ABSTRACT
Mycotoxins are secondary metabolites produced by Aspergillus, Penicillium and other genera which contaminate food and exhibit hazardous effects on humans and animals. Aflatoxins (AFs) caused liver necrosis, fibrosis. In the present study, we planned to evaluate the action of bradykinin potentiating factor (BPF) extracted from Egyptian cobra snake venom and synthetic antioxidants; butylated hydroxytoluene (BHT) and oltipraz (OPZ) on the hepatic tissue of aflatoxicosed rats. Aflatoxicosis caused a significant increase of nitric oxide (NO) and lipid peroxidation as malondialdehyde (MDA) associated with a significant decrease in the level of total thiols, glutathione (GSH), and the activities of glutathione peroxidase (GPx) and transferase (GST). In addition to a decrease in the counts of white blood cells (WBCs), lymphocytes (LYM), monocytes (MONO), eosinophils (EOS), red blood cells (RBCs) and an increase in neutrophil counts (NEUT) as well as a significant decrease in the gene expression of hepatic interleukin-1β (Il-1β). The previous changes were confirmed by histopathological alterations. Treatment of aflatoxicosed rats with any of BPF, BHT or OPZ resulted in amelioration of the oxidative stress parameters, blood picture and hepatic IL-1β with improvement in histological features. In conclusion, either BPF, BHT or OPZ can be used for the treatment of aflatoxicosis.
1. Introduction
Mycotoxins are secondary metabolites produced by Aspergillus, Penicillium and other genera which contaminate food and exhibit hazardous effects on humans and animals mainly malnutrition, immunosuppression, genotoxicity, carcinogenic and teratogenic effects due to inhibition of macromolecule synthesis and metabolism (Nassar et al. Citation1985; Megalla et al. Citation1990; Maresca and Fantini Citation2010; Adejumo and Adejoro Citation2014; Ashiq Citation2015; Tola and Kebede Citation2016). The AFs are referred to four different types and their metabolites contaminating the food of animals and humans (Nassar et al. Citation1982; Hafez et al. Citation1985; Rajendra et al. Citation2014). AFs caused liver necrosis, fibrosis and enhanced increasing the risk of hepatocellular carcinoma (Agag Citation2004; Rotimi et al. Citation2019).
Animal venoms including those from spiders, snakes, cone snails and scorpions contain components that cause rapid death of the animal prey (Yang et al. Citation2016). The Egyptian cobra (Naja haje haje) contains a number of non-toxic low molecular weight basic polypeptides such as bradykinin potentiating peptides (BPPs) that have physiological and immunochemical properties (Ferreira Citation1965; Caldwell et al. Citation2015; Xu et al. Citation2015). It was found that bradykinin potentiating factor (BPF) extracted from the Egyptian scorpion and cobra venom enhanced physiological functions as well as cellular growth of the uterus and the development of the ovarian follicle in mice (Nassar Citation1989, Citation1990; Abdel-Raheim et al. Citation1995). In addition, it generates thymus and spleen cellularity, recovered blood picture and accelerated the healing process in irradiated guinea pigs (Abu-Sinna et al. Citation2005; Salman Citation2009; Sarin Citation2015; Salman Citation2018).
Butylated hydroxy toluene (BHT) is an antioxidant phenolic acid that is used to prevent rancidity of food products containing fats and oils (Lundebye et al. Citation2010). Moreover, BHT has the potential to defend against oxidative stress and protect against cancer and cardiovascular diseases (Weisburger et al. Citation1977; Cohen et al. Citation1984; Jaouad and Torsten Citation2010). Oltipraz (OPZ) [4-methyl-5-(2-pyrazinyl)-1,2-dithiole-3-thione] has been widely studied as a cancer chemopreventive agent due to enhancement of necrosis factor-2 (Nrf2) and the consequent changes in target gene transactivation as well as enhancing the induction of the glutathione-S- transeferase (GST) A2 gene (Ramos et al. Citation2001; Guise Citation2013). Moreover, OPZ has therapeutic effects on cirrhotic liver even in high doses (Dwyer et al. Citation1997; Cho et al. Citation2006).
Accordingly, this work has been designed in order to evaluate the therapeutic effect of BPF, BHT and OPZ against aflatoxicosis in female rats.
2. Materials and methods
2.1. Chemicals used
Butylated hydroxy toluene (W218405), oltipraz (O9389), thiobarbutric acid (T5500), naphthylethylene diamine dihydrochloride (N9125), 5,5- dithiobis-2-nitrobenzoic acid (D8130), sulphanilamide (S9251) and coomassie brilliant, blue R stain (B0149) were purchased from Sigma-Aldrich Company (St. Louis, MO), U.S.A. Interleukin-1β (IL-1β) taqman assay (Rn99999009_m1) was purchased from Thermo Fisher Scientific Company, U.S.A.
2.2. Extraction, purification and identification of AFB1
AFB1 was extracted from harvested media of growing Aspergillus flavus according to the method of Booth (Citation1971), purified by TLC method of El-Kady and Moubasher (Citation1982).
2.3. Extraction, purification and identification of BPF
Venom extraction and dialysis; the collected amount of the Egyptian cobra snake crude venom was dialyzed according to the method of Abdel-Raheim et al. (Citation1995). Isolation and purification of BPF from the collected cobra venom dialysate by the chemical method of Ferreira (Citation1965). Physiological examinations of the isolated BPF; isolated BPF was identified by its hypotensive effect on arterial blood pressure of rabbit by oscillograph in the central lab of Zoology Department, Faculty of Science, Assiut University. The amino acid content of the isolated BPF was analyzed by amino acid analyzer SW (Hydrolisate Separation Method) at Desert Research Center, Ministry of Agriculture and Land Reclamation, Cairo, Egypt.
2.4. Experimental animals
In this study, 120 female Wistar albino rats were used and obtained from the Animal House of the Faculty of Medicine, Assiut University, Assiut, Egypt with mean body weight of 130 ± 10 gm. Rats were housed in cages, kept at room temperature with normal 12 h light/12 h dark cycle and supplemented with standard commercial pellets for feeding, water, ad libtium. All of the animal procedures were performed in accordance with the guidelines for the care and use of experimental animals established by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) and the protocol of the National Institutes of Health (NIH) (Ragab et al. Citation2015).
2.4.1. Design of the experiment
Rats were divided randomly into six groups of 20 rats each as follows:
G I: Reference normal group.
G II0: Intoxicated group with AFB1 for four weeks.
G II1: Self- recovery for the same period after intoxication.
G II2: Treated with BPF for the same period after intoxication.
G II3: Treated with BHT for the same period after intoxication.
G II4: Treated with OPZ for the same period after intoxication.
2.4.2. Route of administration of intoxicating solutions and the other treating agents
The animals of GII0 were individually intoxicated by oral administration three times weekly (500 μg AFB1 suspended in milk/kg b.wt) for successive four weeks according to Raisuddin et al. (Citation1993). GII1 animals after intoxication were left without treatment for self-recovery. GII2 intoxicated animals were treated by intrapetionally three doses weekly for successive four weeks (1 μg BPF in PBS (PH 7.4)/kg b.wt) according to Omar and Meki (Citation1997). GII3 intoxicated animals were orally treated by 0.5 mg BHT in PBS (PH 7.4)/kg b.wt three times weekly for successive four weeks according to Hocman (Citation1988). Similarly to GII3, the animals of GII4 were orally administrated by OPZ (2 mg in PBS (PH 7.4)/kg b.wt) according to Dimitrov et al. (Citation1992).
2.4.3. Collection and preparation of the samples for biochemical determination and histopathological examinations
At the end time of the experiment, blood samples were collected from the heart under an aesthesia by ether and sacrificed by cervical dislocation for sampling of considered tissue organs. At the time of scarifying, the blood was collected in tubes and centrifuged after blood clotting at 4000 rpm for 10 min to separate serum. The liver was quickly removed, washed with saline solution and small slices were fixed in glutraldehyde for electron microscopic examinations and the remnant was imbedded in liquid nitrogen and kept frozen at −80 for complementary cDNA studies. 10% w/v homogenate of liver in 0.1M phosphate buffer (pH 7.4) was prepared, then the homogenate was preserved at −20°C for the subsequent biochemical indices.
2.5. Serological characters
Another portion of blood was immediately introduced into EDTA tubes for complete blood cell counting (CBC) by using automatic blood cell counter (Exigo PM800 blood analyzer) at the Pathology and Clinical Pathology Department, Faculty of Veterinary Medicine, Assiut University.
2.6. Biochemical determinations
Total protein content in the supernatant of hepatic tissue homogenates was performed according to the method of Lowry et al. (Citation1951). Alanine aminotransferase (ALT) activity in serum was determined by ALT-Liquizyme (4 + 1) E.C.2.6.1.2 kit which purchased from Egyptian Company for Biotechnology. Nitric oxide (NO) was determined by Gries reagent according to Ding et al. (Citation1988). Lipid peroxidation as MDA was evaluated according to the method of Wills (Citation1969). Total thiols content was assayed according to the method of Ellman (Citation1959). Glutathione (GSH) was estimated according to the method of Beutler et al. (Citation1963). The activity of GSH-peroxidase (GPx) and transferase (GST) were assayed according to Habig et al. (Citation1974). The interleukin-1β (IL-1β) gene expression in hepatic tissue was evaluated by Real time PCR at the Tissue Culture and Molecular Biology Center Lab in Assiut University according to Livak and Schmittgen (Citation2001).
2.7. Histopathogical features
Semi-thin section and transmission electron microscope observations were done in Electron Microscope Unit (E.M.U) of Assiut University according to Bozzola and Russell (Citation1991).
2.8. Statistical analysis
Statistical differences of parameters are presented as Mean ± SEM, statistical significance was determined using the one way ANOVA test in Graph Pad Prism 5.03 at P < 0.05 where a is a significant of aflatoxicosed GII0 with respect to reference normal GI, b is a significant of treated groups GII1, 2, 3, 4 with respect to reference normal GI and c is a significant of treated groups GII1, 2, 3, 4 with respect to aflatoxicosed GII0.
3. Results
3.1. Laboratory biosynthesized and collected pure sample of AFB1
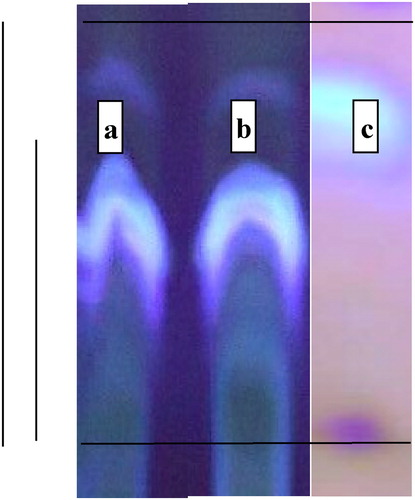
The biologically synthesized aflatoxins (B1, B2, G1 and G2) were characterized, identified and collected AFB1 fraction in pure sample by TLC chromatographic separation by chloroform:methanol solvent (97:3) as presented in .
3.2. Identification of amino acid composition in the isolated BPF
The amino acid composition of the isolated BPF from the venom of Egyptian cobra venom (Naje haje haje) demonstrated high content of Asp, Lys, Cys, Arg, Thr, Glu, Ile and Gly and low content of Val, Phe, His, Ser, Pro, Ala, Met and then Leu, Tyr in a descending order with the absence of Trp, Hyp, Asn and Gln as it is shown in .
Table 1. Amino acid composition of the isolated BPF from the snake venom.
Aspartic (ASP), lysine (LYS), cysteine (CYS), arginine (ARG), threonine (THR), glutamic (GLU), isoleucine (ILE), glycine (GLY), valine (VAL), phenylalanine (PHE), histidine (HIS), serine (SER), proline (PRO), alanine (ALA), methionine (MET), leucine (LEU), tyrosine (TYR), tryptophane (Trp), hydroxyproline (Hyp), asparagine (Asn) and glutamine (Gln).
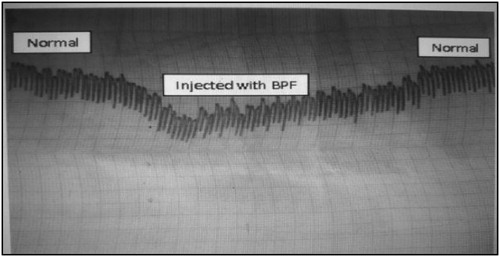
Identification of isolated BPF was confirmed by hypotensive effect on rabbit arterial blood pressure as shown in .
3.3. Serological characters
The blood cell counts in the tested groups showed that; WBCs, LYM, MONO, EOS and RBCs counts were significantly decreased in the intoxicated rats GII0 and self-recovery GII1 but the counts of NEUT was concomitantly increased with respect to the reference normal GI. All of these characters were returned back to be further approached to the reference normal GI in treated rats (BPF GII2, BHT GII3 and OPZ GII4) as shown in .
Table 2. Blood cell counts in the tested groups.
3.4. Biochemical indices
3.4.1. ALT activity (U/l) in serum of experimental groups
ALT in serum was significantly increased in the intoxicated rats (GII0) in comparison to the reference normal (GI) and still elevated in the self-recovery (GII1). However, in treated rats with BPF (GII2), BHT (GII3) and OPZ (GII4), the levels were approximating to those of reference normal (GI) as shown in .
Table 3. ALT activity (U/l) in serum of experimental groups.
3.4.2. Oxidative stress markers in hepatic tissue
NO and MDA in hepatic tissue were significantly increased in the intoxicated rats GII0 in comparison to the reference normal GI and still elevated in the self-recovery GII1. However in treated rats with BPF GII2, BHT GII3 and OPZ GII4, the levels were approximating to those of reference normal GI. The chemical reducing agents; total thiols and GSH were significantly reduced by intoxication GII0 and differentially increased during ageing GII1 and treated with the three tested therapeutic agents; BPF GII2, BHT GII3 and OPZ GII4. The hepatic tissue enzyme activities of GPx and GST were significantly reduced by intoxication GII0 and also differentially elevated by self-recovery mechanisms GII1 and treated by the considered three tested agents (BPF GII2, BHT GII3 and OPZ GII4) as shown in .
Table 4. Oxidative stress markers in hepatic tissue of the tested groups (Mean ± SEM).
3.4.3. Gene expressions of hepatic IL-1β as % fold change:
It is illustrated in that aflatoxicosis in GII0 resulted in a significant reduction in the ability of gene expression for hepatic IL-1β as fold change compared with that of reference normal level GI. Elapsed period for 4-weeks without treatment (self-recovery GII1) permitted the immunological status of the aflatoxicosed rats to enhance such factor for further increase of T-lymphocytic action. Furthermore, any of the treating agents (BPF GII2, BHT GII3 and OPZ GII4) significantly increased IL-1β gene expression to be more promoted than that of the group of self-recovery GII1.
Table 5. Gene expression of hepatic IL-1β as fold change parameter.
3.5. Histopathological features
xx.
4. Discussion
The obtained results for AFB1 biosynthesis showed that the fungus of Aspergillus flavus efficiently produced the well-known four metabolites named B1, B2, G1 and G2 (). Identification, isolation and purification of fraction AFB1 was successfully performed by repeating collection of the chromatographed purified zones of AFB1 according to El-Kady and Moubasher (Citation1982).
The amino acid composition of the chemically isolated BPF presented in was mostly similar to those detected by Fernandez et al. (Citation2004) and Ianzer et al. (Citation2004) who claimed that the structure of some bradykinin potentiating peptides (BPPs) must contain proline that facilitates inhibition of the ACE that prevents formation of angiotensin II (from the parent angiotensin I) initiating vasodilatation and hypotension of exposed animals. The tested hypotensive character of the isolated BPF in this work was practically achieved on rabbit arterial blood pressure as presented in . Moreover, evaluation of BPF activity using isolated guinea pig ileum contraction was also effective in eliciting smooth muscle contraction mediated by endogenous BK which is responsible for the predicted hypotensive effect of the BPF. The results that resembling those detected by Ferreira (Citation1965) in his work on Bothrops jararaca as well as that was isolated BPF from the Egyptian scorpion Buthus occitanus as it was published by Abdel-Raheim et al. (Citation1995) and Sharma et al. (Citation1996).
CBC of AFB1 intoxicated rats (GII0) and self-recovery (GII1) groups indicated a significant decrease in white blood cells, lymphocytes, monocytes, eosinophils and erythrocytes, however, neutrophils were increased (). These results were similar to that published before by Corrier (Citation1991) and Raisuddin et al. (Citation1993) who found that aflatoxicosis is generally associated with immunosuppression in domestic animals, poultry and laboratory animals due to impairment of cellular mediated immunity functions. Moreover, the observed significant increase in neutrophils could be attributed to the elicited inflammatory response to aflatoxicosis (Donmez et al. Citation2011; Nicola and Jason Citation2018). Self-recovery group and treated groups by either BPF, BHT and OPZ showed recovery of CBC by increases of all CBC except neutrophils. This improvement in CBC could be returned to promotion of the activity of pro-inflammatory cascade reactions (Takahashi and Hiraga Citation1984; Cottrell et al. Citation1994; Petricevich and Pena Citation2002; Salman et al. Citation2017).
ALT activity was significantly increased in AFB1 intoxicated rats (GII0) in comparison to control one (GI) and still elevated in the self-recovery (GII1), however, in treated rats with BPF (GII2), BHT (GII3) and OPZ (GII4), the levels were elevated to those of reference normal (GI) and reduced against aflatoxicosis group as in . These results were similarly agreed before by many authors such as Groseva et al. (Citation2014) and Rotimi et al. (Citation2019) who reported that these changes represent the initial protective response of the mitochondria against the aflatoxin toxicity and the increased activity of these enzymes provided evidence for their release from damaged hepatocytes. In the present study, the reduction of serum ALT activity closed to normal levels after treated with BPF, BHT and OPZ suggested healing of hepatic parenchymal cells and regeneration of hepatocytes (Ashry et al. Citation2012; Dassarma et al. Citation2018; Giudice et al. Citation2019).
The hepatic tissue content of NO as in was frankly increased by AFB1 intoxication (GII0) as a first responding agent against inflammation that recognizes oxidative damage of the tissue by providing the superoxide anion derived by AFs that accumulate ROS and RNS simultaneously (Massey et al. Citation1995; Anup and Hartmut Citation2018). All together of derived free radicals initiated what is determined as MDA as an expressing biomarker for accumulated LPO interpret the significant reduction in reducing chemicals; total thiols and GSH associated with reduced enzymatic actions of GPx and GST that respond against AFB1 inflammation (Carlberg and Mannervik Citation1985; Sies Citation1997; Iciek et al. Citation2004). In the present study, the treatment from aflatoxicosis with BPF (GII2) resulted in more improved characters against oxidative stress parameters (). These results were in a great agreement with those reported by Mikrut et al. (Citation2001) after their trial to treat acute hypoglycemic animals with bradykinin agent. Moreover, Salman et al. (Citation2016) reduced the intoxicating parameters initiated by HgCl2 in living animals by therapeutic BPF isolated from scorpion venom Buthus occitanus. The antioxidative effect of BPF in both of acute or chronically inflammed animals be beneficially considered as well as promoting physiological activities and cellular tissue regeneration, proliferation and differentiation (Nassar et al. Citation1990; Nassar Citation1992; Jovcic et al. Citation1996; Őztürk Citation2001; Sancho et al. Citation2007; Ashry et al. Citation2012).
The synthetic antioxidants; BHT (GII3) or OPZ (GII4) showed powerful antioxidative stress action in treated aflatoxicosed rats (GII0) () due to their probable scavenging properties that stimulated the GSH-linked detoxification mechanisms as it was observed by others (Dimitrov et al. Citation1992; Klein et al. Citation2002; Nassar et al. Citation2014). Moreover, the detected hepato-protective effect of BHT is attributed to its predicted antioxidant activity that normalized the abnormal intracellular events involved in fat absorption and maintaining the integrity of the cell membrane of the liver and prevented accumulation of LPO as it was similarly observed by Barsha et al. (Citation2018) and Dassarma et al. (Citation2018) during their identification for hepato-protective effectors against hepatotoxicity in rats. In addition, the diminished GST activity by aflatoxication was mostly prevented by BHT treatment since reduction of GST activity is proportional to the GSH content in the biological system of the intoxicated animals as it was deduced by Philip and Anders (Citation2007), Marcus et al. (Citation2012) and Sohn et al. (Citation2013). The observed therapeutic effect of OPZ in this study can be referred to its potential enhancing regulatory mechanisms for further genetic production of detoxificating enzymes through activation of the ARE- NrF2 pathway as it was detected by Dimitrov et al. (Citation1992), Velayutham et al. (Citation2005) and Giudice et al. (Citation2019).
The gene expression of hepatic IL-1β () was determined by real time PCR as fold change showed considerable reduction in AFB1 intoxicated rats (GII0). This reduction was slightly improved in self-recovery rats (GII1) due to IL-1β could be served as a potent intermediate ligand between tissue injury and the resulting physiological indices of inflammation (Nicola and Jason Citation2018). In this study, it seems that induced aflatoxicosis suppressed the immune system of the exposed animals by negatively affected T-cell dependent immunity.
Dugyala and Sharma (Citation1996), Batey and Wang (Citation2002) and Miao et al. (Citation2016) claimed that cellular components of the immune system which produce various cytokines play a key role in host resistance against intoxicating inflammatory actions. The tested isolated snake venom peptide BPF (GII2) (ACE inhibitor) that potentiates the endogenous BK via activation of B2 receptors; significantly increased the cytokine IL-1β against induced aflatoxicosis (Murakami et al. Citation1997; Fernandes et al. Citation2001; Abu-Amra et al. Citation2015). The authors recorded a profound cell signalling that initiated gene expression for TNFα, phospho-lipase A2 and other pro-inflammatory immune response by such activated bradykinin. The therapeutic synthetic antioxidants BHT (GII3) from phenol families and OPZ treated (GII4) from other thiols significantly elevated the gene expressing affinity for production of IL-1β to activate the immunological respond against inflammatory action of AFs. The antioxidant BHT may enhance regulatory factors that up-regulated the appropriate gene expression of cytochrome-P450 families to overcome the oxidative stress initiated by aflatoxicosis as it was announced by Nassar et al. (Citation2014). Moreover, it was suggested by some workers (Ramos et al. Citation2001; Nisha and Peter Citation2017; Giudice et al. Citation2019) that chemoprotective action of OPZ against hepatic tissue injury or carcinogenesis could be predicted by enhancement of phase II genes that express the antioxidant responsive element necrosis factor-2 (ARE-NF2).
Normal liver rats (GI) showing the normal morphological structure of the hepatic lobule, hepatic cells (h), sinusoids (S) and central vein (C) as well as large vesicular nucleus (N), rough endoplasmic reticulum RER (er), mitochondria (m) as in . The biochemical changes in intoxicated (GII0) or self-recovery (GII1) groups were confirmed by the histological observation in the hepatic tissue () which showed congestion of the portal vein, hyperplasia of the bile duct, increased collagen fibres in the portal area, presence of fat vacuoles and cell nucleus dysplasia. Similar findings were recorded by Preetha et al. (Citation2006) and Devendran and Balasubramanian (Citation2011) who returned these degenerative changes to the increased of oxidative stress and mitochondrial dysfunction by aflatoxin and its metabolites in hepatic tissue. Treatment of intoxicated rats by either BPF (GII2), (), BHT (GII3) or OPZ (GII4), () prominently reduced most of the previously detected histological observations due to reduction of oxidative stress and enhancement in mitochondrial function (Neal et al. Citation1976; Anup and Hartmut Citation2018). In addition, BPF () specifically enhanced a localized appearance of Kupffer cells in the hepatic sinusoids as well as other features of cellular improvements. It is known that Kupffer cells promote the resolution of inflammation and enhancement of hepatic wound healing which BPF acting as acceptable cell signalling peptide (Dixon et al. Citation2013). In previous work, similar BPFs enhanced cellular proliferation and differentiation of gonads in the intact animals (Nassar et al. Citation1990; Nassar Citation1992). In addition, an enriched α-neurotoxic peptide from scorpion with basic arginine in its sequence was assessed as a crucial amino acid residue responsible for its cellular receptor binding affinity (Kharrat et al. Citation1990). Moreover, the rich cysteine residues in the isolated BPF from venom of Bothrops jararaca initiate its physiological activity on cellular B2 receptors of guinea pig ileum (Ferreira Citation1965). In this study, the most abundant content of amino acid residues of lysine and cysteine in the sequence of the tested BPF may considered as efficient condition for its probable cell signalling effect that was predicted by some workers (Omar and Meki Citation1997; El-Saadani Citation2004; Padrissa et al. Citation2009). Similarly, phenolic BHT (GII3) or thiolic one OPZ (GII4) () improved liver damage as direct antioxidant reagents. Sael and Pablo (Citation2015) announced that BHT plays a role in injured hepatic tissue repair that could be revealed by antioxidants as well as workers like Liping et al. (Citation2019) who reported that OPZ can potentiate activated collagenases that hydrolyze accumulated fibres in injured liver.
Figure 3. (a) Light micrograph of semi thin section of liver of normal rats (GI) showing the normal morphological structure of the hepatic lobule, hepatic cells (h), sinusoids (S) and central vein (C). T.B. stain. (b) T.E. micrograph of hepatic cell of normal rats (GI) showing large vesicular nucleus (N), rough endoplasmic reticulum RER (er), mitochondria (m).
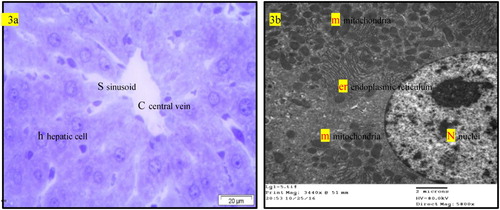
Figure 4. (a) Light micrograph of semi thin section of liver of intoxicated rats with AFB1 (GII0) or that for self-recovery (GII1) showing bile duct hyperplasia (b), congestion of the portal vein (v) and increases of collagen fibres in the portal area (x). T.B. Stain (The same histopathological features in GII0 and GII1). (b) T.E. micrograph of liver of intoxicated rats with AFB1 (GII0) or that for self-recovery (GII1) showing increases of collagen fibre in the portal area (co), dysplasia of the hepatic cell nucleus (N) and presence of fat vacuoles in the hepatic cells (f) (The same histopathological features in GII0 and GII1).
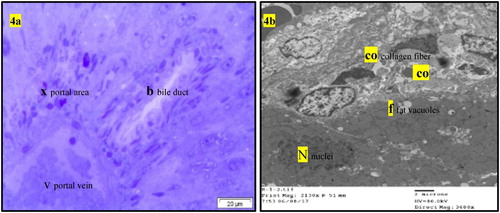
Figure 5. (a) Light micrograph of semi thin section of liver of rats intoxicated with AFB1 for one month then treated with BPF for one month (GII2) showing the hepatic cells arranged in plats contain fat globuls with activation of Kupffer cells (arrow). T.B. Stain. (b) T.E. micrograph of hepatic cells of rats intoxicated with AFB1 for one month then treated with BPF for one month (GII2) showing presence of variable size fat globules (f) and large vesicular nucleus (N). (c) T.E. micrograph of hepatic cells of rats intoxicated with AFB1 for one month then treated with BPF for one month (GII2) showing presence of Kupffer cells (K) and collagen fibre (co) in Dise,s space and RBC,s in the sinusoid (S).
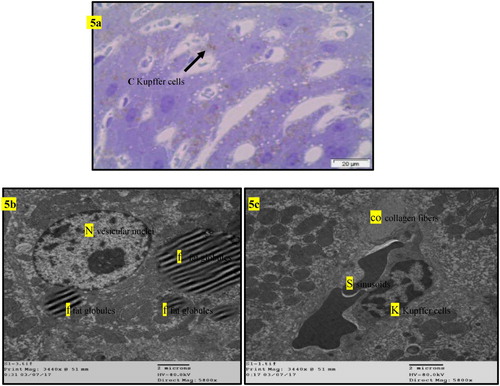
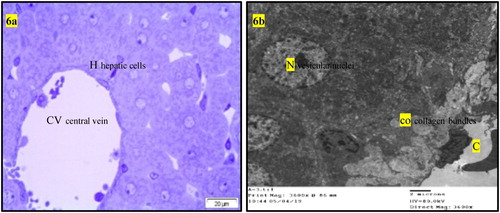
Figure 6. (a) Light micrograph of semi thin section of liver of rats intoxicated with AFB1 then treated with BHT (GII3) or OPZ (GII4) for one month showing hepatic lobules are nearly of normal structures of central vein (CV) and hepatic cells (H) (The same histopathological features in GII3 and GII4). (b) T.E. micrograph of liver of rats intoxicated with AFB1 then treated with BHT (GII3) or OPZ (GII4) for one month showing presence of collagen bundles (co) in the wall of the central vein (C) and the hepatic cells having vesicular nucleus (N) (The same histopathological features in GII3 and GII4).
5. Conclusion
Conclusively, any of the three tested components for injured hepatic treatment from induced aflatoxicosis; BPF from the cobra snake venom and the synthetic antioxidant animal food additives; BHT and OPZ each of which is a valuable therapeutic agent for hepatic tissue repair. Although, the metabolic pathway of BHT in rats and humans can depress growth and may cause lung damage or inflammation and bleeding but it is not classified as carcinogenic for humans. Concomitantly, hepatic metabolic derivatives of OPZ such as pyrrolo-pyrazine structures can induce microRNAs that affect gene transcription of hypoxia inducible factor (HIF-1α) in human colon cancer treatment as well as GST expression. OPZ can be stimulant for GST production as cancer chemopreventive agent. The chemically isolated BPF as a nontoxic and beneficial pharmacologically active peptide isolated from snake venom consisted less than twenty different amino acid residues that could be easily degraded by peptidases in exposed animal when it is used as a therapeutic agent against aflatoxicosis.
No conflict between the authors; the authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Disclosure statement
No conflict of interest was reported by the author(s).
Additional information
Funding
References
- Abdel-Raheim MM, Nassar AY, Rochat H. 1995. A BPP (peptide K12) from the venom of Egyptian scorpion Buthus occitanus. Peptides. 16(8):1359–1365.
- Abu-Amra E, El-Sayed MF, Badr A. 2015. Temperature dependence of cardiac sarcoplasmic reticulum and sarcolemma in the ventricle of catfish (Clarias gariepinus). J Basic Appl Zool. 72:89–95.
- Abu-Sinna G, Kafafy Y, Nassar AY, Salman A. 2005. Synergistic effect of bone marrow transplantation and BPF isolated from venom on thymus and spleen of sublethally irradiated Guinea pig. Egypt J Radiat Sci Appl. 18:249–276.
- Adejumo TO, Adejoro DO. 2014. Incidence of AFs, fumonisins, trichothecenes and ochratoxins in Nigerian foods and possible intervention strategies. Food Sci Qual Manage. 31:127–146.
- Agag BI. 2004. Mycotoxins in foods and feeds, AFs. Assoc Univers Bull Environ Res. 7(1):173–191.
- Anup R, Hartmut J. 2018. Oxidative stress and acute hepatic injury. Curr Opin Toxicol. 7:17–21.
- Ashiq S. 2015. Natural occurrence of mycotoxins in food and feed. Compr Rev Food Sci Food Saf. 14:159–175.
- Ashry O, Moustafa M, Baset AA, Abu Sinna GE, Farouk H. 2012. Outcome of venom bradykinin potentiating factor on rennin-angiotensin system in irradiated mice. Int J Radiat Biol. 88:840.
- Barsha D, Dilip KN, Somnath G, Saptadip S. 2018. Hepatoprotective effect of food preservatives (butylated hydroxyanisole, butylated hydroxytoluene) on carbon tetrachloride-induced hepatotoxicity in rat. Toxicol Rep. 9:31–37.
- Batey RG, Wang J. 2002. Molecular pathogenesis of T lymphocyte induced liver injury in alcoholic hepatitis. Front Biosci. 7:1662–1675.
- Beutler E, Duron O, Kelly BM. 1963. Improvement method for the determination of blood glutathione. J Lab Clin Med. 61:882–888.
- Booth. 1971. Methods in microbiology, vol(4), chapter I; Introduction to general methods: 1-47 and chapter II; Fungal culture media: 49–94.
- Bozzola JJ, Russell LD. 1991. Electron microscopy principles and techniques for biologists: Jones and Bartlitt publishers 20 park plasa Boston Mao. 2116.
- Caldwell MW, Nydam RL, Palci A, Apesteguia S. 2015. The oldest known snakes from the middle Jurassic-lower cretaceous provide insights on snake evolution. Nat Commun. 6:5996.
- Carlberg I, Mannervik B. 1985. Glutathione reductase. Methods Enzymol. 113:484–490.
- Cho IJ, Kim SH, Kim SG. 2006. Inhibition of TGF beta1- mediated π-induction by OPZ through selective interruption of smad activation. Cytokine. 35:284–294.
- Cohen LA, Polansky M, Furuya K, Reddy M, Berke B, Weisburger JH. 1984. Inhibition of chemically induced mammary carcinogenesis in rats by short-term exposure to BHT: interrelationships among BHT concentration, carcinogen dose and diet. J Natl. Cancer Inst. 72:165–174.
- Corrier DE. 1991. Mycotoxicosis: mechansims of immunosuppression. Vet Immunol Immunopathol. 30:73–87.
- Cottrell S, Andrrews CM, Clayton D, Wild BJ, Powell CJ. 1994. Hematological and platelets effect of BHT-E321. Comp Haemat Int. 4:102–107.
- Dassarma B, Nandi DK, Gangopadhyay S, Samanta S. 2018. Hepatoprotective effect of food preservatives (BHA, BHT) on carbon tetrachloride induced hepatotoxicity in rat. Toxicol Rep. 5:31–37.
- Devendran G, Balasubramanian U. 2011. Biochemical and histopathological analysis of aflatoxin induced toxicity in liver and kidney of rat. J Plant Sci Res. 1(4):61–69.
- Dimitrov NV, Bennett JL, McMillan J, Perloff M, Leece CM, Malone W. 1992. Clinical pharmacology studies of OPZ. A potential chemopreventive agent. Invest New Drugs. 10(4):289–298.
- Ding AH, Nathan CF, Stuehr DJ. 1988. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages: comparison of activating cytokines and evidence for independent production. J Immunol. 141:2407.
- Dixon L, Barnes M, Tang H, Pritchard M, Nagy L. 2013. Kupffer cells in the liver. Compr Physiol. 3(2):785–797.
- Donmez N, Donmez HH, Keskin E, Kısadere I. 2011. Effects of aflatoxin on some haematological parameters and protective effectiveness of esterified glucomannan in Merino Rams. Sci World J. 2012:4.
- Dugyala RR, Sharma RP. 1996. The effect of AFB1 on cytokine mRNA and corresponding protein levels in peritoneal macrophages and splenic lymphocytes. Int J Immunopharmacol. 18(10):599–608.
- Dwyer PJ, Clayton M, Halbherr T, Myers CB, Yao KS. 1997. Cellular kinetics of induction by OPZ and its keto derivative of detoxification enzymes in human colon adenocarcinoma cells. Clin Cancer Res. 3:783–791.
- El-Kady IA, Moubasher MH. 1982. Toxigenicity and toxins of Stachybotrys isolates from wheat straw samples in Egypt. Exp Mycol. 6(1):25–30.
- El-Saadani MA. 2004. A scorpion venom peptide fraction induced prostaglandin biosynthesis in Guinea pig kidneys: incorporation of 14C-linoleic acid. J Biochem Tokyo. 135(1):109–116.
- Ellman GL. 1959. Tissue sulfhydryl groups. Arch Biochem Biophys. 82(1):70–77.
- Fernandes L, Fortes Z, Nigro D, Totes R, Santos R, Helena M. 2001. Potentiation of BK by angiotensin (1-7) on arterioles of spontaneously hypertensive rats studied in vivo. Hypertension. 37:703–709.
- Fernandez JH, Neshich G, Camargo AC. 2004. Using BPP structures to develop new antihypertensive drugs. Genet Mol Res. 3(4):554–563.
- Ferreira SH. 1965. BPF present in the venom of Bothrops jararaca. Br J Pharmacol Chemother. 24(1):163–169.
- Giudice A, Barbieri A, Bimonte S, Cascella M, Cuomo A, Crispo A, D'Arena G, Galdiero M, DellaPepa ME, Botti G, et al. 2019. Dissecting the prevention of estrogen-dependent breast carcinogenesis through Nrf2-dependent and independent mechanisms. Onco Targets Ther. 12:4937–4953.
- Groseva N, Valchev I, Binev R, Kanakov D, Hristov T, Lazarov L, Uzunova K, Nikolov Y. 2014. Investigations on liver function in mulards with experimentally induced aflatoxicosis. Istanbul Univ. 40(1):53–62.
- Guise TA. 2013. Breast cancer bone metastases: it’s all about the neighborhood. Cell. 154(5):957–959.
- Habig WH, Pabst MJ, Jakoby WB. 1974. GSTs, the first enzymatic step in mercapturic acid formation. J Biol Chem. 249(22):7130–7139.
- Hafez AH, Megalla SE, Mohran MA, Nassar AY. 1985. Aflatoxin and aflatoxicosis: the kinetic behavior of dietry AFs in colostrum drawn from cow postpartum. Mycopathologia. 89:161–164.
- Hocman G. 1988. Chemoprevention of cancer: phenolic antioxidants (BHT, BHA). Int J Biochem. 20(7):639–651.
- Ianzer D, Konno K, Marques R, Vieira FC, Stöcklin R, Martins AC, Pimenta DC. 2004. Identification of five new BPPs from Bothrops jararaca crude venom by using electrospray ionization tandem mass spectrometry after a two-step liquid chromatography. Peptides. 25(7):1085–1092.
- Iciek M, Chwatko G, Lorenc KE, Bald E, Włodek L. 2004. Plasma levels of total, free and protein bound thiols as well as sulfane sulfur in different age groups of rats. Acta Biochim Pol. 51:815–824.
- Jaouad B, Torsten B. 2010. Exogenous antioxidants – double-edged swords in cellular redox state: Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid Med Cell Longev. 3(4):228–237.
- Jovcic G, Vanovic Z, Biljanovic-Paunovic L, Bugarski D, Stosic-grujicic S, Milenkovic P. 1996. In vivo effects of Il-1 receptor antagonist on hematopoietic bone marrow progenitor cells in normal mice. Eur Cytokine Netw. 7:71–74.
- Kharrat R, Darbon H, Granier C, Rochat H. 1990. Structure- activity relationships of scorpion α- neurotoxins: contribution of arginine residues. Toxicon. 28:509–523.
- Klein PJ, VanVleet TR, Hall JO, Coulombe RA. 2002. Dietary butylated hydroxytoluene protects against aflatoxicosis in Turkeys. Toxicol Appl Pharmacol. 182(1):11–19.
- Liping L, Guangqi L, Guangchuan W, Dongxiao M, Zhen L, Chunqing Z. 2019. Carvedilol improves liver cirrhosis in rats by inhibiting hepatic stellate cell activation, proliferation, invasion and collagen synthesis. Mol Med Rep. 20(2):1605–1612.
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta-C(T)) method. Methods. 25(4):402–408.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. 1951. Protein measurement with the folin phenol reagent. J.Biol.Chem. 193(1):265–275.
- Lundebye AK, Hove H, Måge A, Bohne VJ, Hamre K. 2010. Levels of synthetic antioxidants (ethoxyquin, BHT and BHA) in fish feed and commercially farmed fish. J Food Additiv Contam Part A. 27(12):1652–1657.
- Marcus NY, Blomenkamp K, Ahmad M, Teckman JH. 2012. Oxidative stress contributes to liver damage in a murine model of alpha-1-antitrypsin deficiency. Exp Biol Med. 237(10):1163–1172.
- Maresca M, Fantini J. 2010. Some food-associated mycotoxins as potential risk factors in humans predisposed to chronic intestinal inflammatory diseases. Toxicon. 56:282–294.
- Massey TE, Stewart RK, Daniels JM, Liu L. 1995. Biochemical and molecular aspects of mammalian susceptibility to AFB1 carcinogenicity. Proc Soc Exp Biol Med. 208(3):213–227.
- Megalla SE, Nassar AY, Moharram AM, Abdel-Gawad KM. 1990. Some physiological studies on fungi isolated from poultry feedstuffs. J Basic Microbiol. 30(3):165–180.
- Miao L, Yi Z, Peng L, Shu Y, Wen Z, Jian H, Yuan W, Jian H. 2016. Intervention of grape seed proanthocyanidin extract on the subchronic immune injury in mice induced by AFB1. Int J Mol Sci. 17(4):516.
- Mikrut K, Paluszak J, Kozlik J, Sosnowski P, Krauss H, Grześkowiak E. 2001. The effect of bradykinin on the oxidative state of rats with acute hyperglycaemia. Diabetes Res Clin Pract. 51(2):79–85.
- Murakami M, Kuwata H, Amakasu Y, Shimbara S, Nakatani Y, Atsumi G, Kudo I. 1997. Prostaglandin E2 amplifies cytosolic phospholipase A2- and cyclooxygenase-2-dependent delayed prostaglandin E2 generation in mouse osteoblastic cells. Enhancement by secretory phospholipase A2. J Biol Chem. 272(32):19891–19897.
- Nassar AY. 1989. Isolated fraction from toxins of Egyptian scorpions and cobra, activated smooth muscle contraction and glomerular filtration. Toxicon. 27:57.
- Nassar AY. 1992. BPF isolated from venom of Buthus occitanus promotes spermatogenesis in premature mice. Rec Advan Toxinol Res. 2:119–135.
- Nassar AY, Abu-Sinna G, Abd El-Rahim S. 1990. Effect of a BPF from venom of the Egyptian scorpion Buthus occitanus, on the ovaries and endometrium of mice. Toxicon. 28(5):525–534.
- Nassar AY, Ali AM, Nafady AA, El-Baz A, Mohamed YS, Abdel-Latif FF. 2014. Copper (I) nicotinate complex exhibits more prophylactic effect than BHT against nephrotoxicity in chronically aflatoxicosed rats. Glo Adv Res J Med Sci. 3(9):251–261.
- Nassar AY, Megalla SE, Abd El-Fattah HM, Hafez AH, El-Deap TS. 1982. Binding of AFB1, G1 and M1 to plasma albumin. Mycopathologia. 79(1):35–38.
- Nassar AY, Megalla SE, Hafez AH, Galal AF, Mohamed MA. 1985. The effect of AFB1 on the utilization of serum calcium. Mycopathologia. 91:127–131.
- Neal NGE, Godoy HM, Judah DJ, Butler WH. 1976. Some effects of acute and chronic dosing with AFB1 on rat liver. Cancer Res. 36:1771–1778.
- Nicola RS, Jason JA. 2018. Role of C-reactive protein at Sites of inflammation and Infection. Front Immunol. 9:754.
- Nisha D, Peter S. 2017. Targeting reactive oxygen species in development and progression of pancreatic cancer. Expert Rev Anticancer Ther. 17(1):19–31.
- Omar MH, Meki MA. 1997. A bradykinin potentiating fraction isolated from the venom of Egyptian scorpion Buthus occitanus induced prostaglandin biosynthesis in female Guinea pigs. Comp Biochem Physiol. 3:183–189.
- Őztürk Y. 2001. Kinin receptors and their antagonists as novel therapeutic agents. Curr Pharm. 7(2):135–161.
- Padrissa S, Franco R, Boillot O, Serafín A, Rimola A, Arroyo V, Rodés J, Peralta C, Roselló J. 2009. Effect of angiotensin II and BK inhibition in rat reduced-size liver transplantation. Liver Transpl. 15:313–320.
- Petricevich VL, Pena CA. 2002. The dynamic of cytokine-d NO secretion in mice injected with Tityus serrulatus scorpion venom. Mediat Inflam. 11:173–180.
- Philip GB, Anders MW. 2007. Glutathione transferase omega 1 catalyzes the reduction of s-(phenacyl)glutathiones to acetophenones. Chem Res Toxicol. 20(1):149–154.
- Preetha SP, Kanniappan M, Selvakumar E, Nagaraj M, Varalakshmi P. 2006. Lupeol ameliorates AFB1 induced peroxidative hepatic damage in rats. Comp Biochem Physiol. 143:333.
- Ragab MMS, Sary Kh A, El-Metwally TH, Badr G, Mahmoud MH, Omar HM. 2015. Effect of a high fat, high sucrose diet on the promotion of non-alcoholic fatty liver disease in male rats: the ameliorative role of three natural compounds. Lipids Health Dis. 14:83.
- Raisuddin S, Singh KP, Zaidi SI, Paul BN, Ray PK. 1993. Immunosuppressive effects of AFs in growing rats. Mycopathologia. 124(3):189–194.
- Rajendra DY, Bhagwan SM, Sangeeta AD. 2014. Survey on aflatoxin awareness and assessment of Pune district of Maharashtra, India. Adv Appl Sci Res. 5(2):18–24.
- Ramos M, Kwak MK, Dolan PM, Itoh K, Yamamoto M, Talalay P, Kensler TW. 2001. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc Natl Acad Sci USA. 98(6):3410–3415.
- Rotimi OA, Rotimi SO, Goodrich JM, Adelani IB, Agbonihale E, Talabi G. 2019. Time-course effects of acute AFB1 exposure on hepatic mitochondrial lipids and oxidative stress in rats. Front Pharmacol. 10:467. DOI:10.3389/fphar.2019.00467.
- Sael C, Pablo M. 2015. Antioxidants in liver health. World J Gastrointest Pharmacol Ther. 6(3):59–72.
- Salman MM. 2009. Effect of a single dose of a BPF isolated from scorpion venom (Buthus occitanus) on total protein and albumin in serum of irradiated growing male Guinea pigs. Acad J Biolog Sci. 1(1):33–43.
- Salman MM. 2018. Antioxidant effects of BPF isolated from scorpion venom in liver injury induced by carbon tetrachloride (CCl4) in male albino rats. J Clin Toxicol. 8(6):399–403.
- Salman MM, Kotb AM, Haridy MA, Hammad S. 2016. Hepato- and nephroprotective effects of BPF from scorpion (Buthus occitanus) venom on mercuric chloride treated rats. Excli J. 15:807–816.
- Salman MA, Kotb AM, Mohie AM, Klaus G, Seddik H. 2017. Effect of a BPF isolated from scorpion venom (Leiurus quinquestriatus) on some blood indices and lipid profile in irradiated rats. Mol Cell Biochem. 434:1–6.
- Sancho P, Bataller R, Fernandez G, Moreno M, Ramalho LN, Colmenero J, Marí M, Clària J, Jiménez W, Arroyo V, et al. 2007. BK attenuates hepatocellular damage and fibrosis in rats with chronic liver injury. Gast. 133:2019–2028.
- Sarin H. 2015. Pressuromodulation at the cell membrane as the basis for small molecule hormone and peptide regulation of cellular and nuclear function. J Transl Med. 13:372.
- Sharma JN, Uma K, Noor AR, Rahman AR. 1996. Blood pressure regulation by the kallikrein-kinin system. Gen Pharmacol. 27(1):55–63.
- Sies H. 1997. Oxidative stress, oxidants and antioxidants. Exp Physiol. 82(2):291–295.
- Sohn SW, Jung JW, Lee SY, Kang HR, Park HW, Min KU, Cho SH. 2013. Expression pattern of GSTP1 and GSTA1 in the pathogenesis of asthma. Exp Lung Res. 39(4-5):173–181.
- Takahashi O, Hiraga K. 1984. Effects of dietary BHT on functional and biochemical properties of platelets and plasma. Food Chem Toxicol. 22(2):97–103.
- Tola M, Kebede B. 2016. Occurrence, importance and control of mycotoxins. Cogent Food Agric. 2:1191103.
- Velayutham M, Villamena FA, Fishbein JC, Zweier JL. 2005. Cancer chemopreventive OPZ generates superoxide anion radical. Arch Biochem Biophys. 435(1):83–88.
- Weisburger EK, Evarts RP, Wenk ML. 1977. Inhibitory effect of BHT on intestinal carcinogenesis in rats by azoxymethane. Food Cosmet Toxicol. 15:139–141.
- Wills ED. 1969. Lipid peroxide formation in microsomes; general considerations. Biochem J. 113(2):315–324.
- Xu X, Li B, Zhu S, Rong R. 2015. Hypotensive peptides from snake venoms: structure, function and mechanism. Curr Top Med Chem. 15(7):658–669.
- Yang DC, Deuis JR, Dashevsky D, Dobson J, Jackson TN, Brust A, Xie B, Koludarov I, Debono J, Hendrikx I, et al. 2016. The snake with the scorpion’s sting: novel three-finger toxin sodium channel activators from the venom of the long glanded blue coral snake (Calliophis bivirgatus). Toxins Basel. 8(10):303.