?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The presence of essential amino acids and low anti-nutritional factors (ANFs) in Moringa oleifera leaf meal (MOLM) designates it as a good source of nutrients for poultry. This study investigated the effect of dietary MOLM on the growth performance, semen, hatchability traits and day-old chick weight of Potchefstroom Koekoek (PK) chickens. Two isonitrogenous and isoenergetic diets were formulated by diluting basal layer and grower mash with 0 (MOLM0) and 70% (MOLM70) with MOLM. Eighty PK chickens (40 hens and 40 roosters) aged 22 weeks were used in this study. For growth performance and hatchability traits, 40 laying hens were randomly assigned to two treatments with four replicates having five laying hens per pen. Diets did not significantly affect growth performance. The MOLM70 diet resulted in increased sperm velocity (rapid), semen concentration, semen pH, motility and progressive motility than the control (MOLM0). It is concluded that the inclusion of MOLM in chicken diets did not adversely affect growth performance, but improved sperm motility, hatchability and weight of day-old chicks of PK chickens. The findings recommend that MOLM is nutritionally equipped to enhance both the reproductive and the productive performance of PK.
Introduction
In rural communities of Africa, locally bred chickens are sources of essential amino acids, fatty acids and micronutrients for the general populace (Manyeula et al. Citation2019). Yet, there has been a drop in the number of locally bred chickens, mainly due to their poor productive and reproductive performance (Ben Larbi et al. Citation2013). It has been known that low fertility and poor hatchability are experienced under natural mating conditions due to poor semen quality as results of poor nutrition (Bucak et al. Citation2010). However, Ndegwa et al. (Citation1996) stated that the productivity of locally bred chickens can be increased through improved management, especially targeting nutrition through supplementation. Dietary manipulation has been recommended as a method of improving cockerel semen quality due to the strong relationship between nutrition and overall fertility (Hudson and Wilson Citation2013). Peters et al. (Citation2008) reported semen quality as the measure of the capacity of semen to achieve fertilisation, which is essential in the production of hatching eggs and can be heavily influenced by nutrition. The process of spermatogenesis to produce semen of a good quality requires amino acids such as methionine, cysteine (Fouad et al. Citation2020) and arginine (Wu et al. Citation2009), a fatty acid α-linoleic, vitamin A, C and E as well as zinc and selenium (Cheah and Yang Citation2011).
Recently, leaf meals have been used in animal nutrition for their nutritional and medicinal benefits. Moringa oleifera is one of the plants that contain all of these compounds. Moringa oleifera leaf meal (MOLM) is best known for its high leaf protein (27%) content, adequate amino acid profile, high levels of vitamins A and E, low levels of anti-nutritional compounds, fatty acids (Ogbe and Affiku Citation2012; Sebola et al. Citation2015), phenol (8 μg/ml), flavonoids (27 μg/ml) (Rajanandh and Kavitha Citation2010), an alkaloid (0.07%) (Madukwe et al. Citation2013), ferulic acid (46.8 mg/g) and chlorogenic acid (18.0 mg/g) (Fitri et al. Citation2015). However, the use of MOLM as a reproductive booster in poultry is still limited. Prabsattroo et al. (Citation2015) reported that hydroethanolic extract from M. oleifera significantly increased the number of interstitial Leydig cells in rats. Also, Abu et al. (Citation2013) reported that MOLM had no adverse effect on the testicular morphometry and epididymal sperm quality of rabbit bucks at an inclusion level of up to 15%. Additionally, Syarifuddin et al. (Citation2017) stated that MOLM increased plasma testosterone concentrations, libido and sperm motility of Bali bulls. Therefore, this study was aimed at evaluating the effect of MOLM on the growth performance, semen quality and hatchability traits of Potchefstroom Koekoek (PK) chickens. It was hypothesized that including MOLM in locally bred chicken diets would have no negative effects on growth performance, semen and hatchability traits.
Materials and methods
The study was conducted according to the guidelines of the Ethics. All animal husbandry practices were performed with full consideration of animal welfare.
Study area
During the experimental period, ambient temperatures ranged from 19°C to 25°C.
Feed ingredients and experimental diets
Moringa oleifera leaves were collected from Limpopo, to formulate two isonitrogenous and isoenergetic diets () in a mash form to meet the nutritional requirements of growing chickens. The diets were as follows: MOLM0 = a basal diet without MOLM and MOLM70 = a basal diet with 70 g/kg MOLM (). Sebola et al. (Citation2015) optimized a 70% MOLM inclusion rate which could be used without compromising the objective of meeting the nutritional requirements of growing locally bred chickens. The experiment was conducted in three parts.
Table 1. Ingredients and nutrient composition (g/kg) of Moringa oleifera leaf meal (MOLM) based diets.
Experiment 1A – growth performance
A total of 40 laying hens were raised on a starter mash for four weeks. At five weeks of age, the hens were randomly allotted to two experimental diets until 40 weeks of age. A completely randomized design (CRD) was used for this experiment with a pen holding five birds, replicated four times, resulting in a total of eight battery cages (measuring 60 × 50 × 75 cm). Feed and water were provided ad libitum during the experimental period under continuous lighting throughout the trial. Feed offered to the hens and refusals were weighed daily for nine weeks. Average weekly feed intake (AWFI), average weekly body weight gain (AWG) and feed conversion ratio (FCR) were calculated (Ogbe and Affiku Citation2012) and data were analysed following Model 2.
Experiment 1B – semen and sperm quality
Forty PK roosters were raised on a starter mash for four weeks. At five weeks of age (301 ± 22.70 g), the chickens were randomly allotted to two experimental diets until 40 weeks of age. A CRD was used for this experiment with a cage holding five roosters, replicated four times. Semen was collected from five roosters for six days by means of the dorso-abdominal massage method (Burrows and Quinn Citation1937) with a day interval between 9:00 and 11:00. Average semen (0.39 ± 0.14 ml) was collected from individual roosters into a 15 ml tube, placed in a thermo flask at 41°C and transported to the mobile laboratory.
The motility parameters measured by computer-assisted sperm analyser (CASA) (Microptic, Spain, at a magnification of × 10 – Nikon®, China) included the following: curvilinear velocity (VCL), velocity over the actual sperm track, which included all deviations of sperm head movement; average path velocity (VAP), velocity over a calculated, smoothed path; straight-line velocity (VSL), velocity over the straight-line distance between the beginning and end of the sperm track; and linearity (LIN) (see ). Two hundred microlitres of swim-up media (Kobidil + extender) placed Eppendorf tube and added 10 µl of semen. Five microlitres of the solution was then used for CASA. A total of five fields were captured and saved for further analyses. Semen pH was measured using a calibrated pH metre (Hanna instruments®, Portugal). Twenty microlitres of eosin stain and 7 µl of semen were mixed into the append of the tube and 5 µl mixture was used for morphology evaluation using a fluorescence microscope. An average of 300 sperms per slide per replica per cock was used for morphology assessment (Bakst et al. Citation1994). Undiluted 20 µl of semen was placed into microcuvettes and placed into the spectrophotometer (spermacue) for semen concentration. The sperm output for each cockerel was calculated as the product of semen volume (ml) and sperm concentration (109/ml) (Anderson Citation2001; Cerolini et al. Citation2006) and data were analysed using Model 1.
Experiment 1C – fertility, hatchability and chick weight
At Week 31, a mating ratio of 5 (hens experiment A) to 1 (rooster experiment B) was housed in an eight deep litter pen. They were offered diets shown in for five weeks: the first week was for adaptation and egg collection was done for four weeks. Egg collection was done for three days and stored at 18°C prior to setting. Fifty eggs per treatment were randomly selected, sprayed with disinfectant and placed in an incubator with the broad end pointing upwards set at a temperature of 37.5°C and relative humidity (RH) of 65% for 18 days. Candling was done on the 7th and 18th day of incubation, and the fertile eggs only were then transferred into a hatchery at a temperature of 37.4°C and RH 70% until hatching. After hatching, the chicks were counted and the eggs that were not hatched were counted to calculate the fertility rate following the formula of Ashour et al. (Citation2020).
Hatchability was expressed according to the chicks that hatched from fertile eggs using the formula of Sahin et al. (Citation2009).
The hatched chicks were weighed using a digital weighing scale (Explorer EX224, 0.01 g readability (two decimal places) OHAUS Corp, Parsippany, NJ, USA) and the data collected were analysed following Model 1.
Statistical analyses
Data on egg hatchability, fertility rate, hatching of fertile eggs and weight of day-old chicks were analysed using the GLM procedure of SAS (Citation2010), with diet as the only effect (Model 1). Data on feed intake, weight gain, feed conversion ratio and semen volume and characteristics were measured on a weekly basis and were analysed using the fixed model procedure of SAS (Citation2010), which took the effects of both diet and week of measurement (Model 2) into account.
The statistical models employed were as follows: Model
(1)
(1) where
= response variable,
= general mean,
= the fixed effects of diets,
= random error associated with observation,
= assumed to be normally and independently distributed. Model
(2)
(2)
where = response variable,
= general mean,
= effects of diets,
= effects of time,
= effects of time interacting with diets and
= random error term.
For all the statistical tests, least square means was compared using the probability of difference (PDIFF) option in the LSMEANs statement of SAS. The level of significance was set at P < .05.
Results
Experiment 1A – growth performance
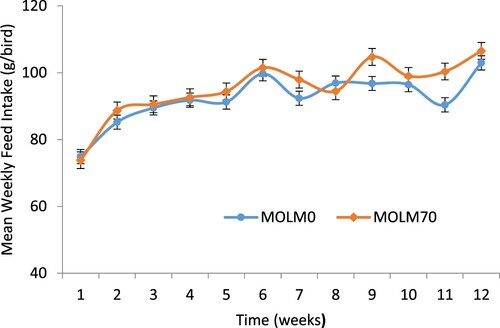
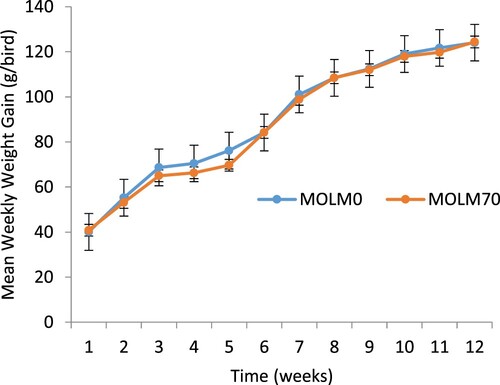
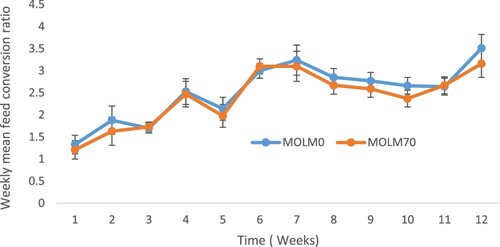
There was no significant dietary effect on the overall weekly feed intake of the PK hens. However, the feed intake was lower in hens fed the MOLM0 diet in all the weeks except in Weeks 1 and 8 compared to those fed the MOLM70 diet (). Feed intake decreased at Weeks 7 and 11 in hens fed MOLM0; for hens fed MOLM70, feed intake decreased in Weeks 7, 8 and 10. Diet had no significant effect on the weight gain of PK hens (). However, there was no indication of weight loss in this study. There was no dietary effect (P > .05) on the FCR of the PK hens (). However, a linear increase in the FCR was observed throughout the experiment for hens fed all the diets.
Experiment 1B – semen and sperm quality
The statistical differences in the effects of diet and day and their interaction on semen characteristics are presented in . Dietary treatment had an effect (P < .05) on semen pH but did not affect semen volume, sperm concentration and morphology (live, dead, mid and tail). Effect of day affect semen volume and morphology (P < .05) (mid and tail), but sperm concentration, pH and number of dead and live sperm in morphology were not affected. The two-way interaction (diet × day) significantly affected semen volume (P < .05) but did not affect other semen characteristics (sperm concentration, semen pH and morphology). No significant difference (P > .05) was noticed in terms of sperm concentration, semen volume, medium and slow velocity percentage, linearly, straightness, wobbles and morphology (). The inclusion of MOLM70 resulted in high (P < .05) semen pH, progressive motility, VCL, VSL and VAP. From shows that diet did not affect (P > .05) the semen volume of the PK cockerels. However, semen volume was higher in cockerels fed MOML70 on all days except Day 2 when the cockerels fed MOLM0 had a higher semen volume.
Figure 4. Effects of replacing soybean meal in chicken diets with graded levels of MOLM on semen volume from Day 1 to Day 6.
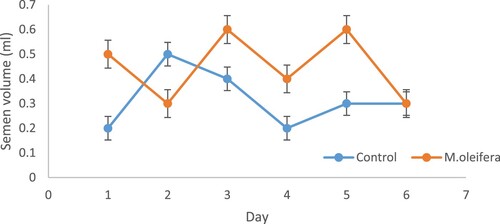
Table 2. Effects of replacing soybean meal in chicken diets with graded levels of MOLM on treatment, day and their interaction (treatment × day) effect on semen quality.
Table 3. Effects of replacing soybean meal in chicken diets with graded levels of MOLM on semen characteristics and quality (mean ± Stdev).
Experiment 1C – fertility, hatchability and chick weight
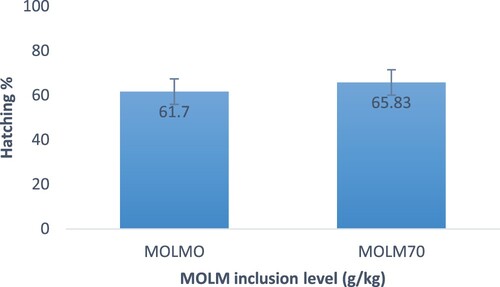
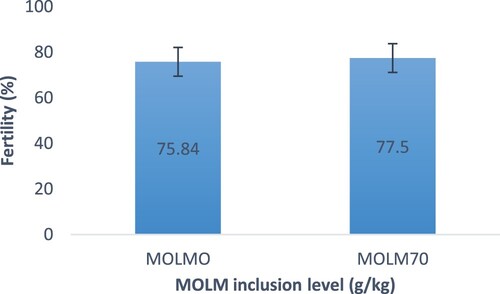
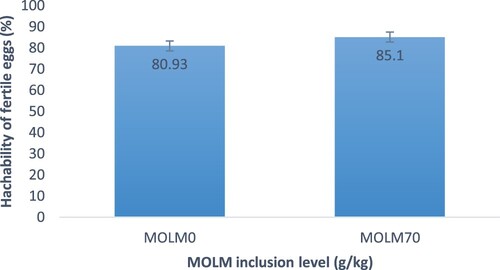
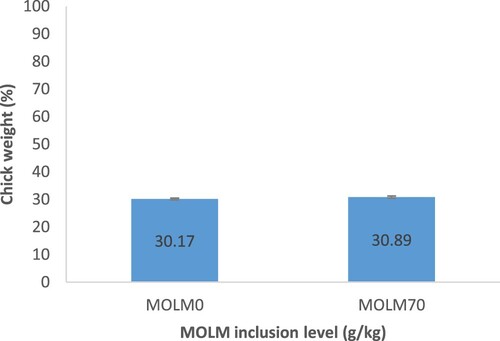
shows that eggs from hens fed MOLM70 experienced the highest (P < .05) hatchability, but eggs from hens fed MOLM0 experienced the lowest. With regards to fertility rate, eggs from hens fed MOLM70 resulted in a higher fertility rate (77.50% ± 6.2%) than their counterparts (75.84% ± 5.7%) (). In terms of hatchability, eggs from hens fed MOLM70 had significantly the highest hatchability of fertile eggs (85.1% ± 7.3%). Eggs from hens fed MOLM0 had the lowest (P < .05) hatchability of fertile eggs (). Heavier day-old chicks were from eggs from hens fed MOLM70 rather than those fed MOLM0 ().
Discussions
Experiment 1A – growth performance
The observation that the inclusion of MOLM in PK hens’ diets had no significant effects on feed intake, weight gain and FCR contrasts with those reported for broilers when MOLM was included (Safa and Tazi Citation2012). The differences observed might be caused by the chicken breed used. The general increase in body weight of PK hens indicates that the treatment had no adverse effect on the growth and body weight of the hens. Vast literatures (Sebola et al. Citation2015; Olugbemi et al. Citation2010) reported similar results with the current results on feed intake and FCR when MOLM is included in the diets of chickens. It is known that MOLM contains bioactive compounds (Mbikay Citation2012). The synergy between each bioactive compounds in MOLM may be an important feature of their action which may affect nutrient absorption and processing, red-ox state, or immunity.
Experiment 1B – semen and sperm quality
Bearden et al. (Citation2004) mention that low sperm concentration has been associated with low fertility. Therefore, the higher sperm concentrations recorded for MOLM70 suggest that testicular development and proper hormone balance in PK roosters were triggered by the inclusion of MOLM. This is supported by the findings of Saalu et al. (Citation2011) who reported that rats fed MOLM showed high sperm production and normal seminiferous epithelium. The non-significant finding of sperm concentration in this study agrees with the report by Olubowale et al. (Citation2014), who recorded a non-significant sperm concentration when dietary lipid sources were included in the diet of Hy-line silver-brown cockerels. However, the study differs from the findings of some authors (Cerolini et al. Citation2006; Yusuf Citation2014) who reported a significant effect on sperm concentration when incorporating fish oil and dietary M. oleifera leaf meal in the diet of turkey roosters.
The pH results obtained with MOLM70 were within the range obtained by other studies in the literature (Gebriel et al. Citation2009; Ogbe and Affiku Citation2012; Orunmuyi et al. Citation2013). Improved mean pH (7.37) in PK roosters offered MOLM70 in this study improved sperm velocity by increasing the pH to alkaline. The literature (Ashizawa and Wishart Citation1987; Ashizawa et al. Citation1997) states that the percentage of motile sperm and sperm velocity was increased at alkaline pH in domestic roosters. Lower pH is detrimental to spermatozoa as shown by the lower metabolic production of lactic acid in active sperm.
The significant differences reported for progressive motility in the present study highlighted the positive effect of MOLM in enhancing sperm motility by providing the substrate (ATP) needed for motility. The increased progressive motility from PK roosters fed MOLM70 may be attributed to vitamin E (Sebola et al. Citation2017; Fuglie Citation2013) and selenium found in MOLM. Reports (Young et al. Citation1986; Hansen and Deguchi Citation1996) state that dietary selenium causes an increase in sperm concentration, sperm motility and sperm capacity in farm animals (including poultry species).
Plant material like MOLM contains substantial amounts of beneficial antioxidants, phytochemicals, minerals and vitamins known to increase growth and stimulate reproduction in animals, including poultry species (Mahfuz and Piao Citation2019).
An indicator of some disorders in spermatogenesis is sperm morphology. The MOLM contains essential antioxidant and phenolic compounds that help in protecting the testes against morphologic, spermatogenic and oxidative changes brought on by toxic materials and certain anti-neoplastic agents (Saalu et al. Citation2011; Orunmuyi et al. Citation2013). However, in the present study, dietary treatment had no significant effect on sperm morphology. But this was not the case with the report of Yusuf (Citation2014), who noted a significant effect on the sperm morphology of turkey fed M. oleifera and Gongronema latifolium leaf meals.
Experiment 1C – fertility, hatchability and chick weight
The most sensitive parameters to genetic and environmental influences are fertility and hatchability (Peters et al. Citation2008). Reports (Stahl et al. Citation1986; Peebles and Brake Citation1987) state that factors affecting hatchability and fertility include bird strain, plane of nutrition, condition and length of storage of eggs, egg quality and mating ratio. Fertility and hatchability of fertile eggs were actually improved in chickens fed MOLM70, compared with those on MOLM0. Moyo et al. (Citation2011) showed that MOLM contains high levels of zinc and vitamin E, but Park et al. (Citation2004) and Amen and Al-Daraji (Citation2011) reported that zinc and vitamin E could play a beneficial role in the hatchability of eggs. Zinc helps with the protection of genetic material structure or deoxyribonucleic acid (DNA) chromatin in the sperm nucleus, which is an important structure for successful fertility. Durmus et al. (Citation2004) noted increased hatchability with increasing zinc in the diets of brown parent stock layers.
Organically bounded selenium supplementation of laying hens’ diets improved the environment of the sperm storage tubules in the hen's oviduct, increasing the interval of time the sperms can be stored, allowing the sperms to live longer and increasing the number of sperm holes in the yolk layer (Agate et al. Citation2000; Davtyan et al. Citation2006; Hanafy et al. Citation2009). Osman et al. (Citation2010) reported that supplementation of plant leaves containing selenium increased fertility and hatchability percentage. Narushin and Michael (Citation2002) state that the physical characteristics of the egg such as the shape index, weight, length and width play a significant role in embryo development and successful hatching. This might be the reasons for improved fertility and hatchability in the group fed MOLM70 in the present study. The hatchability of fertile eggs noted in this study was higher than the 77% reported by Tesfaye et al. (Citation2014) for layer hens fed a diet containing 5% MOLM but similar to the 84% noted by Molekwa and Dennis (Citation2009) for PK chickens artificially inseminated with semen from high performing cocks. The fertility rate and hatchability of the present study were similar to the findings of Wubalem (Citation2006), who reported that the inclusion of MOLM at different levels improved egg fertility and hatchability. Chick weight at one day old was shown to be positively affected by the inclusion of MOLM70. Improved chick weight in the current study agrees with the finding of Etalem et al. (Citation2014), who noted higher chick weight in hens fed 5% MOLM. Lesuisse et al. (Citation2017) observed that chick weight could be improved by additional protein in the hen's diet. MOLM is known to be a good source of protein (Alebachew et al. Citation2016).
Conclusions
In conclusion, the findings of the current study indicate that the inclusion of MOLM significantly improved semen quality parameters such as the progression, velocity and motility of sperm cells. Also, feeding MOLM improved subsequent hatchability traits and chick weight.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
References
- Abu AH, Ahemen T, Ikpechukwu P. 2013. The testicular morphometry and sperm quality of rabbit bucks fed grad-ed levels of Moringa oleifera leaf meal (MOLM). Agrosearch. 13:49–56.
- Agate DD, O’dea EE, Rustad ME. 2000. Effects of dietary selenium on laying hen fertility as assessed by the perivitelline sperm whole assay. Proceedings of the Poultry Research and Production Symposium. Alberta Poultry Research Centre. p. 1–4.
- Alebachew W, Etalem T, Berhan T. 2016. Effects of feeding different dietary levels of Moringa oleifera leaf meal on egg production, fertility and hatchability of dual purpose Koekoek hens. Middle-East J Sci Res. 24:2909–2920.
- Amen HMM, Al-Daraji HJ. 2011. Effects of dietary supplementation with different levels of zinc on sperm egg penetration fertility traits of broiler breeder chicken. . Pak J Nutr. 10:1083–1088.
- Anderson MJ. 2001. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 26:32–46.
- Ashizawa K, Hashimoto K, Tsuzuki Y. 1997. Regulation of fowl sperm flagellar motility by protein phosphatase type 1 and its relationship with dephosphorylation of axonemal and/or accessory cytoskeletal proteins. Biochem Biophys Res Commun. 235:108–112.
- Ashizawa K, Wishart GJ. 1987. Resolution of the sperm motility stimulating principle of fowl seminal plasma into Ca2þ and an unidentified low molecular weight factor. J Reprod Fertil. 81:495–499.
- Ashour EA, El-Kholy MS, Alagawany M, Abd El-Hack ME, Mohamed LA, Taha AE, El Sheikh AI, Laudadio V, Tufarelli V. 2020. Effect of dietary supplementation with Moringa oleifera leaves and/or seeds powder on production, egg characteristics, hatchability and blood chemistry of laying Japanese quails. Sustainability. 12:1–9.
- Bakst MR, Wishart G, Brillard JP. 1994. Oviductal spermatozoa selection, transport, and storage in poultry. Poult Sci Rev. 5:117–143.
- Bearden JJ, Fuguay JW, Willard ST. 2004. Applied animal reproduction. 6th ed. Starkville: Mississippi State University. p. 183–196.
- Ben Larbi M, M’hamdi N, Haddad B. 2013. Indigenous chicken production systems in villages in the south of Tunisia. LRRD. 25:99. [accessed 2021 Jan 12]. http://www.lrrd.org/lrrd25/6/larb25099.htm.
- Bucak MN, Tuncer PB, Sarıözkan S, Başpınar N, Taşpınar M, Çoyan K, Bilgili A, Akalın PP, Büyükleblebici S, Aydos S. 2010. Effects of antioxidants on post-thawed bovine sperm and oxidative stress parameters: antioxidants protect DNA integrity against cryodamage. Cryobiology. 61:248–253. https://www.tandfonline.com/loi/taar20.
- Burrows WH, Quinn JP. 1937. The collection of spermatozoa from the domestic fowl and Turkey. Poult Sci 16:19–24.
- Cerolini S, Zainiboni L, Maldjian A, Gliozzi T. 2006. Effect of docosahexaenoic acid and -tocopherol enrichment in chicken sperm on semen quality, sperm lipid composition and susceptibility to peroxidation. Theriogenology. 66:877–886.
- Cheah Y, Yang W. 2011. Functions of essential nutrition for high quality spermatogenesis. Adv Biosci Biotechnol. 2:182–197.
- Davtyan D, Papazyan T, Nollet L. 2006. Dose response of Se added as sodium selenite or Sel Plex on sperm quality and breeder productivity. 12th European Poultry Conference, Verona, Italy, 10–14. p. 23–28.
- Durmus İ, Ataşoğlu C, Mizrak C, Ertaş S, Kaya M. 2004. Effect of increasing zinc 196 concentration in the diets of brown parent stock layers on various production and 197 hatchability traits. Archiv fuer Tierzucht Dummerstorf. 47:483–489.
- Etalem T, Getachew A, Mengistu U, Tadelle D. 2014. Cassava root chips and Moringa oleifera leaf meal as alternative feed ingredients in the layer ration. J Appl Poult Res. 23:614–624.
- Fouad AM, Kasem HAK, Ruan D, Xia W, Chen W, Wang S, Zheng C. 2020. Nutritional modulation in fertility in male poultry. Poult Sci 99: 5637– 5646.
- Fitri AT, Toharmat DA, Astuti A, Tamura H. 2015. The potential use of secondary metabolites in Moringa oleifera as an antioxidant source. Med Pet. 38:169–175.
- Fuglie LJ. 2013. New uses of Moringa studied in Nicaragua. Echo Development Notes. 68:1–6.
- Gebriel GM, Kalamah MA, El-fiky A, Ali FA. 2009. Some factors affecting semen quality traits in Norfa cocks. Egyp Poult Sci. 29:677–693.
- Hanafy M, El-Sheikh A, Abdalla E. 2009. The effect of organic selenium supplementation on productive and physiological performance in a local strain of chicken. Egyp Poult Sci. 29:1061–1084.
- Hansen JC, Deguchi Y. 1996. Selenium and fertility in animals and man – a review. Acta Vet Scand. 37:19–30.
- Hudson BP, Wilson JL. 2013. Effects of dietary menhaden oil on fertility and sperm quality of broiler breeder males. J Appl Poult Res. 12:341–347.
- Lesuisse J, Li C, Schallier S, Leblois J, Everaert N, Buyse J. 2017. Feeding broiler breeders a reduced balanced protein diet during the rearing and laying period impairs reproductive performance but enhances broiler offspring performance. Poult Sci. 96: 3949–3959.
- Madukwe EU, Ezeugwu JO, Eme PE. 2013. Nutrient composition and sensory evaluation of dry Moringa oleifera aqueous extract. Int J Sci Basic Appl Res. 13:100–102.
- Mahfuz S, Piao XS. 2019. Application of Moringa (Moringa oleifera) as natural feed supplement in poultry diets. Anim. 9:1–9.
- Manyeula F, Mlambo V, Marume U, Sebola NA. 2019. Nutrient digestibility, haemo-biochemical parameters and growth performance of an indigenous chicken strain fed canola meal-containing diets. Trop Anim Health Prod. 51: 2343–2350.
- Mbikay M. 2012. Therapeutic potential of Moringa oleifera leaves in chronic hyperglycemia and dyslipidemia: a review. Front Pharmacol. 3:1–12.
- Molekwa JT, Dennis U. 2009. Relationships between cock semen viability and the fertility of artificially inseminated South African indigenous chicken breeds. S Afr J Anim Sci. 39:24–28.
- Moyo B, Masika PJ, Mar LJ, Hugo A, Muchenje V. 2011. Nutritional characterization of Moringa (Moringa oleifera Lam.) leaves. Afr J Biothnol. 10:12925–12933.
- Narushin V, Michael R. 2002. Egg physical characteristics and hatchability. Worlds Poult Sci J. 58:297–303.
- Ndegwa JM, Kimani CW, Siamba D, Mburu BM. 1996. On farm evaluation of improved management practices on production performance of indigenous chicken. December Quarterly Report, NAHRC, Naivasha, Kenya.
- Ogbe AO, Affiku PJ. 2012. Proximate study, mineral and anti-nutrient composition of Moringa oleifera leaves harvested from Lafia, Nigeria: potential benefits in poultry nutrition and health. JMBFS. 1(3):1296–1308.
- Olubowale OS, Greyling JPC, deWitt FH, Hugo A, Raito MB. 2014. Effects of dietary lipid sources on the semen quality of Hy-line silver-brown cockerels. J Anim Plant Sci. 24:991–997.
- Olugbemi TS, Mutayoba SK, Lekule FP. 2010. Effect of Moringa (Moringa oleifera) inclusion in cassava based diets fed to broilers chickens. Inter J Poult Sci. 9:363–367.
- Orunmuyi M, Akanwa CL, Nwagu BI. 2013. Semen quality characteristics and effect of mating ratio on reproductive performance of hubbard broiler breeders. J Agric Sci. 5:154–159.
- Osman MG, Elhadi EA, Khalafalla MM. 2010. Callus formation and organogenesis of tomatoes (Lycopersicon esculantum meal. C.V Omadurman) induced by thidiazornon. Afr Biotechnol. 9:4407–4413.
- Park S, Kim WK, Birkhold SG, Kubena LF, Nisbet DJ, Ricke SC. 2004. Using a feed-grade zinc propionate to achieve molt induction in laying hens and retain post-molt egg production and quality. Biol Trace Element Res. 101:165–179.
- Peebles ED, Brake J. 1987. Eggshell quality and hatchability in broiler breeder eggs. Poult Sci. 66:596–604.
- Peters SO, Shoyebo OD, Ilori BM, Ozoje MO, Ikeobi CON, Adebambo OA. 2008. Semen quality traits of seven strain of chickens raised in the humid tropics. Int J Poult Sci. 7:949–953.
- Prabsattroo T, Wattanathorn J, Iamsaard S, Somsapt P, Sritragool O , Thukhummee W, Muchimapura, S. 2015. Moringa oleifera extract enhances sexual performance in stressed rats. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 16: 179–190.
- Rajanandh MG, Kavitha J. 2010. Quantitative estimation of β-sitosterol, total phenolic and flavonoid compound in the leaves of Moringa oleifera. Int J Pharmtech Res. 2:1409–1414.
- Saalu LC, Osinubi AA, Akinbami AA, Yama OE, Oyewopo AO, Enaibe BU. 2011. Moringa oleifera Lamarck (drumstick) leaf extract modulates the evidences of hydroxyurea-induced testicular derangement. Int J Appl Res in Natural Products. 4:32–45.
- Safa MA, Tazi EL. 2012. Effect of feeding different levels of Moringa oleifera leaf meal on the performance and carcass quality of broiler chicks. Int J Sci Res. 3:147–151.
- Sahin EH, Sengor E, Yardimci M, Cetingul IS. 2009. Relationship between pre-incubation egg parameters from old breeder hens, egg hatchability and chick weight. J Anim Vet Adv. 8:115–119.
- Sebola NA, Mlambo V, Mokoboki HK, Muchenje V. 2015. Growth performance and carcass characteristics of three chicken strains in response to incremental levels of dietary Moringa oleifera leaf meal. Livest. Sci. 178:202–208.
- Sebola NA, Mlambo V, Mokoboki HK. 2017. Chemical characterisation of Moringa oleifera (MO) leaves and the apparent digestibility of MO leaf meal-based diets offered to three chicken strains. Agroforest Syst. 93:149–160.
- Stahl JL, Cook ME, Sunde ML. 1986. Zinc supplementation: its effect on egg production, feed conversion, fertility and hatchability. Poult Sci. 65:2104–2109.
- Statistical Analysis System Institute Inc. 2010. Users guide. Carry (NC): SAS.
- Syarifuddin A, Toleng AL, Rahardja DP, Ismartoyo I, Yusuf M. 2017. Improving libido and sperm quality of Bali bulls by supplementation of Moringa oleifera leaves. Media Peternakan. 40:88–93.
- Tesfaye E, Animut G, Urge M, Dessie T. 2014. Moringa oleifera leaf meal as an alternative protein feed ingredient in broiler ration. Int J Poult Sci. 12:289–297.
- Wu G, Bazer FW, Davis TA, Kim SW, Li P, Marc Rhoads J, Carey Satterfield M, Smith SB, Spencer TE, Yin Y. 2009. Amino Acids. 7:153–168.
- Wubalem AA. 2006. Effects of different dietary levels of Moringa oleifera leaf meal on egg production, quality, shelf life, fertility and hatchability of dual purpose Koekoek hens [Ph.D. thesis]. Addis Ababa, Ethiopia: Addis Ababa University.
- Young ID, Leymaster KA, Lunstra DD. 1986. Genetic variation in testicular development and its relationship to female reproductive traits in swine. J Anim Sci. 63:17–26.
- Yusuf M. 2014. Semen quality and egg hatchability in local turkey fed diets containing Moringa oleifera and Gongronema latifolium leaf meal [M.Sc. thesis]. Nsukka: Department of Animal Science, University of Nigeria.