 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The effect of flaxseed meal or mixture with rapeseed meal or rice bran on the performance and nutritional quality of the eggs was investigated in a 6-week feeding trial on 120, Tetra SL layers (38 weeks). The hens, 4 groups (30 hens/group; 2 birds/cage) were feed with a basal diet (C); flaxseed meal (5%; E1); flaxseed meal-rapeseed meal (2.5%; 10%; E2) and flaxseed meal-rice bran (2.5%; 10%; E3). At the end of the trial, 18 eggs/group were collected to determine the egg quality and were assayed for the nutritional parameters. The results showed that the polyunsaturated fatty acid (PUFA) content was higher in groups E2 (28.65%) and E3 (29.62%). The index of atherogenicity (AI) decreased significantly (P<0.05) in group E2 (0.53) compared with C group (0.57), while the index of hypercholesterolemic fatty acid registered a higher value (2.69) vs. control group (2.45). In conclusion, use of the dietary flaxseed meal or mixture with rapeseed meal or rice bran had beneficial effects on egg nutritional quality, regarding the decrease in significant SFA content as well as the ΣSFA/ΣUFA ratio and the PUFA ω6:ω3 ratio. Also, the experimental diets significantly increased the yolk PUFA contents, proving a beneficial effect on human health.
1. Introduction
Poultry eggs are a good source of essential nutrients. The egg is considered as nature’s most complete food containing high-quality proteins, a 2:1 ratio of unsaturated fats to saturated fat, an excellent source of iron, phosphorus and other minerals and vitamins (Anton et al. Citation2006; Nau et al. Citation2010; Ruxton Citation2010; Zdrojewicz et al. Citation2016; Heflin et al. Citation2018). In relation to other foods, eggs are also a rich source of phospholipids that can influence the absorption of intestinal cholesterol (Cohn et al. Citation2010; Blesso Citation2015). Although it is the best source of all the vital nutrients, its high cholesterol content (US, Dietary Guidelines for Americans, Citation2015a) and saturated fatty acids (SFA) (US, Dietary Guidelines Advisory Committee, Citation2015b) represented the major constraints for egg consumers (Fard et al. Citation2018; Wen et al. Citation2019).
In human body, the cholesterol level has many important functions (Kim and Campbell Citation2018), such as being a major component of the brain (Dietschy and Turley Citation2001); it's a chemical precursor to steroid hormones and bile acids (Orth and Bellosta Citation2012; Morgan et al. Citation2016) or influences low-density cholesterol increase (Berger et al. Citation2015)
In the egg yolk, cholesterol deposition can be affected sometimes by diet type (Faitarone et al. Citation2013), especially for enriched diets. Stock and Compton (Citation2001) achieved a reduction in egg cholesterol content by using 4% canola oil and 7% canola oil, while Olgun and Yıldız (Citation2014) investigated the effects of Lucerne oil on egg cholesterol levels. Other authors (Kostogrys et al. Citation2017) also reported a decrease in yolk cholesterol content when ω-3 PUFA-enriched diets were fed to laying hens. On the other hand, some authors observed an increase (Hoan and Mai Citation2016) or no change (Petrović et al. Citation2012) at the level of cholesterol concentration in the yolk eggs. But, the ages of laying hens had a significant influence on yolk cholesterol content in response to the diets. Petrović et al. (Citation2012) found yolk cholesterol content decreased in the early stages of laying period and reached gradually the plateau until the end of egg-producing period. No matter what, clinical trials demonstrated no link between egg intake and an increase in serum cholesterol concentrations (Nakamura et al. Citation2006).
Over time, a few studies from the literature (Fraeye et al. Citation2012; Ren et al. Citation2013; Flachs et al. Citation2014) have demonstrated the direct influence of structure and nature of animal feed on the nutritional value of food animal products. The use of some materials rich in polyunsaturated fatty acids, such as oilseeds, oils or oil extracts in the diet formulation, may lead to the enrichment of foods in omega 3 fatty acids and the reduction of egg cholesterol level (Cherian et al. Citation2009; Shafey et al. Citation2015; Aguillón-Páeza et al. Citation2020). Higher amounts of PUFAs in egg yolk fat can be obtained by supplementing the laying hen diets with flaxseed because they are a good source of fatty acids (Turcu et al. Citation2019). In laying hens, the egg yolk fatty acid content depends on the liver lipid synthesis, the lipid components of the diet and the liver uptake of dietary lipids (Sim and Qi Citation1995). PUFA acids (ω-6 and ω-3) have many important functions in the human body, e.g. building and repairing cells and regulating blood pressure, kidney function and our immune system (Livsmedelsverket Citation2016). The PUFA ω-6 and ω-3 ratio has also become a factor in the prevention of many chronic diseases (Omidi et al. Citation2015). Thus, the high ratio value of PUFA ω-6 and ω-3 has been shown to be linked to many chronic diseases (Omidi et al. Citation2015; Livsmedelsverket Citation2016). Therefore, by increasing PUFA ω-3 concentration decreases the ratio of ω6 and ω-3. On the other hand, Omri et al. (Citation2019) showed that SFA are considered pro-atherogenic, while unsaturated fatty acids are considered anti-atherogenic. The index of thrombogenicity (IT), defined as the ratio of the pro-thrombogenetic (saturated) to the anti-thrombogenetic (unsaturated) fatty acids, shows the tendency to form clots in the blood vessels. Alagawany et al. (Citation2018) reported significantly variations depending on ω-3 sources regarding the transfer efficiency to the yolk egg.
The objective of the present study is to evaluate the effect of dietary flaxseed meal or combination with rapeseed meal or rice bran in poultry diets on the layer performance, egg quality, cholesterol content, fatty acid composition and health lipid indexes of eggs yolks of hens.
2. Materials and methods
2.1. Experimental birds and management
The experiment complied with Directive 2010/63/EU on the protection of animals used for scientific purposes and the experimental procedures. The feeding trial was conducted in the experimental halls of The National Research-Development Institute of Animal Biology and Nutrition (IBNA-Balotesti, Romania) according to a protocol approved by the Ethical Commission of the institute (no. 52/30.07.2014). A total of 120 Tetra SL laying hens (aged 38 weeks) were housed in an experimental hall equipped with Zucammi batteries (Z.M.E.C 50-model 2012) dimensioned according to the sanitary-veterinary norms regarding the minimum standards for the protection of laying hens (height of front = 455 mm, height of back = 375 mm, total depth = 550 mm, height between tiers = 582 mm and tilt = 14%) under controlled environmental conditions (average temperature/total period 20.87 ± 1.51°C; 52.11 ± 3.05% humidity; 3.55 ± 0.37% ventilation per layer). The hens were divided randomly into four groups (C, E1, E2 and E3) of thirty hens each (fifteen replicates each of 2 bird repetition). During the experimental period, hens had free access to the feed and water. The feed was administrated once daily at 08:30 and water was available at all times.
2.2. Dietary treatments
The basal diet formulation (control diet) for laying hens was based on corn and soybean meal. Unlike to control C diet (C), the experimental diet formulations used flaxseed meal, rapeseed meal and rice bran, designated as follows: flaxseed meal (E1), flaxseed meal-rapeseed meal (E2), and flaxseed meal-rice bran (E3). The ingredients and chemical composition of diets are given in . The diet formulations () were developed with a dedicated software (HYBRIMIN® Futter 5), in agreement with the feeding requirements of laying hens, as given by NRC (1994). All diets were isocaloric and isonitrogenous, containing 17.8% crude protein (CP) and 2800 kcal /kg diet metabolizable energy (ME).
Table 1. Diet formulation and estimated chemical composition of experimental diet.
2.3. Sample collection and procedures
Samples of diets (about 500 g/group) were collected for the basic chemical composition and fatty acid content. The proximate composition of diets was determined according to standardized methods (AOAC Citation2005), and fatty acid contents were determined by gas chromatography, as described by Panaite et al. (Citation2016).
Throughout the feeding trial, daily feed intake (g/day/layer), feed conversion ratio (g feed/g egg), laying percentage (%) and egg weight (g) were monitored. Feed intake and egg production were recorded daily, egg weight was measured daily, and body weight was measured at the beginning and the end of the experimental period. Laying percentage was calculated as the number of eggs produced per hen divided by the number of days of the experimental period. Data on feed intake and egg weight were used to calculate feed conversion ratio (feed intake/egg weight; g/g).
In order to evaluate egg quality, 18 eggs/group were randomly collected and used to determine the external and internal quality parameters of the eggs. Measurements were performed for egg weight, albumen, yolk and eggshell weight (Kerm scales, 0.001precision); colour intensity (Egg Analyzer TM); Haugh unit (Egg Analyzer TM); eggshell breaking strength (Egg Force Reader, Sanovo engineering A/S, Denmark); albumen and yolk pH (portable pH meter Five Go F2-Food kit with LE 427IP67, Sensor Mettler Tolledo) and albumen height (using stage micrometer manually). After that, six yolk samples (3 eggs/sample) for each group were formed from the collected eggs (18/group) and assayed for the fatty acid and cholesterol content.
2.4. Egg yolk fatty acid analysis
The fatty acid profile from dried yolk samples (65°C) was determined using the fatty acid methyl ester (FAME) gas chromatography according to ISO/TS 17764–2 (2008), described by Panaite et al. (Citation2019). Fatty acids from the total lipid extracts were converted to their methyl esters by transesterification in methanol containing 3% concentrated sulphuric acid at 80°C for 4 h. Methyl esters of fatty acids were analysed in a Perkin Elmer-Clarus 500 chromatograph equipped with a flame ionization detector (FID) and fitted with a BPX70 capillary column (60 m × 0.25 mm i.d., 0.25 μm film thickness). The column temperature was programmed at 5°C/min−1 from 180°C to 220°C. The carrier gas was hydrogen (35 cm/s linear velocity at 180°C) and the splitting ratio was 1:100. The injector and detector temperatures were 250°C and 260°C, respectively. FAME identification was done by comparison with retention times of the known standards. The results were expressed as g fatty acid per 100 g total fatty acids. The average amount of each fatty acid was used to calculate the sum of the total saturated (SFA), total monounsaturated (MUFA) and total PUFA.
Health lipid indexes of eggs yolks: indexes of atherogenicity (AI) and thrombogenicity (TI) were calculated according to Untea et al. (Citation2020) using Equations (1) and (2). The ratio between the hypocholesteronic and hypercholesteronic (HH) fatty acid (Equation 3) was calculated according to Omri et al. (Citation2019).
The following equations were used to calculate these indexes:
(1)
(1)
(2)
(2)
(3)
(3) where Σ = Summatory, MUFA = monounsaturated fatty acids, and PUFA = polyunsaturated fatty acids.
2.5. Yolk cholesterol content
The determination of cholesterol from dried yolk samples (65°C) was performed using a gas chromatographic method in accordance with the method of AOAC Official Method AOAC International 1996 99410. The method involves saponification of the sample by reflux boiling in a solution of methanol and potassium hydroxide (5% KOH in methanol), followed by extraction in petrol ether, concentration in rotavapor, and addition of chloroform, followed by extraction in petroleum ether and pouring on chloroform after concentration. The sample is split in GC (gas chromatograph Perkin Elmer Clarus-500; -FID flame ionization detector), separated by chromatography column (HP-5 capillary 30 m, 0.32 mm ID, 0.1um. df thick film) and then compared with standard chromatograms by measuring peak area, after determining the cholesterol concentration.
2.6. Statistical analysis
One-way analysis of variance (ANOVA), using StatView for WINDOWS (SAS, version 6.0), was carried out to determine the effects of treatments on layer performance, egg quality parameters, fatty acid composition and cholesterol content in eggs. Significance between individual means was identified using the Tukey’s multiple range test. Mean differences were considered significant at P < 0.05.
3. Results
3.1. Layer performance and egg quality
Layer performance and egg quality results are shown in . The use of experimental diets had significant effects (P ≤ 0.05) on both production performance and internal and external quality parameters of egg. The layers from E2 group had a significantly (P < 0.05) higher average daily feed intake than groups C, E1 and E3. In group E3, daily feed intake decreased significantly (P < 0.05) compared to E1 and E2, but insignificant (P > 0.05) compared to the C group. At the same time, feed conversion ratio recorded for the group E3 was the lowest, differing significantly (P < 0.05) only compared to group E2. The highest laying percentage was recorded for E1 and E2 groups. The average weight of eggs was not significantly (P < 0.05) different between groups, but internal and external quality parameters of egg were significantly influenced by the experimental diets (). The yolk weight from the group E2 registered a significant increase (P < 0.05), compared to groups C, E1 and E3. This also led to an increase in the yolk: albumen ratio, maintaining at the same significant differences between groups, also with regard to the yolk weight. As well, the albumen from E3 group registered a pH value increase. The eggs with the most intense yolk colour were from the group E1 compared to groups C, E2 and E3. However, the E1 eggs’ colour difference was significantly (P < 0.05) higher only compared to the C eggs.
Table 2. Effect of dietary flaxseed meal or combination with rapeseed meal or rice bran on layer performance and egg quality (average values/group).
3.2. Egg yolk fatty acid profile
The results, regarding the total fat content and yolk egg fatty acid profile, are shown in . Egg total fat content was significantly (P < 0.05) higher for E3 eggs compared with the other experimental groups (E1 and E2), but did not differ significantly (P > 0.05) from the C group. The new feed formulation tested in this study led to significantly different concentrations (P < 0.05), compared to the C group, of the SFA (myristic, pentadecanoic, palmitic, heptadecanoic), especially in the group E3. Among the listed fatty acids, the content of myristic acid in E1 and E3 groups significantly (P < 0.05) decreased, while the palmitic acid content decreased in E2 and E3 groups, compared to the C group. Although there was an increase in stearic acid concentration, there was no difference (P > 0.05) compared to the C group. Between the MUFA, significantly lower (P < 0.05) was the myristoleic and palmitoleic acid concentration, although the results were lower for the pentadecenoic, heptadecenoic and oleic ones.
Table 3. Effect of dietary flaxseed meal or combination with rapeseed meal or rice bran on fatty acid composition of egg yolk (average values/group, g acid/100 g total FAME).
For PUFA omega-3, the largest improvement observed for α-linolenic acid (ALA) was in the group E1, which included 5% flaxseed meal, compared to the other two experimental groups that had a lower addition level of flaxseed meal, even if it was mixed with another oil raw material. Moreover, E2 and E3 groups were similar in terms of yolk ALA concentration. Significantly higher concentrations (P < 0.05), unlike in the C group, were obtained for docosapentaenoic (DPA) and docosahexaenoic (DHA) PUFA omega-3 fatty acids in all experimental groups. Regarding the DHA concentration, significant differences (P < 0.05) were also between all three experimental groups.
The results regarding the egg fatty acid content, depending on the level of unsaturation, are shown in . As can be seen, the SFA content of eggs was lower (P > 0.05) in all three experimental groups, compared to the C group. However, significant (P < 0.05) was the SFA level determined in the eggs from hens that received the mixture of 2.5% flaxseed meal and 10% rapeseed meal (E2), compared to the C group. Regarding the MUFA content, compared to the control group, significantly lower results were obtained in group E3 and higher values in group E1.
Table 4. Effect of dietary flaxseed meal or combination with rapeseed meal or rice bran on fatty acid content of egg according to the level of unsaturation and health lipid indexes of eggs’ yolks (average values/group, g acid/100 g total FAME).
Regarding the PUFA content, there was a significant (P < 0.05) increase in E2 and E3 groups, which included 2.5% flaxseed meal, compared to the E1 group (5% flaxseed meal). This PUFA content increase is due to 10% rapeseed meal (E2), and 10% rice bran (E3) inclusion, compared to the E1 group which included only flaxseed meal.
The recorded data showed a significant improvement (P < 0.05) with 6.24% of PUFA E3 egg content, group with flaxseed meal and rice bran. At the same time, this group had the highest increase (P < 0.05) of PUFA ω-3 content, although all three new feed formulations led to the optimization of PUFA ω-3 concentration, compared to the C group. The best ratio of ω-6/ω-3 fatty acids was obtained for E1 and E3 eggs, although there was a decrease in the value of their ratio in all tested groups, compared to the group C. From the obtained results (), it can be concluded that the E3 diet formulation, supplemented with a level of 2.5% flaxseed meal and 10% rice bran, has led to obtaining eggs with improved nutritional qualities in terms of essential fatty acid concentration for human nutrition, like PUFA and PUFA ω-3
For the health lipid indexes of egg yolk, the experimental diets led to the decrease of the atherogenic and thrombogenic potential of egg yolk lipid. The index of atherogenicity (AI) decreased significantly (P < 0.05) in the group E2. The E3 group recorded a decrease of AI, but insignificant compared to groups C, E1 and E2. At the same time, E3 had the lowest value of thrombogenic index (TI), similar to that of groups C and E2. Hypercholesterolemic index values were significantly (P < 0.05) higher in the experimental groups, compared to the C group.
3.3. Cholesterol concentration of egg yolk
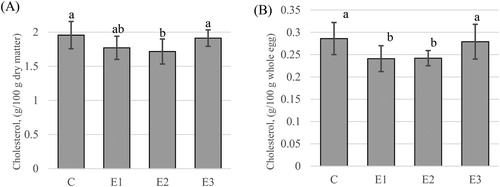
The results on the yolk cholesterol concentration are presented in . The new diet formulations determined a cholesterol level decrease in all the three experimental groups (1.77 ± 0.07 g; 1.72 ± 0.08 g; 1.91 ± 0.05 g/100 g dry matter, or 0.24 ± 0.01 g; 0.24 ± 0.01 g and 0.28 ± 0.02 g/100 g whole egg) compared with the results obtained for the C group (1.96 ± 0.06 g dry matter or 0.29 ± 0.01 g/100 g whole egg). Significant (P < 0.05) decrease compared to the C group was the cholesterol content in E1 and E2 groups expressed in whole egg (g cholesterol/100 g whole egg).
Figure 1. Effect of dietary flaxseed meal or combination with rapeseed meal or rice bran on cholesterol concentration of egg yolk: (A) – dry matter and (B) – whole egg; C – Control diet; E1 – diet supplemented with flaxseed meal (5%); E2 – diet supplemented with flaxseed meal (2.5%) and rapeseed meal (10%); E3 – diet supplemented with flaxseed meal (2.5%) and rice bran (10%); a,bMeans with no common letter differ (P < 0.05).
4. Discussions
4.1. Layer performance and egg quality
The results obtained in this study for growth performance are comparable to those from the specialty literature. A significant increase (P < 0.05) of egg production was obtained by Aziza et al. (Citation2013) by using 10% flaxseed meal in hens’ diet. Cherian and Quezada (Citation2016) used 10% flaxseed meal and obtained similar results for egg production, but there was a significantly increase for average daily feed intake (g/day). Also, Hayat et al. (Citation2010) included 10% flaxseed meal and observed a significant decrease in average daily feed intake, without affecting egg production, egg weight and mass or feed conversion. Other studies have shown that long-term feeding with flax seed reduces the egg production and egg sensory quality (Jia et al. Citation2008).
The recommended levels for rapeseed meal used in laying hens’ diets was optimal, ranging between 4 and 10% (Perez-Maldonado et al. Citation2003), but good results were obtained without affecting the health and performance of the birds at a higher inclusion rate (Ciurescu Citation2009; Gheisari and Ghayor Citation2014; Oryschak et al. Citation2020). However, the Hy-Line International Company (2010) recommends restricting the use of rapeseed meal because it can affect the egg sensory quality by changing their taste, printing a fish hue when the inclusion level in diet is higher than 12%.
For the E3 group fed with flaxseed meal (2.5%) and rice bran (10%), the obtained data are in agreement with those reported by Haghnazar and Rezaei (Citation2004). They used rice bran (0, 5, 10, 15, 20 and 25%) in diets without affecting the production performance, but the eggs weight increased concomitantly with the rice bran level increasing in the experimental diets. Contrary to our results, Samli et al. (Citation2006) showed that by increasing the level of rice bran (5%, 10% and 15%) in birds’ diet, the production performance and egg mass decrease significantly. Thus, the recommendations on the rice bran inclusion in birds’ diets vary from 7.5% to 40% (Nobakht Citation2007), but the best animal performances like feed conversion and economic results seems to be below 10% inclusion level (Rezaei Citation2006).
Specialty literature highlighted a number of research studies on the influence of the egg quality parameters through the flax, rapeseed meal and rice bran. There are a few studies that have shown that using flax in laying hens’ diet has led to a decrease in eggshell weight and eggshell thickness, without affecting the Haugh unit, the yolk: albumen ratio and egg yolk weight (Cherian and Quezada Citation2016). In another study by Aziza et al. (Citation2013) egg weight and Haugh unit increased, and eggshell thickness decreased significantly (P < 0.05). Also, Aziza et al. (Citation2016) stated that using 10% flaxseed meal does not affect the yolk weight, compared to the C group. On the other hand, Riyazi et al. (Citation2009) showed an eggshell weight improvement by using 10% rapeseed meal in chicken diet, but as the rapeseed meal level inclusion in diet increases, the yolk index decreases. In another study, Samli et al. (Citation2006) found that by using 15% rice bran in laying hens’ diets, Haugh units were significantly affected (P < 0.05), but did not affect the albumen and yolk weight or eggshell thickness.
4.2. Egg yolk fatty acid content
Polyunsaturated fatty acids play an important role in both animal and human nutrition, which are considered to be essential for human beings, linoleic acid (LA, C18: 2 ω-6) and α-linolenic acid (ALA, C18: 3 ω-3) (Aguillón-Páeza et al. Citation2020). In this study, the use of vegetable sources (flaxseed meal, rapeseed meal and rice bran) in laying hen diets led to a significant increase (P ≤ 0.05) of yolk PUFA ω-3 fatty acids, as follows: α-linolenic acid (C18: 3n3); eicosatrienoic acid (C20: 3n3), docosapentaenoic acid (C22: 5n3) and docosahexaenoic acid (C22: 6n3).
The α-linolenic acid concentration (C18: 3n3) in the yolk egg from experimental groups was significantly (P≤0.05) higher compared to the group C, but the highest increase, however, was recorded in the group E1. Perić et al. (Citation2019) conducted a comparative study regarding the effect of flax in several forms (flour, cake and oil) on the ratio of PUFA/SFA and ω-6/ω-3 in eggs. The recorded results highlighted the improvement of PUFA/SFA and ω-6/ω-3 ratio when flax was included alone (5%), as well as in the mixture (10% cake and 2% oil). At the same time, the use of a higher level of addition in diets (10% flax flour) has led to obtaining values closer to that of an ideal ratio between fatty acids, compared to the C group.
The results obtained in the present study are in agreement with those reported by Perić et al. (Citation2019) regarding the ratio of PUFA ω-6/ω-3 fatty acids (3.34 ± 0.62) when they included 5% flax flour in the structure of the diet. At the same time, the concentrations obtained by us in this study, when supplementing the hen’s diets with 5% flaxseed meal, are similar to those obtained at an 8% flax seed level (Khan et al. Citation2017). In another study (Cherian and Quezada Citation2016) the reported results showed that using 10% of flax seed in poultry diets significantly increased (P < 0.05) the ALA, DPA and DHA concentration, compared to the C group.
Regarding the rapeseed meal use, the results obtained by Oryschak et al. (Citation2020) showed that including two different varieties of rapeseed, meal or cake (20%) in laying hens’ diet indicated a SFA content decrease in the experimental groups compared to the C group (between 27.00% and 27.44%), while the MUFA content increased, although insignificant (P > 0.05) compared to the C group. The PUFA content decreased in all experimental groups compared to the C group, the tendency was observed also in PUFA ω-3 and ω-6 content.
Compared to the data reported by Oryschak et al. (Citation2020), the results obtained in this study have shown the beneficial effect of using rapeseed meal in combination with flaxseed meal, although the addition level in ratio was lower compared to the tested one in Oryschak et al. (Citation2020) study. In a similar study (Yuan et al. Citation2019), the effects of including two different levels of rapeseed oil (2% and 4%) on production performance, egg quality and biochemical parameters of poultry were evaluated. The reported results showed that using rapeseed oil did not enrich the feed formulation in fatty acids compared to the C group. As a general conclusion of the study, the researchers noted that adding rapeseed oil in hens diet decreased egg production, reduced the total cholesterol (TC) and triglyceride (TG) value in the serum and increased the egg Haugh unit.
In this study, the highest docosahexaenoic acid content increase was registered in the group E3, supplemented with flaxseed meal (2.5%) and rice bran (10%). These results are comparable to those obtained by Habibollahi et al. (Citation2019) in a study on different levels of rice bran (0; 15; 25%) in diets, in the presence of phytase. The obtained results indicated an improvement of ALA content in eggs from experimental groups, compared to the C group, the concentration improvement is proportional to the rice bran inclusion level in diet.
In this study, the health lipid indexes of eggs’ yolks recorded significant decreases for atherogenity index (AI) and thrombogenity index (TI), these results are comparable with the one obtained by Omri et al. (Citation2019) in a study with linseeds (L) and a mixture of linseeds, tomato and sweet red pepper (LTP). The researchers obtained a decrease with regard to the SFA content and PUFA ω6: ω3 ratio, while the yolk PUFA content increased especially the eicosapentaenoic (EPA) and doxosahexaenoic acid (DHA). Moreover, it also notably decreased the thrombogenic index. Untea et al. (Citation2020) recorded a significant TI decrease in the group fed with walnut leaves, but AI was not influenced by bilberry or walnut leave supplements present in laying hens’ diets. It has been shown that eggs with a lower SFA/UFA ratio showed low values of AI, TI, and hypercholesterolemic (HH) indexes, and they have been recommended for a healthy diet (Ulbricht and Southgate Citation1991; Santos-Silva et al. Citation2002), because the AI indicates the relationship between the sum of the main SFA and that of the main classes of unsaturated ones (Omri et al. Citation2019; Untea et al. Citation2020).
4.3. Egg cholesterol content
One of the alternative ways to reduce the potential cholesterolemic effect of eggs is to change the yolk fatty acid composition (Agboola et al. Citation2016). It has been scientifically demonstrated that by using high PUFA diets, the egg cholesterol level can be reduced. Eggs have traditionally been associated with numerous factors unfavourable to health due to high cholesterol (Weggemans et al. Citation2001; Lamas et al. Citation2016), but recent research has shown that healthy people who consume up to three eggs each day have the overall beneficial effect on biomarkers associated with cardiovascular disease risk (DiMarco et al. Citation2017). The results obtained in the present study showed that by using vegetable sources (flaxseed meal, rapeseed meal and rice bran) in laying hens’ diet, the yolk cholesterol concentration can be decreased. Similar results were obtained by Khan (Citation2019) when using a combination of flax seeds (2%), flax cake (5%) and vitamin B6 (40 mg / kg diet) in laying hens’ diets, obtaining a positive effect on the lowering egg cholesterol. In another similar study, Faitarone et al. (Citation2013) evaluated the effects of supplementation with different vegetable oils on egg cholesterol levels and their nutritional composition. The researchers reported a cholesterol content decrease compared to the group C when it was used a mixture of 2.5% rapeseed oil and 2.5% canola oil, resulting in a significant decrease of cholesterol concentration. The data available in the specialized literature (Nobakht Citation2007) have shown that feeding laying hens with degreased rice bran can lead to the yolk cholesterol content decrease, together with the eggshell calcium concentration. A recent study (Habibollahi et al. Citation2019) showed that the inclusion of 15% to 25% rice bran in hens’ diet led to a lower yolk cholesterol level. Also, in the specialized literature, it has been shown that feeding laying hens with 10% rice bran significantly (P <0.05) decreased the yolk cholesterol concentration (Nobakht Citation2007). Other studies have claimed that the source of fat in the hens’ diet does not influence the cholesterol content in egg yolk or the whole egg (Millet et al. Citation2006).
5. Conclusions
The results of this study demonstrate the beneficial effects of dietary flaxseed meal or combination with rapeseed meal or rice bran had on egg nutritional quality, regarding the significant SFA content decrease, as well as the ΣSFA/ΣUFA ratio and therefore the PUFA ω6: ω3 ratio. Also, the experimental diets significantly increased the yolk PUFA contents, namely the total PUFA ω-3 fatty acid content. The best results were obtained in the group fed with 2.5% flaxseed meal and 10% rice bran, showing the lowest values for health lipid indexes of eggs’ yolks proving a beneficial effect on human health.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Agboola AF, Omidiwura BRO, Oyeyemi A, Iyayi EA, Adelani A. 2016. Effects of four dietary oils on cholesterol and fatty acid composition of egg yolk in layers. Int J Biol Biomol Agr Food Biotech Eng. 10(2):43–50.
- Aguillón-Páeza YJ, Romero LA, Diazc GJ. 2020. Effect of full-fat sunflower or flaxseed seeds dietary inclusion on performance, egg yolk fatty acid profile and egg quality in laying hens. Anim Nutr. In Press, Journal Pre-proof. DOI:10.1016/j.aninu.2019.12.005.
- Alagawany M, El-Hack MEA, Farag MR, Sachan S, Karthik K, Dhama K. 2018. The use of probiotics as eco-friendly alternatives for antibiotics in poultry nutrition. Environ Sci Pollut Res. 25(11):10611–10618. DOI:10.1007/s11356-018-1687-x.
- Anton M, Nau F, Nys Y. 2006. Bioactive egg components and their potential uses. J World Poult Sci. 62:429–438. DOI:10.1017/S004393390600105X.
- AOAC. 2005. Official methods of analysis, 18th ed. Washington, DC: Association of Official Analytical Chemists.
- Aziza AE, Orma OA, Awadin WF, Seady YE. 2016. Effects of supplementation of broiler diets with fish oil and linseed oil on growth performance, cytokines, and cecal histopathological changes in broiler chickens infected by eimeria. Int J Agric Vet Med. 4:12–27.
- Aziza AE, Panda AK, Quezada N, Cherian G. 2013. Nutrient digestibility, egg quality, and fatty acid composition of brown laying hens fed camelina or flaxseed meal. J Appl Poult Res. 22(4):832–841. doi:10.3382/japr.2013-00735.
- Berger S, Raman G, Vishwanathan R, Jacques PF, Johnson EJ. 2015. Dietary cholesterol and cardiovascular disease. Am J Clin Nutr. 102(2):276–294. doi:10.3945/ajcn.114.100305.
- Blesso CN. 2015. Egg phospholipids and cardiovascular health. Nutrients. 7:2731–2747. doi:10.3390/nu7042731.
- Cherian G, Campbell A, Parker T. 2009. Egg quality and lipid composition of eggs from hens fed Camelina sativa. J Appl Poult Res. 18(2):143–150. DOI:10.3382/japr.2008-00070.
- Cherian G, Quezada N. 2016. Egg quality, fatty acid composition and immunoglobulin Y content in eggs from laying hens fed full fat camelina or flax seed. J Anim Sci Biotechnol. 7(1):15. DOI:10.1186/s40104-016-0075-y.
- Ciurescu G. 2009. Efficiency of soybean meal replacement by rapeseed meal and/or canola seeds in commercial layer diets. Archiva Zootechnica. 12(1):27–33.
- Cohn JS, Kamili A, Wat E, Chung RW, Tandy S. 2010. Dietary phospholipids and intestinal cholesterol absorption. Nutrients. 2:116–127. doi:10.3390/nu2020116.
- Dietschy JM, Turley SD. 2001. Cholesterol metabolism in the brain. Curr Opin Lipidol. 12(2):105–112.
- DiMarco DM, Missimer A, Murillo AG, Lemos BS, Malysheva OV, Caudill MA, Blesso CN, Fernandez ML. 2017. Intake of up to 3 eggs/day increases HDL cholesterol and plasma choline while plasma trimethylamine-N-oxide is unchanged in a healthy population. Lipids. 52(3):255–263.
- Faitarone ABG, Garcia EA, Roça RDO, Ricardo HDA, de Andrade EN, Pelícia K, Vercese F. 2013. Cholesterol levels and nutritional composition of commercial layers eggs fed diets with different vegetable oils. Braz J Poultry Sci. 15(1):31–37. doi:10.1590/S1516-635X2013000100006.
- Fard DSP, Salari S, Sari M, Zadeh SAM, Zarei M. 2018. Effects of lipid sources and different levels of zinc on fatty acid profile and cholesterol egg yolk, antioxidant status and some blood parameters of laying hens. J Vet Res. 73(1):119–128. https://jvr.ut.ac.ir/article_67431_bb.
- Flachs P, Rossmeisl M, Kopecky J. 2014. The effect of ω-3 fatty acids on glucose homeostasis and insulin sensitivity. Physiol Res. 63(1):S93–S118.
- Fraeye I, Bruneel C, Lemahieu C, Buyse J, Muylaert K, Foubert I. 2012. Dietary enrichment of eggs with omega-3 fatty acids: a review. Food Res Int. 48:961–969. DOI:10.1016/j.foodres.2012.03.014.
- Gheisari A, Ghayor P. 2014. Different dietary levels of rapeseed meal effects on egg quality characteristics in indigenous breeding hens. J Anim Physiol Anim Nutr. 9(1):1–8.
- Habibollahi M, Abousadi MA, Nakhaee P. 2019. The effect of phytase on production performance, egg quality, calcium and phosphorus excretion, and fatty acids and cholesterol concentration in hy-line layers fed diets containing rice bran. J Appl Poultry Res. 28(3):688–698. DOI:10.3382/japr/pfz020.
- Haghnazar A, Rezaei M. 2004. To determine the metabolizable energy of rice bran and the use of it in layer ration. In XII. World’s Poultry Congress, June 8–13, Istanbul.
- Hayat Z, Cherian G, Pasha TN, Khattak FM, Jabbar MA. 2010. Sensory evaluation and consumer acceptance of eggs from hens fed flax seed and 2 different antioxidants. Poult Sci. 89(10):2293–2298. DOI:10.3382/ps.2009-00575.
- Heflin LE, Malheiros R, Anderson KE, Johnson LK, Raatz SK. 2018. Mineral content of eggs differs with hen strain, age, and rearing environment. Poult Sci. 97:1605–1613. DOI:10.3382/ps/pey025.
- Hoan ND, Mai AK. 2016. The effect of different levels of sesame oil on productive performance, egg yolk and blood serum lipid profile in laying hens. Open J Anim Sci. 6:85–93. DOI:10.4236/ojas.2016.61011.
- Jia W, Slominski BA, Guenter W, Humphreys A, Jones O. 2008. The effect of enzyme supplementation on egg production parameters and omega-3 fatty acid deposition in laying hens fed flaxseed and canola seed. Poult Sci. 87(10):2005–2014. DOI:10.3382/ps.2007-00474.
- Khan SA. 2019. Inclusion of pyridoxine to flaxseed cake in poultry feed improves productivity of omega-3 enriched eggs. Bioinformation. 15(5):333–341. DOI:10.6026/97320630015333.
- Khan SA, Khan A, Khan SA, Beg MA, Ali A, Damanhouri G. 2017. Comparative study of fatty-acid composition of table eggs from the Jeddah food market and effect of value addition in omega-3 bio fortified eggs. Saudi J Biological Sci. 24:929–935. DOI:10.1016/j.sjbs.2015.11.00.
- Kim JE, Campbell WW. 2018. Dietary cholesterol contained in whole eggs is not well absorbed and does not acutely affect plasma total cholesterol concentration in men and women: results from 2 randomized controlled crossover studies. Nutrients. 10(9):1272. DOI:10.3390/nu10091272.
- Kostogrys RB, Filipiak-Florkiewicz A, Dereń K, Drahun A, Czyzynska-Cichon I, Cieslik E, Franczyk-Zarów M. 2017. Effect of dietary pomegranate seed oil on laying hen performance and physicochemical properties of eggs. Food Chem. 221:1096–1103. DOI:10.1016/j.foodchem.2016.11.051.
- Lamas A, Anton X, Miranda JM, Roca-Saavedra P, Cardelle-Cobas A, Rodriguez JA, Franco CM, Cepeda A. 2016. Technological development of functional egg products by an addition of ω-3 polyunsaturated-fatty-acid-enriched oil. CyTA-J Food. 14(2):289–295. DOI:10.1080/19476337.2015.1100220.
- Livsmedelsverket 2016. Fleromättat fett, omega-3, omega-6 [online]. [accessed 2016 December 7]. http://www.livsmedelsverket.se/livsmedel-ochinnehall/naringsamne/fett/fleromattat-fett-omega-3-och-omega-6/.
- Millet S, De Ceulaer K, Paemel MV, Raes K, De Smet S, Janssens GPJ. 2006. Lipid profile in eggs of Araucana hens compared with Lohmann Selected Leghorn and ISA Brown hens given diets with different fat sources. Br Poult Sci. 47(3):294–300. DOI:10.1080/00071660600741818.
- Morgan AE, Mooney KM, Wilkinson SJ, Pickles NA, Mc Auley MT. 2016. Cholesterol metabolism: a review of how ageing disrupts the biological mechanisms responsible for its regulation. Ageing Res Rev. 27:108–124. DOI:10.1016/j.arr.2016.03.008.
- Nakamura Y, Iso H, Kita Y, Ueshima H, Okada K, Konishi M, Inoue M, Tsugane S. 2006. Egg consumption, serum total cholesterol concentrations and coronary heart disease incidence: Japan Public Health Center-based prospective study. Br Poult Sci. 96(5):921–928. DOI:10.1017/BJN20061937.
- Nau F, Yamakawa YNY, Réhault-Godbert S. 2010. Nutritional value of the hen egg for humans. Prod Anim Paris Inst National Recherche Agron. 23:225–236.
- Nobakht A. 2007. The effects of inclusion different levels of rice bran in laying hens diets on performance and plasma and egg yolk cholesterol contents. J Anim Vet Adv. 6(9):1120–1124.
- Olgun O, Yıldız AÖ. 2014. Effect of dietary alfalfa meal on performance, egg quality, egg yolk cholesterol and hatchability parameters of quail breeders. Turkish J AgriFood Sci Technol. 3(3):103–106. DOI:10.24925/turjaf.v3i3.103-106.208.
- Omidi M, Rahimi S, Torshizi MAK. 2015. Modification of egg yolk fatty acids profile by using different oil sources. Vet Res Forum. 6(2):37.
- Omri B, Chalghoumi R, Izzo L, Ritieni A, Lucarini M, Durazzo A, Abdouli H, Santini A. 2019. Effect of dietary incorporation of linseed alone or together with tomato-red pepper mix on laying hens’ egg yolk fatty acids profile and health lipid indexes. Nutrients. 11(4):813. DOI:10.3390/nu11040813.
- Orth M, Bellosta S. 2012. Cholesterol: its regulation and role in central nervous system disorders. Cholesterol. Article ID 292598. DOI:10.1155/2012/292598.
- Oryschak MA, Smit MN, Beltranena E. 2020. Brassica napus and Brassica juncea extruded-expelled cake and solvent-extracted meal as feedstuffs for laying hens: lay performance, egg quality, and nutrient digestibility. Poult Sci. 99(1):350–363. DOI:10.3382/ps/pez501.
- Panaite T, Criste RD, Ropota M, Cornescu GM, Alexandrescu D, Criste V, Vasile G, Margareta O, Untea A. 2016. Effect of layer diets enriched in omega-3 fatty acids supplemented with Cu on the feeding value of the eggs. Rom Biotech Lett. 21(4):11754–11762. http://hdl.handle.net/123456789/468.
- Panaite TD, Nour V, Vlaicu PAL, Ropota M, Corbu ALR, Saracila M. 2019. Flaxseed and dried tomato waste used together in laying hens diet. Arch Anim Nutr. 73(3):222–238. DOI:10.1080/1745039X.2019.1586500.
- Perez-Maldonado RA, Mannion PF, Farrell DJ. 2003. Effects of heat treatment on the nutritional value of raw soybean selected for low trypsin inhibitor activity. Br Poult Sci. 44(2):299–308. DOI:10.1080/0007166031000085463.
- Perić J, Drinić M, Mićić N. 2019. Fatty acids in fee of laying hens on the production parameters and the ratio of omega-6 and omega-3 fatty acids. Biotechnol Anim Husb. 35(4):377–386. DOI:10.2298/BAH1904377P.
- Petrović M, Gačić M, Karačić V, Gottstein Ž, Mazija H, Medić H. 2012. Enrichment of eggs in ω-3 polyunsaturated fatty acids by feeding hens with different amount of linseed oil in diet. Food Chem. 135:1563–1568. DOI:10.1016/j.foodchem.2012.06.020.
- Ren Y, Perez TI, Zuidhof MJ, Renema RA, Wu J. 2013. Oxidative stability of omega-3 polyunsaturated fatty acids enriched eggs. J Agr Food Chem. 61(47):11595–11602. DOI:10.1021/jf403039 m.
- Rezaei M. 2006. Utilization of mixed rice bran in laying hen diets. Pakistan J Biol Sci. 9(8):1420–1423.
- Riyazi SR, Ebrahimnezhad Y, Nazeradl K, Maheri-Sis N, Salamatdust R, Vahdatpour T. 2009. The effects of replacing soybean meal with different levels of rapeseed meal on egg quality characteristics of commercial laying hens. Asian J Anim Vet Adv. 4:337–341.
- Ruxton C. 2010. Recommendations for the use of eggs in the diet. Nurs Stand. 24:47–55.
- Samli HE, Senkoylu N, Akyurek H, Agma A. 2006. Using rice bran in laying hen diets. J Cent Eur Agric. 7:135–140.
- Santos-Silva J, Bessa RJB, Santos-Silva F. 2002. Effects of genotype, feeding system and slaughter weighton the quality of light lambs. Fatty acid composition of meat. Livestock Product Sci. 77:187–194. DOI:10.1016/S0301-6226(02)00059-3.
- Shafey TM, Al-Batshan HA, Farhan AM. 2015. The effect of dietary flaxseed meal on liver and egg yolk fatty acid profiles, immune response and antioxidant status of laying hens. Ital J Anim. 14(3):3939. DOI:10.4081/ijas.2015.3939.
- Sim JS, Qi GH. 1995. Designing poultry products using flaxseed. In: Thompson L.U., Cunnane S., editors. Flaxseed in human nutrition. Champaign, IL: American Oil Chemist’s Society Press; p. 315–333.
- Stock RH, Compton JD. 2001. U.S. Patent No. 6,316,041. Washington, DC: U.S. Patent and Trademark Office.
- Turcu RP, Olteanu M, Criste RD, Panaite TD, Ropotă M, Vlaicu PAL, Drăgotoiu D. 2019. Grapeseed meal used as natural antioxidant in high fatty acid diets for Hubbard broilers. Braz J Poultry Sci. 21(2):001–012. DOI:10.1590/1806-9061-2018-0886.
- Ulbricht TLV, Southgate DAT. 1991. Coronary heart disease: seven dietary factors. Lancet. 338:985–992. DOI:10.1016/0140-6736(91)91846-M.
- Untea AE, Varzaru I, Panaite TD, Gavris T, Lupu A, Ropota M. 2020. The effects of dietary inclusion of bilberry and walnut leaves in laying hens’ diets on the antioxidant properties of eggs. Animals. 10(2):191. DOI:10.3390/ani10020191.
- US Department of Health and Human Services. 2015a. US Department of Agriculture. 2015–2020. Dietary guidelines for Americans. 8th ed. Washington, DC: US Dept of Health and Human Services. http://www.health.gov/DietaryGuidelines.
- US Department of Health and Human Services. 2015b. US Department of Agriculture. Scientific report of the 2015 dietary guidelines advisory committee. Part D. Washington, DC: US Dept of Health and Human Services. Chap 6. http://health.gov/dietaryguidelines/2015-scientific-report.
- Weggemans RM, Zock PL, Katan MB. 2001. Dietary cholesterol from eggs increases the ratio of total cholesterol to high-density lipoprotein cholesterol in humans: a meta-analysis. Am J Clin Nutr. 73(5):885–891. DOI:10.1093/ajcn/73.5.885.
- Wen Z, Wu Y, Qi Z, Li X, Li F, Wu X, Yang P. 2019. Rubber seed oil supplementation enriches ω-3 polyunsaturated fatty acids and reduces cholesterol contents of egg yolks in laying hens. Food Chem. 301:125198. DOI:10.1016/j.foodchem.2019.125198.
- Yuan N, Wang JP, Ding XM, Bai SP, Zeng QF, Su ZW, Xuan Y, Peng HW, Fraley GS, Zhang KY. 2019. Effects of supplementation with different rapeseed oil sources and levels on production performance, egg quality, and serum parameters in laying hens. Poult Sci. 98(4):1697–1705. https://www.indexmundi.com/agriculture.
- Zdrojewicz Z, Herman M, Starostecka E. 2016. Hen’s egg as a source of valuable biologically active substances. Postepy Hig Med Dosw. 70:751–759. DOI:10.5604/17322693.1208892.
