?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This study evaluated the effect of Opuntia ficus-indica L. supplementation on gilts during late gestation and lactation, particularly on their biochemical parameters and voluntary feed intake (VFI) at lactation. Thirty-two gilts were randomly distributed into two groups: control group (CG), gilts fed conventionally and experimental group (EG), gilts fed commercial feed plus O. ficus-indica. Glucose concentration was lower (P < 0.05) in the EG. Insulin concentration was higher in the gilts that consumed O. Ficus-indica. Triglyceride’s concentration was lower (P < 0.05) at gestation, farrowing and lactation in the EG. Total cholesterol was higher (P < 0.05) in gestation and lactation in the CG. HDL present higher concentration in lactation in the gilts that consumed O. Ficus-indica. LDL concentration was higher (P < 0.05) in gestation and lactation in the CG. Leptin concentration was higher (P < 0.05) at lactation in the CG. The gilts of the EG had higher VFI (22.6% more) and presented less body weight loss (3.7% less) at weaning (P < 0.05). These findings suggest that O. Ficus-indica favourably regulates the biochemical indicators involved in the development of insulin resistance, and this is reflected in the higher VFI and lesser body weight loss at weaning.
1. Introduction
Insulin resistance (IR) is a metabolic disorder that involves decreases in cellular sensitivity towards insulin and predisposes to hyperglycemia and dyslipidemia (Unger et al. Citation2014). However, in sows, like most female mammals IR is a metabolic adaptation at late gestation and lactation that allows to direct a higher input of nutrients to the uterus (for the higher exponential growth of the fetus) and the udder (to start milk production) (Père and Etienne Citation2007, Citation2018). The importance of IR in sows is that it has an effect on the sow’s productivity, specifically on feed intake in lactation (Père and Etienne Citation2007; Manu et al. Citation2020). Because the effect of IR was observed in lower feed intake, during late gestation this effect is not perceived, since, during this period, feeding schemes are based on sow's restricted feeding, in contrast to ad libitum feeding at lactation (Solà-Oriol and Gasa Citation2017). Also, leptin has no effect on feed intake in gestation since there is resistance to leptin (hyperleptinemia) for a higher input of nutrients to the uterus (Tessier et al. Citation2013). However, in lactation, sows do not experience leptin resistance (Cools et al. Citation2014). Some studies show that there is an increase in the serum leptin concentration gradually until the middle third of the gestation, reaching a high concentration and remaining high until farrowing in swine females (Saleri et al. Citation2015). It was reported that backfat depths were positively associated with leptin concentrations. However, no direct relationship between levels of circulating leptin and sow fertility, if leptin is involved in the control of reproduction, its role is merely permissive. E.g. through the association that leptin has on reduced feed intake in lactating sows. The lower feed intake promotes catabolism of the sow and modifies its metabolic state. This puts at risk the synthesis of hormones involved in reproduction: LH, FSH, IGF-I, estrogens, etc. (De Rensis et al. Citation2005; Mosnier, Etienne, et al. Citation2010; Solé et al. Citation2021).
For the reasons described above, strategies that maximize feed intake in lactating sows should be sought. It has been reported (Serena et al. Citation2007; Quesnel et al. Citation2009; Jha and Berrocoso Citation2015; Li et al. Citation2021) that the addition of dietary fibre to the diet of gestating sows promotes gastric health and increases feed intake during lactation. This is because dietary fibre favours the metabolic profile minimizing insulin resistance and dyslipidemia (Serena et al. Citation2007; Li et al. Citation2021).
In sheep, rabbits, mice, and pigs, the dietary fibre of various foods, including Opuntia spp., has been linked to an improvement in glucose and lipid metabolism (Brahim et al. Citation2012; Halmi et al. Citation2013; Ordaz et al. Citation2017). The lower plasma concentrations of glucose, cholesterol, and triglycerides related to the consumption of O. ficus-indica are associated with: (i) stimulation of insulin secretion and best glucose reabsorption by different tissues and, (ii) modifies lipid biosynthesis by binding to bile acids, this improves cholesterol catabolism (Kritchevsky et al. Citation1988; Fernández et al. Citation1992; Pari and Latha Citation2005; Gouws et al. Citation2019). Consequently, our hypothesis focuses on O. ficus-indica effect on the regulation of biochemical indicators (insulin and leptin mainly), minimizing resistance to insulin and its effects on sow productivity during late gestation and lactation. Hence, this study aimed to evaluate the effect of O. ficus-indica supplementation on gilts during late gestation and lactation, and particularly on their biochemical parameters and voluntary feed intake at lactation.
2. Materials and methods
This research was carried out at the Swine Unit of ‘Posta Zootécnica’ belonging to the Veterinary Medicine and Husbandry Faculty of Universidad Michoacana de San Nicolás de Hidalgo (FMVZ-UMSNH), Tarímbaro, Michoacán, México (Road; 19°46′N, 101°08′W, and altitude of 1855 m). The animals used in this research were bred in accordance with the regulations of the zoo-technical and zoo-sanitary legislation of Mexico for the humanitarian care and use of animals in research SAGARPA-SENASICA.
2.1. Animal diets and husbandry
Forty gilts were directly exposed to mature boars (eight gilts: one boar) for at least 15 min daily from 160 days of age until the last gilt had displayed pubertal estrus. Gilts that presented the second estrus at 21.0 ± 1.0 days (n = 35 gilts) were inseminated (182 ± 1.3 days; 137 ± 8.2 kg) with semen of boar genotype Yorkshire × Pietrain and were housed in groups (n = 7 gilts) in 16 m2 pens during the 110 days of gestation. Eight gilts were removed from the investigation: five during the selection process to be inseminated (sows with a lagged reproductive cycle), one gilt due to abortion and two gilts due to problems related to lameness. Therefore, thirty-two sows were used to carry out the research. All gilts were fed 2.5 kg−1 of commercial feed per day (divided into two portions, at 8:00 and 15:00 h), until day 84 of gestation (). At day 85 of gestation, the gilts were housed in individual pens of 2.0 × 2.0 m2. According to a completely random design, the animals (n = 32 gilts) were divided into two groups (n = 16 gilts·group−1): control group (CG), gilts fed commercial feed only (2.5 kg·day−1) and experimental group (EG), gilts fed commercial feed (2.5 kg·day−1) plus O. ficus-indica (as a source of dietary fibre). O. ficus-indica (fresh base) supplementation was 1.0% with respect of the gilts’ body weight; the body weight of the gilts corresponded to the stage at which the gilts were found (gestation or lactating). Immediately after farrowing, all gilts were fed ad libitum with a commercial diet for lactation (). The only variation in the gilts’ feeding at lactation was the addition of fresh O. ficus-indica to the diet of EG ().
Table 1. Composition of gestation and lactation diets.
Cladodes of O. ficus-indica were offered to the gilts at approximately 90 days of age; their chemical composition is shown in . Cladodes were manually cut (41.0 kg per week) and stored at 4.0°C until they were fed to the gilts. For feeding, cladodes were manually fragmented into pieces of approximately 3.0 × 2.0 cm and were mixed with the commercial feed corresponding to the first meal of the day (8:00 am) procedure performed in both phases, gestation, and lactation.
For farrowing and lactation sows were lodged in cages of stainless steel with plastic slatted floors until the moment of weaning (21 d post-farrowing). During farrowing and lactation, there was artificial light between 8:00 and 15:00 h, and the environmental temperature was 20–24°C. Each cage was provided with a heat source for the piglets. Farrowing occurred naturally on day 115 ± 0.12 of gestation (day 0 of lactation). The sows at farrowing had a litter size of 10.9 ± 1.3 piglets, 9.5 ± 0.8 piglets born alive, 1.3 ± 0.2 stillbirths, and 0.1 ± 0.01 mummies. Litters were balanced by eight piglets within the first 48 h post-farrowing. Piglets that died during lactation were not replaced. During lactation, seven casualties were recorded. In the CG, three died because of crushing, and in EG, four casualties occurred (three because of crushing and one from diarrhea). The casualties caused by crushing occurred during the first week of lactation and the casualty from diarrhea occurred in the second week of lactation.
2.2. Blood sampling
At day 85, 100, and 110 of gestation and at day 1, 3, 7, 14, and 21 of lactation, eight gilts from a group−1 were selected for pre-prandial (12 h fasting) blood sampling. A 10 mL blood sample was taken from the vena jugularis between 7:00 and 7:30 (1 h before the start of the morning meal). Immediately after sampling, each blood sample was divided into two subsamples: 6 mL in tubes with serum cloth activator (for an analysis of glucose, triglycerides, cholesterol, HDL and LDL) and 4 mL in tubes with lithium heparin (for analysis insulin and leptin). The subsamples were stored at 4°C until centrifugation. Subsequently, the tubes were centrifuged (1000 × g for 10 min), and plasma and serum samples were stored at −20°C until further analysis.
2.3. Hormones and metabolites’ analysis
Plasma concentrations of glucose, insulin, triglycerides, cholesterol, HDL, LDL, and leptin were determined. Glucose, triglycerides cholesterol, HDL, and LDL concentrations were determined using enzymatic methods adapted in a Cobas C111 Mira (Roche, Basel, Switzerland). The reagents used were as follows: GLUH2 (ref. 04 657 527 190, E.E.U.U), sensitivity was 2.0 mg·dL−1, the intra- and inter-assay variation coefficient was <1.0 and <1.9% at 128.0 mg·dL−1; TRIGL (ref. 04 657 594 190, E.E.U.U), sensitivity was 9.0 mg·dL−1, the intra-and inter-assay variation coefficient was <8.0 and <14.0% at 600 mg·dL−1; CHOL2 (ref. 04 718 917 190, E.E.U.U), sensitivity was 10.0 mg·dL−1, the intra-and inter-assay variation coefficient was <10.0 and <12.0% at 300 mg·dL−1; HDLC3 (ref. 05 401 488 190, E.E.U.U), sensitivity was 10.0 mg·dL−1, the intra-and inter-assay variation coefficient was <10.0 and <12.0% at 100 mg·dL−1; and LDL3 (ref. 07 005 806 190, E.E.U.U), sensitivity was 10.0 mg·dL−1, the intra- and inter-assay variation coefficient was <8.0 and <12.0% at 120 mg·dL−1. Insulin and leptin concentrations were determined using commercial ELISA kits (SIGMA-ALDRICH, St. Louis, MO, E.E.U.U). The sensitivities for each hormone were as follows: insulin, 4 µIU·mL−1, the intra-and inter-assay variation coefficient was <10 and <12% at 47.5 µIU·mL−1, respectively, and leptin, 2 pg·mL−1, the intra- and intra-assay variation coefficient was <10.0 to <12% at 400 pg·mL−1.
2.4. Gilts’ productive performance
The feed intake, energy balance, loss of bodyweight of the gilts and piglet development were evaluated; for feed intake, the feed supplied and rejected was weighed daily with a digital scale (Dibatec®; capacity of 40 kg and accuracy of ±5 g). The energy imbalance was obtained through the methodology established by Noblet et al. (Citation1990). Gilts were weighed pre-farrowing (day 110 of gestation) and at weaning (day 21 of lactation) using a fixed electronic scale (STG-1500-T1500SL, OCONY®; with a capacity of 1-1500 kg). To estimate the loss in body weight at lactation, the weight of the post-farrowing gilts was estimated using the prediction equation of Mallmann et al. (Citation2018). Piglets were weighed at birth and on days 7, 14, and 21 (weaning) of lactation.
2.5. Statistical analysis
Data were analyzed by ANOVA through repeated measurements by PROC MIXED [SAS Inst. Inc., Cary, NC, EUA] (Littell et al. Citation1998). The gilt represented the experimental unit in the model. The effects of the group, day, and their interaction were evaluated in terms of gilts body weight, piglets’ weight, plasma glucose, insulin, triglyceride, cholesterol, HDL, LDL, leptin, and HOMA-IR index. The model used was:
(1)
(1) where Yijkl = response variable: gilt body weight, piglets’ weight, plasma glucose, insulin, triglyceride, cholesterol, HDL, LDL, leptin, and HOMA-IR index; µ = constant common in the population; Gi = fixed effects of the i-th group, with i = CG, EG; G(G)j(i) = random effect of the j-th gilt, nested within the i-th group, with i = CG, EG; Dk = fixed effects of the k-th day; G*Dik = fixed effects of the interaction of the i-th group with the k-th day; εijkl = random effect associated with each observation (∼NID = 0, σ2e).
The data of feed intake, intake energy, energy balance, body weight loss of the gilts and piglets weaned per gilt were evaluated through ANOVA in PROC GLM (SAS 9.4 Inst. Inc., Cary, NC, USA). The effects of the group, week, and their interaction were evaluated. The model used was:
(2)
(2) where Yijk = response variable: feed intake, intake energy, energy balance, body weight loss of the gilts and piglets weaned per gilt; µ = constant common in the population; Gi = fixed effects of the i-th group, with i = CG, EG; Wj = fixed effects of the j-th week of lactation; G*Wij = fixed effects of the interaction of the i-th group with the j-th week of lactation; εijk = random effect associated with each observation (∼NID = 0, σ2e).
Significant differences among groups were considered at P < 0.05. Normality of distribution and homogeneity of variance for residuals were tested using PROC UNIVARIATE [SAS Inst. Inc., Cary, NC, EUA]. In the case of non-normality, parameters were normalized by log transformation prior to analysis to generate a normal distribution.
Insulin resistance was indirectly estimated using HOMA-IR according to the equation proposed by Matthews et al. (Citation1985):
The values in tables and figures are presented as minimum squares ± SEM.
3. Results
3.1. Metabolic indicators
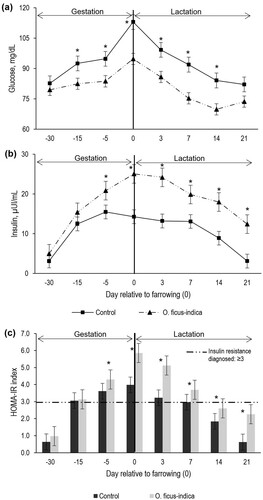
The effects of the supplementation with O. ficus-indica on the biochemical indicators of gilts were estimated per group, sampling day, and group per day interaction. According to the group per day interaction, CG showed higher (P < 0.05) plasma glucose concentrations on each evaluation day (). A plasma glucose peak was found at farrowing day in both groups; however, it was lower in EG gilts (P < 0.05): 94.7 vs. 112.9 ± 3.4 mg·dL−1 of CG. Plasma insulin according to group per day interaction (P = 0.0034) was higher (P < 0.05) at farrowing day and at days 3 and 7 of lactation in both groups (), however, EG showed higher concentration (). HOMA-IR index was higher (P < 0.05) in gilts that consumed O. ficus-indica on each evaluated day (). According to HOMA-IR index, both groups presented IR from day 100 of gestation at day 7 of lactation (). However, IR degree was higher (P < 0.05) in EG gilts (10.8% more from day 100 of gestation to farrowing and 30.6% more in the first week of lactation) than that in CG ().
Figure 1. Plasma concentration of glucose (a), insulin (b), and HOMA-IR index (c) according to the group. Each point or bar represents the mean ± standard error of mean (SEM; n = 16 gilts by group). *Indicates statistically significant difference (P < 0.05) between groups.
Group per day interaction effect (P < 0.001) on the lipid indicators was evaluated. Plasma triglycerides were lower (P < 0.05) at days 100 and 110 of gestation in EG (). At lactation, from the second week, the gilts that consumed O. ficus-indica presented higher (P < 0.05) plasma triglycerides, a pattern that remained consistent until the end of lactation (). Plasma total cholesterol showed a similar trend as triglycerides at gestation, that is, lower (P < 0.05) concentrations in EG (). Day three post-farrowing was the only day that showed significant differences in total cholesterol between groups, with its concentration being higher (P < 0.05) in the CG gilts ().
Figure 2. Plasma concentration of triglycerides (a), cholesterol (b), HDL (c), and LDL (d) according to the group. Each point represents the mean ± standard error of mean (SEM; n = 16 gilts by group). *Indicates statistically significant difference (P < 0.05) between groups.
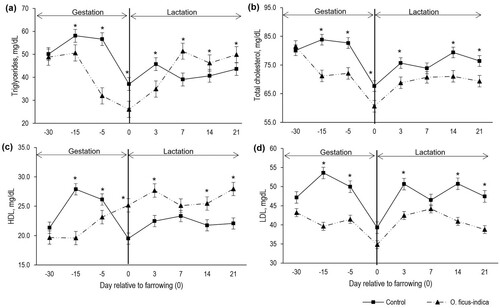
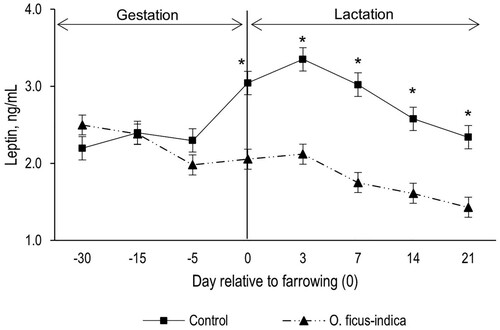
HDL in gestation (day 100 and 110) was lower (P < 0.05) in gilts that consumed O. ficus-indica. At lactation, the gilts that consumed O. ficus-indica (EG) showed higher plasma HDL (P < 0.05; ). Gilts that consumed O. ficus-indica presented lower (P < 0.05) concentration of plasma LDL in both phases (). Group per day interaction effect (P < 0.001) on plasma leptin showed that, at gestation (day 100 and 110) plasma leptin was equal (P > 0.05) in both groups (). However, at lactation, the CG gilts showed higher (P < 0.05) leptin concentrations on each evaluated day ().
3.2. Gilts’ performance
The effects of supplementation gilts with O. ficus-indica on voluntary feed intake, energy balance, loss of bodyweight and piglet development in lactation were estimated by group (P < 0.001), week (P < 0.001), and their interaction. The group per week interaction affected the daily feed intake (P = 0.037) and energy balance (P = 0.012). Commercial feed intake on an average per day at lactation was higher (P < 0.05) in the EG gilts: 5.4 vs. 4.2 ± 0.06 kg·day−1 in CG gilts (). Energy balance on average showed a similar trend as feed intake; increased energy balance in gilts that consumed O. Ficus-indica (0.43 ± 1.19 MJ·day−1) compared with the control (−7.33 ± 1.19 MJ·day−1). The loss of bodyweight in lactation was lower (P < 0.05) in EG gilts: 1.1 vs. 4.8 ± 1.2% in CG gilts (). Group per week interaction had a similar trend to the feed intake and energy balance, being higher in the gilts that consumed O. ficus-indica, in each evaluated week (). O. ficus-indica supplementation had no effect (P > 0.05) on the weight of piglets at birth and at weaning ().
Table 2. Least squares mean for feed intake, energy intake and energy balance of sows according to the group and week of lactation.
Table 3. Least squares mean for the productive performance of the sow and piglets according to the group.
4. Discussion
In sows, the gradual development of IR promotes the presence of dyslipidemia (Mosnier, Etienne, et al. Citation2010). IR detected at the end of gestation is accentuated at the first week of lactation because the sow physiologically requires more glucose for lactose synthesis (Pari and Latha Citation2007). In the presence of IR, the sow mobilizes body reserves (fat and protein) and thus increases the concentration of energy substrates (e.g. total cholesterol, triglycerides, leptin) because the sows do not yet present optimal feed intake to satisfy their nutritional requirements (Mosnier, Etienne, et al. Citation2010). An increase in lipid indicators was observed in CG gilts in both late gestation and lactation. The behaviour of these biochemical indicators (total cholesterol, triglycerides, LDL, and leptin) in CG is associated with a higher concentration of glucose at the late gestation stage and during the first week of lactation and this relationship with the feed intake at lactation. In addition to the high glucose concentration during the peripartum period, high concentrations of endogenous opioid peptides are also present during this period, essential peptides to stimulate the production of endorphins, reduce the concentrations of GnRH, FSH and LH and synthesize prolactin to start lactation (Barb et al. Citation1986; Farmer Citation2016). The higher the synthesis of prolactin and its interaction with IR, the glucose concentration increases for the formation of milk components, in this case, lactose. In addition to the effect of the opioid peptides described above, they also have action on feed intake due to their interaction with proopiomelanocortin (POMC). It has been established that POMC has several post-translational metabolic pathways, not only giving rise to β-endorphins. At the same time, it synthesizes corticotropic hormone (ACTH), hormone that participates in the inhibition of feed intake (González et al. Citation2006).
Quesnel et al. (Citation2009) report that the administration of fibrous diets (>30% NDF) favours the feed intake at lactation. With respect to using O. ficus-indica as a source of dietary fibre in the present research, it has been reported in rats (Nuñez et al. Citation2013) that the consumption of this cactus decreases plasma glucose and increases plasma insulin. Soluble fibre in monogastric is not digested by the gastrointestinal enzymes, therefore, they modify the absorption of bile salts, cholesterol, and glucose (Morán et al. Citation2012). In addition, a diet rich in soluble fibre increases the viscosity of food bolus and propitiates the absorption of energy substrates (Shapiro and Gong Citation2002). Haber et al. (Citation1977) showed that, when carbohydrates are present intracellularly in plant foods, their release in the intestine is slower and glucose-insulin responses in the blood decreases.
HOMA-IR index for IR diagnoses in humans and sows is ≥ 3.0 (Matthews et al. Citation1985; Tan et al. Citation2015). The gilts that consumed O. ficus-indica had a higher HOMA-IR index with respect to the CG, this is due to the higher synthesis of insulin in the gilts of the EG. It should be noted that regardless of the highest HOMA-IR index in the EG the hyperinsulinemia is not a product of IR, it was due to the over-production of insulin by the consumption of O. ficus-indica. This behaviour is justified analysing glucose behaviour, where EG had no concomitant glucose-insulin increase. The consumption of O. ficus-indica reduced glucose concentration per direct action of insulin because this cactus stimulates insulin secretion, improves the insulin-stimulated phosphorylation of IRS-2 and Akt in the liver, and thus normalizes the excessive production of hepatic glucose (Pari and Latha Citation2005). O. ficus-indica can act the same way as oral antidiabetics, by shutting down the K+/ATP channels, depolymerizing membrane and stimulating the Ca2+ channels for insulin secretion (Halmi et al. Citation2013).
Changes in glucose kinetics due to the consumption of O. ficus-indica propitiates lower catabolism (indirectly assessed in loss of bodyweight), which is conducive to less dyslipidemia because of the higher feed intake at lactation. With regard to the greater dyslipidemia found in the CG gilts, it has been reported that regardless of the sows’ genotype (lean or fat), the highest degree of dyslipidemia in the CG respect to EG is mainly associated with the IR by which the sow passes during peripartum (Mosnier, Le Floc’h, et al. Citation2010; Torres-Rovira et al. Citation2011). IR cause an increase in glucose concentrations, which limits feed intake. The low feed intake favours body catabolism in the sow, therefore the release of NEFA's, this is reflected in a higher concentration of cholesterol, LDL, and leptin ( and ).
Increased triglycerides and HDL from the seventh-day post-farrowing at the end of lactating in EG gilts could be associated with the beginning of ovarian reactivation (Barb et al. Citation2008). Ovarian reactivation is characterized by an increase in reproductive hormones FSH, LH, and estrogens – hormones that are synthesized through cholesterol precursors (Barb et al. Citation1991). During ovarian reactivation, the dietary fibre of O. ficus-indica could act favourably by its effect on non-starch polysaccharides, that when subjected to fermentation by the colon microbiota lead to a higher production of volatile fatty acids (Cani et al. Citation2006). Volatile fatty acids intervene on energetic contribution of the organism and can facilitate the synthesis of precursors of cholesterol (Molist et al. Citation2009). Volatile fatty acids that are monomers in the luminal aqueous phase are absorbed by any segment of the digestive tract, therefore have a total digestibility (Berruezo et al. Citation2011), and this propitiates the increase in triglycerides and HDL from the second week of lactation in EG gilts ().
Leptin is a mediator of the regulation of the long-term energy balance, as it has an effect on the suppression of feed intake and induces weight loss (Martínez et al. Citation2014). Leptin resistance has been reported during the last third of gestation, however, it has no effect on feed intake (Saleri et al. Citation2015; Szczesna and Zieba Citation2015). Such behaviour was also observed in the present investigation, since no difference was found between groups in the leptin concentration at the last third of gestation. As far as the lactation phase is concerned, it has been reported (Tessier et al. Citation2013) that there is no resistance to leptin, since, in this phase, the loss of the intracellular signal of the function of leptin receptors. Therefore, in lactation, leptin does have an effect on food consumption. This could be observed in the feed intake of the sows, the sows from the CG had lower feed intake and higher leptin concentrations with respect to the EG ( and ).
Leptin concentration is higher in sows of Asian genotypes than in European genotypes (Guay et al. Citation2001). Farmer et al. (Citation2007) report a difference in leptin concentration on the eighteenth day of lactation. The Landrace genotype showed a higher leptin concentration with respect to the Yorkshire, Duroc, and synthetic lines genotypes. This behaviour is associated with the greater thickness of dorsal fat in Landrace sows and their association (r = 0.67) with leptin (Estienne et al. Citation2000). Kolaczynski et al. (Citation1996), established that, the increase in 10% of bodyweight results in a 300% increase in the leptin concentration; a phenomenon that is observed during the last third of gestation and propitiates lower feed intake at lactation. Quesnel et al. (Citation2009) report that a diet rich in fibre during gestation leads to a reduction in leptin concentration and an increase in feed intake at lactation. This behaviour was also observed in the present research: the gilts that consumed O. ficus-indica presented a lower concentration of leptin in lactation and a greater feed intake, in addition, energy balance was lower as was the loss of bodyweight at weaning.
Finally, among the hypotheses that were had about the use of O ficus-indica in the feeding of pregnant and lactating sows to reduce the glucose concentration was the possible effect on the development of the piglet and the quantity and quality of the sow's milk. About it, the research group has already reported that the consumption of O ficus-indica does not affect the quality and quantity of the milk produced by the sow or the development of the piglet (Ortiz et al. Citation2017, Citation2020). However, there are some limitations that must be considered when interpreting the results of this study. The study was carried out in sows of hybrid genotype, it would be necessary to carry out more investigations in the genetic lines of current hyperprolific sows which are more susceptible to a lower feed intake due to their higher energy demand to meet the requirements of a larger litter and higher milk production. It should be noted that when evaluating the energy balance in hybrid sows (with n = 8 piglets/litter) fed O ficus-indica, it was higher (−2.1 ± 3.5 MJ·day−1) with respect to conventionally fed sows: −9.1 ± 2.7 MJ·day−1 (Ordaz et al. Citation2019). Reason for which, it is expected that the productive behaviour of hyperprolific sows fed with nopal as part of the diet is positive, since it would generate greater feed consumption which would reflect a better metabolic state of the animals. In addition, strategies must be sought to process and store O ficus-indica that facilitate its incorporation into the sow's diet without losing its properties, in order to be implemented in intensive swine production systems.
5. Conclusion
IR is an inherent physiological process of sows, however in current swine production systems IR limits the productive potential of the sow because it has effects on sow productivity: low feed intake in lactation, greater body weight loss in lactation, low productivity in the next production cycle, less longevity, etc. The intake of O. ficus-indica at late gestation and during lactation in gilts favourably modulates the regulation of biochemical indicators that participate in the development of insulin resistance and dyslipidemia, which is reflected in higher voluntary feed intake and less body weight loss at weaning.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Barb CR, Estienne MJ, Kraeling RR, Marple DN, Rampacek GB, Rahe CH. 1991. Endocrine changes in sows exposed to elevated ambient temperature during lactation. Domest Anim Endocrinol. 8:117–127. doi:10.1016/0739-7240(91)90046-m.
- Barb CR, Hausman GH, Lents CA. 2008. Energy metabolism and leptin: effects on neuroendocrine regulation of reproduction in the gilt and sow. Reprod Domest Anim. 43:324–330. doi:10.1111/j.1439-0531.2008.01173.x.
- Barb CR, Kraeling RR, Rampacek GB, Whisnant C. 1986. Opioid inhibition of luteinizing hormone secretion in the postpartum lactating sow. Biol Reprod. 35:368–371. doi:10.1095/biolreprod35.2.368.
- Berruezo GR, Graciá CM, Valencia JA. 2011. Bioavailability of short-chain fatty acids: absorption mechanisms. Analesz. 24:125–134. https://helvia.uco.es/handle/10396/9453.
- Brahim KL, Abdelkader D, Miloud H, Kheira G. 2012. Effect of incorporation of the spineless Opuntia ficus Indica in diets on biochemical parameters and its impact on the average weight of ewes during the maintenance. Glob Vet. 8:352–359. https://idosi.org/gv/GV8(4)12/7.pdf.
- Cani PD, Knauf C, Iglesias MA, Drucker DJ, Delzenne NM, Burcelin R. 2006. Improvement of glucose tolerance and hepatic insulin sensitivity by oligofructose requires a functional glucagon-like peptide 1 receptor. Diabetes. 55:1484–1490. doi:10.2337/db05-1360.
- Cools A, Maesb D, Decaluwéa R, Buysec J, Kempend TAGT, Liesegange A, Janssens GPJ. 2014. Ad libitum feeding during the peripartal period affects bodycondition, reproduction results and metabolism of sows. Anim Feed Sci Technol. 145:130–140. doi:10.1016/j.anireprosci.2014.01.008.
- De Rensis F, Gherpelli M, Superchi P, Kirkwoodc RN. 2005. Relationships between backfat depth and plasma leptin during lactation and sow reproductive performance after weaning. Anim Reprod Sci. 1:95–100. doi:10.1016/j.anireprosci.2005.01.017.
- Estienne MJ, Harper AF, Barb CR, Azain MJ. 2000. Concentrations of leptin in serum and milk collected from lactating sows differing in body condition. Domest Anim Endocrinol. 19:275–280. https://www.ncbi.nlm.nih.gov/pubmed/11118791.
- Farmer C. 2016. Altering prolactin concentrations in sows. Domest Anim Endocrinol. 56:S155–S164. doi:10.1016/j.domaniend.2015.11.005.
- Farmer C, Charagu P, Palin MF. 2007. Influence of genotype on metabolic variables, colostrum and milk composition of primiparous sows. Can J Anim Sci. 87:511–515. doi:10.4141/CJAS07041.
- Fernández ML, Lin ECK, Trejo A, McNamara DJ. 1992. Prickly pear (Opuntia sp.) pectin reverses low density lipoprotein receptor suppression induced by hypercholesterolemic diet in Guinea pigs. J Nutr. 122:2330–2340. doi:10.1093/jn/122.12.2330.
- González HME, Ambrosio MKA, Sánchez ES. 2006. Regulación neuroendócrina del hambre, la saciedad y mantenimiento del balance energético. Artemiza. 8:191–200. https://www.redalyc.org/articulo.oa?id=14280309.
- Gouws CA, Georgousopoulou EN, Mellor DD, McKune A, Naumovski N. 2019. Effects of the consumption of prickly pear cacti (Opuntia spp.) and its products on blood glucose levels and insulin: a systematic review. Medicina. 55(5):138. doi:10.3390/medicina55050138.
- Guay F, Palin MF, Jacques MJ, Laforest JP. 2001. Effects of breed, parity, and folic acid supplement on the expression of leptin and its receptors’ genes in embryonic and endometrial tissues from pigs at day 25 of gestation. Biol Reprod. 3:921–927. doi:10.1095/biolreprod65.3.921.
- Haber GB, Heaton KW, Murphy D, Burroughs LF. 1997. Depletion and disruption of dietary fibre: effects on satiety, plasma-glucose and serum insulin. Lancet. 1:679–682. doi:10.1016/S0140-6736(77)90494-9.
- Halmi BS, Benlaksira B, Bechtarzi K, Berouel K, Serakta M, Richi F, Djaalab H, Maameri Z, Djerrou Z, Hamdipacha Y. 2013. Pharmaco-toxicological study of Opuntia ficus indica L. aqueous extract in experimental animal. Int J Medic Arom Plants. 3:375–381. https://pdfs.semanticscholar.org/52da/faaeb7ae59ece5b3703bfd9410780a6efd71.pdf.
- Jha R, Berrocoso JFD. 2015. Dietary fiber and protein fermentation in the intestine of swine and their interactive effects on gut health and on the environment: a review. Anim Feed Sci Technol. 212:18–26. doi:10.1016/j.anifeedsci.2015.12.002.
- Kolaczynski JW, Considine RV, Ohannesian J, Marco C, Opentanova I, Nyce MR, Myint M, Caro JF. 1996. Responses to leptin in short-term fasting and refeeding in humans: a link with ketogenesis but not ketones themselves. Diabetes. 45:1511–1515. doi:10.2337/diab.45.11.1511.
- Kritchevsky SA, Satchithanandasm T, Cassidym M, Vahouny GV. 1988. Dietary fiber supplements: effects on serum and liver lipids and on liver phospholipid composition in rats. Lipids. 23:318–321. doi:10.1007/bf02537341.
- Li H, Yin J, Tan B, Chen J, Zhang H, Li Z, Ma X. 2021. Physiological function and application of dietary fiber in pig nutrition: a review. Anim Nutrit. 7:259–267. doi:10.1016/j.aninu.2020.11.011.
- Littell RC, Henry PR, Ammerman CB. 1998. Statistical analysis of repeated measures data using SAS procedures. J Anim Sci. 76:1216–1231. doi:10.2527/1998.7641216x.
- Mallmann L, da Silva OG, Zacarias RJ, Basquera BF, Pereira FD, Ghiggi FJE, Andretta I, da Rosa UR, Gonçalves MAP, Pandolfo BF. 2018. Proposal of equations for predicting post-farrowing sow weight. Acta Sci Vet. 46:1574. doi:10.22456/1679-9216.83867.
- Manu H, SuHyup L, Baidoo SK. 2020. PSVIII-5 effect of glycemic and endocrine response to feeding time in pregnant sows. J Anim Sci. 98:210–211. doi:10.1093/jas/skaa054.364.
- Martínez ME, Miana M, Jurado LR, Bartolomé MV, Souza NF. 2014. The potential role of leptin in the vascular remodeling associated with obesity. Int J Obes. 38:1565–1572. doi:10.1038/ijo.2014.37.
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. 1985. Homeostasis model assessment: insulin resistance and B-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 28:412–419. doi:10.1007/bf00280883.
- Molist F, de Segura AG, Gasa J, Hermes RG, Manzanilla EG, Anguita M, Pérez JF. 2009. Effects of the insoluble and soluble dietary fibre on the physicochemical properties of digesta and the microbial activity in early weaned piglets. Anim Feed Sci Technol. 149:346–353. doi:10.1016/j.anifeedsci.2008.06.015.
- Morán RS, Ávila A, Tovar AR, Pedraza CJ, López RP, Torres N. 2012. Opuntia ficus indica (nopal) attenuates hepatic steatosis and oxidative stress in obese Zucker (fa/fa) rats. J Nutr. 42:1956–1963. doi:10.3945/jn.112.165563.
- Mosnier E, Etienne M, Ramaekers P, Pére MC. 2010. The metabolic status during the peri partum period affects the voluntary feed intake and the metabolism of the lactating multiparous sow. Livest Sci. 127:127–136. doi:10.1016/j.livsci.2009.06.023.
- Mosnier E, Le Floc’h N, Etienne M, Ramaekers P, Sève B, Père MC. 2010. Reduced feed intake of lactating primiparous sows is associated with increased insulin resistance during the peripartum period and is not modified through supplementation with dietary tryptophan. J Anim Sci. 88:612–625. doi:10.2527/jas.2008-1768.
- Noblet J, Dourmad JY, Etienne M. 1990. Energy utilization in pregnant and lactating sows: modelling of energy requirements. J Anim Sci. 68:562–572. doi:10.2527/1990.682562x.
- Nuñez LMA, Paredes LO, Reynoso CR. 2013. Functional and hypoglycemic properties of nopal cladodes (O. ficus-indica) at different maturity stages using in vitro and in vivo tests. J Agric Food Chem. 61:10981–81096. doi:10.1021/jf403834x.
- Ordaz G, Juárez A, Vargas K, Pérez RE, Ortiz R. 2019. Effects of dietary inclusion of Opuntia ficus-indica on the glycemia and productive performance in lactating sows. S Afr J Anim Sci. 49:824–834. doi:10.4314/sajas.v49i5.5.
- Ordaz OG, Juárez CA, Pérez SRE, Román BRM, Ortiz RR. 2017. Effect of spineless cactus intake (Opuntia ficus-indica) on blood glucose levels in lactating sows and its impact on feed intake, body weight loss, and weaning-estrus interval. Trop Anim Health Prod. 49:1025–1033. doi:10.1007/s11250-017-1295-7.
- Ortiz R, López M, Pérez RE, Ramírez PP, Ordaz G. 2020. Effect of the inclusion of different levels of dietary cactus (Opuntia ficus-indica) on gilts’ biochemical parameters and feed intake during lactation. Animals. 10:1881. doi:10.3390/ani10101881.
- Ortiz RR, Orozco GA, Val AD, Portillo ML, Pérez SRE. 2017. Effect of addition of prickly pear (Opuntia ficus-indica) to the diet of lactating sows on the production and quality of milk. Nova Sci. 9:290–312. doi:10.21640/ns.v9i18.765.
- Pari L, Latha M. 2005. Antidiabetic effect of Scoparia dulcis: effect on lipid peroxidation in streptozotocin diabetes. Gen Physiol Physiol. 24:13–26. http://www.gpb.sav.sk/2005_01_13.pdf.
- Père MC, Etienne M. 2007. Insulin sensitivity during pregnancy, lactation, and postweaning in primiparous gilts. J Anim Sci. 85:101–110. doi:10.2527/jas.2006-130.
- Père MC, Etienne M. 2018. Nutrient uptake of the uterus during the last third of pregnancy in sows: effects of litter size, gestation stage and maternal glycemia. Anim Reprod Sci. 188:101–113. doi:10.1016/j.anireprosci.2017.11.014.
- Quesnel H, Meunier SMC, Hamard A, Guillemet R, Etienne M, Farmer C, Dourmad JY, Père MC. 2009. Dietary fiber for pregnant sows: influence on sow physiology and performance during lactation. J Anim Sci. 87:532–543. doi:10.2527/jas.2008-1231.
- Saleri R, Sabbioni A, Cavalli V, Superchi P. 2015. Monitoring blood plasma leptin and lactogenic hormones in pregnant sows. Animal. 9:629–634. doi:10.1017/S1751731114003085.
- Serena A, Hedemann MS, Bach KE. 2007. Feeding high fibre diets changes luminal environment and morphology in the intestine of sows. Livest Sci. 109:115–117. doi:10.1016/j.livsci.2007.01.105.
- Shapiro K, Gong W. 2002. Natural products used for diabetes. J Am Pharm Assoc. 42:217–226. doi:10.1331/108658002763508515.
- Solà-Oriol D, Gasa J. 2017. Feeding strategies in pig production: sows and their piglets. Anim Feed Sci Technol. 233:34–352. doi:10.1016/j.anifeedsci.2016.07.018.
- Solé E, Ros-Freixedes R, Tor M, Reixach J, Pena RN, Estany J. 2021. Antagonistic maternal and direct effects of the leptin receptor gene on body weight in pigs. PLoS ONE. 16(1):e0246198. doi:10.1371/journal.pone.0246198.
- Szczesna M, Zieba DA. 2015. Phenomenon of leptin resistance in seasonal animals: the failure of leptin action in the brain. Domeest Anim Endocrinol. 52:60–70. doi:10.1016/j.domaniend.2015.03.002.
- Tan C, Wei H, Sun H, Ao J, Long G, Jiang S, Peng J. 2015. Effects of dietary supplementation of oregano essential oil to sows on oxidative stress status, lactation feed intake of sows, and piglet performance. Biomed Res Int. 2015:525218. doi:10.1155/2015/525218.
- Tessier DR, Ferraro ZM, Gruslin A. 2013. Role of leptin in pregnancy: consequences of maternal obesity. Placenta. 34:205–211. doi:10.1016/j.placenta.2012.11.035.
- Torres-Rovira L, Pallares P, Gonzalez-Añover P, Perez-Solana ML, Gonzalez-Bulnes A. 2011. The effects of age and reproductive status on blood parameters of carbohydrate and lipid metabolism in Iberian obese sows. Reprod Biol. 11:165–171. doi:10.1016/S1642-431X(12)60053-9.
- Unger G, Fabiana SB, Perruzza FG, Pennacchiotti L. 2014. Triglycerides and glucose index: a useful indicator of insulin resistance. Endocrinol Nutr. 61:533–540. doi:10.1016/j.endonu.2014.06.009.