ABSTRACT
The present study aimed to determine the effects of dietary energy levels on follicular development and in vitro maturation of oocytes in pre-pubertal goats. Thirty-six female six-month-old stall-fed Black Bengal goats were divided into three equal groups that were randomly assigned to receive three iso-nitrogenous diets containing different levels of metabolizable energy (8.67, 10.20, and 11.73 MJ/kg DM). After 150 days of rearing, the weight, length and width of the ovaries increased significantly in medium and high levels of dietary energy groups than in the low-energy group. Medium and high-energy levels in the diet significantly increased the number of visible antral follicles and large antral follicles than the low level of dietary energy. The number of antral follicles per ovary was higher in high-energy fed goats than medium- and low-energy groups. The low-energy group showed more non-growing primordial follicles and degenerated follicles than the other groups. The medium- and high-dietary energy levels significantly increased the maturation rate of oocytes to metaphase II than the low-energy group. These results reveal that the level of dietary energy positively influences follicular development and meiotic competence of goat oocytes.
1. Introduction
The ovarian follicle is an integral part of the reproductive system in mammalian species. Primordial follicles are the most abundant and least developed follicles of the ovary. Each follicle consists of a primary oocyte surrounded by a single layer of crescent-shaped simple squamous follicular cells. When they start growing, their granulosa cells transform into a cuboidal form. Then the follicles were developed to secondary and finally Graafian follicles or pre-ovulatory (antral) follicles. However, thousands of primordial follicles present at birth, a large number of them become atretic during growth, and only a small number achieve maturity and ovulate (Baker Citation1963).
Oocytes are the fundamental unit of female reproduction. Fertilization of oocytes is mandatory for development into embryos. Before fertilization occurs, oocytes must be matured. Their maturation involves a series of steps that start from the germinal vesicle (GV) stage and end the second meiotic division forming a polar body (Maller and Krebs Citation1980). Maturation of oocytes depends on several factors, including diameters of oocytes and follicles, cumulus morphology and overall reproductive status of animals (Vassena et al. Citation2003; Yuan et al. Citation2005; Duarte et al. Citation2008). The maturation of oocytes and their subsequent development may be affected by numerous factors, including nutritional status and age of the animal (Skinner Citation2005). It has been reported that the lower plane of nutrition affects ovarian follicle development in ewes (Alexander et al. Citation2007). Butler and Smith (Citation1989) have revealed that lower dietary intake often shows failure or delay in ovarian cyclicity, follicular growth and ovulation in dairy cattle. Luteinizing hormone pulsatility and follicular development are affected in energy-deficient pigs (Zak et al. Citation1997; Quesnel et al. Citation1998). However, little is known regarding the influence of dietary energy levels on ovarian development, oocyte maturation and degeneration of oocytes and ovarian follicles in ruminants.
Black Bengal goat is a promising dwarf genotype distributed in Bangladesh and India. They are popular for their high prolificacy, high-quality meat and skin and adaptability in hot and humid environments. They often have infertility due to abnormalities in their reproductive organs. Amin et al. (Citation2005) reported that the infertility of these goats is caused by lower numbers of ovarian follicles. Low energy intake is a severe problem in goat production in tropical countries (McGregor Citation1985). Poor farmers usually raise their goats by grazing on harvested fallow lands, along the roads and canal sides without any supplementation. This system of rearing cannot satisfy the nutrient requirements for growth and reproduction. However, the effects of dietary energy on reproduction especially ovarian follicular development and oocyte maturation of these goats are not well known. Therefore, the present study was designed to investigate the effect of dietary energy levels on follicular development and in vitro maturation status of oocytes in Black Bengal goats.
2. Materials and methods
2.1. Chemicals
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA), unless otherwise specified.
2.2. Animals and their management
The experiment was conducted with thirty-six female pre-pubertal six-month old Black Bengal goats. Their initial body weight ranged from 6 to 8 kg. The goats were identified with ear tags. They were grouped and housed in three separate pens with 14.67 square feet of floor space for individual goats. Anthelmintic drug (Dovenix, Advance Pharmaceuticals Company Limited, Hong Kong) was administered according to the manufacturer’s instructions to de-worm the goats. Goats were vaccinated against the Peste des Petis Ruminants (PPR) supplied by the local Livestock department. Roughage and concentrate feeds were supplied in separate troughs. Every day the floor, feeder and water trough were cleaned using phenyl. Goats were allowed for 4 weeks of adjustment with the experimental conditions and diets.
2.3. Experimental diets and feeding of goats
Three diets were formulated using commonly available feed ingredients (). The medium energy diet (a standard requirement for goats, control group) was formulated to supply 10.2 MJ ME/Kg dry matter (DM) (NRC, Citation1981). Other diets contained high energy (11.73 MJ ME/Kg DM) and low energy (8.67 MJ ME/Kg DM). These diets were iso-nitrogenous, containing 14% crude protein on a DM basis. High and low energy differed from medium one by 15%. The ingredients’ proportions in each type of diet and their energy content are given in .
Table 1. Ingredients and chemical composition of the experimental diets (DM basis).
All the goats were stall-fed. Diets were adjusted for the required nutrient based on the live weight changes of the animals in all treatments at the end of every fifteen days. The concentrate mixture was supplied first, and then green grass was offered. The ratio was supplied in two equal parts in the morning and the evening. Fresh drinking water was supplied in buckets at all times for ad-libitum drinking. Every morning and every evening before feeding the animals, each feed was weighed carefully, and the total quantity of feed supplied to the animals was recorded. Before supplying the feed to the animals, the quantity of leftovers of feed of the previous day was collected, weighed and recorded. The leftover feed by an individual animal during 24 h was deducted from the feed supplied, and was recorded as the daily feed intake.
2.4. Collection of ovaries
The goats were sacrificed after 150 days of feeding them on three different diets. After collection, the ovaries were transported to the laboratory within 1 h. Adipose tissues surrounding the ovaries were removed carefully. Slide Calipers measured the length and width of each ovary, and the weight was also recorded. Visible antral follicles and follicles with more than 4 mm in diameter were counted and recorded. One ovary from each goat was prepared for histological examination, and the other was used to collect oocytes for in vitro maturation.
2.5. Preparation of histological section
Histological examination was conducted following the procedure described previously with some modifications (Sarker et al. Citation2015). Briefly, ovaries were fixed in Bouin’s solution for 8 h. After washing in Dulbecco’s phosphate-buffered saline (DPBS), the ovarian tissues were dehydrated in alcohol, cleared in xylene and embedded in paraffin. Serial sections of 5 μm diameter were prepared by a rotatory microtome. Sections were de-paraffinized in xylene and rehydrated by alcohol. Then, the sections were stained with haematoxylin and eosin. Finally, the stained sections were permanently mounted with a coverslip using a mixture of distyrene, a plasticizer and xylene (DPX mountant for histology; Merck Specialities Private Limited, Mumbai, India).
2.6. Microscopic observations
From the serial sections, every fifth section was observed by light microscope. The numbers of different stages of follicles were recorded. The follicles were counted in the section where the oocyte nucleus was seen. Double counting was avoided. The follicles were classified into four categories according to the number and morphology of granulosa cell layers as follows:
Primordial follicles with a single layer of flattened granulosa cells surrounding the oocyte.
Primary follicles with a single layer of cuboidal granulosa cells.
Secondary follicles with two or more layers of granulosa cells but no antrum.
Antral follicles with an antral cavity with multiple layers of granulosa cells
A follicle was considered degenerated if its oocyte showed the sign of pyknosis, large vacuoles, condensed cytoplasm, disappearance of nuclear membranes, shrinkage of a nucleus, swollen granulosa cells, or loss of granulosa cells.
2.7. In vitro maturation of oocytes
Ovaries were washed once in DPBS containing gentamicin sulphate (0.08 mg/mL) and followed three times in DPBS. Large antral follicles (4–6 mm in diameter) were dissected from ovaries using a pair of surgical blades (No.11, Keisei Medical Industrial Co., Ltd, Tokyo, Japan) and placed in 20 mM2-4[4-(2-Hydroxyethyl)-1-piperazinyl] ethane sulphonic acid (HEPES) buffered (pH 7.4) medium 199 (TCM-199: Nissui Pharmaceutical, Tokyo, Japan) containing 0.85 mg/mL NaHCO3and 0.08 mg/mL gentamicin sulphate. The follicles were opened using a pair of forceps in HEPES under a dissection microscope, and cumulus-oocyte complexes (COCs) were collected. COCs with abnormal oocyte cytoplasm partially expanded cumulus cells, or degenerated/abnormal cumulus cells were discarded from the experiment. In vitro maturation was done according to Islam et al. (Citation2020) with some modifications. Briefly, the basic medium for oocyte maturation was TCM-199 supplemented with 5% (v/v) fetal bovine serum (FBS), 100 ng/mL follicle-stimulating hormone (FSH; NIDDK, Washington, DC, USA), 0.1 mg/mL sodium pyruvate and 0.08 mg/mL gentamicin sulphate. COCs were incubated at 38.5°C under 5% CO2 in humidified air for 24 hrs.
2.8. Assessment of meiotic progress
The oocytes were denuded mechanically using a small-bore glass pipette by adding 0.1% (w/v) hyaluronidase. Oocytes were fixed in aceto-ethanol (acetic acid: ethanol = 1:3) for 48 h and stained with 1% (w/v) aceto-orecin. The stages of meiotic division were assessed using a differential interference contrast microscope (Olympus, USA). Oocytes were classified based on the morphology of the chromatin and nuclear envelope according to previous reports (Motlik et al. Citation1978; Alam et al. Citation2018). In this experiment, chromosomal configurations after germinal vesicle breakdown were classified as early diakinesis, late diakinesis, metaphase I and metaphase II. Oocytes with abnormalities in the cytoplasm or chromatin configuration were designated as degenerated ones.
2.9. Statistical analysis
Data are presented as mean ± SD (Standard Deviation). Data were analysed using ‘MSTAT’ statistical programme to compute the analysis of variance (ANOVA) for CRD. The means were compared with Duncan’s Multiple Range Test (DMRT). Differences at P< .05 were considered statistically significant.
3. Results
3.1. Morphology of ovaries
The morphological features of ovaries are shown in . The weight of ovaries increased significantly in the goats-fed medium and higher than the low level of dietary energy (). Similarly, medium and high levels of dietary energy significantly increased the length and width of ovaries than the low level of dietary energy-fed goat group. During the morphological assessment of ovaries, visible differences were observed in the number of antral follicles in ovaries of goats fed different levels of dietary energy. Thus, all visible antral follicles and large antral follicles (>4 mm in diameter) were further examined. The visible antral follicles and large antral follicles (>4 mm in diameter) were significantly higher in ovaries of the goats-fed medium and high levels of dietary energy than the low level of dietary energy.
Table 2. Morphology of ovaries from goats fed different levels of dietary energy.
3.2. Development of ovarian follicles
Typical histological sections of ovaries from goat-fed different dietary energy levels are shown in . Histological examination of ovaries revealed that the proportion of primordial follicles was higher (P < .05) in goats consuming the low-energy diet compared to other diets (). The percentage of primary follicles was higher (P < .05) in the medium energy-fed group than others. The percentage of secondary follicles was higher in high-energy-fed goats than others although those did not differ between medium- and low-energy groups. Similarly, a significantly increased number of antral follicles were obtained from the high-energy-offered goats than medium- and low-energy diet groups.
Figure 1. Typical histological sections from the ovaries of goats fed different levels of dietary energy. From top to down, it represents ovaries from low-energy diet (A), medium-energy diet (B), and high-energy diet (C), respectively. Scale bars represent 200 µm.
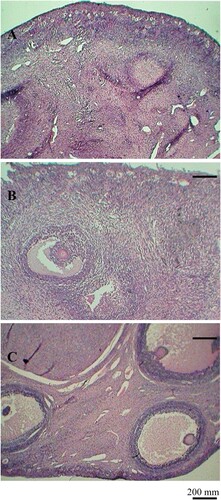
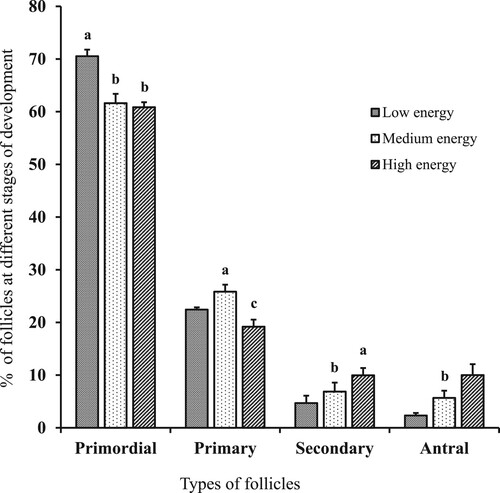
Figure 2. Percentages of different types of follicles in the ovaries of goats fed different levels of dietary energy. Ovaries were fixed in Bouin’s solution, dehydrated in alcohol, cleared in xylene and embedded in paraffin. Serial sections of five µm thickness from the ovarian tissue were de-paraffinized in xylene, rehydrated by alcohol and stained with haematoxylene and eosine. The follicles were counted in the section where the oocyte nucleus avoided double counting. a-cValues with different superscripts are significantly different within the same follicular stage (P< .05).
3.3. Degeneration of ovarian follicles
The degenerated primordial, secondary and antral follicles were significantly higher in goats that received a low-energy diet than a high-energy diet (). Typical morphologies of different types of degenerated follicles in goats from the low-energy group, are shown in . However, there were no significant differences in the number of degenerated primary follicles among the treatment groups.
Figure 3. Typical morphologies of degenerated follicles in ovaries of goats fed different levels of dietary energy. Arrows indicated degenerated primary (A), secondary (B) and antral follicles (C), respectively. Scale bars represent 40 (A), 100 (B) and 200 µm(C), respectively.
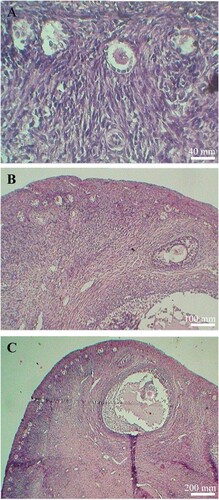
Table 3. Effect of dietary energy levels on the degeneration of ovarian follicles in goats.
3.4 In vitro maturation of the oocyte
Since the level of dietary energy significantly influences the follicular development of goats, oocytes collected from these goats were further subjected to in vitro maturation to examine their maturation competence. Here, oocytes from all groups resumed meiosis and progressed their meiotic division in vitro. However, the percentage of oocytes matured to the metaphase II (MII) stage was lower in low-energy-offered goats than medium- and high-energy groups ( and ).
Figure 4. In vitro meiotic competence of oocytes collected from goats fed different levels of dietary energy. From top to down, it represents low (A), medium (B) and high (C) level of energy in the experimental diet. Large antral follicles (4–6 mm in diameter) were dissected from ovaries, and follicles were opened using a pair of forceps, and cumulus-oocyte complexes (COCs) were collected. COCs were cultured in TCM-199 supplemented with FBS, FSH, sodium pyruvate and gentamicin sulphate at 38.5°C under 5% CO2 in humidified air for 24 hrs. After denudation, oocytes were fixed in aceto-ethanol (acetic acid: ethanol = 1:3) for 48 h and stained with 1% (w/v) aceto-orecin. Chromosomes were indicated by arrows. Scale bars represent 10 µm.
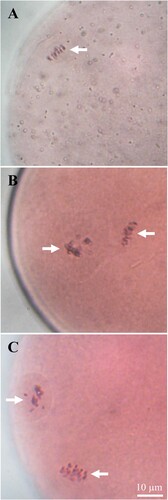
Table 4. In vitro maturation of oocytes collected from goats offered different levels of dietary energy.
4. Discussion
In mammals, ovarian folliculogenesis is an essential step of reproduction. At the beginning of this process, primordial follicles change the shape of their granulosa cell from flattened to cuboidal to form primary follicles. Additional granulosa cell layers are further added to the primary follicles to produce secondary follicles. An antrum (space between the granulosa cell layers) is formed inside the follicles to form the antral follicle stage. Several factors regulate ovarian folliculogenesis in domestic animals (Webb et al. Citation2003; Hernandez-Medrano et al. Citation2012). Several reports reveal the effects of nutrition on ovarian follicular development. Dietary intake positively regulates the development of bovine ovarian follicles (Murphy et al. Citation1991; Gutiérrez et al. Citation1997; Mackey et al. Citation1999). The present study demonstrated that a higher level of dietary energy increased follicular development in goats. A high level of dietary energy increased ovarian antral follicle count in heifer (Maurasse et al. Citation1985). A more increase in dietary energy level (12.5%) than a normal requirement in gilt has shown greater follicular development (Zhou et al. Citation2010). A schematic model for the regulation of folliculogenesis by nutrition has been proposed by Scaramuzzi et al. (Citation2006). According to that model, high energy creates static and acute effects that control ovarian follicular development. In the static condition, high-energy diet increases leptin concentration in the blood of the animals. Leptin induces gonadotropin hormone secretion by hypothalamic and pituitary actions in vivo and in vitro conditions (Yu et al. Citation1997). Gonadotropin hormones are the primary regulatory hormones for folliculogenesis. In the present study, increased follicular development in goats of high-energy diet might be associated with the actions of leptin and gonadotropins.
Here, a low level of dietary energy significantly increased degenerated follicles in goat ovaries. Underfeeding goats for 24 weeks increased the follicular atresia (degeneration) rate (Rondina et al. Citation2017). They suggested that underfeeding reduced blood flow to the follicular part, which induced follicular atresia. Reduced ovarian stromal blood flow affects perifollicular vascularization resulting in oxidative stress and follicular atresia (Chan et al. Citation2015). This supports the degeneration of follicles in low dietary energy-consuming goats.
The morphological study revealed that ovaries’ weight, length and width increased in goats of medium- and high-dietary energy groups. A low level of dietary energy decreased antral follicles in the ovaries of beef cows (Perry et al. Citation1991) during the postpartum period. In our study, the antral follicles decreased in the low-energy group. The average volume of follicular fluid in a large antral follicle in human ovaries before ovulation is 2.7 mL (Simonetti et al. Citation1985). Since most of the antral follicles remain in the cortex (outer part of an ovary) and protrude from ovaries they accompany a significant amount of follicular fluid. Thus, in the present study, the ovaries’ length, width and weights of low-energy-fed goats were lower than those of the high- and medium-energy groups.
The present study showed that in vitro maturation of oocytes depends on the levels of dietary energy in goats. Increased proportions of oocytes from medium- and high-energy groups reached to metaphase II (MII) stage compared to the low-energy-fed goats. This indicated that oocytes from the medium- and high-energy receiving goats had greater meiotic competence than those from low dietary treatment groups. High-energy diet increases the concentration of insulin-like growth factor-1 (IGF-I) in blood and ovarian follicular fluids and IGF-I significantly contributes to the nuclear maturation of oocytes (Zhou et al. Citation2010). In the present study, energy deficiency might decrease the IGF-I level in blood and follicular fluid of goats that ultimately affect in vitro maturation oocytes. Further study is required to elucidate this poor developmental competence of goat oocytes induced by energy deficiency. Moreover, an appropriate culture method is important to obtain the desired rate of oocyte development in vitro. During the last few decades tremendous efforts have been made to develop culture methods for oocyte development in vitro. So far the methods used for in vitro maturation of oocytes have low reproducibility. Recently it has been shown that 3D in vitro maturation improves nuclear and cytoplasmic maturation of oocytes, which can be used for clinical and toxicological applications (Mastrorocco et al. Citation2020). Thus, such a 3D in vitro maturation system might be used as an alternative to overcome low reproducibility in goat feeding in the case of constrained resources.
5. Conclusion
The results show that the dietary level of energy positively influences ovarian morphological parameters, follicular development and subsequent in vitro maturation of oocytes in goats.
Acknowledgements
The authors are thankful to Dr. Ralph Behrendt, Senior Research Scientist-Livestock system, Agriculture Research & Development, Department of Economic Development, Jobs, Transport and Resources, 915 Mt. Napier Road, Private Bag 105, Hamilton, Victoria 3300, Australia, for his critical suggestions for improvement of this manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Alam MH, Lee J, Miyano T. 2018. Inhibition of PDE3A sustains meiotic arrest and gap junction of bovine growing oocytes in in vitro growth culture. Theriogenology. 118:110–118. https://doi.org/10.1016/j.theriogenology.2018.05.028
- Alexander BM, Kiyma Z, McFarland M, Van Kirk EA, Hallford DM, Hawkins DE, Kane KK, Moss GE. 2007. Influence of short-term fasting during the luteal phase of the estrous cycle on ovarian follicular development during the ensuing proestrus of ewes. Anim Reprod Sci. 97:356–363. https://doi.org/10.1016/j.anireprosci.2006.01.012
- Amin MR, Salim MS, Khandoker MAMY, Hossain MM. 2005. Causes of infertility in Black Bengal does investigated by anatomical and histological tools. Prog Agri. 16:117–124.
- Baker TG. 1963. A qualitative and cytogenetical study of germ cells in human ovaries. Proc. Reprod. Soc. London (Biology). 158:417–433.
- Banerjee GC. 1978. Feeds and Principles of animal nutrition. Calcutta, New Delhi: Oxford and IBH Publishing Company. https://www.amazon.com/Feeds-Principles-Animal-Nutrition-Banerjee/dp/8120401913.
- Butler WP, Smith RD. 1989. Interrelationship between energy balance and postpartum reproductive function in dairy cattle. J Dairy Sci. 72:767–783. https://doi.org/10.3168/jds.S0022-0302(89)79169-4
- Chan KA, Bernal AB, Vickers MH, Gohir W, Petrik JJ, Sloboda DM. 2015. Early life exposure to undernutrition induces ER stress, apoptosis, and reduced vascularization in ovaries of adult rat offspring. Biol Reprod. 92(110):1–14.
- Duarte G, Flores JA, Malpaux B, Delgadillo JA. 2008. Reproductive seasonality in female goats adapted to a subtropical environment persists independently of food availability. Domest Anim Endocrinol. 35:362–370. https://doi.org/10.1016/j.domaniend.2008.07.005
- Gutiérrez CG, Oldham J, Bramley TA, Gong JG, Campbell BK, Webb R. 1997. The recruitment of ovarian follicles is enhanced by increased dietary intake in heifers. J Anim Sci. 75:1876–1884. https://doi.org/10.2527/1997.7571876x
- Hernandez-Medrano JH, Campbell BK, Webb R. 2012. Nutritional influences on folliculogenesis. Reprod Domest Anim. 47 (Suppl. 4):274–282. https://doi.org/10.1111/j.1439-0531.2012.02086.x
- Islam MN, Alam MH, Khatun A, Akter I, Modak AK, Hashem MA, Moniruzzaman M. 2020. Effects of stem cell factor on in vitro growth of buffalo oocytes. Theriogenology. 142:114–119. https://doi.org/10.1016/j.theriogenology.2019.09.044
- Khan J, Wangehuk K, Sampath KT, Poadyal SM, Habib G, Samarasinghe K. 2008. Best practices in animal feed production and management in SAARC countries. Bangladesh, Dhaka: SAARC Agriculture Centre.
- Mackey DR, Sreenan JM, Roche JF, Diskin MG. 1999. Effect of acute nutritional restriction on incidence of anovulation and periovulatory estradiol and gonadotropin concentrations in beef heifers. Biol Reprod. 61:1601–1607. https://doi.org/10.1095/biolreprod61.6.1601
- Maller JL, Krebs EG. 1980. Regulation of oocyte maturation. Curr Top Cell Regul. 16:271–311. https://doi.org/10.1016/B978-0-12-152816-4.50012-1
- Mastrorocco A, Cacopardo L, Martino NA, Fanelli D, Camillo F, Ciani E, Roelen BA, Ahluwalia A, Dell’Aquila ME. 2020. One-step automated bioprinting-based method for cumulus-oocyte complex microencapsulation for 3D in vitromaturation. PloS one. 15(9):e0238812. https://doi.org/10.1371/journal.pone.0238812
- Maurasse C, Matton P, Dufour JJ. 1985. Ovarian follicular populations at two stages of an estrous cycle in heifers given high energy diets. J Anim Sci. 61:1194–1200. https://doi.org/10.2527/jas1985.6151194x
- McGregor BA. 1985. Growth, development and carcass composition of goats: a review, in goat production and research in the tropics: proceedings of a workshop held at the University of queensland, brisbane, Australia, 6-8 February 1984. Canberra: Australian Centre for International Agricultural Research. A. C. T., 82-90. http://dro.deakin.edu.au/view/DU: 30065951.
- Motlik J, Koefoed-Johnsen HH, Fulka J. 1978. Breakdown of the germinal vesicle in bovine oocytes cultivated in vitro. J Expt Zool. 205:377–383. https://doi.org/10.1002/jez.1402050306
- Murphy MG, Enright WJ, Crowe MA, McConnell K, Spicer LJ, Boland MP, Roche JF. 1991. Effect of dietary intake on pattern of growth of dominant follicles during the oestrous cycle in beef heifers. J Reprod Fertil. 92:333–338. https://doi.org/10.1530/jrf.0.0920333
- NRC. 1981. Nutrient Requirements of domestic animals. No. 15. Nutrient Requirements of goats: angora, dairy, and meat goats in temperate and tropical countries. Washington, DC: National Academy Press. http://zarrinamravan.com/files/bgallery/Nutrient%20Requirements%20of%20Goats%20(1981).pdf.
- Perry RC, Corah LR, Cochran RC, Beal WE, Stevenson JS, Minton JE, Simms DD, Brethour JR. 1991. Influence of dietary energy on follicular development, serum gonadotropins, and first postpartum ovulation in suckled beef cows. J Anim Sci. 69:3762–3773. https://doi.org/10.2527/1991.6993762x
- Quesnel H, Pasquier A, Mounier AM, Prunier A. 1998. Influence of feed restriction during lactation on gonadotropic hormones and ovarian development in primiparous sows. J Anim Sci. 76:856–863. https://doi.org/10.2527/1998.763856x
- Ranjan SK. 1980. Animal nutrition in the tropics. Ghajiabod, V.P., India: Vikas Publishing house Pvt. Ltd. Vikas house. Pp 63-167.
- Rondina D, Freitas VJ, Bruno JB, Celestino JJ, Santos RR. 2017. Mitotic index and morphological characteristics of ovarian small follicles from goats submitted to nutritionally unbalanced regimens. Zygote. 25:567–574. https://doi.org/10.1017/S0967199417000351
- Sarker MB, Alam MH, Saha BK, Amin MR, Moniruzzaman M. 2015. Effects of soybean milk replacer on growth, meat quality, rumen and gonad development of goats. Small Rumin Res. 130:127–135. https://doi.org/10.1016/j.smallrumres.2015.07.018
- Scaramuzzi RJ, Campbell BK, Downing JA, Kendall NR, Khalid M, Muñoz-Gutiérrez M, Somchit A. 2006. A review of the effects of supplementary nutrition in the ewe on the concentrations of reproductive and metabolic hormones and the mechanisms that regulate folliculogenesis and ovulation rate. Reprod Nutr Dev. 46:339–354. https://doi.org/10.1051/rnd:2006016
- Simonetti S, Veeck LL, Jones HW. 1985. Correlation of follicular fluid volume with oocyte morphology from follicles stimulated by human menopausal gonadotropin. Fertil Steril. 44:177–180. https://doi.org/10.1016/S0015-0282(16)48731-5
- Skinner J. 2005. Regulation of primordial follicle assembly and development. Hum Reprod Update. 11:461–471. https://doi.org/10.1093/humupd/dmi020
- Vassena R, Mapletoft RJ, Allodi S, Singh J, Adams GP. 2003. Morphology and developmental competence of bovine oocytes relative to follicular status. Theriogenology. 60:923–932. https://doi.org/10.1016/S0093-691X(03)00101-8
- Webb R, Nicholas B, Gong JG, Campbell BK, Gutierrez CG, Garverick HA, Armstrong DG. 2003. Mechanisms regulating follicular development and selection of the dominant follicle. Reprod. Suppl. 61:71–90.
- Yu WH, Kimura M, Walczewska A, Karanth S, McCann SM. 1997. Role of leptin in hypothalamic–pituitary function. Proc Natl Acad Sci USA. 94:1023–1028. https://doi.org/10.1073/pnas.94.3.1023
- Yuan YQ, Van Soom A, Leroy JL, Dewulf J, Van Zeveren A, de Kruif A, Peelman LJ. 2005. Apoptosis in cumulus cells, but not in oocytes, may influence bovine embryonic developmental competence. Theriogenology. 63:2147–2163. https://doi.org/10.1016/j.theriogenology.2004.09.054
- Zak LJ, Xu X, Hardin RT, Foxcroft GR. 1997. Impact of different patterns of feed intake during lactation in the primiparous sow on follicular development and oocyte maturation. J Reprod Fertil. 110:99–106. https://doi.org/10.1530/jrf.0.1100099
- Zhou DS, Fang ZF, Wu D, Zhuo Y, Xu SY, Wang YZ, Zhou P, Lin Y. 2010. Dietary energy source and feeding levels during the rearing period affect ovarian follicular development and oocyte maturation in gilts. Theriogenology. 74:202–211. https://doi.org/10.1016/j.theriogenology.2010.02.002
