ABSTRACT
The present study investigates Vibrio outbreak in the hatchery system designed for larval rearing of shovel-nosed lobster Thenus unimaculatus in India. High larval mortalities during the episode led to an almost complete loss of the larval stock during the first 10 days. Microscopic examination revealed heavy fouling of the phyllosoma appendages. Histopathological analysis showed the presence of bacteria in larval tissues. Investigation by scanning electron microscopy revealed the formation of bacterial plaques on appendages and adhesion of bacteria on fine setae in heavily infected larvae. Bacteria isolated from larvae and identified by 16S rRNA sequencing and phylogenetic analysis indicated that the strains were closely related to luminescent Vibrio campbellii and Vibrio harveyi. The virulence potential of Vibrio strains isolated from larvae was evaluated through an experimental infection. The study helps to understand microbial infection and its impact on phyllosoma survival and may allow a more effective health management regime to improve larval survival in the hatchery phase.
Introduction
The hatchery phase represents a critical point in closing the life cycle of any cultured species. Generally, in commercially viable crustacean aquaculture ventures, the success depends on species with shorter larval phases (Wickins and Lee Citation2008). The shovel-nosed lobster, Thenus unimaculatus (Burton and Davie Citation2007), is emerging as a preferred candidate species of aquaculture interest because of its shorter larval phase (30 days), hardiness and reasonable market value (Kizhakudan et al. Citation2004; Kizhakudan Citation2005; Vijayakumaran and Radhakrishnan Citation2011; Kizhakudan and Krishnamurthy Citation2014). Though the Indian species was earlier known as Thenus orientalis, studies by Burton and Davie (Citation2007) confirmed the presence of a different species, Thenus unimaculatus. It is one of India's important commercially exploited lobster (Radhakrishnan et al. Citation2005). The larval phase of T. unimaculatus consists of four stages of phyllosoma I–IV, followed by a post-larval, non-feeding, nisto stage that metamorphose to juveniles. Phyllosoma is dorsoventrally flat, leaf-like, transparent, and is known as the first seed. The complete larval cycle of T. unimacultus has been studied in detail (Kizhakudan and Krishnamurthy Citation2014), but its seed production is yet to be developed commercially. Periodic mass mortalities in the early stages of hatchery-reared larvae hamper the commercial-scale rearing of T. unimaculatus. The major technological challenge for T. unimaculatus culture involves maintaining good health throughout the larval phase.
Though an array of pathogenic bacteria are known to induce mortality in hatcheries, Vibrio species represent the major pathogenic group affecting crustacean larvae, juveniles and other aquatic organisms (Goulden et al. Citation2012; Prado et al. Citation2015; Rojas et al. Citation2015; Dubert et al. Citation2016). Vibrio are widely distributed in aquatic environments (Vandenberghe et al. Citation2003), in commensal or pathogenic relations, thereby playing a significant role in aquaculture (Beaz-Hidalgo et al. Citation2010; Romalde et al. Citation2014). They can seriously hamper the survival of larvae, especially when the larvae are stressed due to poor hatchery conditions (Lacoste et al. Citation2001; Tseng and Chen Citation2004). Despite the development of efficient rearing techniques, T. unimaculatus often suffers recurrent episodes of high mortality during the larval phases. These episodes are mainly associated with high Vibrio load. This study provides the details of our investigation on the Vibrio outbreak in the hatchery system designed for larval rearing of T. unimaculatus. We focused on identifying, characterization, and infection dynamics of Vibrio strains in phyllosoma of T. unimaculatus. Understanding this microbial aspect in the larval-rearing system and its impact on phyllosoma may allow a more effective health management regime and thus improve larval survival.
Materials and method
Larval rearing
Wild T. unimaculatus brooders were collected from local fishers of Chennai-Mahabalipuram stretch in North Tamil Nadu, India and transported to the hatchery in Kovalam Field Laboratory, Central Marine Fisheries Research Institute, Chennai, India. Animals were kept in black coloured rectangular tanks with in situ sand bed and filter recirculatory system. Water quality parameters in the tanks like temperature, salinity and pH were maintained at 28–30°C, 33–35 ppt and 8-8.2, respectively. During the breeding season in winter (December to March), females were examined weekly for the estimated date of hatching. The brooders were held in separate tanks, with daily water exchange done manually at the hatching date. Healthy larvae hatched from the brooders and showing positive phototaxis were used for larval rearing in horizontal raceways with filtered seawater with 37–39 ppt salinity. Water exchange with 50% tank volume was carried out daily and aeration was restricted to mild disturbance of water using small airstones. The larvae were fed on freshly chopped hepatopancreas of the backwater clam, Meretrix casta.
Phyllosoma survival
Phyllosoma survival percentage of the whole population was measured over three standard larval-rearing trials. Larval survival estimates were calculated based on counts of dead animals siphoned out daily from the bottom of each larval-rearing tank.
Microscopic examination
Larvae were examined daily under a light microscope (Carl Zeiss, Germany) for any fouling or change in the typical morphology of different tissues.
Histology
Samples of live phyllosoma were randomly selected from larval-rearing tanks, though during die-off periods, samples were a mixture of living and moribund animals. During the disease outbreak, approximately five larvae were examined on days 1, 3, 5, 7 and 10. Phyllosoma larvae were fixed in Davidson's fixative (Hasson et al. Citation1997) for 48 h before processing for routine histological procedures (Bell and Lightner Citation1988). Briefly, the fixed tissues were dehydrated in graded ethanol series (70%, 90%, and 100%) for 60 min each. After dehydration, tissues were treated with xylene for 60 min (twice) and paraffin blocks were made using a tissue embedding system. Tissue sections (4–5 μm) were cut, further processed, stained with haematoxylin, and counter-stained with eosin following standard procedures (Bell and Lightner Citation1988). The stained tissue sections were mounted in dibutyl phthalate polystyrene xylene and visualized with light microscopy.
Scanning electron microscopy (SEM)
The larvae were washed three times with sterile seawater, fixed in 3% glutaraldehyde (prepared in filtered seawater) for 4 h. Following this, the larvae were washed thoroughly and post-fixed in 1% osmium tetroxide in 0.1 M cacodylate buffer for 2 h (Bourne et al. Citation2004). After fixation, the larvae were washed and dehydrated in ethanol series and dried in a polaron critical point drying apparatus, mounted on carbon tabs and sputter-coated with platinum at 25 mA for 150 s in a Balzars MFD 020 sputter coating unit. The coated specimens were examined using a TESCAN VEGA3 scanning electron microscope at 30 kV.
Isolation of the pathogen
The bacteria were isolated from larvae on days 1, 3, 5, 7 and 10. Ten moribund phyllosoma larvae were washed in sterile saline to remove loosely attached epibionts and excess detritus. Samples were homogenized in 10-fold dilution of sterile saline, and serial dilutions of 1 × 10−3 were prepared. From the different dilutions thus obtained, 100 µl from each was plated in triplicates on Zobell Marine Agar (ZMA) (Himedia) and Thiosulfate Citrate Bile Sucrose Agar (TCBS) (Himedia). The plates were incubated at 37°C for 48 h. Fifteen dominant and unique morphotypes were sub-cultured to purity and subsequently characterized by biochemical and phylogenetic analyses (Garrity et al. Citation2004).
Biochemical tests
Phenotypic characterization of bacterial isolates was done following a schematic procedure (Alsina and Blanch Citation1994; Ottaviani et al. Citation2003; Noguerola and Blanch Citation2008). Bacterial isolates were tested for the following phenotypic characters: cell morphology and mobility; Gram staining; Voges-Proskaur test; oxidase and catalase activity; oxidation/fermentation; hydrolysis of gelatin. Catalase activity was determined by bubble formation in 3% H2O2 solution (Reiner Citation2010) and oxidase activity using cytochrome oxidase paper (Himedia) (Rameshkumar et al. Citation2011). Growth at various temperatures (4–40°C), NaCl concentrations (0–10%) using Tryptic Soy Broth (Himedia) with 1.5% NaCl at 28°C was also examined. Additional biochemical characterization was performed using standardized API 20E identification systems (bioMérieux) with incubation at 28°C according to the manufacturer's instructions, except that sterile 1.5% (w/v) NaCl was used to prepare the inoculum. Sensitivity to the vibriostatic agent 2, 4-diamino-6, 7-diisopropylpteridine (O/129) was determined using Oxoid discs containing 10 and 150μg O/129 per disc (Supplementary Table 1).
Extraction of bacterial genomic DNA
The total bacterial DNA was extracted from single bacterial colonies using the HiPurA™ Multi-Sample DNA Purification Kit (Himedia) as per the manufacturer's instructions. The 260/280 and 260/230 OD ratios were found to be 1.8 and 2-2.2, respectively, confirming the purity of the DNA for downstream sequencing.
PCR amplification of 16S rRNA genes
The bacterial 16S rRNA gene was amplified by 16S rRNA gene sequencing using universal bacterial primers, NP1F 5′-GAGTTTGATCCTGGCTCA-3′ and NP1R 5′-ACGGCTACCTTGTTACGACTT- 3′ (Nair et al. Citation2012). All PCR reactions were performed in an Eppendorf Mastercycler (Eppendorf, Hamburg, Germany). Equal quantity (50 ng) of DNA template was used in 25 µl reaction consisting of 25 picomoles (0.5 µl) each of forward and reverse primer, 10X PCR buffer (2.5 µl), 25 mM MgCl 2 (2 µl), 10 mMdNTPs (0.5 µl), 1U Taq Polymerase (0.25 µl) and nuclease-free water. A negative control without the DNA template was also included for the PCR reaction. The following thermal cycling parameters were used: initial denaturation: 5-min at 95°C; denaturation: 35 cycles, 1 min at 94°C; annealing: 55°C for1 min; extension: 72°C for 1 min; final extension of 10 min at 72°C.
Sequencing and phylogenetic analysis
Sanger sequencing of the PCR products was performed by Eurofins Genomics India Pvt. Ltd Bengaluru, India. The NCBI BLAST database was used to analyse sequence homology (98–100%) for extracted 16S rRNA gene sequences. The overlapping 820 bp fragment of 16S rRNA gene from sequencing reactions was then aligned using ClustalW. A phylogenetic tree was constructed by the neighbour-joining method (MEGA version 7 software) using Kimura 2-parameter model (Kimura Citation1980) and tested by bootstrap with 1000 repetitions (Kumar et al. Citation2008).
Inoculum preparation for experimental infection
Bacterial isolates obtained during the luminous outbreak in the hatchery were used for the challenge study. Isolates were sub-cultured on TCBS at 28°C and suspended in 10 ml of seawater sterilized by filtration (0.22 µm pore size). The final suspension was adjusted to an optical density at 600 nm (OD) of 0.1. Corresponding total viable counts were determined by plating on ZMA (Himedia). The mean of 3 replicate counts was used for the immersion challenge. Phyllosoma larvae (stage 1) were distributed into 12 plastic trays each with 30 larvae at a density of 6 per litre. The animals were acclimated in darkness at 28°C for 8 h. The final concentrations used in the trays were prepared by adding appropriate volumes of bacteria to final concentrations of 108, 105 and 103 CFU ml−1, representing a high, medium and low level of bacterial dose, respectively. Each treatment was run in triplicate. Experimental control was not exposed to any bacteria. The number of dead or luminous phyllosoma was assessed every 24 h for five days. Phyllosoma not displaying any active movement after prolonged inspection being recorded as dead. The water temperature and salinity were maintained at 28°C and 35 ppt respectively throughout the experiment, and aeration was not provided to any of the trays. Vibrio strains were reisolated from moribund phyllosoma and confirmed by PCR with the same conditions as mentioned above in the PCR section.
Ethics statement
We adhered to the ethical guidelines and the biosafety rules and regulations followed by the institution ICAR-Central Marine Fisheries Research Institute, India.
Results
Phyllosoma survival
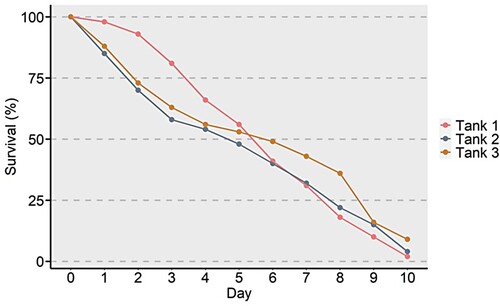
Larval rearing was characterized by mass mortality events due to the luminescent Vibrio outbreak. Phyllosoma appeared whitish-opaque, lethargic and poorly phototactic. Survival graphs of three tanks showed a similar pattern in 10 days. Initially, there was a mortality of 10–20% in the stock of larvae in the tanks, which increased over a period of 10 days. Survival on the 10th day was 0–10% in the three tanks ().
Microscopic observation
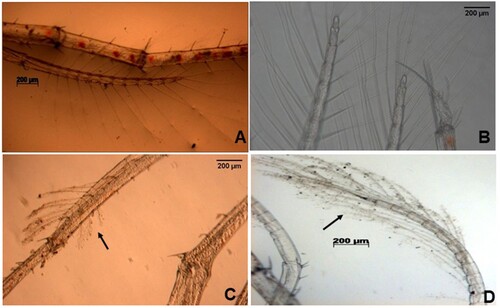
The microscopic examination of infected phyllosoma showed changes as compared to healthy individuals. Heavy fouling was observed on larval setae in infected phyllosoma. The fouling was observed both on appendages and setae ().
Histopathological examination
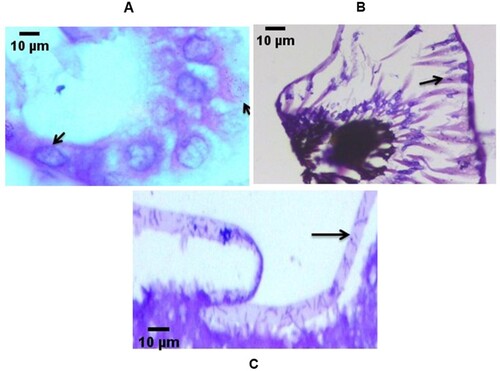
The hepatopancreatic tubules of moribund larvae was atrophied with necrosis of hepatopancreatocytes and (2 or 3/5) larvae contained bacteria in the lumen. Examination of eyes also indicated bacterial infiltration. Severe filamentous bacterial infections on the setae of the appendages were also identified ().
SEM analysis
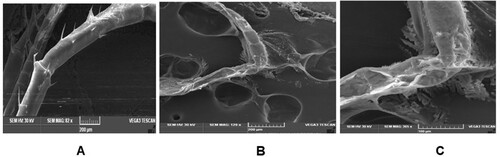
Examination of P1 phyllosoma larvae by SEM revealed the presence of numerous bacteria over the appendage surface. These bacteria were present in large numbers surrounding small spines protruding from appendages ().
Biochemical characters
All strains isolated were Gram-negative, oxidase-positive and negative for vibriostatic agent O/129 (10 µg); showed no growth in 0% and 10% sodium chloride, and positive growth in medium supplemented with 3% sodium chloride. Further analysis revealed facultative anaerobism in all strains. Vigorous growth was also observed on ZMA and Tryptic soy agar supplemented with 2.0% NaCl (w/v) at 28°C for 12 h. Luminescence was observed on plates in a dark room. Growth on TCBS agar was also checked after 72 h by yellow, convex, round colonies at incubation temperature of 28°C. This indicated that all the isolates were halophilic Vibrio species. The strains were negative for arginine dihydrolase reaction, and positive for indole production and gelatin production, establishing close identity with V. harveyi-related organisms. Majority of strains showed variable results in the ornithine decarboxylase test, which is considered as the key test for differentiating V. campbellii from V. harveyi (Alsina and Blanch Citation1994) (Supplementary Table 1).
Identification by 16S rRNA
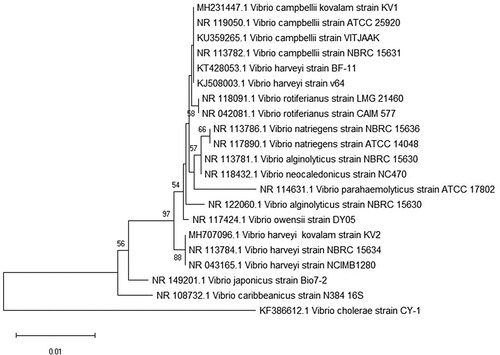
All isolates exhibited 99% sequence identity to the 16S rRNAgene of Vibrio species in the NCBI database. The strains V. campbellii and V. harveyi were repeatedly isolated from both ZMA and TCBS media and were dominant at different time points in a larval-rearing run. The nucleotide sequence data from strains have been submitted to GenBank database under accession numbers, MH231447.1, MH628047.1, MH707096.1 and MH707097. The lowest E value was considered for all comparisons. Construction of a phylogenetic tree with sequences of 16S rRNA with MEGA7 software using NJ analysis revealed a similar identification pattern. Constructed phylogenetic tree showed that the isolated bacteria were closely related to V. harveyi and V. campbellii and were farthest from V. cholera which acted as outgroup ().
Figure 5. Phylogenetic tree of isolated strains and other reference Vibrio strains based on concatenated sequences of 16S rRNA. The phylogenetic tree was constructed based on sequences of 16S rRNA gene using Neighbour-joining (NJ) analysis of MEGA 7 software. Vibrio cholerae was used as an outgroup. Bootstrap analysis with 1000 trials was used to provide confidence estimates for the phylogenetic tree topologies. The scale bar represents 0.01 substitutions per nucleotide site.
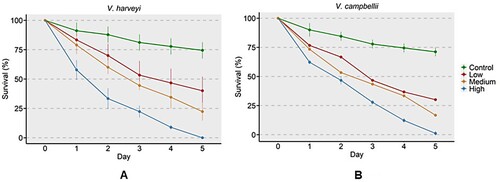
Experimental infection of phyllosoma
Experimental infection at higher concentration (108 CFU ml−1) of V. harveyi and V. campbellii resulted in about 40% mortality of phyllosoma on the first day, and by the fifth day, there was almost 100% mortality. At medium (105 CFU ml−1) and low (103 CFU ml−1) bacterial concentrations, survival of about 25% and 40%, respectively, was observed on the 5th day. In the control group, up to 75% survived on the 5th day ().
Discussion
Vibriosis is a global disease in marine aquaculture (Austin et al. Citation2005). Outbreaks of vibriosis have been observed in several lobster species causing mass mortality of the larvae (Diggles et al. Citation2000; Handlinger et al. Citation2000; Webster et al. Citation2006). Although, microbial community dynamics of lobster larvae have been studied in detail (Bourne et al. Citation2004; Citation2007; Payne et al., Citation2006; Goulden et al., Citation2012), the studies are largely centred on Panulirus ornatus. The present study represents the first attempt to identify, characterize and understand the infection dynamics of Vibrio campbellii and Vibrio harveyi during phyllosoma stage of Thenus unimaculatus. We employed a polyphasic approach incorporating direct microscopic analysis, scanning electron microscopy, culture-based microbiological, biochemical, and molecular methods to understand the impact of Vibriosis on phyllosoma larvae.
Estimation of larval survival in large volumes with unevenly mixed larvae is difficult. Our previous attempts at harvesting live phyllosomas to obtain accurate counts led to appendage injury and increased mortality which could not be distinguished from mortality due to bacterial outbreak. To this end, an indirect method of survival estimation based on the count of dead larvae was employed. This method has been also followed by Bourne et al. (Citation2004). Increased mortalities are often observed in early developmental stages around the time of moulting, which occurs approximately every 7–9 days (Nguyen et al., Citation2018). We found a reduction in the survival percentage by about 50% during days 7–9 of the outbreak, consistent with the notion that phyllosoma is more susceptible to infection during the moulting stages. Survival patterns were consistent across the three larval-rearing runs, with about 90% mortality within 10 days. Diggles et al. (Citation2000) observed about 75% mortality over 4 weeks in Jasus verreauxi reared at 20–23°C, and infected with Vibrio harveyi. Among other factors, the temperature is a critical factor that determines the rate of a bacterial outbreak (Travers et al., Citation2009). In our study, the larvae were reared at 28–30°C, which could have significantly accelerated the bacterial growth in the rearing system.
Routine microscopy revealed heavy fouling of the phyllosoma appendages. Phyllosoma death has been reported to increase due to heavy epibiont growth, increasing the difficulty of moult shedding, and hampering respiration which makes it difficult to meet the oxygen requirements (Diggles, Citation1999, Handlinger et al., Citation1999, Bourne et al., Citation2007). This shortage of oxygen is all the more exacerbated during the moulting stage when demand for oxygen increases (Carvalho & Phan, Citation1998). Further, the epibiont growth is reported to interfere with the ability of phyllosoma to process and masticate feed, which leads to a progressive decline of the nutritional status and increases susceptibility to opportunistic pathogenic bacteria (Handlinger et al., Citation2000; Fernandez-Leborans et al., Citation2006). Similar observations have been made on phyllosomas of Panulirus ornatus (Bourne et al., Citation2007) and Jasus edwardsii (Handlinger et al., Citation1999) infected with the filamentous bacteria. Although we did not observe fouling around the mouth area of the infected phyllosoma, it is possible that fouling may still have hindered the nutrition of phyllosoma by compromising its locomotion, thereby restricting capture of feed. SEM analysis confirmed the nature of fouling as bacterial plaques on the appendages and adhesion of bacteria on the fine setae in heavily infected larvae. Bourne et al. (Citation2007) also observed the adhesion of bacteria on the surface of mouth and anus. These entangled appendages are believed to render the animal unable to feed adequately (Bourne et al., Citation2004; Payne et al., Citation2006). This may also have significantly contributed to the observed mortality that we report here. The epibiont associated with the fouling of phyllosoma appendages is predominantly Leucothrix mucor and the heavy infestation of this epibiont can cause larval mortality (Sadusky & Bullis, Citation1994; Kitancharoen, Hatai, & Hara, Citation1997). Although in the present study, we did not mainly focus on the epibionts which caused fouling, characterization of the fouling bacteria is an important aspect that should be considered as the nature of epibionts is known to, at least partly, determine the welfare of phyllosoma (Shields, Citation2011).
Histopathological analysis demonstrated that the bacterial infestation was not only restricted to the body surface of phyllosoma but had also affected the internal tissues of the larvae. Proliferation of bacteria was observed in the tubules of the hepatopancreas. Similar pathologies associated with Vibrio infections have also been reported in cultured phyllosomas of packhorse rock lobster, Jasus verreauxi (Diggles et al., Citation2000), southern rock lobster, Jasus edwardsii (Handlinger et al., Citation1999) and P. ornatus (Bourne Citation2004; Citation2007), and in different life stages of penaeid shrimp (Lavilla-Pitogo et al., Citation1998; Soonthornchai et al. Citation2010). Larval feeds and cannibalism of dead larvae can contribute to Vibrio transmission during phyllosoma stages (Goulden, Hall, Bourne, Pereg, & Høj, Citation2012). Both these factors may be responsible for the occurrence of bacteria in the hepatopancreas (de Souza Valente & Wan, Citation2021). Infestation of the hepatopancreas also underlines the importance of research towards development of formulated larval feeds which can at least partly reduce the dependence on animal tissues (Gora et al., Citation2018). Heavily infested moribund or dead phyllosoma exhibit fluorescence that attracts healthy phyllosoma for cannibalism leading to rapid spread of vibriosis (Goulden et al., Citation2012; de Souza Valente & Wan, Citation2021). Among other factors, light is an important regulator of cannibalism in crustaceans (Romano & Zeng, Citation2017). Therefore, proper light conditions in the rearing facility which ensure cessation of cannibalism could be a strategy to reduce larval mortality (Gardner & Maguire, Citation1998). Examination of eyes indicated bacterial infiltration which suggests the progression to systemic infection in the late stages of moribidity or postmortem. The hepatopancreas, appendages and the eye region are the major organs of the phyllosoma and we were able to find Vibrio infection in these tissues. The systemic nature of Vibrio infection in phyllosoma indicates that although diet may be the major route for entry of Vibrio in the phyllosoma, it rapidy spreads to different organs making it difficult to ascertain the major predilection site for Vibrio in the phyllosoma. This development of rapid systemic infection in phyllosoma advocates for antibiotics to control the Vibrio infection, however, several studies have reported the development of resistance in Vibrio against antibiotics (Kitiyodom et al., Citation2010). Therefore, prophylactic approach like using natural bioactive compounds is a more sustainable and environment-friendly strategy to control Vibrio outbreaks in the hatcheries (Hall et al., Citation2013; Rossi et al., Citation2021).
In recent years, sequencing and comparison of the 16S rRNA gene have become an important tool for identifying bacterial species (Srinivasan et al., Citation2015). Dominant strains isolated from the culture included luminescent V. campbellii and V. harveyi and this showed a mixed dominance for disease outbreaks. Isolation of more than one species of Vibrio during disease occurrences have been previously reported in the other crustaceans (Davis & Sizemore, Citation1982; Harrison et al., Citation2022). Both V. harveyi and V. campbellii are widespread in marine environments. They are responsible for diseases in many wild and reared aquatic organisms, most notably penaeid shrimp, several fish species, and mollusks (de Souza Valente & Wan, Citation2021). V. campbellii has been isolated from diseased farm-shrimps from south India and has become a major emerging pathogen (Haldar et al., Citation2011). Furthermore, phylogenetic analysis of 16S rRNA gene sequences of isolated sequences clustered with known V. harveyi-related clade, including V. campbellii, V. harveyi and V. rotiferianus isolates. V. campbellii is closely related to several other Vibrio species, i.e. V. harveyi, V. rotiferianus, V. alginolyticus and V. parahaemolyticus (Thompson et al. Citation2004, Citation2007). The phyllosoma larvae are particularly vulnerable to opportunistic bacteria as their immune system is still developing (Gollas-Galvan et al. Citation2017) and culture conditions can further predispose larvae to a variety of stressors that may compromise the immune system (Rehman et al. Citation2017). Although through experimental infection we were able to ascertain the causative agents as Vibrio spp., it has been reported that Vibrio spp. in crustaceans are opportunistic bacteria which become pathogenic under stressful conditions (de Souza Valente and Wan Citation2021). Therefore, the contribution from the growth of other opportunistic pathogens to further deteriorate the health of phyllosoma and increase mortality cannot be ruled out. This aspect of bacterial disease outbreaks has not been thoroughly investigated in lobsters. Furthermore in our study, we focused on the culture-dependent technique to determine the causative agent. Future studies should also focus on the culture-independent high-throughput techniques like metagenomics to understand the contribution of other opportunistic pathogens towards disease outbreaks. Unfortunately, there is also no information regarding the microbiota profile of the phyllosoma larvae of T.unimaculatus in the wild and hatcheries. Understanding the shift in the microbiota profile of the phyllosoma during a disease outbreak is fundamental in maintaining the health of the phyllosoma (Holt et al. Citation2020).
The virulence potential of Vibrio strains isolated from luminous phyllosoma was evaluated and Vibrio strains were reisolated from moribund experimentally infected phyllosomas. Since we were able to find two species of Vibrio during the outbreak, we used three different doses of both species for the experimental challenge to ascertain which of the two Vibrio sp. predominantly accounts for the observed mortality. The selection of three doses of Vibrio sp. for the experimental challenge of phyllosoma in the present study was based on previous reports (Diggles et al. Citation2000; Goulden et al. Citation2012). We observed mortalities of 100% with dose 108 CFU ml−1 on 5th day at 28°C by V.harveyi and V. campbellii while as Diggles et al. (Citation2000) reported mortality of 100% with dose 107 CFU ml−1 at 24°C on 7th day in the phyllosoma of Jasus verreauxi by V.harveyi. On the other hand, there was 60% mortality with dose 107 at 28°C in the phyllosoma of P.ornatus by V.owensii (Goulden et al. Citation2012). Employing different doses for the experimental challenge helped us to understand a dose-dependent relationship of Vibrio spp. with the mortality. It appeared that both the species contributed equally to phyllosoma mortality.
The route of the experimental infection is an essential factor that determines the outcome of any bacterial challenge experiment. Goulden et al. (Citation2012) reported a high interindividual variation during the immersion challenge in the phyllosoma of P.ornatus by V.owensii while a consistent mortality pattern was reported in the phyllosoma when they were challenged with the same bacteria using artemia as vector. We used immersion challenge as the route of infection to confirm the susceptibity with different doses, as it allows us more control in regulating the bacterial load exposed to larvae. However, future studies should also confirm the susceptibility using different transmission routes to ensure the phyllosoma susceptibility.
Conclusion
We report the investigation on a disease outbreak in a lobster hatchery. The pathogens were identified to a species level based on biochemical and molecular methods. The study revealed that Vibrio spp. are the primary pathogens which can lead to a wipe out of almost the whole stock. The findings of this study will help in the development of prophylactic measures which will improve the larval survival in the hatchery phase.
TAAR_2050736_SupplementaryTable1
Download MS Word (19.7 KB)Acknowledgements
The authors are grateful to Director, ICAR-Central Marine Fisheries Research Institute, India for providing support and facilities to carry out this research.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Alsina M, Blanch AR. 1994. Improvement and update of a set of keys for biochemical identification of Vibrio species. J Appl Bacteriol. 77(6):719–721.
- Austin B, Austin D, Sutherland R, Thompson F, Swings J. 2005. Pathogenicity of vibrios to rainbow trout (Oncorhynchus mykiss, Walbaum) and Artemia nauplii. Environ Microbiol. 7(9):1488–1495.
- Beaz-Hidalgo R, Balboa S, Romalde JL, Figueras MJ. 2010. Diversity and pathogenicity of Vibrio species in cultured bivalve molluscs. Environ Microbiol Rep. 2:34–43.
- Bell TA, Lightner DV.. 1988. A handbook of normal penaeid shrimp histology. Baton Rouge, LA, USA: World Aquaculture Society.
- Bourne D, Høj L, Webster N, Payne M, Skindersøe M, Givskov M, Hall M. 2007. Microbiological aspects of phyllosoma rearing of the ornate rock lobster Panulirus ornatus. Aquaculture. 268(1-4):274–287.
- Bourne DG, Young N, Webster N, Payne M, Salmon M, Demel S, Hall M. 2004. Microbial community dynamics in a larval aquaculture system of the tropical rock lobster, Panulirus ornatus. Aquaculture. 242(1-4):31–51.
- Burton TE, PJF Davie. 2007. A revision of the shovel-nosed lobsters of the genus Thenus (Crustacea: Decapoda: Scyllaridae), with descriptions of three new species. Zootaxa. 1429(1):1–38.
- Carvalho PSM, Phan VN. 1998. Oxygen consumption and ammonia excretion during the moulting cycle in the shrimp Xiphopenaeus kroyeri. Comp Biochem Physiol A Mol Integr Physiol. 119(3):839–844.
- Davis JW, Sizemore RK. 1982. Incidence of Vibrio species associated with blue crabs (Callinectes sapidus) collected from Galveston Bay, Texas. Appl Environ Microbiol. 43(5):1092–1097.
- de Souza Valente C, Wan AHL. 2021. Vibrio and major commercially important vibriosis diseases in decapod crustaceans. J Invertebr Pathol. 181:107527.
- Diggles BK. 1999. Diseases of spiny lobsters in New Zealand. In Conference Proceedings of the International Symposium on Lobster Health Management, Curtin University of Technology, Perth, Australia. p. 18–34.
- Diggles BK, Moss GA, Carson J, Anderson CD. 2000. Luminous vibriosis in rock lobster Jasus verreauxi (Decapoda: Palinuridae) phyllosoma larvae associated with infection by Vibrio harveyi. Dis Aquat Org. 43(2):127–137.
- Dubert J, Nelson N, Spinard E, Kessner L, Gomez-Chiarri M, da Costa F, Prado S, Barja JL. 2016. Following the infection process of vibriosis in Manila clam (Ruditapes philippinarum) larvae through GFP-tagged pathogenic Vibrio species. J Invertebr Pathol. 133:27–33.
- Fernandez-Leborans G, Zitzler K, Gabilondo R. 2006. Protozoan ciliate epibionts on the freshwater shrimp Caridina (Crustacea, Decapoda, Atyidae) from the Malili Lake system on Sulawesi (Indonesia). J Nat Hist. 40(35-37):1983–2000.
- Gardner C, Maguire GB. 1998. Effect of photoperiod and light intensity on survival, development and cannibalism of larvae of the Australian giant crab Pseudocarcinus gigas (Lamarck). Aquaculture. 165(1):51–63.
- Garrity GM, Bell JA, Lilburn TG. 2004. Taxonomic outline of the procaryotes, Bergey's manual of systematic bacteriology, Rel. 5.0, 2nd ed. New York: Springer. DOI: 10.1007/bergeysoutline200405.
- Gollas-Galvan T, Cabanillas-Gámez M, Hernández-López J, Coronado-Molina D, Martínez-Porchas M. 2017. Transcriptional expression of immune system genes in Litopenaeus vannamei during ontogenetic development. Aquac Res. 48(3):1110–1118.
- Gora A, Jayasankar V, Rehman S, Kizhakudan JK, Laxmilatha P, Vijayagopal P. 2018. Biochemical responses of juvenile rock spiny lobster Panulirus homarus under different feeding regimes. J Appl Anim Res. 46(1):1462–1468.
- Goulden EF, Hall MR, Bourne DG, Pereg LL, Høj L. 2012. Pathogenicity and infection cycle of Vibrio owensii in larviculture of the ornate spiny lobster (Panulirus ornatus). Appl Environ Microbiol. 78(8):2841–2849.
- Haldar S, Chatterjee S, Sugimoto N, Das S, Chowdhury N, Hinenoya A, Asakura M, Yamasaki S. 2011. Identification of Vibrio campbelliiii isolated from diseased farm-shrimps from south India and establishment of its pathogenic potential in an Artemia model. Microbiology. 157:179–188.
- Hall MR, Kenway M, Salmon M, Francis D, Goulden EF, Høj L. 2013. 9 - Palinurid lobster larval rearing for closed-cycle hatchery production. In: G. Allan, G. Burnell, editors. Advances in aquaculture hatchery technology. Cambridge, UK: Woodhead Publishing; p. 289–328.
- Handlinger J, Carson J, Ritar A, Crear B. 2000. A study of diseases in cultured phyllosoma larvae and juveniles of southern rock lobster (Jasus edwardsii). J Shellfish Res. 19(1):676.
- Handlinger J, Carson J, Ritar AJ, Crear BJ, Taylor DP, Johnston D. 1999. Disease conditions of cultured phyllosoma larvae and juveniles of the southern rock lobster (Jasus edwardsii, decapoda; palinuridae). In Proceedings of the International Symposium on Lobster Health Management, Curtin University of Technology, Perth, Australia. p. 75–87.
- Harrison J, Nelson K, Morcrette H, Morcrette C, Preston J, Helmer L, Wagley S. 2022. The increased prevalence of Vibrio species and the first reporting of Vibrio jasicida and Vibrio rotiferianus at UK shellfish sites. Water Res. 211:117942.
- Hasson KW, Hasson J, Aubert H, Redman RM, Lightner DV. 1997. A new RNA-friendly fixative for the preservation of penaeid shrimp samples for virological detection using cDNA genomic probes. J Virol Methods. 66(2):227–236.
- Holt CC, van der Giezen M, Daniels CL, Stentiford GD, Bass D. 2020. Spatial and temporal axes impact ecology of the gut microbiome in juvenile European lobster (Homarus gammarus). ISME J. 14(2):531–543. DOI: 10.1038/s41396-019-0546-1.
- Kimura M. 1980. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 16(2):111–120.
- Kitancharoen N, Hatai K, Hara N. 1997. Filamentous bacterium, Leucothrix mucor from phyllosoma of spiny lobster, Panulirus japonicus. Aquac Sci. 45(2):231–239.
- Kitiyodom S, Khemtong S, Wongtavatchai J, Chuanchuen R. 2010. Characterization of antibiotic resistance in Vibrio spp. isolated from farmed marine shrimps (Penaeus monodon). FEMS Microbiol Ecol. 72(2):219–227. DOI: 10.1111/j.1574-6941.2010.00846.x.
- Kizhakudan JK. 2005. Culture potential of the sand lobster Thenus orientalis (Lund). In: Proceedings of the International Symposium on improved sustainability of fish production by appropriate technologies for utilization - Sustain Fish. p. 16–18.
- Kizhakudan JK, Krishnamurthy S. 2014. Complete larval development of Thenus unimaculatus Burton & Davie, 2007 (Decapoda, Scyllaridae). Crustaceana. 87(5):570–584.
- Kizhakudan JK, Thirumilu P, Rajapackiam S, Manibal C. 2004. Captive breeding and seed production of scyllarid lobsters - opening new vistas in crustacean aquaculture. Mar Fish Inf Serv Tech Ext Ser. 181:1–4.
- Kumar S, Nei M, Dudley J, Tamura K. 2008. MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief Bioinform. 9(4):299–306.
- Lacoste A, Jalabert F, Malham SK, Cueff A, Poulet SA. 2001. Stress and stress-induced neuroendocrine changes increase the susceptibility of juvenile oysters (Crassostrea gigas) to Vibrio splendidus. Appl Environ Microbiol. 67(5):2304–2309.
- Lavilla-Pitogo CR, Leaño EM, Paner MG. 1998. Mortalities of pond-cultured juvenile shrimp, Penaeus monodon, associated with dominance of luminescent vibrios in the rearing environment. Aquaculture. 164(1-4):337–349.
- Nair AV, Vijayan KK, Chakraborty K, Leo Antony M. 2012. Diversity and characterization of antagonistic bacteria from tropical estuarine habitats of Cochin, India for fish health management. World J Microbiol Biotechnol. 28:2581–2592.
- Nguyen NH, Fitzgibbon QP, Quinn J, Smith G, Battaglene S, Knibb W. 2018. Can metamorphosis survival during larval development in spiny lobster Sagmariasus verreauxi be improved through quantitative genetic inheritance? BMC Genet. 19(1):27. DOI: 10.1186/s12863-018-0621-z.
- Noguerola I, Blanch AR. 2008. Identification of Vibrio spp. with a set of dichotomous keys. J Appl Microbiol. 105(1):175–185.
- Ottaviani D, Masini L, Bacchiocchi S. 2003. A biochemical protocol for the isolation and identification of current species of Vibrio in seafood. J Appl Microbiol. 95(6):1277–1284.
- Payne MS, Hall MR, Bannister R, Sly L, Bourne DG. 2006. Microbial diversity within the water column of a larval rearing system for the ornate rock lobster (Panulirus ornatus). Aquaculture. 258(1-4):80–90.
- Prado S, Dubert J, Barja JL. 2015. Characterization of pathogenic vibrios isolated from bivalve hatcheries in galicia, NW Atlantic coast of Spain. Description of Vibrio tubiashii subsp. Europaensis subsp. nov. Syst Appl Microbiol. 38:26–29.
- Radhakrishnan EV, Deshmukh VD, Manisseri MK, Rajamani M, Kizhakudan JK, Thangaraja R. 2005. Status of the major lobster fisheries in India. N Z J Mar Freshw Res. 39:723–732.
- Rameshkumar N, Gomez-Gil B, Spröer C, Lang E, Dinesh Kumar N, Krishnamurthi S, Nair S, Roque A. 2011. Vibrio plantisponsor sp. nov., a diazotrophic bacterium isolated from a mangrove associated wild rice (Porteresia coarctata tateoka). Syst Appl Microbiol. 34(7):487–493.
- Rehman S, Gora AH, Ahmad I, Rasool SI. 2017. Stress in aquaculture hatcheries: source, impact and mitigation. Int J Curr Microbiol Appl Sci. 6(10):3030–3045.
- Reiner K. 2010. Catalase test protocol. Washington, DC: American Society for Microbiology. p. 1–6.
- Rojas R, Miranda C, Romero J, Asenjo F, Valderrama K, Segovia C, Ugalde JA, Santander J. 2015. Genome sequence of Vibrio VPAP30, isolated from an episode of massive mortality of reared larvae of the scallop Argopecten purpuratus. Genome Announc. 3(4):e00745–15.
- Romalde JL, Diéguez AL, Lasa A, Balboa S. 2014. New Vibrio species associated to molluscan microbiota: a review. Front Microbiol. 4:413.
- Romano N, Zeng C. 2017. Cannibalism of Decapod crustaceans and implications for their aquaculture: a review of its prevalence, influencing factors, and mitigating methods. Rev Fish Sci Aquac. 25(1):42–69. DOI: 10.1080/23308249.2016.1221379.
- Rossi B, Esteban MA, García-Beltran JM, Giovagnoni G, Cuesta A, Piva A, Grilli E. 2021. Antimicrobial power of organic acids and nature-identical compounds against two Vibrio spp.: an in vitro study. Microorganisms. 9(5):966.
- Sadusky TJ, Bullis RA. 1994. Experimental disinfection of lobster eggs infected with Leucothrix mucor. Biol Bull. 187(2):254–255.
- Shields J. 2011. Diseases of spiny lobsters: a review. J Invertebr Pathol. 106(1):79–91.
- Soonthornchai W, Rungrassamee W, Karoonuthaisiri N, Jarayabhand P, Klinbunga S, Söderhäll K, Jiravanichpaisal P. 2010. Expression of immune-related genes in the digestive organ of shrimp, Penaeus monodon, after an oral infection by Vibrio harveyi. Dev Comp Immunol. 34(1):19–28.
- Srinivasan R, Karaoz U, Volegova M, MacKichan J, Kato-Maeda M, Miller S, Nadarajan R, Brodie EL, Lynch SV, Heimesaat MM. 2015. Use of 16S rRNA gene for identification of a broad range of clinically relevant bacterial pathogens. PloS one. 10(2):e0117617.
- Thompson FL, Gomez-Gil B, Vasconcelos ATR, Sawabe T. 2007. Multilocus sequence analysis reveals that Vibrio harveyi and V. campbellii are distinct species. Appl Environ Microbiol. 73(13):4279–4285.
- Thompson FL, Iida T, Swings J. 2004. Biodiversity of vibrios. Microbiol Mol Biol Rev. 68(3):403–431.
- Tong LJ, Moss GA, Pickering TD, Paewai MP. 2000. Temperature effects on embryo and early larval development of the spiny lobster Jasus edwardsii, and description of a method to predict larval hatch times. Mar Freshw Res. 51(3):243–248.
- Travers M-A, Basuyaux O, Le Goic N, Huchette S, Nicolas J-L, Koken M, Paillard C. 2009. Influence of temperature and spawning effort on haliotis tuberculata mortalities caused by Vibrio harveyi: an example of emerging vibriosis linked to global warming. Glb Chg Biol. 15(6):1365–1376.
- Tseng IT, Chen JC. 2004. The immune response of white shrimp Litopenaeus vannamei and its susceptibility to Vibrio alginolyticus under nitrite stress. Fish Shellfish Immunol. 17(4):325–333.
- Vandenberghe J, Thompson FL, Gomez-Gil B, Swings J. 2003. Phenotypic diversity amongst Vibrio isolates from marine aquaculture systems. Aquaculture. 219(1-4):9–20.
- Vijayakumaran M, Radhakrishnan EV. 2011. Slipper Lobsters. In: Fotedar R, Phillips B, editors. Recent advances and new species in aquaculture. Chichester, UK: John Wiley & Sons; p. 85–114.
- Webster NS, Bourne DG, Hall M. 2006. Vibrionaceae infection in phyllosomas of the tropical rock lobster Panulirus ornatus as detected by fluorescence in situ hybridization. Aquaculture. 255(1-4):173–178.
- Wickins JF, Lee DOC. 2008. Crustacean farming: ranching and culture. Chichester: John Wiley & Sons.