, and LDL cholesterol by interfering the recycling of lipids in the gastrointestinal tract (Navid Citation2010). Some specific bacteria such as B. subtilis could prevent bile salts from reabsorption and convert them to the second type. At the same time they could synthesize esterase enzymes converting free fatty acids to their esterified forms, which differ from triglyceride in the intestinal tract. Therefore, cholesterol and triglyceride were less likely of being absorbed into the serum (Zhang et al. Citation2012).
Plasma gamma-glutamyl transpeptidase and alanine aminotransferase concentrationsenhanced in chicks fed P. indica. The presence of intracellular enzymes in the blood plasma relates to either hepatic malfunction or hepatocyte membrane leakage (Peebles et al. Citation1997). In medicine, an examination of serum enzymes is an important method for assessing damage to liver cells. Serum enzymes are mainly from the liver as the liver parenchyma is compressed, which can lead to inflammation of the liver cells and an increase in enzyme synthesis, resulting in an increase in serum enzyme concentrations. From our point of view, long-term P. indica inclusion maybe induced inflammation of the liver cells; however, there was no enlargement of liver and no significant differences in the relative weight of the liver between the treatments. As there are no reports on the effect of P. indica in animal and poultry to support the opinion that P. indica per se or its metabolites induces hepatic inflammation needs to be further illustration.
The histological examinations of ileum showed that supplementation of broilers diets with P. indica increased the villus height and villus height:crypt depth ratio and decreased crypt depth in ileum considerably, suggesting an increased epithelial surface due to feeding of P. indica. In particular, the distinguished increase of villus height due to P. indica inclusion may be considered beneficial for the ileal absorptive capacity. Additionally, broilers fed with control or lactobacilli showed deeper crypt depths. Shorter villi and deeper crypts may lead to poor nutrient absorption and lower animal performance (Xu et al. Citation2003). Moreover, deeper crypts may be indicative of an increase in the proliferative pool of enterocytes, which might be needed for rapid epithelial turnover in response to inflammation from pathogens or their toxins, as there was shown high population of E. coli in control and lactobacilli-fed chicks. We observed relatively long villi length and thinner crypt and also increased CP digestibility and lower E. coli population in the group supplemented with P. indica and yeast culture. This effect may resulted from reduced toxicity or better protection of villi and is likely related to better nutrient absorption. Probiotics have been shown to be protective to the intestinal epithelium by prohibiting gut pathogens proliferations inducing expression of intestinal protective factors (Lutgendorff et al. Citation2009), strengthening tight junctions and fixing the cytoskeleton of epithelial cells (Sherman et al. Citation2005). Researches have reported that P. indica enhances resistance to toxins, pathogenic microorganisms, and increases seed biomass yield (Waller et al. Citation2008).
Piriformospora indica inclusion significantly increased thymus and bursa of Fabricius relative weight and also a numerical increased in antibody response against SRBC. This effect might have resulted from reduced toxicity or better protection of villi and is likely related to better nutrient absorption. It has been also shown that P. indica enhances stress tolerance based on general and nonspecific plant-species mechanism (Ghabooli et al. Citation2013). Mass spectrometry analysis resulted in the identification of 45 differentially accumulated proteins involved in reactive oxygen scavenging, metabolisms, signal transduction, and plant defense responses. Interestingly, P. indica increases the level of proteins involved in photosynthesis, antioxidative defense system and energy transport (Ghabooli et al. Citation2013). Recent works demonstrated that probiotics resulted in an enhancement of broiler humoral immune response (Kabir et al. Citation2004). The enhancement of the humoral immune response by probiotics maybe resulted from defense against specific stress induced factors (Kabir et al. Citation2004) and could therefore be regarded as an improved capacity of the humoral and cellular immune system of birds to cope with foreign antigens. In addition, probiotics have been shown to have immunomodulatory activity at a cellular level resulting in an enlargement of immune related organs that protected against pathogens (Dalloul et al. Citation2005). However, an in-depth investigation of P. indica effects on broiler cellular and humoral immunity was beyond the scope of this study, but it will be interesting subject for evaluation of that in clos future.
The results of the present study showed that supplementation of the diet with P. indica increased gizzard pH and reduced ileal pH. Increased gizzard pH may be resulted from increased digestibility and increased removal of anti-nutrient factors such as phytate, which increases gastrointestinal pH. Conflicting reports regarding differences in gastrointestinal pH and probiotics supplementation may arise from the large variation in microbial species and level of inclusion rate of these products. It is suspected that addition of antibiotics, probiotics, or prebiotics may be efficient at reducing the pathogen load and so that reducing gastro intestinal pH. Result of experiments was shown that reducing salmonella population and increase in desirable microorganism in gastro intestinal tract is accompanied by pH reduction (Spring et al. Citation2000).
5. Conclusions
In conclusion, the current trial clearly demonstrates that P. indica is a promising prebiotic candidate because of high mannan content and could help broilers to achieve high levels of performance criteria, suitable gut health and morphology, boost immunity and immune related organs growth, and has no obvious side effects for poultry species. Piriformospora indica also showed greater performance and gut health in comparison with the L. acidophilus and yeast culture. Therefore, a more comprehensive framework is needed to reflect and help to further evaluation and finding the mechanisms by which P. indica make its beneficial effects in poultry.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- AOAC. 2003. Official methods of analysis of the AOAC International, 17th ed. Gaithersburg, MD: AOAC Int.
- AOAC (Association of Official Analytical Chemists). 2000. Official method of analysis, 17th end (gaithersburg, MD, AOAC).
- Apajalahti J, Kettunen A, Graham H. 2004. Characteristics of the gastrointestinal microbial communities with special reference to the chicken. Worlds Poult Sci J. 60(223):232.
- Applegate TJ, Klose V, Steiner T, Ganner A, Schatzmayr G. 2010. Probiotics and phytogenics for poultry: Myth or reality? J Appl Poult Res. 19:194–210. doi: 10.3382/japr.2010-00168
- Banerjee G, Ray AK. 2017. The advancement of probiotics research and its application in fish farming industries. Res Vet Sci. 115:66–77. doi: 10.1016/j.rvsc.2017.01.016
- Bryant MP, Burkey LA. 1953. Cultural methods and some characteristics of some of the more numerous groups of bacteria in the bovine rumen. J Dairy Sci. 36:205–217. doi: 10.3168/jds.S0022-0302(53)91482-9
- Carnevali O, Maradonna F, Gioacchini G. 2017. Integrated control of fish metabolism, well being and reproduction: the role of probiotic. Aquaculture. 472:144–155. doi: 10.1016/j.aquaculture.2016.03.037
- Chesson A. 1994. Probiotics and other intestinal mediators. In: D. J. A. Cole, J. Wiseman, M. A. Varley, editor. Principles of pig science. Loughborough: Nottingham University Press; p. 197–214.
- Cole CB, Fuller R, Newport MJ. 1987. The effect of diluted yogurt on the gut microbiology and growth of piglets. Food Microbiol. 4:83–85. doi: 10.1016/0740-0020(87)90021-9
- Dalloul RA, Lillehoj HS, Tamim NM, Shellem TA, Doerr JA. 2005. Induction of local protective immunity to Eimeria acervulina by a Lactobacillus-based probiotic. Comp Immunol Microbiol Infect Dis. 28:351–361. doi: 10.1016/j.cimid.2005.09.001
- Donsbough AL, Powell S, Waguespack A, Bidner TD, Southern LL. 2010. Uric acid, urea, and ammonia concentrations in serum and uric acid concentration in excreta as indicators of amino acid utilization in diets for broilers. Poult Sci. 89:287–294. doi: 10.3382/ps.2009-00401
- Fritts CA, Kersey JH, Motl MA, Kroger EC, Yan F, Si J, Jiang Q, Campos MM, Waldroup AL, Waldroup PW. 2000. Bacillus subtilis C-3102 (Calsporin) improves live performance and microbiological status of broiler chickens. J Appl Poult Res. 9:149–155. doi: 10.1093/japr/9.2.149
- Fuller R. 1989. Probiotics in man and animals. J Bacteriol. 66:365–378.
- Ghabooli M. 2014. Effect of piriformospora indica inoculation on some physiological traits of barely (hurdeum vulgare) under salt stress. Chem Nat Compd. 50:1082–1087. doi: 10.1007/s10600-014-1164-9
- Ghabooli M, Khatabi B, Ahmadi FS, Sepehri M, Mirzaei M, Amirkhani A. 2013. Proteomics study reveals the molecular mechanisms underlying water stress tolerance induced by Piriformospora indica in barley. J Proteom. 94:289–301. doi: 10.1016/j.jprot.2013.09.017
- Ghaffari M, Ghabooli M, Khatabi B, Hajirezaei M, Schweizer P, Hosseini Salekdeh G. 2016. Metabolic and transcriptional response of central metabolism affected by root endophytic fungus Piriformospora indica under salinity in barley. J Plant Mol Biol. 90:699–717. doi: 10.1007/s11103-016-0461-z
- Hutsko S. L., Meizlisch K., Wick M., Lilburn M. S. 2016. Early intestinal development and mucin transcription in the young poult with probiotic and mannan oligosaccharide prebiotic supplementation. Poult Sci. 95:1173–1178. doi: 10.3382/ps/pew019
- Jones GH, Ballou CE. 1969. Studies on the structure of yeast mannan. J Biol Chem. 244:1043–1051.
- Kabir SML, Rahman MM, Rahman MB, Rahman MM, Ahmed SU. 2004. The dynamics of probiotics on growth performance and immune response in broilers. Int J Poult Sci. 3:361–364. doi: 10.3923/ijps.2004.361.364
- Knap IA, Kehlet B, Bennedsen M, Mathis GF, Hofacre CL, Lumpkins BS, Jensen MM, Raun M, Lay A. 2011. Bacillus subtilis (DSM17299) significantly reduces salmonella in broilers. Poult Sci. 90:1690–1694. doi: 10.3382/ps.2010-01056
- Latorre JD, Hernandez-Velasco X, Kallapura G, Menconi A, Pumford NR, Morgan MJ, Layton SL, Bielke LR, Hargis BM, Tellez G. 2014. Evaluation of germination, distribution, and persistence of Bacillus subtilis spores through the gastrointestinal tract of chickens. Poult Sci. 93:1793–1800. doi: 10.3382/ps.2013-03809
- Li LL, Hou ZP, Li TJ, Wu GY, Huang RL, Tang ZR, Yang CB, Gong J, Yu H, Kong XF. 2008. Effects of dietary probiotic supplementation on ileal digestibility of nutrients and growth performance in 1- to 42-day-old broilers. J Sci Food Agric. 88:35–42. doi: 10.1002/jsfa.2910
- Lutgendorff F, Nijmeijer RM, Sandström PA, Trulsson LM, Magnusson KE, Timmerman HM, van Minnen LP, Gooszen HG, Akkermans LM, Söderholm JD. 2009. Probiotics prevent intestinal barrier dysfunction in acute pancreatitis in rats via induction of ileal mucosal glutathione biosynthesis. PLOS One. 4:e4512. doi: 10.1371/journal.pone.0004512
- Navid HM. 2010. Effect of probiotic bacteria utilization on serum cholesterol and triglycerides contents and performance of broiler chicks. Global Vet. 5:184–186.
- Ng SC, Hart AL, Kamm MA, Stagg AJ, Knight SC. 2009. Mechanisms of action of probiotics: Recent advances. Inflamm Bowel Dis. 15:300–310. doi: 10.1002/ibd.20602
- Palmer MF, Rolls BA. 1983. The activities of some metabolic enzymes in the intestines of germ free and conventional chicks. Br J Nutr. 50:783–790. doi: 10.1079/BJN19830149
- Peebles ED, Cheaney JD, Brake JD, Boyle CR, Latour MA, McDaniel CD. 1997. Effects of added lard fed to broiler chickens during the starter phase. 2. Serum lipids. Poult Sci. 76:1648–1654. doi: 10.1093/ps/76.12.1648
- Santoso U, Ohtani S, Tanaka K, Sakaida M. 1999. Dried Bacillus subtilis culture reduced ammonia gas release in poultry house. Asian-Australas J Anim Sci. 12:806–809. doi: 10.5713/ajas.1999.806
- SAS. 2003. SAS users guide: Statistics. Version 9.1 ed. Cary, NC: SAS Inst. Inc.
- Sherman PM, Johnson-Henry KC, Yeung HP, Ngo PS, Goulet J, Tompkins TA. 2005. Probiotics reduce enterohemorrhagic Escherichia coli O157:H7- and enteropathogenic E. coli O127:H6-induced changes in polarized T84 epithelial cell monolayers by reducing bacterial adhesion and cytoskeletal rearrangements. Infect Immun. 73:5183–5188. doi: 10.1128/IAI.73.8.5183-5188.2005
- Singh LP, Gill SS, Tuteja N. 2011. Unraveling the role of fungal symbionts in plant abiotic stress tolerance. Plant Signal Behav. 6:175–191. doi: 10.4161/psb.6.2.14146
- Sohail M. U., Hume M. E., Byrd J. A., Nisbet D. J., Ijaz A., Sohail A., Shabbir M. Z., Rehman H. 2012. Effect of supplementation of prebiotic mannan-oligosaccharides and probiotic mixture on growth performance of broilers subjected to chronic heat stress. Poult Sci. 91:2235–2240. doi: 10.3382/ps.2012-02182
- Solis De Los Santos F., Donoghue A. M., Farnell M. B., Huff G. R. 2007. Gastrointestinal maturation is accelerated in turkey poullts supplemented with a mannan-oligosaccharide yeast extract (Alphamune). Poult Sci. 86:921–930. doi: 10.1093/ps/86.5.921
- Spring P, Wenk C, Dawson KA, Newman KE. 2000. The effects of dietary mannanoligosaccharides on cecal parameters and the concentrations of enteric bacteria in the ceca of Salmonella-challenged broiler chicks. Poult Sci. 79:205–211. doi: 10.1093/ps/79.2.205
- Sun C, Johnson JM, Cai D, Sherameti I, Oelmuller R, Lou B. 2010. Piriformospora indica confers drought tolerance in Chinese cabbage leaves by stimulating antioxidant enzymes, the expression of drought-related genes and the plastid localized CAS protein. J Plant Physiol. 167:1009–1017. doi: 10.1016/j.jplph.2010.02.013
- Thompson KL, Applegate TJ. 2006. Feed withdrawal alters small-inetstinal morphology and mucus of broilers. Poult Sci. 85:1535–1540. doi: 10.1093/ps/85.9.1535
- U.S. Food and Drug Administration (FDA). 1992. Method #196. In: Bacteriological analytical manual, 7th ed. Arlington, VA: FDA. p. 506.
- Varma A, Verma S, Sahay N, Bütehorn B, Franken P. 1999. Piriformospora indica, a cultivable plant-growth-promoting root endophyte. Appl Environ Microbiol. 65:2741–2744. doi: 10.1128/AEM.65.6.2741-2744.1999
- Waller F, Mukherjee K, Deshmukh SD, Achatz B, Sharma M, Schäfer P, Kogel KH. 2008. Systemic and local modulation of plant responses by Piriformospora indica and related Sebacinales species. Plant Physiol Biochem. 165:60–70.
- Xu ZR, Hu CH, Xia MS, Zhan XA, Wang MQ. 2003. Effects of dietary fructooligosaccharide on digestive enzyme activities, intestinal microflora and morphology of male broilers. Poult Sci. 82:1030–1036. doi: 10.1093/ps/82.6.1030
- Yokota H, Coates MH. 1982. The uptake of nutrients from the small intestine of gnotobiotic and conventional chicks. Br Poult Sci. 47:349–356.
- Zhang JL, Xie QM, Ji J, Yang WH, Wu YB, Li C, Ma JY, Bi YZ. 2012. Different combinations of probiotics improve the production performance, egg quality, and immune response of layer hens. Poult Sci. 91:2755–2760. doi: 10.3382/ps.2012-02339