ABSTRACT
Efficacy of soy isoflavones to promote semen quality by improving antioxidant parameters in Xinong Saanen goats was explored. Soy isoflavones (SI), regulator of steroid hormone synthesis and antioxidant in organism, was supplemented in a total mixed ration of male Xinong Saanen dairy goats to study their effects on intake, body weight, level of testosterone and oestradiol, antioxidant performance, and semen quality. Supplementation with SI increased (P < 0.05) dry matter intake expressed as kg/day or % of body weight, with body weight remaining unchanged. In animals receiving SI supplementation, we observed increases (P < 0.05) in serum superoxide dismutase and total antioxidant capacity and decreases in oestradiol and malondialdehyde concentrations in comparison to the Control, while testosterone and glutathione peroxidase remained unchanged. An increase (p < 0.05) in plasma membrane integrity of sperm were observed in bucks received SI supplementation with no other effects on semen quality such as seminal pH, ejaculate volume, viability, motility, and acrosome integrity. The results indicated that SI supplementation improved semen characteristics via enhancing antioxidant parameters.
Highlights
Supplementation of soy isoflavones increased feed intake in Xinong Saanen bucks.
Soy isoflavones decreased serum oestradiol but not testosterone concentrations.
Soy isoflavones could be used as a safe, effective, and natural antioxidant in dairy goats. It alleviated oxidative stress, without negative influence on sperm quality of Xinong Saanen bucks.
1. Introduction
Breeding bucks with desired characteristics are useful for improving the productive performance in dairy goats. Semen quality has economic significance to assure efficient and successful breeding via artificial insemination. Manipulations to increase semen quality in goats may be helpful if the semen quantity is not adversely affected.
Oxidative stress causes tissue injury by invading of inflammatory cell when the animals suffer from various harmful stimulations resulting in breaking the balance of the oxidation and antioxidation. Vital biological activities rely on ATP produced by oxidative phosphorylation involving metabolic processes of oxygen metabolites and peroxided molecule, which are described as reactive oxygen species (ROS). Oxidative stress occurs when ROS or oxidants exceed the capability of the cell to mount an effective antioxidant response. Consequently physiological functions of cells are impaired that could result in eventual death of cells (Ray et al. Citation2012; Sarikhani et al. Citation2018). There are sufficient evidences (Clyne Citation2011; Ranganathan et al. Citation2018) indicated the importance of low level of endogenous ROS in the regulation of the sperm functions such as capacitation, acrosome reaction, and the fusion of sperm and oocyte (Chandra et al. Citation2012). Over production of ROS may also damage sperm and the antioxidant system of seminal plasma and cause oxidative stress reaction with pathological effects (Homa et al. Citation2015). Oxidative stress influenced male reproductive function negatively by impairing structure and function of semen, increasing the apoptosis of germ cells, and disrupting the synthesis of steroid hormone (Lanzafame et al. Citation2009).
Soy isoflavones (SI), the polyphenol mixture with 3-chromone as the mother nucleus, found in the cotyledon and plumular axis of soybean seed principally, includes daidzein, genistin and glycitin and their corresponding binding forms of glycoside, acetyl and malonyl glucoside (Munro Citation2003). Positive effects of SI, such as antioxidant performance (Wei et al. Citation1995; Wang and Wu Citation2017), antitumor action (Ono et al. Citation2017; Imai-Sumida et al. Citation2017), antiaging effect (Wang et al. Citation2013), prevention of osteoporosis (Akhlaghi et al. Citation2019), obesity (Wang et al. Citation2017) and cardiovascular disease (Hanwell et al. Citation2009; Lu et al. Citation2019), and improving climacteric symptoms (Chedraui Citation2011) had been reported.
The application of SI had implications in livestock and poultry production, especially in the enhancement of immune function, bone metabolism, antioxidant ability, and reducing heat stress (Lee et al. Citation2005; Nur’aini et al., Citation2017; Zhang et al. Citation2017). The SI contains phenolic hydroxyl groups which bind with free radical to enhance antioxidative function as well as chelate metal ions and promote antioxidative ability via the catalytic function of metal ions (Chun et al. Citation2005). The supplement of SI in fodder increased the expression of antioxidative protein and the activity of antioxidase (Barbosa et al. Citation2011). SI, the physiological activator with function of weakening oestrogen, affected the level of hormone in vivo by influencing synthesis of protein and secretion of sex hormone (Zhu et al. Citation2016).
The objectives of the reported research were to study the effects of incorporation of SI into the diet as antioxidants and active substances of oestrogen on feed intake, body condition, secretion of steroid hormone, antioxidant ability and sperm quality in dairy goats.
2. Materials and methods
2.1. Animals and experimental diets
Twelve male Xinong Saanen dairy goats (2–3 years old, 72.5 ± 6.33 kg body weight) at Xinong Experimental Station of Northwest A&F University in Yangling (34°14′ N; 107°59′ E), Shaanxi, China, were equally divided into two groups and randomly assigned to one of the two experimental diets. The study was carried out from August to October. After analysis of the SI amount reported in animals (Masilamani et al. Citation2012), we confirmed that supplementation of additional 100 mg of SI could be effective without adverse effect/nontoxic. The control group (SI−) received a basic total mixed ration () while the treatment group (SI+) received the same TMR plus a supplement of SI (Taiyuan Yongyao Biotechnological Limited Company, Taiyuan, Shanxi, China) at the rate of 100 mg/day. The TMR was formulated according to NRC (2007). The animals were adjusted to the feeding pens and TMR for two weeks prior to the experimental period and the experimental period lasted for 10 weeks. The goat pens were disinfected thoroughly prior to the initiation of the experiment while animals went through immunoprophylaxis and disinsectization. The inactivated vaccines of goats (caprine infectious pleuro pneumonia, combined with ovine/caprine clostridial diseases vaccine) and abamectin (0.2 mg/kg) were subcutaneously injected. Animals were screened for quality of semen with emphasis on sperm counts, mobility and abnormality prior to the initiation of the experiment. There was no difference (P > 0.05) in age, weight and sperm quality between the two groups as confirmed by the statistical analysis prior to the initiation of the experiment.
Table 1. Composition and nutrient content of the concentrate (air-dry basis)
2.2. Feeding, intake and body weight measurements
Animals were divided into two groups and fed twice daily at 08:30 and 15:30 in two pens. The residual feed was recorded prior to the morning feeding daily. Average daily feed dry matter (DM) intake was calculated. Weekly empty body weight was recorded for each animal prior to the morning feeding.
2.3. Steroid hormone secretion and antioxidant index analysis
The jugular blood samples were collected from each animal once every two weeks. Alternate sampling from bilateral jugular vein was employed to minimize animal stress, reaction and vasculitis. The blood samples were incubated at 37°C for 0.5 h then centrifuged (3000 rpm, 10 mins, 4°C). The separated serum was stored at −20°C for further analyses.
Level of testosterone, oestradiol, glutathione peroxidase (GSH-Px), superoxide dismutase (SOD), total antioxidant capacity (T-AOC) and malondialdehyde (MDA) in the serum were analysed by an ELISA kit (Nanjing Jiancheng Bioengineering Institute, Jiangsu, China).
2.4. Semen quality analysis
Semen was collected every two weeks after morning feeding via artificial vagina. Does in oestrus were used as teasers. The volume and pH of semen were assessed immediately after collection. The volume was measured by a disposable syringe and pH was determined using pH test papers. Fresh semen was diluted in semen diluent (375 nM tromethamine, 124 mM citric acid and 41 nM glucose) at 1–4 at 37°C then transferred to vacuum cup within 1 h for further analysis.
Sperm sample (10 μl) was observed under the microscope (X400) to assess the semen viability and motility with three randomly selected microscopic fields and repeated three times.
The percent of sperm with intact plasma membranes (VIA) was detected by Hypoosmotic Swelling Test (HOST) as described (Irez et al. Citation2012) with minor modification. The sperms were placed in the glass dish containing hypoosmotic solution. Then the dish was placed in the incubator at 37°C for 30 min. In our study, HOST 3 and HOST 4 subgroups were considered as sperms with intact plasma membrane. At least 200 sperms were counted using microscope (X400) and replicated three times. The differentiation was based on the criteria that the sperms with intact plasma membranes had bended and swelling tail and took in water to keep cellular balance of osmotic pressure. The sperms with no morphological alteration and inability to balance osmotic pressure indicated the damage of plasma membranes.
The acrosome integrity of sperms was evaluated by Trypan-blue Giemsa stain (Serafini et al. Citation2013) of sperm morphology. After smearing and staining preparation, acrosome integrity was detected under the microscope using oil immersion. The number of total sperms would have to be at least 200. Every analysis was repeated three times.
2.5. Statistical analysis
Data obtained from six animals were expressed as mean ± SD. One-way ANOVA for repeated measures was used to determine the treatment effects of SI in body weight and dry matter intake for each week. For the time course of the hormone and semen quality parameters, a two-way ANOVA with repeated measures was used. The Tukey–Kramer post hoc analysis was used to identify significant differences between treatments (SPSS 19.0). The significant differences between the means were at P < 0.05.
3. Results
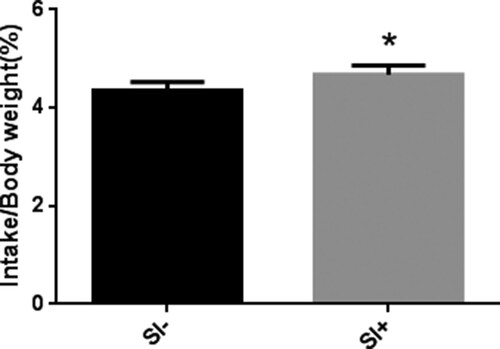
Feed DM intakes of SI+ group were higher (P < 0.05) than those in SI-group during Week 2, 4, 5, 6, 7, and 8 ((A)), while there is no difference (P > 0.05) during Week 1 between groups. There were no differences (P > 0.05) in empty body weight between groups throughout the entire experiment period ((B)). Average feed DM intake expressed as % of empty body weight was higher (P < 0.05) in SI+ than SI−, 5.03 and 4.65%, respectively ().
Figure 1. Mean (±SD) for empty body weight (kg) (A) and dry matter intake (kg) (B) of male Xinong Saanen dairy goats fed diet with (SI+) or without (SI−)_supplementation of soy Isofalvones. * p < 0.05
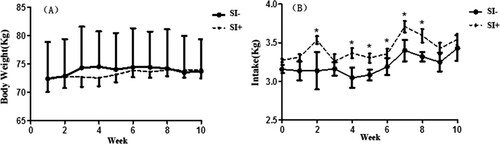
Figure 2. Mean (±SEM) for intake/weight for male Xinong Saanen dairy goats fed diet with supplementation of soy isoflavones (SI+) versus control (SI−). The treatment value was different (P < 0.05) as compared to the control.
There were no differences (P > 0.05) in serum testosterone between groups during the entire experimental period (). Serum oestradiol were lower (P < 0.05) in SI+ than SI− during Week 2, 6, 8, 10 of the experimental period ().
Table 2. Effect of supplementation of soy isoflavones on serum testosterone and oestradiol concentrations
The oestradiol concentration also decreased in Week 2, 4, 6, 8, 10 in SI+ group.
There were no differences (P > 0.05) in serum GSH-Px between SI+ and SI− during the entire experimental period (). The SOD was higher (P < 0.05) in SI+ than SI− during Week 6 and 10 of the experimental period. The T-AOC was higher (P < 0.05) while MDA was lower (P < 0.05) in SI+ than SI− during Week 4, 6, 8, and 10 of the experimental period.
Table 3. Effect of supplementation of soy isoflavones (SI+) versus control (SI−) on serum glutathione peroxidase (GSH-Px), superoxide dismutase (SOD), total antioxidant capacity (T-AOC) and malondialdehyde (MDA) concentrations
Supplementation of 100 mg/day had no effect (P > 0.05) on serum volume and pH, and viability, motility and acrosome integrity of sperm (). However, the data seemed to indicate that SI promoted (P < 0.05) plasma membrane integrity during Week 4 and 10 () of the experimental period.
Table 4. Effect of supplementation of soy isoflavones (SI+) versus control (SI−) on semen quality
4. Discussion
Our study demonstrated that supplementation of soy isoflavones at the rate of 100 mg/day increased feed DM intake and improved oxidation resistance and semen quality in Xinong Saanen bucks.
It has been reported that supplementation of SI increased animal (pigs and rats) intake (Greiner et al. Citation2001; Payne et al. Citation2001; Watkins et al. Citation2005). We found similar increases in feed DM intake with the supplementation of 100 mg/day in Xinong Saanen bucks. Specifically the increases were observed in Week 2, 4, 5, 6, 7, and 8. The soy flavones with soy fragrance stimulate animals’ appetite to induce the increase of intake. In bucks, the situation would potentially occur as goats had a higher threshold for bitter tastes (Robertson et al. Citation2006) to overcome the bitter tastes from SI (Meinlschmidt et al. Citation2016).
Palatability of fodder, genetic environment, feeding, management, and disease all could influence animals’ intake which was one of the major factors affecting animal reproductive performance (Decruyenaere et al. Citation2009). The onset of the experimental period was in August and completed in October, which might be a factor of intake fluctuation (Average temperature were 23–33°C in Yangling in August, 18–27°C in September, and 12–21°C in October). High temperature in August might have resulted in a lower feed DM intake in the early part of the experiment (Lu Citation1989). The strong libido exhibited by the bucks during breeding period prior to and in during the initial period of the experiment might have resulted in the loss of appetite (Walkden-Brown et al. Citation1994; Zarazaga et al. Citation2009).
The significant difference observed in intake expressed as % of empty body weight between SI+ and SI− could also be attributed to the beneficial effect of SI on reducing oxidative stress and accelerating immunity (Ryan-Borchers et al. Citation2006). SI supplement kept animals from oxidative stress and heat stress that was induced by intensive feeding under higher ambient temperature (Xu et al. Citation2009).
SI, a promoter of glucose and lipid metabolism, could help animals maintain the body weight (Silva et al. Citation2018). Body weights of dairy goats were influenced by factors such as age, dietary energy density, dietary protein, age, breed, environment and management (Lu and Potchoiba Citation1990). In our study, the variability of body weight in SI+ bucks was visible. The bucks selected in our study were mature adult goats with relatively stable body weight that should not be influenced easily by environmental factors. However, the animals’ unstable appetite contributed to the variability in body weight which was influenced by animals’ libido in breeding time and heat stress caused by high temperature. Because of the relative large variability in body weight among animals, we did observe a treatment effect. However, we felt that further investigations are merited to reconcile the difference in the effect of SI supplementation on body weight reported by various studies.
Generally, it is known that reproductive hormones that regulate reproductive process can have a direct effect on animal reproductive performance. Testosterone promotes the development of sexual organs and secondary sex characteristic, accelerates the synthesis of protein and reduces urinary nitrogen (Eidelsberg et al. Citation1942; Wilson et al. Citation1973), and maintains normal sexual function (Walker Citation2011). Testosterone was an important hormone which could be used to measure breeding stock reproductive performance (Tyagi et al. Citation2017).
It has been reported that SI, with a weak oestrogen function (Barnes Citation2004), affected male neuroendocrine system, promoted the synthesis of GH in pituitarium and IGF-1 in liver (Antonella Citation2007), via oestradiol receptors in hypothalamus and pituitarium (Setchell Citation2001; Sá Citation2014). In our study we did not observe any changes in testosterone concentration as a result of SI supplementation. Other studies also reported no effects of SI on testosterone concentrations in roosters (Gjorgovska and Filev Citation2013) and rabbits (Yousef et al. Citation2003; Yousef et al. Citation2004). A seasonal variation in reproductive performance can be observed in goats, which accompanied the changes in hormone secretion. Seasonality of buck’s reproductive performance was obviously reflected by changes detectable behaviour, testicular morphologic characteristics and hormone secretion, which followed the breeding activity of the ewes (Sarlós et al. Citation2013). Our study was carried out during breeding period when the blood plasma testosterone concentration remained relatively high level. The testosterone concentration was directly influenced by frequently stimulation from female effect. Oestradiol, enzymatic product of testosterone, was converted from testosterone by cytochrome P450 aromatase in testis (Liguori et al. Citation2018). In our study, supplement of SI inhibited oestradiol secretion which would produce feedback effect on testosterone. We considered that further molecular experiment in testicular cells should be applied to explain the SI effect on the relationship of testosterone and oestradiol.
It had been reported that effects of supplementation of SI on serum oestradiol concentration was inhibitory (Kao et al. Citation1998; Xu et al. Citation1998; Kumar Rajan et al. Citation2017). With 1/10−5 to 1/10−3 activity as that of oestradiol, SI bond with oestradiol receptor beta to inhibit the intact function and feedback regulation of oestradiol (Setchell Citation2001). Another reason for the reduction of oestradiol by SI supplementation is that SI act as inhibitors of steroidogenic enzymes (aromatase and 17β-hydroxysteroid oxidoreductase) which caused oestradiol level to decrease (Deluca et al. Citation2005; Wang et al. Citation2008). In our study we observed a consistent decrease in serum oestradiol concentration with SI supplementation throughout the experimental period, with the exception of Week 4. The data showed the obvious inhibition effect of SI on oestradiol following but no influence on testosterone. The supplement of SI occupied the oestradiol receptor, weakening activity of aromatase to cause the decrease of oestradiol (Carreau et al. Citation2006). It is also possible that effects of SI supplementation on serum testosterone and oestradiol concentrations in male bucks could be multifactorial, as the interactions may permeate throughout reproductive organs. In addition, it could also be related to animal species, age, sex, the amount of SI supplementation, and the length of the experiment.
The antioxidative factors in serum, such as T-AOC, SOD, MDA, GSH-Px, were important parameters to evaluate oxidation resistance of SI in vivo (Tao et al. Citation2005). It has been proved that dietary SI enhanced antioxidant properties in different species (Wei et al. Citation1995; Amigo-Benavent et al. Citation2008; Dixit et al. Citation2012). In our study, we observed SI intake stimulated accumulation of SOD and T-AOC and inhibited the serum MDA concentration. MDA, end product from fatty peroxide formation, caused cross-linking polymerization of protein resulting in cytotoxicity effect (Ulbrecht et al. Citation2019). SOD was a highly conserved enzyme that played an important role in the balance of oxidation and anti-oxidation by dismutation of superoxide into oxygen and hydrogen peroxide (Tsang Citation2014). T-AOC was the parameter which reflected the whole anti-oxidation performance including antioxidant and antioxidase (Pisoschi et al. Citation2015). It seemed to confirm that SI supplementation enhanced enzymatic action of antioxidant system and reduced the degree of lipid peroxidation (Akitha Devi and Giridhar Citation2015). There were reports that the likely mechanism of which SI intake influenced antioxidant action of male bucks was increasing the expression of antioxidant genes via oestrogen receptors, ERK1/2 (Kang et al. Citation2007), and NFκB (Davis Citation2001). It is advisable that further study on molecular mechanism of SI oxidation resistance could be carried out in vivo and in vitro. It had been reported that after treated with high dose SI for 12 months, morphological and histopathologic assessment of testis, sperm quality remained unchanged in rats (Faqi et al. Citation2004), humans (Beaton Citation2010), rabbit (Yousef et al. Citation2004; Cardoso Citation2007), mouse (Jung et al. Citation2004), and monkey (Sharpe et al. Citation2002). In our study, there were no changes observed in pH, volume, viability, motility and acrosome integrity in bucks with SI supplementation. However, plasma membrane integrity of sperm was significantly increased in bucks with SI supplementation. Several studies reported that SI had no adverse effects on reproductive performance of animals (Faqi et al. Citation2004; Cardoso Citation2007; Cardoso and Báo Citation2009). Similar results were also obtained in our study. We concluded that increase of plasma membrane integrity of sperm had a high correlation with antioxidant performance induced by SI (Paul and Smk Citation2017). The HOST was descried as a classical assay to evaluate function of sperm and integrity of physiological function of sperm plasma membrane (Lechniak et al. Citation2003). Sperm plasma membrane, an important part of spermatozoan, is related to most of reproductive process, such as energy metabolism, capacitation, acrosomal reaction and fusion of sperm and oocyte (Jeyendran et al. Citation1984; Grieblova et al. Citation2017).
In conclusion, the study indicated that SI affected the levels of the endogenous hormone and antioxidant parameters and can be considered as a safe, effective, and natural antioxidant with no negative influence on reproductive organs and sperm quality of bucks.
Acknowledgements
This work was funded by the ‘National Key Research and Development Program of China (2018YFD0501900) andthe National Natural Science Foundation of China (31672398).’.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Akhlaghi M, Nasab M, Riasatian M, Sadeghi F. 2019. Soy isoflavones prevent bone resorption and loss, a systematic review and meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr. 5:1–15. doi:10.1080/10408398.2019.1635078.
- Akitha Devi MK, Giridhar P. 2015. Variations in physiological response, lipid peroxidation, antioxidant enzyme activities, proline and isoflavones content in Soybean varieties subjected to drought stress. Proc Natl Acad Sci India. 85:35–44. doi:10.1007/s40011-013-0244-0.
- Amigo-Benavent M, Silván JM, Moreno FJ, Villamiel M, Del Castillo MD. 2008. Protein quality, antigenicity, and antioxidant activity of Soy-based foodstuffs. J Agric Food Chem. 56:6498–6505. doi:10.1021/jf800697n.
- Antonella D. 2007. Relationship of dietary protein and Soy isoflavones to serum IGF-1 and IGF binding proteins in the prostate Cancer lifestyle trial. Nutr Cancer. 58:35–42. doi:10.1080/01635580701308034.
- Barbosa AC, Lajolo FM, Genovese MI. 2011. Effect of free or protein-associated soy isoflavones on the antioxidant status in rats. J Sci Food Agric. 91:721–731. doi:10.1002/jsfa.4242.
- Barnes S. 2004. Soy isoflavones–phytoestrogens and what else? J Nutr. 134:1225–1228. doi:10.1038/sj.ijo.0802613.
- Beaton LK. 2010. Soy protein isolates of varying isoflavone content do not adversely affect semen quality in healthy young men. Fertil Steril. 94:1717–1722. doi:10.1016/j.fertnstert.2009.08.055.
- Cardoso JR. 2007. Effects of chronic exposure to soy meal containing diet or soy derived isoflavones supplement on semen production and reproductive system of male rabbits. Anim Reprod Sci. 97:237–245. doi:10.1016/j.anireprosci.2006.01.014.
- Cardoso JR, Báo SN. 2009. Morphology of reproductive organs, semen quality and sexual behaviour of the Male Rabbit exposed to a Soy-containing diet and Soy-derived isoflavones during gestation and lactation. Reprod Domest Anim. 44:937–942. doi:10.1111/j.1439-0531.2008.01121.x.
- Carreau S, Delalande C, Silandre D, Bourguiba-Hachemi S, Lambard S. 2006. Aromatase and estrogen receptor in male reproduction. Mol Cell Endocrinol. 246:65–68. doi:10.1016/j.mce.2005.11.021.
- Chandra G, Aggarwal A, Singh A, Singh AK, Kumar M, Kushwaha R, Singh YK. 2012. Oxidative stress in sperm biology – a review. Reprod Domest Anim Zuchthyg. 33:54–61. http://www.i-scholar.in/index.php/AR/article/view/65311.
- Chedraui P. 2011. The effect of soy-derived isoflavones over hot flushes, menopausal symptoms and mood in climacteric women with increased body mass index. Gynecol Endocrinol. 27:307–313. doi:10.3109/09513590.2010.490614.
- Chun HS, Chang H-J, Choi EH, Kim HJ, Ku KH. 2005. Molecular and absorption properties of 12 Soy isoflavones and their structure–activity relationship with selected biological activities. Biotechnol Lett. 27:1105–1111. doi:10.1007/s10529-005-8457-9.
- Clyne M. 2011. Male factor infertility: effects of ROS and vitamin E on sperm. Nat Rev Urol. 9:62–62. doi:10.1038/nrurol.2011.227.
- Davis JN. 2001. Soy isoflavone supplementation in healthy men prevents NF-kappa B activation by TNF-alpha in blood lymphocytes. Free Radical Biol Med. 30:1293–1302. doi:10.1016/S0891-5849(01)00535-4.
- Decruyenaere V, Buldgen A, Stilmant D. 2009. Factors affecting intake by grazing ruminants and related quantification methods: A review. biotechnologie, agronomie. Soc Environ. 13:559–573. http://www.pressesagro.be/base/text/v13n4/559.pdf.
- Deluca D, Krazeisen A, Breitling R, Prehn C, Möller G, Adamski J. 2005. Inhibition of 17beta-hydroxysteroid dehydrogenases by phytoestrogens: comparison with other steroid metabolizing enzymes. J Steroid Biochem Mol Biol. 93:285–292. doi:10.1016/j.jsbmb.2004.12.035.
- Dixit AK, Bhatnagar D, Kumar V, Chawla D, Fakhruddin K, Bhatnagar D. 2012. Antioxidant potential and radioprotective effect of soy isoflavone against gamma irradiation induced oxidative stress. J Funct Foods. 4:197–206. doi:10.1016/j.jff.2011.10.005.
- Eidelsberg J, Bruger M, Lipkin M. 1942. Some metabolic Effects of testosterone implants. J Clin Endocrinol. 2:329–331. doi:10.1210/jcem-2-5-329.
- Faqi AS, Johnson WD, Morrissey RL, McCormick DL. 2004. Reproductive toxicity assessment of chronic dietary exposure to soy isoflavones in male rats. Reprod Toxicol. 18:605–611. doi:10.1016/j.reprotox.2004.02.013.
- Gjorgovska N, Filev K. 2013. Effects of isoflavones on testicular weight and testosterone secretion in ISA Brown roosters. Ann Anim Sci. 13:295–301. doi:10.2478/aoas-2013-0010.
- Greiner LL, Stahly TS, Stabel TJ. 2001. The effect of dietary soy genistein on pig growth and viral replication during a viral challenge. J Anim Sci. 79:1272. doi:10.2527/2001.7951272x.
- Grieblova A, Pintus E, Ros-Santaella JL. 2017. Integrity of head and tail plasmalemma is associated with different kinetic variables in boar sperm. Anim Reprod Sci. 184:218–227. doi:10.1016/j.anireprosci.2017.07.020.
- Hanwell HEC, Kay CD, Lampe JW, Holub BJ, Duncan AM. 2009. Acute fish oil and Soy isoflavone supplementation increase postprandial serum (n-3) polyunsaturated fatty acids and isoflavones but do not affect triacylglycerols or biomarkers of oxidative stress in overweight and obese hypertriglyceridemic men. J Nutr. 139:1128–1134. doi:10.3945/jn.109.105171.
- Homa ST, Vessey W, Perez-Miranda A, Riyait T, Agarwal A. 2015. Reactive oxygen species (ROS) in human semen: determination of a reference range. J Assist Reprod Genet. 32:757–764. doi:10.1007/s10815-015-0454-x.
- Imai-Sumida M, Majid S, Dasgupta P, Kulkarni P, Yamamura S. 2017. Abstract 3449: Genistein inhibits renal cancer progression through long non-coding RNA HOTAIR suppression. Cancer Res. 77:3449–3449. doi:10.1158/1538-7445.AM2017-3449.
- Irez T, Usta TA, Zebitay G, Oral E, Senol H, Sahmay S. 2012. Evaluation of subgroups of the human sperm hypoosmotic swelling test in normozoospermic male cases with recurrent fertilization failure: a prospective case-controlled study. Arch Gynecol Obstet. 287:797–801. doi:10.1007/s00404-012-2634-6.
- Jeyendran RS, Van der Ven HH, Perez-Pelaez M, Crabo BG, Zaneveld LJD. 1984. Development of an assay to assess the functional integrity of the human sperm membrane and its relationship to other semen characteristics. J Reprod Fertil. 70:219–228. doi:10.1530/jrf.0.0700219.
- Jung EY, Lee B-J, Yun YW, Kang J-K, Baek I-J, Jurg M-Y, Lee Y-B, Sohn H-S, Lee J-Y, Kim K-S, et al. 2004. Effects of exposure to Genistein and estradiol on reproductive development in immature male mice weaned from dams adapted to a Soy-based commercial diet. J Vet Med Sci. 66:1347–1354. doi:10.1292/jvms.66.1347.
- Kang NJ, Lee KW, Rogozin EA, Cho Y-Y, Heo Y-S, Bode AM, Lee HJ, Dong Z. 2007. Equol, a metabolite of the Soybean isoflavone daidzein, inhibits neoplastic cell transformation by targeting the MEK/ERK/p90RSK/activator protein-1 pathway. J Biol Chem. 282:32856–32866. doi:10.1074/jbc.m701459200.
- Kao YC, Zhou C, Sherman M, Laughton CA, Chen S. 1998. Molecular basis of the inhibition of human aromatase (estrogen synthetase) by flavone and isoflavone phytoestrogens: a site-directed mutagenesis study. Environ Health Perspect. 106:85–92. doi:10.1289/ehp.9810685.
- Kumar Rajan R, Muthusamy S, Balaji B. 2017. Soy isoflavones exert beneficial effects on letrozole-induced rat polycystic ovary syndrome (PCOS) model through anti-androgenic mechanism. Pharm Biol. 55:242–251. doi:10.1080/13880209.2016.1258425.
- Lanzafame FM, Vignera SL, Vicari E, Calogero AE. 2009. Oxidative stress and medical antioxidant treatment in male infertility. Reprod Biomed Online. 19:638–659. doi:10.1016/j.rbmo.2009.09.014.
- Lechniak D, Kedzierski A, Stanislawski D. 2003. The Use of HOS test to evaluate membrane functionality of boar sperm capacitated in vitro. Reprod Domest Anim. 37:379–380. doi:10.1046/j.1439-0531.2002.t01-1-00381.x.
- Lee YB, Lee HJ, Sohn HS. 2005. Soy isoflavones and cognitive function. J Nutr Biochem. 16:641–649. doi:10.1016/j.jnutbio.2005.06.010.
- Liguori G, Pelagalli A, Assisi L, Squillacioti C, Costagliola A, Mirabella N. 2018. Effects of orexins on 17β-estradiol synthesis and P450 aromatase modulation in the testis of alpaca (Vicugna pacos). Anim Reprod Sci. 192:313–320. doi:10.1016/j.anireprosci.2018.03.032.
- Lu CD. 1989. Effects of heat stress on goat production. Small Ruminant Res. 2:151–162. doi:10.1016/0921-4488(89)90040-0.
- Lu CD, Potchoiba MJ. 1990. Feed intake and weight gain of growing goats fed diets of various energy and protein levels. J Anim Sci. 68:1751–1759. doi:10.2527/1990.6861751x.
- Lu Y, Zhao A, Wu Y, Zhao Y, Yang X. 2019. Soybean soluble polysaccharides enhance bioavailability of genistein and its prevention against obesity and metabolic syndrome of mice with chronic high fat consumption. Food Funct. 10:4153–4165. doi:10.1039/C8FO02379D.
- Masilamani M, Wei J, Sampson HA. 2012. Regulation of the immune response by soybean isoflavones. Immunol Res. 54:95–110. doi:10.1007/s12026-012-8331-5.
- Meinlschmidt P, Schweiggert-Weisz U, Eisner P. 2016. Soy protein hydrolysates fermentation: effect of debittering and degradation of major soy allergens. Food Sci Technol. 71:202–212. doi:10.1016/j.lwt.2016.03.026.
- Munro IC. 2003. Soy isoflavones: a safety review. Nutr Rev. 1:1–33. doi:10.1301/nr.2003.janr.1-33.
- Nur’aini F, Rahayu S, Rifa'i M. 2017. NFКB activity decreased in BALB/c mice with high fat diet and fructose. 2ND International Conference on Composite Materials and Material Engineering (ICCMME 2017). AIP Publishing LLC, p. 020005. https://doi.org/10.1063/1.4983416.
- Ono M, Ejima K, Higuchi T, Takeshima M, Wakimoto R, Nakano S. 2017. Equol enhances apoptosis-inducing activity of Genistein by increasing Bax/Bcl-xL expression ratio in MCF-7 human breast Cancer cells. Nutr Cancer Int J. 69:1–8. doi:10.1080/01635581.2017.1367945.
- Paul K, Smk N. 2017. Antioxidants protect proteins’ anchorage to the bilayer by improving plasma membrane integrity of ram spermatozoa during liquid preservation in a soya lecithin-based diluent. Reprod Domest Anim Zuchthyg. 52:1052–1060. doi:10.1111/rda.13023.
- Payne RL, Bidner TD, Southern LL, Geaghan JP. 2001. Effects of dietary soy isoflavones on growth, carcass traits, and meat quality in growing-finishing pigs. J Anim Sci. 79:1230–1239. doi:10.1016/S0739-7240(01)00099-6.
- Pisoschi A, Cimpeanu C, Predoi G. 2015. Electrochemical methods for total antioxidant capacity and its main contributors determination: a review. Open Chem. 13:824–856. doi:10.1515/chem-2015-0099.
- Ranganathan P, Rao KA, Sudan JJ, Balasundaram S. 2018. Cadmium effects on sperm morphology and semenogelin with relates to increased ROS in infertile smokers: an in vitro and in silico approach. Reprod Biol. 18:189–197. doi:doi.org/10.1016/j.repbio.2018.04.003.
- Ray P, Huang B-W, Tsuji Y. 2012. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 24:981–990. doi:10.1016/j.cellsig.2012.01.008.
- Robertson E, Gordon I, Pérez-Barbería F. 2006. Preferences of sheep and goats for straw pellets treated with different food-flavouring agents. Small Ruminant Res. 63:50–57. doi:10.1016/j.smallrumres.2005.02.007.
- Ryan-Borchers TA, Park JS, Chew BP, McGuire MK, Fournier LR, Beerman KA. 2006. Soy isoflavones modulate immune function in healthy postmenopausal women. Am J Clin Nutr. 83:1118. doi:10.1093/ajcn/83.5.1118.
- Sá SI. 2014. Role of plasma membrane estrogen receptors in mediating the estrogen induction of progesterone receptors in hypothalamic ventromedial neurons. J Comp Neurol. 522:298–307. doi:10.1002/cne.23396.
- Sarikhani M, Mishra S, Desingu P, Kotyada C, Wolfgeher D, Mahesh P, Singh M, Sundaresan N. 2018. SIRT2 regulates oxidative stress-induced cell death through deacetylation of c-Jun NH 2 -terminal kinase. Cell Death Differ. 25:1. doi:10.1038/s41418-018-0069-8.
- Sarlós P, Egerszegi I, Balogh O, Molnár A, Cseh S, Rátky J. 2013. Seasonal changes of scrotal circumference, blood plasma testosterone concentration and semen characteristics in Racka rams. Small Ruminant Res. 111:90–95. doi:10.1016/j.smallrumres.2012.11.036.
- Serafini R, Longobardi V, Spadetta M, Neri D, Ariota B, Gasparrini B, di palo R. 2013. Trypan blue/Giemsa staining to assess sperm membrane integrity in salernitano stallions and its relationship to pregnancy rates. Reprod Domest Anim Zuchthyg. 49:1–7. doi:10.1111/rda.12221.
- Setchell KDR. 2001. Soy isoflavones—benefits and risks from nature’s selective estrogen receptor modulators (SERMs). J Am Coll Nutr. 20:354S–362S. doi:10.1080/07315724.2001.10719168.
- Sharpe RM, Bronwen M, Keith M, Irene G, Chris M, McNeilly AS, Walker M. 2002. Infant feeding with soy formula milk: effects on the testis and on blood testosterone levels in marmoset monkeys during the period of neonatal testicular activity. Hum Reprod. 7:1692–1703. doi:10.1093/humrep/17.7.1692.
- Silva P, Ribeiro TA, Tófolo LP, Prates KV, Francisco FA, da Silva Silveira S, Malta A, Lopes DA, Miranda RA, Palma-Rigo K, et al. 2018. Treatment with soy isoflavones during early adulthood improves metabolism in early postnatally overfed rats. Nutr Neurosci. 21:25–32. doi:doi.org/10.1080/1028415X.2016.1213007.
- Tao X, Xu ZR, Han XY, Wang YZ, Zhou LH. 2005. Effects of fluoride levels on lipid peroxidation and antioxidant systems of growing/finishing pigs. Asian-Australas J Anim Sci. 18:552–556. doi:10.5713/ajas.2005.552.
- Tsang CK. 2014. Superoxide dismutase 1 acts as a nuclear transcription factor to regulate oxidative stress resistance. Nat Commun. 5:1–11. doi:10.1038/ncomms4446.
- Tyagi V, Scordo M, Yoon R, Liporace F, Greene L. 2017. Revisiting the role of testosterone: are we missing something? Rev Urol. 19:16. doi:10.3909/riu0716.
- Ulbrecht dO, Oliveira M, Gonçalves DA, Zanoni LZG, do Nascimento VA. 2019. Association between selenium and malondialdehyde as an efficient biomarker of oxidative stress in infantile cardiac surgery. Biol Trace Elem Res. 187:74–79. doi:doi.org/10.1007/s12011-018-1378-y.
- Walkden-Brown S, Norton B, Restall B. 1994. Seasonal variation in voluntary feed intake and growth in cashmere bucks fed ad libitum diets of low or high quality. Aust J Agric Res. 45:355–366. doi:10.1071/AR9940355.
- Walker WH. 2011. Testosterone signaling and the regulation of spermatogenesis. Spermatogenesis. 1:116–120. doi:10.4161/spmg.1.2.16956.
- Wang Q, Ge X, Tian X, Zhang Y, Zhang J, Zhang P. 2013. Soy isoflavone: the multipurpose phytochemical (review). Biomed Rep. 1:697–701. doi:10.3892/br.2013.129.
- Wang Y, Man Gho W, Chan FL, Chen S, Leung LK. 2008. The red clover (Trifolium pratense) isoflavone biochanin A inhibits aromatase activity and expression. Br J Nutr. 99:303–310. doi:10.1017/s0007114507811974.
- Wang S, Wang Y, Pan M-H, Ho C-T. 2017. Anti-obesity molecular mechanism of soy isoflavones: weaving the way to new therapeutic routes. Food Funct. 8:3831–3846. 10.1039/C7FO01094J.
- Wang B, Wu C. 2017. Dietary soy isoflavones alleviate dextran sulfate sodium-induced inflammation and oxidative stress in mice. Exp Ther Med. 14:276–282. doi:10.3892/etm.2017.4469.
- Watkins BA, Reinwald S, Li Y, Seifert MF. 2005. Protective actions of soy isoflavones and n-3 PUFAs on bone mass in ovariectomized rats. J Nutr Biochem. 16:479–488. doi:10.1016/j.jnutbio.2005.01.019.
- Wei HC, Bowen R, Cai QY, Barnes S, Wang Y. 1995. Antioxidant and antipromotional effects of the soybean isoflavone genistein. Proc Soc Exp Biol Med. 208:124–130. doi:10.3181/00379727-208-43844.
- Wilson MJ, Spaziani E, Sigvardt KA. 1973. The scrotum as target organ for testosterone: hormonal control of amino acid distribution. Endocrinology. 93:743–747. doi:10.1210/endo-93-3-743.
- Xu X, Duncan AM, Merz BE, Kurzer MS. 1998. Effects of soy isoflavones on estrogen and phytoestrogen metabolism in premenopausal women. Cancer Epidemiol Biomarkers Prev. 7:1101–1108. doi:10.1023/A:1008831802805.
- Xu SZ, Zhong W, Ghavideldarestani M, Saurabh R, Lindow SW, Atkin SL. 2009. Multiple mechanisms of soy isoflavones against oxidative stress-induced endothelium injury. Free Radical Biol Med. 47:167–175. doi:10.1016/j.freeradbiomed.2009.04.021.
- Yousef MI, El-Demerdash FM, Al-Salhen KS. 2003. Protective Role of isoflavones against the toxic effect of cypermethrin on semen quality and testosterone levels of rabbits. J Environ Sci Health Part B. 38:463–478. doi:10.1081/PFC-120021666.
- Yousef MI, Esmail AM, Baghdadi HH. 2004. Effect of isoflavones on reproductive performance, testosterone levels, Lipid Peroxidation, and seminal plasma Biochemistry of male rabbits. J Environ Sci Health Part B. 39:819–833. doi:10.1081/LESB-200030880.
- Zarazaga LA, Guzmán JL, Domínguez C, Pérez MC, Prieto R. 2009. Effects of season and feeding level on reproductive activity and semen quality in Payoya buck goats. Theriogenology. 71:1316–1325. doi:10.1016/j.theriogenology.2009.01.007.
- Zhang QY, Ma FC, Wang S, Wu CM, Guo P. 2017. Pharmacological activities of genistin: research advances. J Int Pharm Res. 44:315–325. doi:10.13220/j.cnki.jipr.2017.04.004.
- Zhu Y, Xu H, Li M, Gao Z, Huang J, Liu L, Huang X, Li Y. 2016. Daidzein impairs leydig cell testosterone production and sertoli cell function in neonatal mouse testes: an in vitro study. Mol Med Rep. 14:5325–5333. doi:10.3892/mmr.2016.5896.
