ABSTRACT
Transferrin plays an important role in iron metabolism and has been reported to be involved in the immune response to environmental stress. In this study, the transferrin gene (LwTf) from Amur ide (Leuciscus waleckii) was cloned and characterized. The cDNA was observed to contain an open reading frame (ORF) of 1998bp and encode a 665-amino-acid-long protein that shares 73.04% identity with the transferrin from silver carp (Hypophthalmichthys molitrix). Amino acid alignment analysis showed that LwTf contained an initial peptide and two lobes (N- and C-lobes). Quantitative real-time PCR (qPCR) analyses showed that the expression levels of LwTf were abundant in the liver of the fish but were also significant in the brain and spleen, with lower expression observed in six other tissues. The temporal expression profiles were detected during the alkaline challenge experiment in alkali-water and freshwater populations of healthy adult Amur ide. LwTf expression was significantly upregulated in the main immune organs (spleen, kidney, and head kidney) of the freshwater Amur ide population compared to that in the alkali-water Amur ide population. Taken together, these results indicate that in Amur ide, LwTf might play an important role in the innate immune response to a high alkaline stress.
Introduction
Transferrin (Tf) is a multi-functional protein that plays a central role in iron metabolism, and is also thought to be involved in the innate immune system response (Liu et al. Citation2010, Citation2012). Through the binding and transport of iron, transferrin participates in a wide variety of metabolic processes, including immune regulation, antimicrobial and antioxidant activity, DNA synthesis, cytoprotection, and electron transport (Stafford and Belosevic Citation2003; Ong et al. Citation2006). Transferrin has been characterized in a variety of vertebrates, including mammals, reptiles, and birds (Graham and Williams Citation1975; Schreiber et al. Citation1979; Yang et al. Citation1984; Ciuraszkiewicz et al. Citation2007). Cloning, structural, and functional studies have also been extended in various fish species (Denovan-Wright et al. Citation1996; Tange et al. Citation1997; Lee et al. Citation1998; Sahoo et al. Citation2009). The complete sequence of transferrin in Nile tilapia (Oreochromis niloticus) and functional studies in relation to saltwater resistance have been reported (Rengmark and Lingaas Citation2007). Structure and expression of the transferrin gene in channel catfish (Ictalurus punctatus) have also been reported (Liu et al. Citation2010). Neves et al. (Citation2009) reported the response of transferrin from sea bass to bacterial infection. Transferrin gene expression in response to LPS challenge and heavy metal exposure in roughskin sculpin (Trachidermus fasciatus) has also been described (Liu et al. Citation2012). As an acute-phase protein in fish, the concentration of transferrin closely mirrors the condition of infection or stress, although its rise or fall varies with infectious microorganisms or tissue where the injury has occurred (Neves et al. Citation2009; Liu et al. Citation2010). Similar to the results obtained in mammals, transferrin is most abundantly expressed in the liver of fish, and its expression in the brain is species-specific (Tu et al. Citation1991; Denovan-Wright et al. Citation1996; Sahoo et al. Citation2009). However, the expression and function of transferrin in the fish innate immune response to high alkaline stress have not been reported.
Amur ide (Leuciscus waleckii) is a cold freshwater fish (Chang et al. Citation2014) that usually inhabits freshwater environments but can also survive in saline and alkaline lakes (Xu et al. Citation2017); it is primarily distributed in water regions around the Amur river, a few areas in the Liao and Yellow rivers, and inland lakes in Inner Mongolia around China (Chang et al. Citation2014). As an extreme example, Amur ide can survive in the highly alkaline (pH up to 9.6) water of Dali-Nor lake (116°25′−116°45′E, 43°13′−43°23′N), an extremely alkaline lake with HCO3-/CO32- concentration over 53.57 mmol/L (Chang et al. Citation2013). Amur ide is an important source of income for local Mongolians who live around Dali-Nor lake, and it is an important food source for birds migrating from Siberia to the South (Xu et al. Citation2017). Despite the economic and ecological importance of Amur ide in Dali-Nor lake, the mechanisms underlying its high tolerance to alkalinity are still largely unknown (Xu et al. Citation2017). Recently, Chinese scholars have carried out a series of exploratory studies on the molecular mechanism of the adaptation of this fish to high alkalinity in terms of its physiology and biochemistry, population genetics, transcriptome, and genomic adaptation (Cui et al. Citation2015; Chen et al. Citation2019). In particular, the expression of genes related to osmotic pressure regulation, acid–base balance, ion transport, and immunity was found to have significantly increased under high alkaline stress using second-generation sequencing technology (RNA-Seq) (Xu, Ji, et al. Citation2013; Xu, Li, et al. Citation2013).
In this study, we first cloned and characterized the cDNA sequence of the transferrin gene (LwTf) and then examined the expression of LwTf in different tissues of Amur ide originating from Dali-Nor lake and the Songhuajiang River under the same alkaline stress conditions. Simultaneously, we investigated the changes in the expression of LwTf in seven tissues associated with immunity after high alkaline challenge. This study will help in enhancing our understanding of the role of LwTf in the innate immune defense during the adaptation of Amur ide to high alkalinity.
Material and methods
Fish maintenance and sampling
The F3 offspring of alkali-water species (abbreviated hereafter as AW) produced in the laboratory using the broodstock collected from the Dali-Nor lake in 2009 and F1 progenies of freshwater species (abbreviated hereafter as FW) found in the Songhuajiang River in 2016 were collected for this experimentation. Approximately 1000 AW and FW individuals were reared in different outdoor ponds spanning one acre in the Hulan Experimental Station of the Heilongjiang River Fisheries Research Institute (126.63°E, 45.97°N) for three months. A total of 240 fish with an average body weight of 48.72 ± 16.89 g were distributed into two 650 L aquaria equipped with a recirculating water supply for a week. All fish were fed at 1% body weight (BW) with commercial pellets (Shandong Shengsuo feed Technology Co. Ltd, China) twice a day, and no feeding was done 48 h prior to the experimentation. Half of the total water was exchanged twice a day to remove uneaten food and minimize the build-up of nitrates; water quality was monitored by using a YSI water quality analyzer (YSI Incorporated, Yellow Springs, OH, USA). In addition, water was maintained at a temperature of 17–18°C, dissolved oxygen level 7.24–8.21 mg/L, pH 7.29–7.44, ammonia level 0.02–0.12 mg/L, salinity 1.1–1.7 ppt, and alkalinity 0.44–0.48 mmol/L.
Fish used in this study were euthanized in diluted MS-222 at the final concentration of 75 mg/L before dissection. To clone the cDNA sequence of the LwTf gene and examine the expression profiles in various tissues of AW and FW, samples of nine tissues, including gill, liver, heart, spleen, intestine, kidney, head kidney, brain, and muscle, of Amur ide were isolated, flash-frozen in liquid nitrogen, and stored at −80°C for RNA extraction.
Alkaline challenge experiment
This experiment was executed by placing Amur ide into a three-system design that consisted of control groups and alkaline challenge groups. Control groups consisted of AC (AW without alkaline challenge) and FC (FW without alkaline challenge) in one replicate, whereas alkaline challenges were implemented by exposing AW and FW to 50 mmol/L bicarbonate in triplicates for a week (7 days). One hundred juveniles each of AW and FW were selected randomly and distributed equally with 25 fishes in eight 200 l experimental tank equipped with recirculation; each group consisted of three randomly assigned tanks.
To achieve 50 mmol/L bicarbonate alkalinity, a total of 839 g of NaCHO3 (Tianjin Kemiou Chemical Reagent Co. Ltd., China) was dissolved and added into the system. The concentration of bicarbonate was monitored daily by using the titration method with 0.02 mmol/L HCl. The water quality was monitored using a YSI analyzer during the experiments with the temperature at 23.76 ± 0.87°C, salinity 2.16 ± 0.06 mg/L, dissolved oxygen 11.73 ± 0.39 mg/L, ammonia 0.53 ± 0.12 mg/L, and pH 9.61 ± 0.07. Sampling for the time course experiment started at 24 h (1 d) after the alkaline challenge and continued at 48 h intervals (e.g. 3, 5, and 7 d) with 3 fish sampled from each tank (9 fish for each group), while fish from the control group were collected at the end of the experiment and were recorded as 0 d sample. The sampled fish were anesthetized with 75 mg/L MS-222, and seven tissues associated with immunity, including liver, spleen, intestine, kidney, head kidney, brain, and heart tissues, were rapidly collected for RNA extraction. All tissue samples were immediately frozen in liquid nitrogen and then stored at −80°C until RNA extraction. All animal procedures in this study were conducted according to the guidelines for the care and use of laboratory animals of Heilongjiang River Fisheries Research Institute, CAFS.
RNA isolation and first-strand cDNA synthesis
Total RNA was extracted using Trizol® Reagent (Invitrogen, USA) according to the manufacturer’s protocol. The quality and quantity of RNA of each sample were measured using a NanoDropTM 8000 spectrophotometer (Thermo Scientific, USA). All extracted samples had an A260/280 ratio greater than 1.8 and were diluted to 250 ng/μL. One microgram of total RNA from each sample was used as the template for reverse transcription reaction using the PrimeScript® RT Reagent Kit With gDNA Eraser (TaKaRa, Dalian, China). Genomic DNA elimination reaction was performed in a total volume of 10 μL containing 2 μL 5×gDNA Eraser Buffer, 2μL gDNA Eraser, 1μg total RNA, and 5μL RNase free dH2O. The reaction was carried out at 42°C for 2 min. Reverse-transcription reaction was performed in a 20 μL reaction volume containing 10 μL of upper reaction solution, 4 μL 5×PrimeScript Buffer 2, 1 μL PrimeScript RT Enzyme Mix I, 1μL RT PrimerMix, and 4μL RNase free dH2O. The reactions were carried out at 37°C for 15 min followed by 85°C for 5 s. The reaction products were stored at −20°C till further analysis.
Cloning of full-length cDNA of LwTf
To amplify the core sequence of LwTf gene, gene-specific primer pairs (Tf-F1/Tf-R1) were designed using Primer Premier 5.0 based on the previous transcriptome data of Amur ide (Chang et al. Citation2013). PCR was performed in a 25 μL reaction volume containing 18.3 μL of double-distilled water, 2.5 μL of 10× Ex Taq buffer, 1.0 μL of dNTP mix (2.5 μM), 1.0 μL of each primer (10 μM), 0.2 μL Ex Taq (TaKaRa), and 1.0 μL of cDNA (1300 ng/μL) as template. The PCR program was as follows: denaturation at 95°C for 1 min, followed by 35 cycles at 95°C for 15 s, 55°C for 1 min, and 72°C for 2 min. The reaction was ended by the final extension step for 7 min at 72°C. The full-length cDNA sequence was cloned using the SMARTer® RACE 5′/3′ Kit (TaKaRa), according to the core sequence of LwTf gene. The gene-specific nested primers for 5′-RACE (Tf-5′-out and Tf-5′-inner) and 3′-RACE (Tf-3′-outer and Tf-3′-inner) were designed based on the partial cDNA fragment (). The RACE reactions were conducted according to the manufacturer’s instructions. The PCR products were isolated using the DNA Gel Extraction Kit (OMEGA, USA), cloned into the pMD18-T vector (TaKaRa), and sequenced.
Table 1. Primers used for transferrin (Tf) gene cloning and expression analysis in Amur ide.
Bioinformatics analysis
The LwTf cDNA sequence was analyzed using BLAST (http://blast.ncbi.nlm.nih.gov/Blast), and then deposited to GenBank under accession number MN379464. The ORF of LwTf was predicted using ORF finder (http://www.ncbi.nlm.nih.gov/gorf/orfig.cgi). Protein domain features were predicted by SMART (http://smart.embl-heidelberg.de/). Signal peptides were predicted using SignalP5.0 (http://www.cbs.dtu.dk/services/SignalP/). The protein families, domains, and functional sites were determined using InterPro software (http://www.ebi.ac.uk/interpro/). The physical and chemical properties of the proteins were predicted by ProtParam (http://web.expasy.org/protparam/). Transferrin coding sequences from Amur ide and various species were retrieved from GenBank for multiple sequence alignment using Clustal Omega multiple alignment program (http://www.ebi.ac.uk/Tools/msa/clustalo/). A phylogenetic tree was reconstructed by the neighbour-joining method implemented in MEGA version 7.0 based on the sequence alignment using Clustal W and DNAMAN software. The branch supports were assessed with 10,000 bootstrap replications.
Quantitative real-time PCR (qPCR) and statistical analysis
Quantitative real-time PCR (qPCR) was used to determine the expression of LwTf in nine different tissues as well as its expression pattern in seven tissues associated with immunity after high alkaline challenge. First-strand cDNA was synthesized using PrimeScript® RT Reagent Kit with gDNA Eraser (TaKaRa). The cDNA was diluted to 1:10 and stored at −20°C. Primers used for qPCR are given in . Two LwTf gene-specific primers were used to amplify a 142 bp target product. In addition, primers for 18S ribosome RNA gene were used to amplify a 183 bp product as an internal control (Brown et al. Citation1983). qPCR was performed in triplicates for each sample using an ABI 7500 (Applied Biosystems, USA). The reaction was performed in a 20 μL reaction volume containing 10 μL of 2×TB Green Premix Ex TaqII (TaKaRa), 0.4 μL of 50×ROX Reference DyeII, 0.8 μL each of 10 μM forward and reverse primers, 2.0 μL of cDNA, and 6.0 μL of RNase-free water. qPCR was performed as follows: 95°C for 30 s, followed by 40 cycles of 95°C for 5 s, 60°C for 30 s, and a melt curve step (95°C for 15 s, 60°C for 1 min, and 95°C for 15 s). All samples were analyzed in triplicates. The variance of each replicate was tested in one group in order to sure there were no tank effects. The relative levels of LwTf were calculated using the 2(−ΔΔCT) method (Livak and Schmittgen Citation2001). Statistical analysis was performed with one-way ANOVA and Duncan’s variance significance tests for pairwise comparison, using SPSS 13.0 software. Differences were considered significant at P < 0.05.
Results
Identification and sequence analysis of LwTf
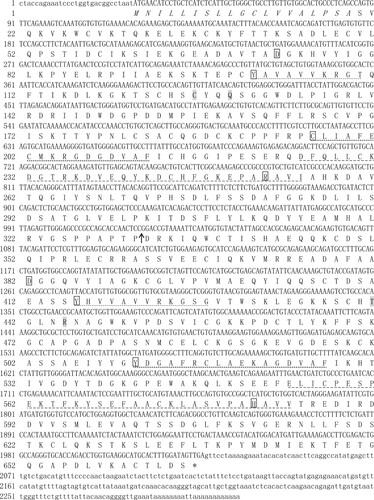
The full-length LwTf cDNA sequence (accession no. MN379464) contained an open reading frame (ORF) of 1998bp encoding 665 amino acids (aa), a 5′- untranslated region (UTR) of 29 bp, and a 3′-UTR of 279 bp with a stop codon (TAA) as well as a stop polyadenylation signal (AATAAA). The deduced protein possessed 665 residues with a putative 20-amino-acid N-terminal signal peptide. The mature transferrin protein had a theoretical molecular mass of 72.9 kDa and an isoelectric point of 6.36. In contrast to the mammalian transferrin gene, LwTf did not contain potential N-glycosylation sites, similar to that observed in cyprinid fish (Lin et al. Citation2007; Sahoo et al. Citation2009), but not in salmonid fish (Lee et al. Citation1998). LwTf contained two lobes (N-lobe: residues 24–331 and C-lobe: residues 335–658) of approximately 300–330 amino acids each (). There was a 31.34% homology between the two lobes of LwTf. InterPro software analysis showed two structural domains (N-lobe and C-lobe) of LwTf contained four iron-binding sites, two anion sites, and the signature features of the transferrin family, namely, transferrin signatures 1, 2, and 3, which suggested that LwTf also had the necessary structural properties to serve as an iron transport protein (Tu et al. Citation1991). The LwTf iron-binding sites at Asp73/Asp382, Tyrl01/Tyr416, Cys196/Tyr511, and Arg252/His581 corresponded to those of Homo sapiens transferrin in Asp82/Asp4ll and Tyrll5/Tyr445, Tyr207/Tyr536, and His268/His604, respectively, which are highly conserved. In addition to the iron binding sites in these two domains (N-lobe and C-lobe), there were several completely conserved amino acid sites ().
Figure 1. The mRNA and predicted amino acid sequence of Leuciscus waleckii transferrin (abbreviated as LwTf). The nucleotides and amino acids are indicated in the upper and lower row, respectively. The signal peptide sequence is in italics. The regions of the N-lobe and the C-lobe are indicated by an arrow. The four iron-binding sites in each lobe of transferrin are indicated by the panes, and the two anion sites in each lobe are shaded. The characteristic transferrin motifs are underlined as follows: transferrin signature 1, transferrin signature 2, and transferrin signature 3.
Homology and phylogenetic analysis
Analysis of the deduced amino acid sequence by multiple sequence alignment indicated that LwTf had the highest, viz. 73.04%, identity match with the transferrin protein sequence of silver carp (Hypophthalmichthys molitrix). LwTf shared 64–74% identity with transferrin protein sequences from cyprinid fish, including grass carp (Ctenopharyngodon idella), goldfish (Carassius auratus), common carp (Cyprinus carpio), bighead carp (Hypophthalmichthys nobilis), and zebrafish (Danio rerio), and 43–45% identity with mammalian (human and mouse) serum transferrin and lactoferrin (). BLAST homology search indicated that the transferrin gene is moderately conserved through evolution. Multiple sequence alignment showed more similarities in the C-lobe when aligned with seven other homologous sequences (). The number of the conserved amino acid sequences that are shaded at the end of the N-lobe was lower than that in the C-terminal region. To analyze the LwTf gene in the larger context of vertebrate genomes, a phylogenetic tree was constructed based on the amino acid sequences of 24 species (). The results of the evolutionary analysis showed that LwTf and genes encoding mammalian (Homo sapiens and Mus musculus) serum transferrin and lactoferrin, which are the two main members of the transferrin super-family, evolved from one common ancestor. LwTf was most closely related to the transferrin gene of cyprinid fish, which formed a separate sub-cluster relative to other fish, as the result of their identity BLAST. An NJ phylogenetic tree was constructed with other reported transferrin families (such as those from cyprinid fish, Ictalurus punctatus, Paralichthys olivaceus, Oryzias latipes, salmonid, amphibians, and mammals) to determine the evolutionary relationship between LwTf and transferrin genes from other species.
Figure 2. Alignment of amino acid sequences of the vertebrate transferrin genes. The conserved and identical residues are represented by black shading, and conservative substitutions are represented by gray shading. The N-lobe and C-lobe regions, which were predicted by the SMART program (http://www.smart.embl-heidelberg.de/), are indicated by arrows. Four iron-binding sites of each lobe of transferrin are indicated by plus signs, and two anion sites of each lobe are indicated by stars. Ol: Oryzias latipes, BAF81983.1; Cc: Cyprinus carpio, ACD99639.1; Hn: Hypophthalmichthys nobilis, ADJ67810.1; Ca: Carassius auratus, AAM90972.1; Dr: Danio rerio, AAH64001.2; Hs: Homo sapiens, NP_001054.2; Mm: Mus musculus, AAH92046.1; Lw: Leuciscus waleckii, MN379464.

Figure 3. Phylogenetic analysis of Leuciscus waleckii and other vertebrate transferrin genes. The phylogenetic tree was constructed based on ClustalW-generated multiple sequence alignment of amino acid sequences using the neighbor-joining method within the MEGA 7.0 package. The topological stability of the neighbor-joining trees was evaluated by 1000 bootstrapping replications, and the bootstrapping percentage values are indicated by numbers at the nodes. The GenBank accession number for each sequence is given after the species name.
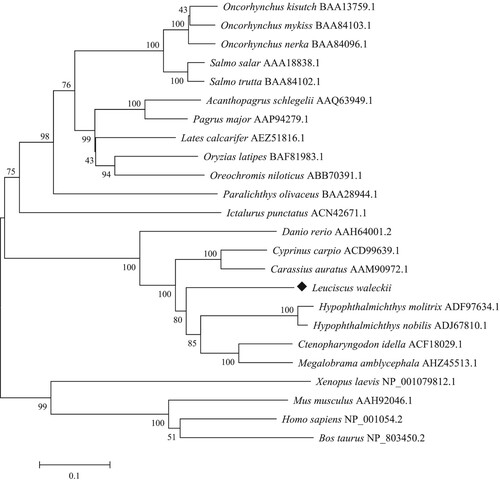
Table 2. Percent identity and similarity of transferrin amino acid sequences of Leuciscus waleckii to those of other organisms.
Expression analysis of LwTf in different tissues
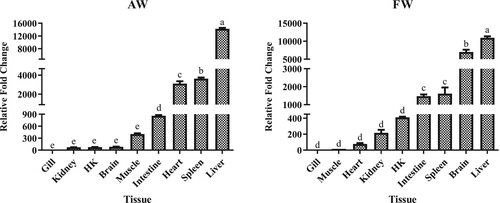
In AW, LwTf was expressed in all examined tissues and was particularly highly expressed in the liver, followed by spleen, heart, and intestine (, P < 0.05). Moderate expression levels were found in the muscle, brain, head kidney, and kidney. The lowest expression level was found in the gill. In FW as well, LwTf was expressed in all examined tissues and was found to be highly expressed in the liver, followed by the brain, spleen, and intestine (, P < 0.05). Moderate expression levels were found in the head kidney, kidney, heart, and muscle. A low expression level was also observed in the gill. Taken together, LwTf was highly expressed in classical immune-related organs (liver and spleen).
Figure 4. Gene expression analysis of LwTf in different tissues of alkali-adapted species (abbreviated as AW) and freshwater species (abbreviated as FW) in Amur ide. Expression levels were calculated against the tissue that had the lowest expression level, and the 18S rRNA housekeeping gene was used as a reference gene. HK is the abbreviation for head kidney. Significant differences are indicated by different letters (n = 3, P < 0.05).
Expression profiles of LwTf in AW after high alkaline stress
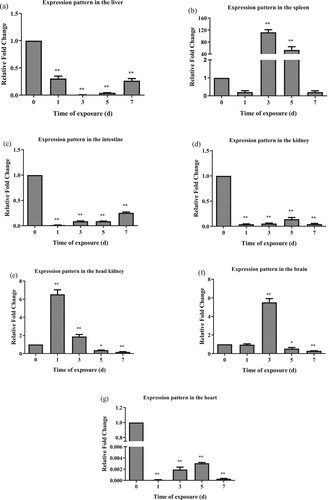
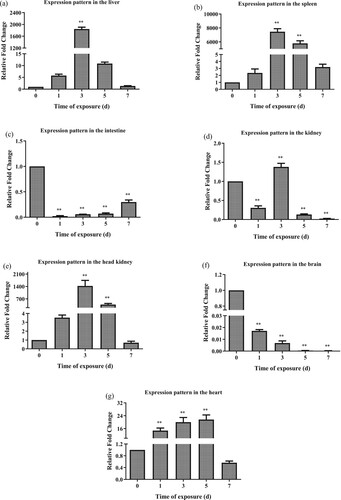
Transferrin modulation in seven tissues associated with immunity was explored to determine its involvement in the immune response of AW against high alkaline stress. In the liver, LwTf expression was significantly downregulated from day 1 to day 3 post-challenge compared with that in the control group (P < 0.01). Afterward, LwTf expression gradually recovered to the level observed on day 1. During the whole process of high alkaline stress, the expression level of LwTf was significantly downregulated ((a)). In the spleen, LwTf expression was decreased on day 1 post-challenge compared with that in the control group (P > 0.05). However, at 3 d to 5 d post-challenge, LwTf expression was significantly upregulated. The expression levels then returned to normal on day 7 post-challenge ((b)). During the whole process of high alkaline stress (from day 1–7), the expression level of LwTf was significantly downregulated in the intestine and kidney, as compared with that in the control group ((c,d)). In the head kidney, LwTf expression was significantly upregulated from day 1 to day 3 post-challenge as compared with that in the control group. However, on 5–7 days post-challenge, LwTf was significantly downregulated compared with that in the control group ((e)). In the brain, LwTf expression was significantly upregulated on day 3 post-challenge compared with that in the control group. However, on 5–7 days post-challenge, LwTf was significantly downregulated compared with that in the control group ((f)). In the heart, LwTf expression was significantly downregulated throughout the whole duration of high alkaline stress, although there was a slow recovery on day 3 and day 5 ((g)).
Figure 5. Time-course analysis of the LwTf expression patterns in alkali-adapted species (abbreviated as AW) after high alkaline stress treatment in the liver, spleen, intestine, kidney, head kidney, brain and heart using qRT-PCR. The samples were analyzed at 1, 3, 5, and 7 d post-treatment. Fold change was calculated by the change in expression at a given time point relative to the untreated control and normalized by the change in the 18S rRNA housekeeping gene. The results are presented as the mean ± SE of fold changes, and significant differences in different time points compared to the control (0 d) are indicated by asterisks (*P < 0.05, **P < 0.01).
Expression profiles of LwTf in FW after high alkaline stress
The expression profiles of LwTf in seven tissues associated with immunity in FW were determined to show the response of transferrin after high alkaline stress. In the liver, LwTf expression was significantly upregulated at day 3 post-challenge compared with that in the control group (P < 0.01). The expression levels then returned to normal on day 7 post-challenge ((a)). In the spleen, LwTf expression was found to have increased on day 1 post-challenge compared with that in the control group (P > 0.05). Furthermore, on days 3–5 post-challenge, LwTf expression was significantly upregulated. The expression levels then returned to normal on day 7 post-challenge ((b)). In the intestine and brain, during the whole duration of high alkaline stress (from days 1–7), the expression level of LwTf was significantly downregulated compared with that in the control group ((c,f)). In the kidney, LwTf expression was significantly downregulated on day 1 post-challenge compared with that in the control group. However, on day 3 post-challenge, LwTf expression was significantly upregulated compared with that in the control group. On day 5 post-challenge, a second decrease in the expression occurred. LwTf expression then returned to normal on day 7 post-challenge ((d)). In the head kidney and heart, LwTf expression was significantly upregulated on day 3 and day 5 post-challenge compared with that in the control group, although there was a slow recovery on day 7 post-challenge compared with that in the control group ((e,g)).
Figure 6. Time-course analysis of the LwTf transferrin expression patterns in freshwater species (abbreviated as FW) after high alkaline stress treatment in the liver, spleen, intestine, kidney, head kidney, brain and heart using qRT-PCR. The samples were analyzed at 1, 3, 5, and 7 d post-treatment. Fold change was calculated by the change in expression at a given time point relative to the untreated control and normalized by change in the 18S rRNA housekeeping gene. The results are presented as the mean ± SE of fold changes, and significant differences in different time points compared to the control (0 d) are indicated by asterisks (*P < 0.05, **P < 0.01).
Discussion
In this study, the transferrin gene of Amur ide was identified, cloned, sequenced, and characterized. LwTf was found to be highly conserved when compared with transferrin encoding genes from other species with two typical lobes (N- and C-lobes) and typical iron-binding domains, suggesting that it has the necessary structural properties to serve as an iron transport protein involved in normal physiological metabolism (Liu et al. Citation2010). The N- and C-lobes of transferrin can bind to iron ions alone and have been thought to be formed by gene duplication during evolution to enhance the transport of iron ions (Zak et al. Citation1995; He et al. Citation1997; Zak and Aisen Citation2003; Zhang et al. Citation2007). Homology analysis showed that there was 31.34% similarity between the two lobes of transferrin in Amur ide, compared with 40% and 36% in Homo sapiens and Pagrus major, respectively (Cai et al. Citation2005; Wang and Huang Citation2007). The LwTf iron-binding sites at Asp73/Asp382, Tyrl01/Tyr416, Cys196/Tyr511, and Arg252/His581 correspond to those of Homo sapiens transferrin at Asp82/Asp4ll, Tyrll5/Tyr445, Tyr207/Tyr536, and His268/His604, respectively, which are highly conserved (Liu et al. Citation2010, Citation2012). Of the four iron-binding sites, Tyr511 and His581 in the C-lobe were found to have conserved; in the N-lobe, Tyr and His were substituted with Cys and Arg, amino acids with chemical properties similar to those of Tyr and His, respectively, implying a selective preference that might indicate a functional advantage (Lambert et al. Citation2005). The results of the similarity study indicated that LwTf had high similarity with transferrin and lactoferrin in other animals at the nucleotide and amino acid levels. Among them, LwTf shared 64–74% identity with transferrin protein sequences from cyprinid fish and 43–45% identity with mammalian (human and mouse) serum transferrin and lactoferrin, which indicated that the transferrin gene has been moderately conserved through evolution (Dietrich et al. Citation2010; Gao et al. Citation2013; Herath et al. Citation2015; Perera et al. Citation2017). The phylogenetic tree constructed based on the amino acid sequences of 24 species demonstrated significant divergence between teleost fish and other vertebrates, which have been believed to evolve from one common ancestor (Park et al. Citation1985; Ong et al. Citation2006).
The constitutive expression of transferrin gene has also been identified in the previous work, with the highest expression in the liver of Cirrhinus mrigala in AW and FW (Sahoo et al. Citation2009). This finding may be related to the fact that transferrin is mainly synthesized in the liver tissue (Liu et al. Citation2010, Citation2012; García-Fernández et al. Citation2011). There was also a relatively high level of LwTf expression in the spleen, intestine, and heart in AW compared with that in the spleen, intestine, and brain in FW (P < 0.05). Previous studies have reported that transferrin expression is species-specific in the brains of teleost fish and mammals (Tu et al. Citation1991; Denovan-Wright et al. Citation1996; Sahoo et al. Citation2009). Different results for transferrin expression were observed in the brain tissues among various species (Kvingedal et al. Citation1993; Gao et al. Citation2013). In this study, the LwTf expression patterns in the brain were found to be opposite in AW and FW. Due to their long-term geographical isolation and environmental adaptability, Amur ide have formed two different geographic populations (AW and FW), which may be a reason for the different expression levels of LwTf in the brain. In vertebrates, with a relatively independent environment owing to the blood–brain barrier, transferrin is likely synthesized locally (Liu et al. Citation2012; Chen et al. Citation2014). However, delineating the underlying mechanism could contribute to a better understanding of why transferrin expression is observed in the brain of some fish species but not in other closely related species.
Amur ide usually inhabits freshwater environments but can also survive in the highly alkaline (up to pH 9.6) water of Dali-Nor lake, an extremely alkaline lake with HCO3–/CO32– concentration over 53.57 mmol/L. The fish has evolved special mechanisms to adapt to changes in alkalinity, although the mechanisms underlying its high tolerance to alkalinity are still largely unknown. Recently, a series of exploratory studies on the molecular mechanism of Amur ide′s adaptation to high alkalinity have been carried out in the context of several aspects of physiology and biochemistry, population genetics, and transcriptome (Xu, Li, et al. Citation2013; Cui et al. Citation2015). Many of these studies found that the expression of genes related to osmotic pressure regulation, acid–base balance, ion transport, and immunity was significantly increased under high alkaline stress (Xu et al. Citation2017; Chen et al. Citation2019). Interestingly, LwTf expression was closely related to the osmotic pressure. The FW Amur ide needs more LwTf expression to resist high alkalinity compared to the AW. Hence, the LwTf expression in FW was significantly upregulated from days 1–3 post-challenge. This finding suggested that although transferrin is constitutively expressed in the liver, its expression can be induced throughout the body under high alkaline stimulation, which is related to the extensive involvement of transferrin in body's immune defense function (Liu et al. Citation2012; Yin et al. Citation2018). As described above, the patterns of LwTf expression in seven tissues associated with the immunity of AW and FW were complicated. Consequently, further studies and functional analyses of the gene regulatory network of LwTf will help to provide important insights into the mechanisms that regulate LwTf expression upon high alkaline stress.
In conclusion, the full-length cDNA of LwTf was cloned and characterized. The results showed a high degree of conservation of gene and protein structure when compared with other fish species. The expression analyses demonstrated that LwTf was ubiquitously present in seven tested tissues associated with immunity and was highly expressed in classical immune organs (liver and spleen). Finally, it was interesting to find a significant upregulation of LwTf in the liver, spleen, intestine, kidney, head kidney, and heart in FW after high alkaline challenge. Thus, this protein might play a role in the immune system and help fish adapt to high alkalinity. The mechanisms involved in the function of LwTf during the adaptation of L. waleckii to high alkalinity need further investigation.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Brown JA, Taylor SM, Gray CJ. 1983. Glomerular ultrastructure of the trout, Salmo gairdneri. Cell Tissue Res. 230(1):205–218.
- Cai Z, Song L, Gao C, Wu L. 2005. Molecular cloning and expression characterization of transferrin (TF) gene in red seabream (Pagrus major). High Technol Lett. 15(5):105–110 (Abstract in English only).
- Chang Y, Tang R, Sun X, Liang L, Chen J, Huang J, Dou X, Tao R. 2013. Genetic analysis of population differentiation and adaptation in Leuciscus waleckii. Genetica. 141(10–12):417–429.
- Chang YM, Tang R, Dou XJ, Tao R, Sun XW, Liang LQ. 2014. Transcriptome and expression profiling analysis of Leuciscus waleckii: an exploration of the alkali-adapted mechanisms of a freshwater teleost. Mol Biosyst. 10(3):491–504.
- Chen B, Xu J, Cui J, Pu F, Peng W, Chen L, Xu P. 2019. Transcriptional differences provide insight into environmental acclimatization in wild Amur ide (Leuciscus waleckii)during spawning migration from alkalized lake to freshwater river. Genomics. 111(3):267–276.
- Chen JH, Wang CH, Li YL, Wang HM, Zhang XJ, Yan BL. 2014. cDNA cloning and expression characterization of serum transferrin gene from oriental weatherfish Misgurnus anguillicaudatus. J Fish Biol. 84(4):885–896.
- Ciuraszkiewicz J, Biczycki M, Maluta A, Martin S, Wątorek W, Olczak M. 2007. Reptilian transferrins: evolution of disulphide bridges and conservation of iron-binding center. Gene. 396(1):28–38.
- Cui J, Xu J, Zhang S, Wang K, Jiang Y, Mahboob S, Al-Ghanim KA, Xu P. 2015. Transcriptional profiling reveals differential gene expression of Amur ide (Leuciscus waleckii) during spawning migration. Int J Mol Sci. 16(6):13959–13972.
- Denovan-Wright EM, Ramsey NB, McCormick CJ, Lazier CB, Wright JM. 1996. Nucleotide sequence of transferrin cDNAs and tissue-specific expression of the transferrin gene in Atlantic cod (Gadus morhua). Comp Biochem Physiol B Biochem Mol Biol. 113(2):269–273.
- Dietrich MA, Zmijewski D, Karol H, Hejmej A, Bilińska B, Jurecka P, Irnazarow I, Słowińska M, Hliwa P, Ciereszko A. 2010. Isolation and characterization of transferrin from common carp (Cyprinus carpio L) seminal plasma. Fish Shellfish Immunol. 29(1):66–74.
- Gao J, Ding S, Huang X, Shi X. 2013. Cloning and expression characterization of the serum transferrin gene in the Chinese black sleeper (Bostrichthys sinensis). Gene. 515(1):89–98.
- García-Fernández C, Sánchez JA, Blanco G. 2011. Characterization of the gilthead seabream (Sparus aurata L.) transferrin gene: genomic structure, constitutive expression and SNP variation. Fish Shellfish Immunol. 31(4):548–556.
- Graham I, Williams J. 1975. A comparsion of glycopeptides from the transferrins of several species. Biochem J. 145(2):263–279.
- He QY, Mason AB, Woodworth RC. 1997. Iron release from recombinant N-lobe and single point Asp63 mutants of human transferrin by EDTA. Biochem J. 328(2):439–445.
- Herath HM, Elvitigala DA, Godahewa GI, Whang I, Lee J. 2015. Molecular insights into a molluscan transferrin homolog identified from disk abalone (Haliotis discus discus) evidencing its detectable role in host antibacterial defense. Dev Comp Immunol. 53(1):222–233.
- Kvingedal AM, Rørvik KA, Alestrøm P. 1993. Cloning and characterization of Atlantic salmon (Salmo salar) serum transferrin cDNA. Mol Mar Biol Biotechnol. 2(4):233–238.
- Lambert LA, Perri H, Halbrooks PJ, Mason AB. 2005. Evolution of the transferrin family: conservation of residues associated with iron and anion binding. Comp Biochem Physiol B Biochem Mol Biol. 142(2):129–141.
- Lee JY, Tada T, Hirono I, Aoki T. 1998. Molecular cloning and evolution of transferrin cDNAs in salmonids. Mol Mar Biol Biotechnol. 7(4):287–293.
- Lin B, Chen S, Cao Z, Lin Y, Mo D, Zhang H, Gu J, Dong M, Liu Z, Xu A. 2007. Acute phase response in zebrafish upon Aeromonas salmonicida and Staphylococcus aureus infection: striking similarities and obvious differences with mammals. Mol Immunol. 44(4):295–301.
- Liu H, Takano T, Abernathy J, Wang S, Sha Z, Jiang Y, Terhune J, Kucuktas H, Peatman E, Liu Z. 2010. Structure and expression of transferrin gene of channel catfish, Ictalurus punctatus. Fish Shellfish Immunol. 28(1):159–166.
- Liu Y, Yu S, Chai Y, Zhu Q. 2012. Transferrin gene expression in response to LPS challenge and heavy metal exposure in roughskin sculpin (Trachidermus fasciatus). Fish Shellfish Immunol. 32(1):223–229.
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 25(4):402–408.
- Neves JV, Wilson JM, Rodrigues PNS. 2009. Transferrin and ferritin response to bacterial infection: the role of the liver and brain in fish. Dev Comp Immunol. 33(7):848–857.
- Ong ST, Ho JZS, Ho B, Ding JL. 2006. Iron-withholding strategy in innate immunity. Immunobiology. 211(4):295–314.
- Park I, Schaeffer E, Sidoli A, Baralle FE, Cohen GN, Zakin MM. 1985. Organization of the human transferrin gene: direct evidence that it originated by gene duplication. Proc Natl Acad Sci USA. 82(10):3149–3153.
- Perera NC, Godahewa GI, Hwang JY, Kwon MG, Hwang SD, Lee J. 2017. Molecular, structural, and functional comparison of N lobe and C lobe of the transferrin from rock bream, Oplegnathus fasciatus, with respect to its immune response. Fish Shellfish Immunol. 68:299–309.
- Rengmark AH, Lingaas F. 2007. Genomic structure of the Nile tilapia (Oreochromis niloticus) transferrin gene and a haplotype associated with saltwater tolerance. Aquaculture. 272(1–4):146–155.
- Sahoo PK, Mohanty BR, Kumari J, Barat A, Sarangi N. 2009. Cloning, nucleotide sequence and phylogenetic analyses, and tissue-specific expression of the transferrin gene in Cirrhinus mrigala infected with Aeromonas hydrophila. Comp Immunol Microbiol Infect Dis. 32(6):527–537.
- Schreiber G, Dryburgh H, Millership A, Matsuda Y, Inglis A, Phillips J, Edwards K, Maggs J. 1979. The synthesis and secretion of rat transferrin. J Biol Chem. 254(23):12013–12019.
- Stafford JL, Belosevic M. 2003. Transferrin and the innate immune response of fish: identification of a novel mechanism of macrophage activation. Dev Comp Immunol. 27(6–7):539–554.
- Tange N, Jong-Young L, Mikawa N, Hirono I, Aoki T. 1997. Cloning and characterization of transferrin cDNA and rapid detection of transferrin gene polymorphism in rainbow trout (Oncorhynchus mykiss). Mol Mar Biol Biotechnol. 6(4):351–356.
- Tu GF, Achen MG, Aldred AR, Southwell BR, Schreiber G. 1991. The distribution of cerebral expression of the transferrin gene is species specific. J Biol Chem. 266(10):6201–6208.
- Wang F, Huang LY. 2007. Cloning and sequence analysis of human transferrin gene. J Jinan Univ. 28(2):111–119 (Abstract in English only).
- Xu J, Ji P, Wang B, Zhao L, Wang J, Zhao Z, Zhang Y, Li J, Xu P, Sun X. 2013. Transcriptome sequencing and analysis of wild Amur ide (Leuciscus waleckii) inhabiting an extreme Alkaline-Saline lake reveals insights into stress adaptation. PLoS One. 8(4):e59703.
- Xu J, Li JT, Jiang Y, Peng W, Yao Z, Chen B, Jiang L, Feng J, Ji P, Liu G, et al. 2017. Genomic basis of adaptive evolution: the survival of Amur ide (Leuciscus waleckii) in an extremely alkaline environment. Mol Biol Evol. 34(1):145–159.
- Xu J, Li Q, Xu L, Wang S, Jiang Y, Zhao Z, Zhang Y, Li J, Dong C, Xu P, Sun X. 2013. Gene expression changes leading extreme alkaline tolerance in Amur ide (Leuciscus waleckii) inhabiting soda lake. BMC Genom. 14(1):682.
- Yang F, Lum JB, McGill JR, Moore CM, Naylor SL, van Bragt PH, Baldwin WD, Bowman BH. 1984. Human transferrin: cDNA characterization and chromosomal localization. Proc Natl Acad Sci USA. 81(9):2752–2756.
- Yin X, Mu L, Bian X, Wu L, Li B, Liu J, Guo Z, Ye J. 2018. Expression and functional characterization of transferrin in Nile tilapia (Oreochromis niloticus) in response to bacterial infection. Fish Shellfish Immunol. 74:530–539.
- Zak O, Aisen P. 2003. Iron release from transferrin, its C-Lobe, and their complexes with transferrin receptor: Presence of N-lobe accelerates release from C-Lobe at endosomal pH. Biochemistry. 42(42):12330–12334.
- Zak O, Aisen P, Crawley JB, Joannou CL, Patel KJ, Rafiq M, Evans RW. 1995. Iron release from recombinant N-lobe and mutants of human transferring. Biochemistry. 34(44):14428–14434.
- Zhang XJ, Qu G, Zhu WL, Zhang L, Wang J, Wu J, Liu HY, Chen F, Xu H. 2007. Construction of intestinal cDNA library and analysis of some expressed sequence tags sequencing of Ctenopharyngodon idellus. Acta Hydrobiol Sin. 31(2):251–258 (Abstract in English only).
