ABSTRACT
The study objective was to examine effect of age at castration on stress indicators and performance of 40 crossbred suckler beef calves. Calves were assigned to two age groups and two castration treatments; calves 2.5- or 5.0-mo-old (mean body weight (SD) = 120.8 (29.3), 218.1 (30.8) kg, respectively) were sham (control) or Burdizzo castrated (n = 10 per treatment) in a 2 × 2 factorial design. Following castration, peak plasma cortisol concentrations were greater in 2.5-mo-old and 5.0-mo-old calves compared with corresponding controls, while peak cortisol concentrations in control animals were greater in 5-mo-old compared with 2.5-mo-old calves. The integrated cortisol responses for the first 4 h after castration were not different between 2.5- and 5.0-mo-old calves. However, the integrated cortisol response was greater in 5.0-mo-old calves from 4 to 9 h post treatment. The increase in scrotal circumference after castration was greater in 5.0-mo-old calves, and abnormal postures were observed more often in 5-mo-old castrates. There was no effect of castration on haematology profiles, haptoglobin, metabolites, body temperature and growth performance. In conclusion, the physiological stress, inflammation and pain-related behaviours caused by Burdizzo castration were reduced when castrating calves at 2.5- than at 5.5-mo-old.
1. Introduction
Castration of male calves for beef production worldwide is a common livestock husbandry management practice (Coetzee Citation2013; Canozzi et al. Citation2017). The benefits of castration include reduced aggression and mounting behaviour of animals resulting in less injury during handling management and less dark-cutting beef (Mahmood et al. Citation2017). Burdizzo castration is a bloodless method of castration, which causes marked atrophy of the testes and complete absence of functional testicular tissue (Thüer et al. Citation2007; Pang et al. Citation2011; Pieler et al. Citation2013; Lambertz et al. Citation2014). It is a recommended castration method over rubber ring and surgical castration due to reduced stress induced by this method (King et al. Citation1991; Molony et al. Citation1995; Stafford et al. Citation2002; Stafford and Mellor Citation2005, Citation2011; Thüer et al. Citation2007).
While the benefits of castration are widely accepted, all castration methods induce physiological, neuroendocrine, and behavioural changes associated with pain and distress (Coetzee Citation2013; Canozzi et al. Citation2017). The severity of the response depends on the age of the calf and the castration method (Robertson et al. Citation1994; Molony et al. Citation1995; Thüer et al. Citation2007). Plasma cortisol concentration has been widely used as a measurement of castration-induced distress since the magnitude of the response, as indicated by peak height, interval to peak and integrated response, is proportional to the degree of severity of different castration procedures (Canozzi et al. Citation2017).
Limited literature indicates that it is beneficial to castrate calves at younger ages in order to minimize the adverse effects of castration. Recommendations on the appropriate age to castrate calves are inconsistent. Studies assessing the stress post castration at different ages suggests that castration of calves at a younger age is advantageous as they have a reduced cortisol response (King et al. Citation1991; Ting et al. Citation2005), reduced pain-related behaviours (Robertson et al. Citation1994), and increased weight gain (Petherick et al. Citation2015; Norring et al. Citation2017) compared with calves castrated at older ages. Conversely, other studies suggest that younger calves may be more sensitive to wound palpation and have increased wound healing time after castration compared with older calves (Norring et al. Citation2017). Previous studies that addressed the effects of age at castration were either limited to very young calves less than 2 months of age (Robertson et al. Citation1994) or had measured plasma cortisol too infrequently (n = 5 in the first 12 h after castration) to be reliably used as an indicator of acute stress in calves (King et al. Citation1991). Furthermore, most of the work to date has focused on castration of dairy bull calves, mainly older than 6-mo-old (Coetzee Citation2013) which makes it difficult to draw recommendations for beef farmers and veterinary practitioners concerning the best age to castrate suckler beef calves (Canozzi et al. Citation2017). The objective of the study was to compare the age-related differences in plasma cortisol, acute phase protein, metabolite concentrations, haematological variables, behaviour and performance of suckler beef calves in response to Burdizzo castration. The study hypothesis was that the acute stress, inflammatory reactions, and haematology changes caused by Burdizzo castration would be reduced by performing the procedure on younger calves.
2. Materials and methods
2.1. Animal ethics
All animal procedures performed in this study were reviewed and approved by the Teagasc Animal Ethics Committee (TAEC-147-2017) and were conducted under experimental licence from the Health Products Regulatory Authority, Dublin, Ireland (AE19132-P068) in accordance with the protection of animals used for scientific purposes i.e. Directive 2010/63/EU and S.I. No. 543 of 2012, as amended by S.I. No. 434 of 2013 and S.I. No. 174 of 2014.
2.2. Animal management and treatments
Forty crossbred (Aberdeen Angus, Charolais, Limousin and Simmental) beef calves from the suckler herd at the research centre were assigned to two age groups; 2.5- and 5.0-mo-old on the day of castration. The study was structured as a factorial design (2 × 2) with two ages at castration, and two castration treatments (). Calves were blocked within treatments by bodyweight, sire breed, dam breed, and dam parity.
Table 1. Calves and age per treatment.
All calf-dam pairs were grazed together at pasture before the experiment. Ten calves (5 castrated; 5 sham) were randomly selected each week of the experiment, starting with 2.5-mo-old calves (May/June) followed by the 5.0-mo-old calves (August). The timing of experimental procedures is detailed in .
Table 2. Description of experimental procedures and timing pre- and post-treatment.
At 48 h before treatment (castration or control), the calf-dam pairs (n = 10) were housed and penned together for an acclimatization period. Each calf-dam pair was allocated to a pen in which the dams were restrained in headlocks for 11 h each day with access to feed and water, and adequate space to lay down and stand up. Each calf was tethered behind it’s dam and had free access to suckle the dam and to feed. Treatments were applied at random across the 10 pens containing the dam-calf pairs.
These procedures were replicated during each of the 4 weeks of the study (10 calves per week). After the last blood sampling on the castration day (), the calf-dam pair were free in their respective pens until 48 h after castration.
Table 3. Blood samples sampling time for each variable collected relative to castration (0 h (h)).
2.3. Diet
For the first two weeks of the experiment, cows and 2.5-mo-old calves were zero-grazed. For two weeks prior to castration of the 5-mo-old calves, a change in diet was implemented as grass supply was affected due to the drought conditions, and cows and calves were fed grass silage for 2 weeks. Subsequently, 5.0-mo-old calves and their dams, when housed, had access to grass silage during the experiment.
2.4. Castration procedure
As part of the castration procedure, gentle manual restraint of the calves was used to facilitate the veterinarian to conduct the castration procedure. A closed castration procedure was performed by crushing the spermatic cord of each testis once for 10 s using a 23-cm Burdizzo clamp (Agrihealth Castrator; Agrihealth Ltd., Monaghan, Ireland) as described previously (Ting et al. Citation2003a). The control calves were sham-handled by holding the testes to the side of the scrotum, and they were restrained for the time normally required for completing the castration procedure (∼2 min/calf). The castration procedures were performed between 0900 and 0930 h on day 0.
2.5. Blood sample collection
Calves were fitted with indwelling jugular catheters as described in Ting et al. (Citation2003a) for all blood collections. The catheters were kept in place until the last blood sample was collected at 48 h post-castration. Heparinized blood samples (9 mL) were collected for plasma cortisol determination. Serum samples were collected in SST II (Serum Separator Tubes; 8.5 mL) for haptoglobin, non-esterified fatty acids (NEFA) and beta-hydroxybutyrate (BHB) determination. Whole blood was collected in K3-EDTA anticoagulant (Ethylene Diamine Tetra-acetic acid) (4.0 mL) for haematology profiles ().
Plasma was separated from blood, for cortisol analysis, by centrifugation at 1600 × g at 8°C for 15 min and subsequently stored at −20°C until assayed. Plasma cortisol concentrations were determined using the commercial cortisol ELISA kit (ADI-900-071, Enzo Life Sciences, Inc, Farmingdale, U.S.A.) according to the manufacturers’ instructions. The intra-assay and inter-assay coefficients of variation were 3.8% and 18.4%, respectively. Serum samples were separated from blood by centrifugation at 1600 × g at 8°C for 10 min and subsequently stored at −20°C until assayed. The concentration of serum haptoglobin, NEFA and BHB were measured using an automatic analyser (AU400, Beckman Coulter Ireland. Ltd, O’Callaghans Mills, Co. Clare, Ireland) and commercial assay kits (haptoglobin: Tridelta Development Ltd., Wicklow, Ireland; NEFA and BHB: Randox Laboratories, Crumlin, Antrim, UK). Unclotted whole K3-EDTA blood samples for haematological variables (white blood cell, neutrophil (N), lymphocyte (L), monocyte, basophil, and red blood cell numbers, haemoglobin concentration, haematocrit percentage and platelet number) were analysed using an ADVIA haematology analyser (AV ADVIA 2120, Bayer Healthcare, Siemens, UK) equipped with software for bovine blood, within 1–2 h of blood collection. The N:L ratio was also calculated.
2.6. Scrotal measurements
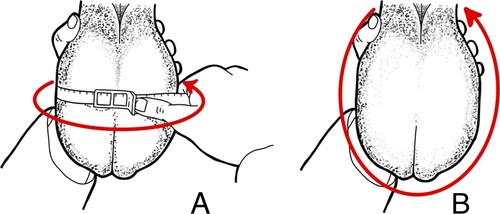
Scrotal latitudinal and longitudinal circumferences () were measured in cm using circumference measuring tape (Reliabull, Lane Manufacturing, Denver, CO) and a flexible tape (cm), respectively.
2.7. Rectal body temperature
Rectal body temperature was recorded using a digital thermometer (accuracy 0.1°C) (Jørgen Kruuse A/S model VT-801 BWC, Marslev, Denmark).
2.8. Behavioural assessment
A CCTV camera, connected to a network video recorder (DS-9608/9616/9632NI-ST; Hikvision, No.555 Qianmo Road, Binjiang District, Hangzhou, China), was installed 3 m above each pen and was set to record behaviour on a continuous basis. Pain-related behavioural observations () were conducted when the calves were in the pens, retrospectively, by reviewing video recordings.
Table 4. Ethogram used for categorizing behavioural observations of suckled beef calves after castration.
Behavioural observations were recorded by scan observations every 10 min for 3 h at the following time-spans: from 0500 h to 0800 h; from 1900h to 2200 h (T1) on the day of castration; and from 0500 h to 0800 h on the first (T2) and second (T3) days following treatment. Continuous behavioural observations were conducted for 3 h periods (T1: first hour, T2: second hour, T3: third hour) immediately after the treatment (castration or control) being applied to each calf, starting 1 min after the castration time (recorded previously). The behaviours from the video recordings were analysed by the same observer using a behavioural ethogram () adapted from previous castration studies (Robertson et al. Citation1994; Molony et al. Citation1995; Thüer et al. Citation2007; Boesch et al. Citation2008; Pieler et al. Citation2013; Lambertz et al. Citation2014). The data from the videos were coded using BORIS – Behavioural Observation Research Interactive Software (Friard and Gamba Citation2016).
The frequency of allo-grooming and self-grooming states were very low. Therefore, they were pooled in one analysis and results are showed as ‘grooming’. The frequency of lying lateral posture during scan observations was very low. Therefore, lying lateral and lying ventral abnormal were pooled in one analysis and results are represented as ‘lying abnormal’.
2.9. Growth performance
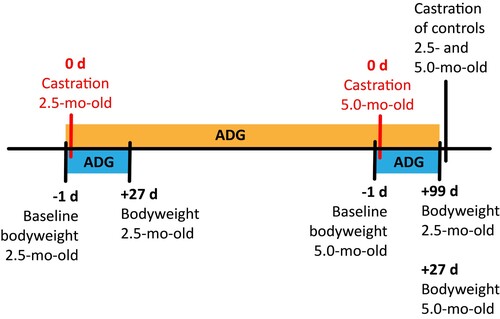
All calves were weighed at −1 d relative to castration (day 0) using a digital platform scale (accuracy 0.1 kg) (Gravitation, Meath, Ireland) for a baseline bodyweight. Calves in the control group were castrated 99 days (2.5-mo-old) and 27 days (5.0-mo-old) after treatment. A final bodyweight of calves was recorded at 27 days post treatment for each age group (). An additional bodyweight of 2.5-mo-old calves was recorded before castration of control calves 99 days after castration of this age group. An average daily gain (ADG) was calculated using the baseline (−1 d) and the final bodyweight (+27 d) for both age groups. An additional ADG was calculated for 2.5-mo-old calves using the baseline and bodyweight at 99 days after castration.
2.10. Statistical analysis
One calf and its data were excluded from the study because the calf and dam pair were very agitated at the planned time of castration, preventing the application of treatment.
A mean value of plasma cortisol concentration at baseline (−0.5, −0.25 and 0 h) relative to castration was used as baseline (0 h). For each animal, the interval to peak, increase to peak and the first peak cortisol concentration was recorded. The area under the cortisol response curve (area under the curve; AUC) was calculated from 0 to 4 h (primary response; AUC1), and from 4 to 9 h after treatment (secondary response; AUC2) using the linear trapezoidal rule as described by Ting et al. (Citation2003a). The difference in cortisol AUC was expressed as the difference between the AUC of the treatment group and the respective control from 0 to 4 h (dAUC1) and from 4 to 9 h (dAUC2). Data for scrotal circumference was expressed as the difference between the measurements taken at 24 and 48 h after treatment minus the pre-treatment baseline (−24 h).
All data were analyzed in accordance with the factorial nature of the design (2 × 2). Data for plasma cortisol, cortisol AUC, haptoglobin, NEFA and BHB concentration, haematology variables, body temperature, and scrotal measurements were analyzed using repeated measures mixed models ANOVA (PROC MIXED, SAS v 9.4) with an unstructured covariance matrix. Age, treatment, sampling time and their interactions were included as fixed effects.
Data for cortisol dAUC, peak, interval to peak, and ADG were analyzed using mixed models ANOVA (PROC MIXED, SAS v 9.4) with an unstructured covariance matrix. Age, treatment and their interactions were included as fixed effects.
There were 8 missing focal samples for behavioural analysis (4 control and 4 treatment calves 2.5-mo-old) due to technical issues with the cameras during the first 6 h after treatment. Data for behaviours (continuous and scans) were analyzed using repeated measures generalized linear mixed models ANOVA (PROC GLIMMIX, SAS v 9.4) with a compound symmetry covariance matrix. Data for scan observations and events (counts) were analyzed with a specified Poisson distribution. Age, treatment, sampling time and their interactions included as fixed effects. The state grooming, the posture lying lateral, the events head turning and kicking (continuous observations), and the frequency of standing abnormal behaviours (scans observations) occurred too infrequently for statistical analysis.
Animal was the experimental unit in all analyses. The main effects of week, dam parity and calf breed were tested previously in all models. No effect was found for any of the variables measured; therefore, they were not included in the final model. All data were examined for adherence to a normal distribution (PROC UNIVARIATE, SAS v 9.4). Variables that were not normally distributed (plasma cortisol concentration data) were log transformed. Data subjected to transformations were used for analysis and hypothesis testing. The corresponding non-transformed least squares means (Lsmeans) with standard error of the mean (SEM) are presented to facilitate interpretation of results. Differences between the means were tested using the PDIFF option and a Tukey post hoc analysis was employed. Means were considered significantly different at a probability level of P < 0.05.
3. Results
3.1. Plasma cortisol concentration
The total cortisol concentration increased after treatment until 0.5 h and decreased until 4 h post treatment (primary response) (; ). There was a secondary increase in plasma cortisol concentration 4 h after treatment, which remained high until at least 9 h post castration (second response) (; ). Calves 5.0-mo-old had a greater cortisol concentration than 2.5-mo-old calves, regardless of castration treatment, from baseline (0 h) to 4 h and from 4 h to 9 h after treatment. In addition, castrated calves had a greater cortisol concentration than control calves, regardless of age ( and ).
Figure 3. Effect of Burdizzo castration at 2.5- and 5.0-mo-old on the difference in AUC (dAUC) plasma cortisol concentrations in calves. The area under the cortisol response curve (area under the curve; AUC) was calculated from 0 to 4 h (primary response; AUC1), and from 4 to 9 h after treatment (secondary response; AUC2) using the linear trapezoidal rule as described by Ting et al. (Citation2003a). The difference in cortisol AUC was expressed as the difference between the AUC of the treatment group and the respective control from 0 to 4 h (dAUC1) and from 4 to 9 h (dAUC2).
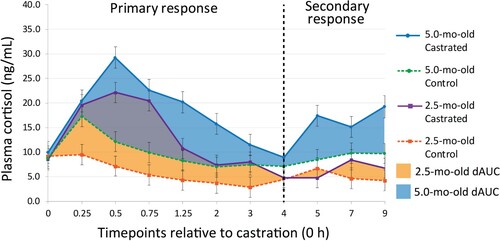
Table 5. Effect of Burdizzo castration at 2.5- and 5.0-mo-old on changes in plasma cortisol concentrations in calves during primary response, from 0 to 4 h post treatment.
Table 6. Effect of Burdizzo castration at 2.5- and 5.0-mo-old on changes in plasma cortisol concentrations in calves during secondary response, from 4 to 9 h post treatment.
Before treatment, there was no difference in plasma cortisol concentration among all groups at baseline (0 h) (P < 0.05). There was a treatment effect on interval to peak (), which was slightly longer for castrated calves (0.6 (0.07) h) compared with control calves (0.4 (0.07) h) (P < 0.01).
Table 7. Effect of Burdizzo castration at 2.5- and 5.0-mo-old on changes in plasma cortisol concentrations in calves.
There was an age and a treatment effect on the peak cortisol concentration and increase to peak. Cortisol peak and increase to peak were increased in calves castrated at 2.5-mo-old and in control and castrated calves 5.0-mo-old compared with 2.5-mo-old control calves ().
The adjusted area under the curve from 0 to 4 h (AUC1) and from 4 to 9 h (AUC2) was greater in castrated calves 5.0-mo-old compared with 2.5-mo-old castrates (P < 0.05). In addition, 5.0-mo-old control calves had a greater AUC1 and AUC2 compared with 2.5-mo-old control calves (P < 0.05) ().
Table 8. Effect of Burdizzo castration of 2.5- and 5.0-mo-old calves on the adjusted area under the curve from 0 to 4 h (AUC1) and from 4 to 9 h (AUC2) for plasma cortisol concentration.
The relative difference in the area under the curve from 0 to 4 h (dAUC1) was not different between 2.5- and 5.0-mo-old calves. However, the relative difference (dAUC2) was greater in 5-mo-old calves than 2.5-mo-old from 4 to 9 h post treatment (; ).
Table 9. Effect of Burdizzo castration at 2.5- and 5.0-mo-old in difference in the area under the curve (AUC) of cortisol concentration in calves.
3.2. Scrotal circumference
Before treatment (−24 h), the mean latitudinal scrotal circumferences was greater for 5.0-mo-old calves compared with 2.5-mo-old calves (P < 0.0001). Both latitudinal and longitudinal scrotal circumferences were increased in castrated calves at 24 and 48 h after treatment compared with their respective baseline. Calves castrated at 5.0-mo-old had a greater increase in latitudinal scrotal circumference at 24 and 48 h compared with 2.5-mo-old castrates (). No differences were observed in longitudinal scrotal circumference between castrated calves 2.5- and 5.0-mo-old.
Table 10. Effect of Burdizzo castration at 2.5- and 5.0-mo-old on the changes in latitudinal and longitudinal scrotal circumferences relative to pre-treatment baseline.
3.3. Behavioural changes - continuous observations
3.3.1. States
There were main effects of treatment and time on time spent standing (). Castrated calves spent more time standing compared with control calves (P < 0.05). Post-treatment, calves spent more time standing during the first hour after treatment (P < 0.001), regardless of the treatment. Duration spent lying was only affected by time (P < 0.001) which was decreased in the first hour after treatment. Although there was no effect of age, treatment or time on time spent eating, the age at castration affected time spent ruminating (P < 0.05). Calves 5.0-mo-old spent more time ruminating compared with 2.5-mo-old calves.
Table 11. Effect of Burdizzo castration at 2.5- and 5.0-mo-old on behaviour of calves during 3 h of continuous observations post treatment.
3.3.2. Postures
Time spent standing in abnormal position was affected by an age × treatment × time interaction (P < 0.001) (). There was no difference in time spent standing in abnormal position between 2.5- or 5.0-mo-old control calves for any sampling time (P > 0.05). However, calves castrated at 5.0-mo-old spent more time standing in abnormal position (928 (47.6) s) compared with calves castrated at 2.5-mo-old (358 (71.4) s), in the first hour post treatment (P < 0.0001). No significant differences were found at 2 and 3 h after treatment (P > 0.05). There were no differences in time spent in standing abnormal position between control and castrated 2.5-mo-old calves at any sampling time or across time (P > 0.05).
There was a treatment × time interaction on time spent lying in ventral abnormal posture (P < 0.01). Castrated calves spent more time lying in ventral abnormal posture (643 (42.9) s) compared with control calves (88 (42.9) s) in the first hour after treatment (P < 0.0001). However, no differences were found between treatments after this time (P > 0.05). Age at castration also had an effect on time spent lying in ventral abnormal posture (P < 0.01). Calves 2.5-mo-old spent more time lying in ventral abnormal position compared with 5.0-mo-old calves. Time spent lying in ventral normal position was affected by treatment (P < 0.05) and by time (P < 0.001). Time spent lying in ventral normal position was greater in control calves compared with castrated calves; and also greater during the first hour after castration, regardless of the treatment.
Duration spent standing in normal position was affected only by time (P 0.001). Calves spent more time standing in normal position in the first hour after treatment, regardless of the treatment.
3.3.3. Events
Number of foot stamping was affected by treatment (P < 0.0001) and time (P < 0.01). Castrated calves showed greater number of foot stamping compared with control calves, regardless of the age at castration. In addition, a greater number of foot stamping events was observed in the first hour after treatment.
The occurrence of tail wagging events was affected by age (P < 0.001). Calves 5.0-mo-old showed more tail wagging events compared with 2.5-mo-old calves.
3.4. Behavioural changes - scan observations
There was no effect of treatment or age on any of the behavioural variables observed before treatment, from 0500 h to 0800 h (data not shown).
3.4.1. States
There was an age × time interaction in the number of eating (P < 0.001), standing (P < 0.001), and grooming (P < 0.05) behaviours, but no effect of treatment (). Calves 5.0-mo-old showed more states of eating, grooming, and standing in the first sampling time compared with 2.5-mo-old calves. In addition, eating states were more frequent in calves 5.0-mo-old in the second day after treatment (T3).
Table 12. Effect of Burdizzo castration at 2.5- and 5.0-mo-old on behaviour of calves during scan observations (3 h blocks).
There was a treatment × time interaction in the number of lying and ruminating behaviours (P < 0.05), however, no differences were observed between treatments within each sampling time. Age at castration had an effect on frequencies of lying and ruminating behaviours (P < 0.0001), which were greater in 5.0-mo-old compared with 2.5-mo-old calves.
3.4.2. Postures
There was a main effect of age in the frequency of standing normal postures (P < 0.0001), which was greater in 5.0-mo-old calves compared with 2.5-mo-old calves. Frequency of lying in abnormal posture was affected by age (P < 0.0001) and treatment (P < 0.01), but no interaction was found. Lying in abnormal posture was more frequent in 2.5-mo-old calves compared with 5.0-mo-old calves, as well as in castrated calves compared with control calves. Frequency of lying in ventral normal position was not affected by age, treatment or time (P > 0.05).
3.5. Haematological profiles
There was a main effect of time on white blood cell, platelet and basophil number, and plasma haemoglobin concentration (). A treatment × time interaction was detected for red blood cell (P < 0.01), neutrophil (P = 0.01), neutrophil:lymphocyte ratio (P < 0.05) and haematocrit percentage (P < 0.01). However, no differences were found between control and castrated calves within each sampling time for any of those variables (P > 0.05). There was an age × time interaction on lymphocytes (P < 0.05) and monocyte (P < 0.01) number, but no castration effect (P > 0.05). However, no differences were found between 2.5- and 5.0-mo-old calves within each sampling time for any of those variables (P > 0.05).
Table 13. Effect of Burdizzo castration at 2.5- and 5.0-mo-old on changes on haematological profiles of bull calves.
3.6. Haptoglobin and metabolites
No changes were found in plasma haptoglobin concentration from −0.5–48 h (). There was a main effect of time for NEFA and BHB concentrations (). Plasma NEFA concentration was greater at 0.5 h post treatment and lower at 24 and 48 h, compared with baseline (P < 0.001). Plasma BHB concentration was lower at 48 h (P < 0.001).
Table 14. Effect of Burdizzo castration at 2.5- and 5.0-mo-old on changes on serum haptoglobin, non-esterified fatty acids (NEFA) and beta-hydroxybutyrate (BHB) concentrations in calves.
3.7. Body temperature
Only main effects of age (P = 0.05) and time were observed (P < 0.001) for body temperature. Calves at 2.5-mo-old had slightly greater body temperature (39.1 (0.08) °C) compared with 5.0-mo-old calves (38.8 (0.08); P = 0. 05). Body temperature was also slightly greater 24 h (39.0 (0.07)) (P < 0.05) and 48 h (39.1 (0.07)) (P < 0.001) after castration compared with 24 h before castration (38.7 (0.07)). Three castrated animals (one animal castrated at 2.5-mo-old at 24 h; one castrated and one control at 5.0-mo-old, both at 48 h) had rectal temperatures greater than 40°C after treatment.
3.8. Average daily gain
There was a main effect of age on ADG from d −1 to d 27. Calves 5.0-mo-old had greater ADG (1.6 (0.07) kg/d) compared with 2.5-mo-old calves (1.3 (0.07) kg/d; P < 0.05). However, no effect of treatment was found (P > 0.05). The ADG was 1.5 and 1.4 (0.07) kg/d for control and castrated calves, respectively. There was no effect of castration on 2.5-mo-old calves ADG from d −1 to d 99 (P > 0.05). The ADG was 1.4 (0.05) for both control and castrated calves.
4. Discussion
Plasma cortisol concentration
Circulating plasma cortisol concentration is commonly measured to assess stress and pain in animals (Thun et al. Citation1981; Molony and Kent Citation1997; Moberg and Mench Citation2000; Mormède et al. Citation2007; Prunier et al. Citation2013). It is suggested that the maximum cortisol plasma concentration post castration could be reduced by castrating animals at younger ages, regardless of the castration method (Bretschneider Citation2005). The cortisol area under the curve (AUC) represents the integrated cortisol response over a given time period. In the present study, Burdizzo castration was shown to be stressful across both ages, as indicated by the increase in cortisol response (AUC) for the first 4 h after treatment (primary response) relative to control animals. A secondary response was observed from 4 to 9 h after treatment in 5.0-mo-old calves, in which the AUC induced by castration was 1.9 times greater in castrates and persisted up to 9 h post treatment. Similarly to the present study, Ting et al. (Citation2005) found a marked reduction on cortisol AUC from 0 to 3 h after Burdizzo castration in 1.5- and 4.5-mo-old Holstein bull calves (54% and 34%, respectively) compared to 5.5-mo-old castrates. In their study, however, they were only able to compare the effect of age at castration only between castrates. In the present study, subtracting the effect of handling (sham castration), there was no difference in the relative difference in cortisol response (dAUC) for the first 4 h after treatment (primary response) of 2.5- and 5.0-mo-old calves. However, a secondary response was observed, from 4 to 9 h after treatment, in which the dAUC induced by castration was 1.8 times greater in 5.0-mo-old castrates and persisted up to 9 h post treatment. These findings suggest that the effect of castration alone on the primary cortisol response is not different in 2.5- and 5.0-mo-old calves. However, handling older calves contributed to the stress response in those animals, as observed by the increased cortisol response in 5.0-mo-old control calves. In fact, it is suggested that young calves may be less distressed by handling and castration compared to older calves (Dockweiler et al. Citation2013). Stressful events performed at the same time (e.g. castration and dehorning) cause an additional stress response when performed together (Sutherland et al. Citation2013). Likewise, the stress response induced by handling older calves may accentuate the stress caused by castration, which could in turn increase the cortisol response. King et al. (Citation1991) reported that the cortisol responses to surgical or Burdizzo castration were reduced in beef calves castrated at 78- compared to 167-d-old. This difference could be due to additional stressors imposed on calves in their study as older calves had an elevated baseline cortisol concentration due to handling, restraint and temporary removal from their dams. It is possible that this pre-treatment management could have resulted in a cumulative stress response, especially in older calves. In the present study, calves were also tethered by a head halter in close proximity to their dams during castration and sampling. While the calves had access to their dams for suckling, there was no separation of the cow-calf pair during the 10 h sampling period. The findings suggest that older calves were experiencing more stress due to restraint, handling and castration and over a longer time period compared with younger calves. These findings should be considered when deciding when to castrate calves and the timing for anesthetic and/or analgesics administration. The use of analgesics needs to be planned to avoid the prolonged secondary cortisol response in older calves, depending on the onset of action and efficacy of the medicine being used.
Scrotal swelling following tissue trauma is a sign of local inflammation (Sidhu et al. Citation2003). In the present study, scrotum swelling was observed in both 2.5- and 5.0-mo-old castrated calves up to 48 h after the procedure, and it was more pronounced in older calves. An increase in scrotal circumference was expected since swelling following Burdizzo castration was observed in previous studies (Ting et al. Citation2005; Pang et al. Citation2008). In agreement with the present study, a reduced swelling was observed in Holstein calves castrated by Burdizzo at 1.5-mo-old compared with 5.5-mo-old castrates (Ting et al. Citation2005). However, they found no differences in scrotal swelling when calves were castrated at 2.5-, 3.5-, 4.5-mo-old. Possibly the swelling may be reduced in very young calves since they had the least genital tissue development and consequently reduced damaged tissue and resultant inflammation, compared to older calves. Performing Burdizzo castration at younger ages may produce less swelling, which might be contributing to tissue healing and to reduced local pain.
Observation of individual behaviours has previously been used to measure pain following castration (Robertson et al. Citation1994; Molony et al. Citation1995; Ting et al. Citation2003a, Citation2003b, Citation2010; Thüer et al. Citation2007; Petherick et al. Citation2015). An increase in time spent standing abnormally is a sign of acute stress observed following Burdizzo castration (Molony et al. Citation1995) and a rise in the frequency of this behaviour can be observed in the first hour after castration (Robertson et al. Citation1994). In the present study, Burdizzo castration at 2.5-mo-old did not increase the time spent standing in abnormal position. However, this behaviour was present in calves castrated at 5.0-mo-old and their frequency in the first hour after the procedure was 2.6 times greater in calves castrated at 5.0-mo-old compared to 2.5-mo-old castrates. In contrast to the present findings, time spent standing abnormally was greater in calves Burdizzo castrated at 1-wk-old during 3 h post castration (Molony et al. Citation1995). Robertson et al. (Citation1994) also reported a sharp rise in abnormal standing behaviour which was sustained for 24 min in calves castrated by Burdizzo at 6 days old, while castrated calves at 21 and 42 days showed observations of abnormal standing up to 120 and 180 min following the procedure. Similarly, Ting et al. (Citation2003a) observed a greater incidence of abnormal standing posture in 13-mo-old Burdizzo castrated animals than in control calves, and the use of local anaesthetic or analgesia reduced, but not completely, the incidence of those behaviours. It is not clear why the young calves in the present study did not show an increase in abnormal behaviour immediately after castration. It is possible that the presence of the dam may attenuate pain-related behaviours in younger calves, but not in older. If this increase in abnormal standing seen in older calves indicates that they suffered more pain than younger calves, then this is evidence that the Burdizzo castration is less stressful for younger calves.
In this study, control calves spent 86% less time lying in abnormal posture than castrated calves in the first 3 h after treatment, regardless of the age group. Molony et al. (Citation1995) found no differences in abnormal lying after Burdizzo castration of 7-day-old calves during 48 d of observations. In addition, few abnormal lying behaviour was observed in calves 6, 21 and 42 days of age following Burdizzo castration (Robertson et al. Citation1994). In this study, compared to control calves, castrates spent more time standing. This is in agreement with Lambertz et al. (Citation2014) who observed an increase in time spent standing/walking in 7-mo-old calves castrated by Burdizzo compared with control calves. Ting et al. (Citation2003b) also observed a greater incidence of standing postures in Burdizzo castrated animals at 13-mo-old compared with controls.
The increased frequency of behaviours such as tail wagging, foot stamping and kicking may be considered as attempts to escape from or remove a painful stimulus (Robertson et al. Citation1994). In the present study, castrated calves of both ages showed a greater number of foot stamping events compared with control calves within 3 h after treatment. Similarly, foot stamping was frequently observed in the first 30 min after Burdizzo castration of calves at 6, 21 and 42 days of age (Robertson et al. Citation1994), as well as in bulls castrated by Burdizzo at 13-mo-old compared with controls (Ting et al. Citation2003b).
An increased frequency of tail wagging has previously been observed following castration (Fisher et al. Citation2001; Sutherland et al. Citation2002, Citation2013) and is suggested to be due to irritation or pain (Fisher et al. Citation2001; Sylvester et al. Citation2004; Sutherland et al. Citation2013). However, we found no difference in tail wagging frequency between castrated and control calves, only between age groups. Tail wagging in cattle might be also a sign of discomfort other than pain. All calves in the present study were tethered and frequently blood sampled during the first 9 h after treatment. This experimental condition might have caused discomfort and irritability in the calves, which can explain the greater frequency of tail wagging in older calves but no differences between castration treatments.
The classical stress response is also accompanied by an increase in haematocrit % and neutrophil number (neutrophilia) and a decrease in lymphocyte number (lymphopaenia) (Hulbert and Moisá Citation2016). The neutrophil:lymphocyte ratios (NLR) is also used as a welfare indicator since alterations in the blood leukogram is an indicator of inflammation, disease, distress, and painful events in cattle (Broom Citation2000), and sheep (Aguayo-Ulloa et al. Citation2014). Burdizzo castration of calves is reported to cause leukocytosis primarily due to neutrophilia (Pang et al. Citation2008). In the present study, red blood cell number, haematocrit %, neutrophil number and NLR were slightly increased in castrated calves regardless of the age but remained within the normal physiological range for cattle (Knowles et al. Citation2000). These findings indicate that the immune system of calves was not compromised, at least until 2 d after castration. This is in agreement with previous studies which found no changes in haematological profiles following Burdizzo castration (Ting et al. Citation2003a; Pang et al. Citation2006, Citation2008). In contrast to the findings of the present study, Ting et al. (Citation2005) found leukocytosis on day 2 in Holstein calves castrated by Burdizzo at 1.5-, 2.5-, 3.5-, 4.5- and 5.5-mo-old and white blood cell count was greater on day 2 in 1.5-, 2.5-, 4.5- and 5.5-mo-old castrates.
The concentration of acute-phase proteins, such as haptoglobin, increase as part of the inflammatory cascade associated with tissue damage or in response to a stressor. In previous studies, an increase in plasma haptoglobin concentration was reported within 1–3 days after castration in young animals (Fisher et al. Citation1997; Earley and Crowe Citation2002; Ting et al. Citation2003b; Pang et al. Citation2006). Although swelling was observed in the present study, no effect of age or castration on serum haptoglobin concentrations up to 48 h after castration was observed. The present finding suggests that the stress induced by Burdizzo castration did not produce an acute inflammatory response by 48 h after the procedure.
The concentration of non-esterified fatty acids (NEFA), a negative energy balance biomarker that increases when the supply of glucose is not sufficient to the animal’s current energy requirements (Adewuyi et al. Citation2005), can be used as a welfare biomarker (Aguayo-Ulloa et al. Citation2014). Burdizzo castration did not affect NEFA and BHB concentrations of calves in both age groups in the present study. Likewise, Pang et al. (Citation2008) found no difference in plasma BHB concentrations in 12-mo-old calves after band and Burdizzo castration 28 d post castration. However, NEFA concentrations were reduced in band and Burdizzo castrated calves. Similar to our study, no differences were observed in plasma NEFA concentrations following surgical castration of calves after weaning up to 72 h post castration (Roberts et al. Citation2015). Furthermore, a change in the diet of the calves in the older group was necessary in the present study, and yet, there were no differences in plasma NEFA and BHB concentrations between the two ages. In addition, serum NEFA and BHB concentrations were within normal reference ranges (Knowles et al. Citation2000). This indicates that the metabolic needs of the calves were met even after the changes in feed and after castration.
There were no major changes in rectal body temperature of calves during the study. Even though there was a difference in body temperature between the two ages, the body temperature was within the normal range of 38–39°C (Bellows R and Lammoglia Citation2000). Similarly, Roberts et al. (Citation2015) reported no change in rectal temperature up to 72 h post surgical castration and sham handling of calves. Pieler et al. (Citation2013) found no increase in rectal temperature of 56 d old calves following surgery, Burdizzo or orchidectomy castration.
In the present study, Burdizzo castration did not affect ADG in both age groups up to 27-d or on 2.5-mo-old calves up to 99 d post treatment. Previous studies reported no difference in ADG (King et al. Citation1991; Pang et al. Citation2006, Citation2008) and lower ADG (Ting et al. Citation2003b) associated with Burdizzo castration compared to sham castrated calves. Moreover, Ting et al. (Citation2005) found that there was no effect of Burdizzo castration on the overall 42-day growth trends of calves castrated at different ages (1.5-5.5-mo-old). The findings of the present study showed that Burdizzo castration had no impact on growth performance.
5. Conclusion
The increased plasma cortisol concentration following castration observed in this study suggests that Burdizzo castration is stressful for both 2.5- and 5.0-mo-old suckler beef calves. However, an increase in abnormal postures, scrotal swelling and a prolonged plasma cortisol response observed in older calves suggest that these calves suffered more stress induced by handling and castration. Therefore, castration of younger suckler calves is preferable from an animal welfare point of view, although pain relief is also recommended.
These age-related differences in stress response found in this study need to be considered when recommending the use of anaesthesia and analgesia during castration, especially for older calves. The timing of pain relief administration should be planned to effectively reduce the stress response according to the age of the calf. Further research investigating the efficacy of anaesthesia and analgesia to mitigate pain during Burdizzo castration of suckler calves at different ages is needed.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Adewuyi AA, Gruys E, van Eerdenburg FJ. 2005. Non esterified fatty acids (NEFA) in dairy cattle. A review. Vet Q. 27:117–126.
- Aguayo-Ulloa LA, Villarroel M, Lonso MPA, La Lama GCMD, María GA. 2014. Finishing feedlot lambs in enriched pens using feeder ramps and straw and its influence on behavior and physiological welfare indicators. J Vet Behav. 9:347–356.
- Bellows R A, Lammoglia MA. 2000. Effects of severity of dystocia on cold tolerance and serum concentrations of glucose and cortisol in neonatal beef calves. Theriogenol. 53:803–813.
- Boesch D, Steiner A, Gygax L, Stauffacher M. 2008. Burdizzo castration of calves less than 1-week old with and without local anaesthesia: short-term behavioural responses and plasma cortisol levels. Appl Anim Behav Sci. 114:330–345.
- Bretschneider G. 2005. Effects of age and method of castration on performance and stress response of beef male cattle: A review. Livest Prod Sci. 97:89–100.
- Broom DM. 2000. Welfare assessment and problem areas during handling and transport. In: Grandin T, editor. Livestock handling and transport. 2nd ed.. Wallingford, CABI Publishing; p. 43–61.
- Canozzi ME, Mederos AA, Manteca X, Turner S, McManus C, Zago D, Barcellos JOJ. 2017. A meta-analysis of cortisol concentration, vocalization, and average daily gain associated with castration in beef cattle. Res. Vet. Sci. 114:430–443.
- Coetzee JF. 2013. Assessment and management of pain associated with castration in cattle. Vet Clin North Am Food Anim Pract. 29:75–101.
- Dockweiler JC, Coetzee JF, Edwards-Callaway LN, Bello NM, Glynn HD, Allen KA, Theurer ME, Jones ML, Miller KA, Bergamasco L. 2013. Effect of castration method on neurohormonal and electroencephalographic stress indicators in Holstein calves of different ages. J Dairy Sci. 96:4340–4354.
- Earley B, Crowe MA. 2002. Effects of ketoprofen alone or in combination with local anesthesia during the castration of bull calves on plasma cortisol, immunological, and inflammatory responses. J Anim Sci. 80:1044–1052.
- Fisher AD, Crowe MA, Nualláin EM Ó, Monaghan ML, Prendiville DJ, O’Kiely P, Enright WJ. 1997. Effects of suppressing cortisol following castration of bull calves on adrenocorticotropic hormone, In vitro interferon-γ production, leukocytes, acute-phase proteins, growth, and feed intake. J Anim Sci. 75:1899–1908.
- Fisher AD, Knight TW, Cosgrove GP, Death AF, Anderson CB, Duganzich DM, Mattews LR. 2001. Effects of surgical or banding castration on stress responses and behaviour of bulls. Aust Vet J. 79:279–284.
- Friard O, Gamba M. 2016. BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol Evol. 7:1325–1330.
- Hulbert LE, Moisá SJ. 2016. Stress, immunity, and the management of calves 1. J Dairy Sci. 99:3199–3216.
- King BD, Cohen RDH, Guenther CL, Janzen ED. 1991. The effect of age and method of castration on plasma cortisol in beef calves. Can J Anim Sci. 770:257–263.
- Knowles TG, Edwards LE, Bazeley KJ, Brown SN, Butterworth A, Warriss PD. 2000. Changes in blood biochemical and haematological profile of neonatal calves with age. Vet. Rec. 147:593–598.
- Lambertz C, Farke-Röver A, Moors E, Gauly M. 2014. Comparison of the effects of weaning and castration when conducted separately or in combination on the behaviour of crossbred beef cattle. Appl Anim Behav Sci. 161:28–33.
- Mahmood S, Roy BC, Larsen IL, Aalhus JL, Dixon WT, Bruce HL. 2017. Understanding the quality of typical and atypical dark cutting beef from heifers and steers. Meat Sci. 133:75–85.
- Moberg GP, Mench JA. 2000. The biology of animal stress: basic principles and implications for animal welfare. 1st ed. (G. P. Moberg and J. A. Mench, editors.). Davis: CABI Publishing.
- Molony V, Kent JE. 1997. Assessment of acute pain in farm animals using behavioral and physiological measurements. J Anim Sci. 75:266–272.
- Molony V, Kent JE, Robertson IS. 1995. Assessment of acute and chronic pain after different methods of castration of calves. Appl Anim Behav Sci. 46:33–48.
- Mormède PS, Andanson B, Aupérin B, Beerda D, Guémené J, Malmkvist X, Manteca G, Manteuffel P, Prunet CG, van Reenen K, et al. 2007. Exploration of the hypothalamic–pituitary–adrenal function as a tool to evaluate animal welfare. Physiol Behav. 92:317–339.
- Norring M, Mintline EM, Tucker CB. 2017. The age of surgical castration effects the healing process in beef calves. Transl Anim Sci. 1:1–21.
- Pang WY, Earley B, Gath V, Crowe MA. 2008. Effect of banding or burdizzo castration on plasma testosterone, acute-phase proteins, scrotal circumference, growth, and health of bulls. Livest Sci. 117:79–87.
- Pang WY, Earley B, Murray M, Sweeney T, Gath V, Crowe MA. 2011. Banding or Burdizzo castration and carprofen administration on peripheral leukocyte inflammatory cytokine transcripts. Res Vet Sci. 90:127–132.
- Pang WY, Earley B, Sweeney T, Crowe MA. 2006. Effect of carprofen administration during banding or burdizzo castration of bulls on plasma cortisol, in vitro interferon- γ production, acute-phase proteins, feed intake, and growth. J Anim Sci. 84:351–359.
- Petherick JC, Small AH, Reid DJ, Colditz IG, Ferguson DM. 2015. Welfare outcomes for 3- and 6-month-old beef calves in a tropical environment castrated surgically or by applying rubber rings. Appl Anim Behav Sci. 171:47–57.
- Pieler D, Peinhopf W, Becher AC, Aurich JE, Rose-Meierhöfer S, Erber R, Möstl E, Aurich C. 2013. Physiological and behavioral stress parameters in calves in response to partial scrotal resection, orchidectomy, and Burdizzo castration. J Dairy Sci. 96:6378–6389.
- Prunier A, Mounier L, Le Neindre P, Leterrier C, Mormède P, Paulmier V, Prunet P, Terlouw C, Guatteo R. 2013. Identifying and monitoring pain in farm animals: A review. Animal. 7:998–1010.
- Roberts SL, Hughes HD, Burdick Sanchez NC, Carroll JA, Powell JG, Hubbell DS, Richeson JT. 2015. Effect of surgical castration with or without oral meloxicam on the acute inflammatory response in yearling beef bulls. J Anim Sci. 93:4123–4131.
- Robertson IS, Kent JE, Molony V. 1994. Effect of different methods of castration on behaviour and plasma cortisol in calves of three ages. Res Vet Sci. 56:8–17.
- Sidhu P, Shojaee Aliabadi F, Andrews M, Lees P. 2003. Tissue chamber model of acute infl ammation in farm animal species. Res Vet Sci. 74:67–77.
- Stafford KJ, Mellor DJ. 2005. The welfare significance of the castration of cattle: A review. N. Z Vet J. 53:271–278.
- Stafford KJ, Mellor DJ. 2011. Addressing the pain associated with disbudding and dehorning in cattle. Appl Anim Behav Sci. 135:226–231.
- Stafford KJ, Mellor DJ, Todd SE, Bruce RA, Ward RN. 2002. Effects of local anaesthesia. or local anaesthesia plus a non-steroidal anti-inflammatory drug on the acute cortisol response of calves to five different methods of castration. Res Vet Sci. 5288:61–70.
- Sutherland MA, Ballou MA, Davis BL, Brooks TA. 2013. Effect of castration and dehorning singularly or combined on the behavior and physiology of Holstein calves. J Anim Sci. 91:935–942.
- Sutherland MA, Mellor DJ, Stafford KJ, Gregory NG, Bruce RA, Ward RN. 2002. Effect of local anaesthetic combined with wound cauterisation on the cortisol response to dehorning in calves. Aust Vet J. 80:165–167.
- Sylvester SP, Stafford KJ, Mellor DJ, Bruce RA, Ward RN. 2004. Behavioural responses of calves to amputation dehorning with and without local anaesthesia. Aust Vet J. 82:697–700.
- Thüer S, Mellema S, Doherr MG, Wechsle B, Nuss K, Steiner A. 2007. Effect of local anaesthesia on short- and long-term pain induced by two bloodless castration methods in calves. Vet J. 173:333–342.
- Thun R, Eggenberger E, Zerobin K, Lüscher T, Vetter W. 1981. Twenty-four-hour secretory pattern of cortisol in the bull: evidence of episodic secretion and circadian rhythm. Endocrinology. 109:2208–2212.
- Ting STL, Earley B, Crowe MA. 2003a. Effect of repeated ketoprofen administration during surgical castration of bulls on cortisol, immunological function, feed intake, growth, and behavior. J Anim Sci. 81:1253–1264.
- Ting STL, Earley B, Hughes JML, Crowe MA. 2003b. Effect of ketoprofen, lidocaine local anesthesia, and combined xylazine and lidocaine caudal epidural anesthesia during castration of beef cattle on stress responses, immunity, growth, and behavior. J Anim Sci. 81:1281–1293.
- Ting STL, Earley B, Veissier I, Gupta S, Crowe MA. 2005. Effects of age of Holstein-Friesian calves on plasma cortisol, acute-phase proteins, immunological function, scrotal measurements and growth in response to Burdizzo castration. Anim Sci. 80:377–386.
- Ting STL, Earley B, Veissier I, Gupta S, Crowe MA. 2010. Effects of Burdizzo castration on CO2 laser induced thermal nociception of Holstein–Friesian calves of different ages. Appl. Anim. Behav. Sci. 126:12–18.