 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The present study is the first record of the effect of cereal bait formulated with papaya seed powder (PSP) on reproductive functions of female Bandicota bengalensis. Treatment baits prepared using WSO (wheat, sugar, oil mix) bait and PSP in ratios 97:3, 95:5 and 90:10 were fed to three groups of rats for 30 days in no-choice with one group as untreated control. The average weight of rats used was 235 g. Rats were evaluated immediately and after 30 days of treatment withdrawal. Acceptance of treatment baits by different groups was 79.06–89.45%, leading to the ingestion of 68.36–186.23 g/kg bwt of PSP. Results revealed significant (P < .05) anti-fertility effects of 10% PSP based bait in the reduction in the relative weight of the uterus; the number of primordial and pre-antral ovarian follicles, breeding success, litter size, duration of the estrous stage, and prolongation of proestrous and diestrous stages further indicated by significantly (P < .05) reduced levels of estradiol, 17β-HSD, ACP and ALP. The present study revealed anti-reproductive effects of 10% PSP-based cereal bait, which were recovered after 30 days of treatment withdrawal, thus suggesting further studies using higher concentrations of PSP for long-term irreversible effects.
1. Introduction
The lesser bandicoot rat, Bandicota bengalensis found throughout Southeast Asia (Sidhu et al. Citation2020), causes severe damage to agriculture (Singla and Babbar Citation2010) besides being implicated in the transmission of various zoonotic diseases (Singla et al. Citation2008, Citation2016). A growing concern, regarding environmental hazards caused due to excessive use of rodenticides (Smith and Shore Citation2015), has focused interest on using environment-friendly management approaches (Nattrass et al. Citation2019).
The use of biologically active plant components in controlling fertility (Dhar and Singla Citation2014; Singla Citation2015) is a promising approach that can limit rodenticides (Mukhtar et al. Citation2018). Different parts of the papaya (Carica papaya) plant, such as leaves, seeds, fruit, latex and bark, have been investigated for developing effective contraceptives (Chinoy et al. Citation1997; Udoh et al. Citation2005; Nwaehujor et al. Citation2014; Ekhator and Shelu Citation2015; Julaeha et al. Citation2015; Odirichukwu Citation2015; Punitha et al. Citation2015; Odirichukwu et al. Citation2016; Kumari et al. Citation2017; Satriyasa et al. Citation2018). These plant parts contain enzymes, lycopenes, carotenoids, alkaloids, monoterpenoids, flavonoids, minerals, vitamins, etc. (Sindhu et al. Citation2019) that cause infertility (Adebiyi et al. Citation2003, Citation2004; Estella et al. Citation2020; Hakameri CS and Usman Citation2020). Ingestion of papaya seeds during pregnancy has been unsafe (Adebiyi et al. Citation2002). They affect the reproductive ability of female rats by disrupting the estrous cycle, increasing atresia in ovarian follicles, affecting androgens and reducing litter size (Dosumu et al. Citation2008; Memudu and Oluwole Citation2021).
Most of the previous studies have reported anti-fertility effects of different extracts of papaya seeds when administered orally to laboratory rats and mice, keeping in view the development of human contraceptives (Raji et al. Citation2005; Chinoy et al. Citation2006; Naik et al. Citation2015; Novitasari et al. Citation2018). No study has yet reported the effect of papaya seeds on the reproduction of wild female rodent species. Also, to use the anti-reproductive property of papaya seeds to manage the wild rodent population, there is a need to develop a bait formulation that can be easily applied under field conditions.
The present study was conducted to investigate the effect of cereal bait formulation based on papaya seed powder (PSP) as an ingredient on various reproductive functions such as estrous cycle, fertility, biochemicals and histology of reproductive organs in female B. bengalensis to provide a technology for integrated rodent pest management programme.
2. Materials and methods
2.1. Experimental design
2.1.1. Study duration and location
The present study was carried out in the Department of Zoology, Punjab Agricultural University (PAU), Ludhiana, Punjab, India, from September 2018 to August 2019.
2.1.2. Plant material and treatment bait preparation
The papaya fruits (C. papaya var. Honey dew) available in the local market were identified by the Department of Fruit Science, College of Horticulture and Forestry, PAU, Ludhiana. Fruits weighing 1.5–2.0 kg were elongated and oval with dark yellow skin and ridges on the surface (Gunnannavar et al. Citation2017). Their seeds were collected, washed gently under tap water, shade dried and pulverized into a fine powder using a domestic grinder (Kaur et al. Citation2021). The required quantity of treatment baits was prepared using WSO (wheat, sugar, oil mix) bait and PSP in ratios 97:3, 95:5 and 90:10, respectively.
2.1.3. Animal collection and maintenance
Animals were collected and maintained in the laboratory, as per the protocol described by Sidhu et al. (Citation2020). Mature male (with scrotal testes) and female (with perforated vaginal opening) B. bengalensis with body weight of 200–300 g were live-trapped from crop fields and fish market, Ludhiana in Punjab province of India and kept in individual metallic cages (36 × 23 × 23 cm) for acclimatization under laboratory conditions for 10–15 days. Food and water were provided ad libitum. Food consisted of WSO bait (a loose mixture of cracked wheat, powdered sugar and refined groundnut oil in 96: 2: 2). The metallic trays were kept under each cage for daily collection and disposal of urine and faecal pellets. To prevent chances of any zoonotic disease transmission from wild rats, a full-sleeved apron, face mask and hand gloves were used while handling the animals.
2.1.4. Cyclicity of female rats
Before experimentation, the cyclicity of female rats was determined by observing their vaginal fluid for the presence, absence or proportional numbers of different types of cells such as leucocytes, epithelial cells and cornified cells (Goldman et al. Citation2007). Vagina was flushed with 0.2 ml of 0.9% saline solution and gently aspirated using a Pasteur pipette. A drop of vaginal fluid was then placed on a glass slide for immediate evaluation under a light microscope (Magnus, CH20i, Olympus (India) Pvt. Ltd., Noida, India), as per the method described in Sandhu and Singla (Citation2019). Vaginal fluid was examined every 24 h and continued till the completion of one cycle. Three consecutive cycles were studied for each rat to determine cyclicity (Sahu and Maiti Citation1978). Non-cyclic female rats were not used for the experiment. All the rats were housed singly in laboratory cages for experimentation. The average body weight of all the cyclic female rats used was 235 g.
2.1.5. Animal grouping and treatment
Cyclic female rats were weighed and divided into four groups each (three treated and one control). Each group consisted of six replicated rats. During treatment, rats of treated groups I, II and III of all the four sets were fed on treatment baits containing different concentrations of PSP (3, 5 and 10%) for 30 days in a no-choice feeding test. Rats of group IV, kept as untreated control, were fed on WSO bait only. The treatment dosage was based on our earlier study (Kaur et al. Citation2021) on using a maximum 5% PSP in bait against male rats under bi-choice conditions where the anti-fertility effect was not much significant. Soin studied against female rats, the concentration of PSP was increased to 10% in bait in a no-choice condition. Before and after the treatment, rats in all the groups were fed on WSO bait. A 40 g formulated bait was fed to rats daily, which was replenished every 24 h. Bait consumption (g) by rats in all the sets and groups was recorded daily to calculate the mean daily consumption (g/100 g body weight (bwt)) of bait and the total amount of PSP ingested (g/kg bwt) in 30 days, as per the method described in Sidhu et al. (Citation2020). Percent acceptance of treatment bait over untreated bait consumed during the pre-treatment period was determined as per the formula described in Singla and Parshad (Citation2002) and given below:
where Y = Total amount (g/100 g bwt) of treated bait containing a particular concentration of PSP consumed in 30 days by rats.
2.1.6. Animal ethics clearance
Approval from the Institutional Animal Ethics Committee (IAEC) for using animals (B. bengalensis of both sexes) was obtained vide memo no. IAEC/2018/1153-1188 under Protocol no. GADVASU/2018/IAEC/46/16. The procedures used in this study adhere to the Committee for the Purpose of Control and Supervision of Experiments on Animals, GADVASU (Guru Angad Dev Veterinary and Animal Sciences, University), Ludhiana, India.
2.1.7. Animal sacrifice and tissue and blood collection
Animals in different sets and groups were evaluated per the protocol given in . As per CPCSEA guidelines (The Committee for the Purpose of Control and Supervision of Experiments on Animals), rats were sacrificed by cervical dislocation after sedation with pentobarbitone sodium. Rats of all the four groups in the first set were sacrificed immediately after treatment withdrawal and that of the second set after 30 days of treatment withdrawal. Rats were dissected to take out both the ovaries and uterus. These were cleared of fatty tissues, soaked on filter papers and weighed (g/100 g bwt) using an electronic balance to determine their relative weights, as described in Dhar and Singla (Citation2014). The whole ovary (left) and piece of the uterus of three female rats in each set and group were collected for histological study. Blood (up to 1 ml) of all the female rats was collected by a cardiac puncture. A clean tube containing 0.1 ml anticoagulant (3.2% tri-sodium citrate solution) was used to collect 0.9 ml of blood. Blood was centrifuged at 3000 rotations per minute (rpm) for 15 min. The supernatant plasma obtained after centrifugation was stored at −20°C in a separate tube until analysis (Sidhu et al. Citation2020).
Table 1. Experimental protocol for animal evaluation.
2.2. Histological analysis
The ovary and uterus of rats were processed and evaluated for histological alterations as per the method described in Dhar and Singla (Citation2014). The whole ovary and piece of the uterus were fixed in Bouins’s fluid for 48 h, processed and embedded in paraffin. The paraffin sections of 6 μm thickness were obtained on glass slides with the help of a rotary microtome (Medimeas Instruments, Ambala Cantt, Haryana, India) and stained with haematoxylin and eosin (H&E) for histomorphological studies. Serial sections of the ovary were taken, and the number of different types of follicles was counted. In each section, only those follicles were counted in which the oocyte’s nucleus was visible, as described by Tilly (Citation2003). In the section of the uterus, the number of endometrial glands was counted, and epithelial cell height (µm) and myometrium thickness (µm) were measured using Magvision Image Analysis Software under a light microscope.
2.3. Biochemical analysis
2.3.1. Total soluble proteins
Total soluble proteins (TSP) were estimated using the method given by Lowry et al. (Citation1951). A spectrophotometer was used to record absorbance at 520 nm against a reagent blank. Protein concentration (mg/g) in the tissue sample was determined by preparing a standard curve of bovine serum albumin.
2.3.2. 17β-HSD and 3β-HSD activities
Activities of 17β-Hydroxysteroid dehydrogenase (17β-HSD) and 3β-Hydroxysteroid dehydrogenase (3β-HSD) were estimated in plasma with some modifications in the method of Agular et al. (Citation1992). Specific activity was calculated in units/min/mg of total proteins.
2.3.3. Alkaline and acid phosphatases
Alkaline phosphatase (ALP) and acid phosphatase (ACP) activity in plasma were estimated per the method given by Bessey et al. (Citation1946). The rate of formation of p-Nitrophenol was measured as absorbance at 410 nm as it is directly proportional to the activity of ALP and ACP in the sample. The μ moles read off from the standard curve corresponding to the measured optical density were multiplied by 20 to obtain units of ALP and multiplied by 4.68 to obtain units of ACP. The activity was measured in μ moles of p-Nitrophenol lib/min/mg protein.
2.3.4. Estradiol
The level of estradiol (ES) in plasma (ng/ml) was estimated using an ELISA kit, as per the manufacturer’s (Xema Medica Co. Ltd., Russia) protocol.
2.4. Fertility assessment
The fertility assessment of rats was done per the procedure described in Kaur et al. (Citation2021). Immediately after treatment withdrawal, six female rats in each group of the third set were paired with untreated mature male B. bengalensis rats in 1:1 in large laboratory pens. Six female rats in each group of the fourth set were paired with mature male rats in a ratio of 1: 1 after 30 days of treatment withdrawal. During pairing, food and water were provided ad libitum. Food consisted of a loose mixture of cracked wheat, powdered sugar, refined groundnut oil and milk powder in the ratio of 91: 2: 2: 5. Pre-soaked black gram seeds (50 g) were also provided in a separate bowl to eliminate the chances of any nutritional deficiency. Male rats were kept with female rats for up to 15 days of pairing to ensure successful mating, after which the males were separated. Female rats were observed for pregnancy and delivery of pups. The number and sex of pups delivered were also recorded. Percent breeding success was determined as per the formula given below:
The proportion of male and female pups delivered was determined as per the formula given below:
2.5. Effect on estrous cyclicity
The duration of the estrous cycle and its stages was determined as per the method described in Dhar and Singla (Citation2014). Immediately after treatment withdrawal, the vaginal fluid of six rats in each group of the fourth set was observed daily for up to 30 days to record the type of stage. The average duration (h) of one estrous cycle and that of its different stages (proestrous, estrous, metestrous and diestrous) was determined separately for the first 15 days and after 16–30 days of treatment withdrawal for comparison and reversibility. The total duration of the estrous cycle was studied from the length of all the stages, and the number of cycles completed was determined from the number of metestrous periods experienced.
2.6. Statistical analysis
Values were determined as Mean ± SD (standard deviation). General linear model in statistical analysis software (SAS) version 9.3 was used for the statistical analysis of data obtained by using a factorial, completely randomized design. Tukey’s HSD (honestly significant difference) test at a 5% level of significance was used to obtain all pair-wise treatment comparisons.
3. Results
3.1. Bait consumption and acceptance of treatment bait
Mean daily consumption (g/100 g bwt) of treatment bait by rats of groups I, II and III treated with 3, 5 and 10% PSP was 7.62, 7.59 and 6.18 g/100 g bwt, respectively, leading to mean total ingestion of 68.36, 114.40–186.23 g/kg bwt of PSP, respectively in 30 days’ treatment. Percent acceptance of treatment bait over untreated bait consumed before treatment by rats of all the treated groups varied from 79.06 to 89.45% (). There was no significant (P > .05) difference in mean daily consumption of treated and untreated baits among different groups of rats.
Table 2. Mean daily consumption and acceptance of bait containing different concentrations of papaya seed powder and active ingredient ingested during 30 days of treatment in no-choice by female B. bengalensis.
3.2. Effect on reproductive organ weights
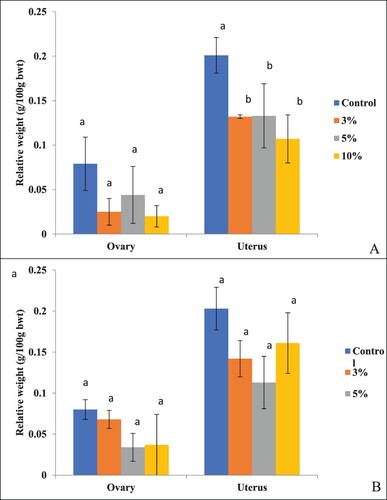
In rats sacrificed immediately and after 30 days of treatment withdrawal, the relative weight (g/100 g bwt) of ovaries didn't differ significantly (P > .05) among groups of treated and control rats at all the three concentrations of PSP ((A)). However, the relative weight of the uterus was to be reduced significantly (P = .003) in rats of the group treated with 10% PSP and sacrificed immediately after treatment withdrawals compared to rats of the control group and those treated with 3% and 5% PSP. In rats treated with different concentrations of PSP and sacrificed after 30 days of treatment withdrawal, no significant (P > .05) effect was observed on the relative weights of ovaries and uterus ((B)).
Figure 1. Effect on the relative weight of the ovary and uterus of B. bengalensis treated with different concentrations of papaya seed powder and sacrificed (A) immediately after treatment withdrawal, and (B) after 30 days of treatment withdrawal. Bars with different superscripts (a–b) differ significantly (P < .05) and bars with similar superscript (a) do not differ significantly (P > .05).
3.3. Effect on histomorphology
3.3.1. In the uterus
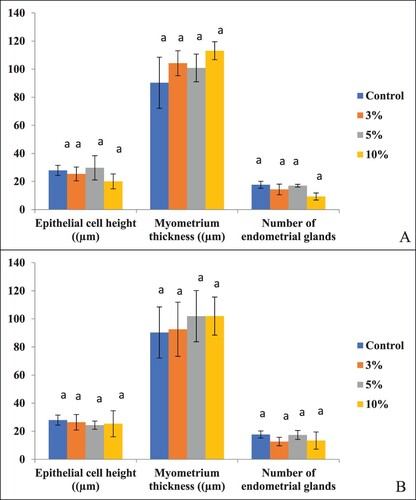
In rats treated with different concentrations of PSP for 30 days and sacrificed immediately after treatment withdrawal, an effect was found on histomorphology of the uterus (). Loss of cellular integrity was there. The number of endometrial glands, luminal epithelium cell height and myometrium thickness were found to be reduced, but the difference between the treated and control groups of rats was not found to differ significantly (P > .05) ((A)). In rats sacrificed after 30 days of treatment withdrawal also, no significant (P > .05) difference was found in many endometrial glands, luminal epithelium cell height and myometrium thickness among the control and treated groups ((B)).
Figure 2. H&E stained sections of the uterus of B. bengalensis treated with different concentrations of papaya seed powder and sacrificed immediately after the withdrawal at 200× magnification showing histomorphological changes, (A) control rat, (B) 3% treated rat, (C) 5% treated rat, and (D) 10% treated rat (LE-Luminal epithelium, L-Lumen, M-Myometrium, EG-Endometrial gland).
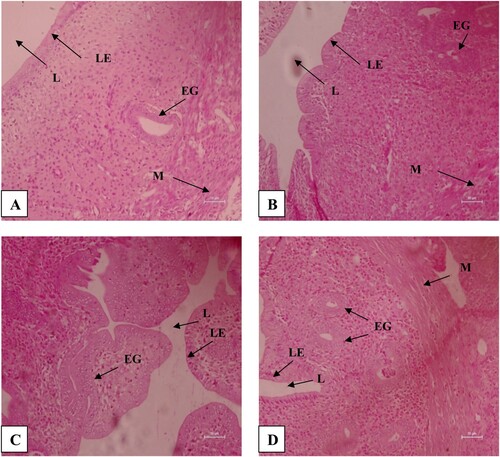
Figure 3. Effect on epithelial cell height, myometrium thickness and the number of endometrial glands in the uterus of B. bengalensis treated with different concentrations of papaya seed powder and sacrificed (A) immediately after treatment withdrawal and (B) after 30 days of treatment withdrawal. Bars with similar superscript (a) do not differ significantly (P > .05).
3.3.2. In the ovary
In rats treated with different concentrations of PSP for 30 days and sacrificed immediately after treatment withdrawal, an effect was found on histomorphology of the ovary (). Follicular cells’ proliferation was grossly affected. In rats sacrificed immediately after treatment withdrawal, the number of primordial (P = .003) and pre-antral follicles (P = .004) was found to decrease significantly in rats treated with 10% concentration compared to rats of the control group and those treated with 3% and 5% concentrations of PSP. However, no significant (P > .05) difference was found in the number of primary, secondary and antral follicles among treated and control groups of rats (). In rats sacrificed after 30 days of treatment withdrawal, no significant (P > .05) difference was found in many primordial, primary, secondary, preantral and antral follicles among rats of treated and control groups, indicating reversibility in effects ().
Figure 4. H&E stained sections of the ovary of B. bengalensis treated with different concentrations of papaya seed powder and sacrificed immediately after the withdrawal at 200× magnification showing the effect on follicular development, (A) control rat, (B) 3% treated rat, (C) 5% treated rat, and (D) 10% treated rat (PrF-Primordial follicle, SF-Secondary normal follicle, AnF-Antral follicle, AtF-Atretic follicle).
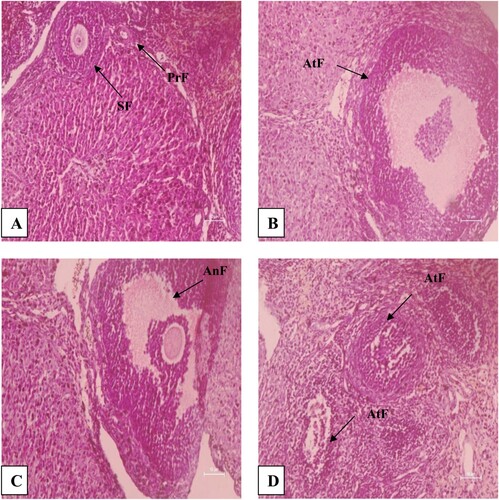
Table 3. Effect of treatment with different concentrations of papaya seed powder for 30 days on the number of different types of follicles in the ovary of B. bengalensis.
3.4. Effect on biochemicals
The plasma levels of 17β-HSD, ES, ALP and ACP were significantly (P < .001–.007) low in rats of the group treated with 10% PSP and sacrificed immediately after treatment withdrawal from the control group and groups treated with 3% and 5% PSP. No significant (P > .05) difference was observed in plasma levels of TSP and 3β-HSD among rats in the treated and control groups ().
Table 4. Effect of treatment with different concentrations of papaya seed powder for 30 days on different biochemical parameters in the plasma of female B. bengalensis.
In rats sacrificed after 30 days of treatment withdrawal, no significant (P > .05) difference was observed in plasma levels of 3β-HSD, 17β-HSD, ALP and ACP among rats of treated and control groups, indicating reversibility in effects; however, the level of ES was still found reduced in rats treated with 10% PSP, indicating no reversibility in the effect caused (). No significant effect of treatment was observed on the plasma level of TSP immediately and after 30 days of treatment withdrawal.
3.5. Effect on fertility
In the present study, the breeding success of female rats of the control group was only 66.67%. Breeding success in female rats of the group treated with 10% PSP and evaluated immediately after treatment withdrawal was to be reduced to 50%, which was increased to 66.67% in rats evaluated after 30 days of treatment withdrawal, indicating partial reversibility in effects. No breeding of rats was observed in the group of rats treated with 3% PSP and evaluated immediately and after 30 days. Breeding success in rats treated with 5% PSP was 83.33% and 100% immediately and after 30 days of treatment, withdrawal indicating not much effect (). The average number of pups (4.00–5.00) delivered by female rats treated with 5% and 10% PSP and evaluated immediately and after 30 days of treatment withdrawal was, however, found to be significantly (P < .05) less than those delivered by control rats (7.00). However, no significant difference in the proportion of male and female pups delivered was observed among treated and control rats ().
Table 5. Effect of treatment with different concentrations of papaya seed powder for 30 days on breeding success of female B. bengalensis.
3.6. Effect on the estrous cycle
During the first 15 days after treatment withdrawal (), the duration of one estrous cycle (117.12 h) was found to be significantly (P < .0001) prolonged and that of the estrous stage (10.67 h) significantly (P < .0001) reduced in rats treated with 10% concentration of PSP compared to rats of the control group and those treated with 3% and 5% PSP. The duration of the diestrous stage in the first 15 days was significantly (P < .0001) prolonged in rats treated with all the three concentrations of PSP, with a significantly higher effect on rats treated with 10% PSP. The duration of the proestrous stage was significantly (P < .001) prolonged in rats treated with 3% and 10% PSP compared to control rats and rats treated with 5% PSP, while the duration of the metestrous stage was reduced significantly(P < .001) in rats of all the three treated groups compared to the control group.
Table 6. Effect of treatment with different concentrations of papaya seed powder for 30 days on estrous cyclicity of female B. bengalensis.
Between 16 and 30 days of treatment withdrawal (), no significant (P > .05) difference was found in the duration of one estrous cycle and metestrous stage among rats of treated and control groups, indicating partial reversibility in effects. The duration of proestrous (11.89 h) and diestrous (71.89 h) stages was still found to increase significantly (P < .001) and that of the estrous stage (9.67 h) decreased significantly (P < .0001) in rats treated with 10% PSP, indicating no reversibility.
4. Discussion
The overall control of the female reproductive system is a complex mechanism involving numerous neuroendocrine factors and hormones that affect the successful production of mature gametes. The present study investigated the effect of cereal bait formulation based on PSP as an ingredient on various reproductive functions, such as histology of reproductive organs, biochemicals, reproductive hormones, estrous cycle and fertility of B. bengalensis, to provide a technology for integrated rodent pest management programme. The study revealed dose-dependent adverse effects of PSP on the histomorphology of the ovary and uterus, biochemicals, reproductive hormones, estrous cycle and fertility of B. bengalensis thus justify its reported use as a female anti-fertility agent (Chinoy et al. Citation1995, Citation2006; Dosumu et al. Citation2008; Julaeha et al. Citation2015; Memudu and Oluwole Citation2021).
In the present study, a significant decrease in the relative weight of the uterus of treated B. bengalensis was found. On the contrary, a significant increase in the weight of the uterus of rats treated with papaya seed extract has been reported by Novitasari et al. (Citation2018). The hormonal and histological changes caused due to papaya seed treatment may be the reason for the reduction in uterine weights (Memudu and Oluwole Citation2021).
Follicles are the basic functional units of reproduction in the ovary as the development, maturation and ovulation of female gamete occur within the ovarian follicle. The present study revealed a significant decrease in the number of primordial and pre-antral follicles in the ovary of rats treated with 10% PSP, which can be correlated with a decrease in the plasma level of estradiol found in the present study. A similar decrease in the serum levels of estradiol in rats treated with papaya seed extract was also observed by Raji et al. (Citation2005). Such changes in hormone levels affect the histological appearance of female reproductive organs (Aritonang et al. Citation2017). The decline in the level of estradiol results in unbalanced ovarian functions, which invariably affect the follicular development, uterine glands, endometrium and fertility index (Biswal Citation2014). In the present study, a non-significant decrease in luminal epithelial cell height, myometrium and number of endometrial glands was observed in the uterus of rats treated with different concentrations of PSP.
The pituitary-gonadal axis controls the pattern and frequency of the estrous cycling through the gonadotropin hormones i.e. follicle-stimulating hormone (FSH) and luetinizing hormone (LH). Evaluation of the estrous cycle in rats is an index to estimate the functional status of the hypothalamic-pituitary-ovarian axis (Cora et al. Citation2015). Change in the estradiol level may also be the cause of irregularity in estrous cyclicity of treated female rats(Aritonang et al. Citation2017). In the present study, treated rats showed significantly prolonged proestrous and diestrous stages and reduced estrous stage. Similar irregularity in the estrous cycle in rats treated with oral doses of papaya seed extract was also observed earlier (Raji et al. Citation2005; Dosumu et al. Citation2008; Naik et al. Citation2015; Odirichukwu et al. Citation2016). The estrous stage is the period of heat or sexual receptivity and a decrease in its duration suggests a reduction in receptivity and fertility (Nayaka et al. Citation2014). The rate of ovulation also decreases due to a prolonged diestrous phase (Naik et al. Citation2015).
In the present study, fertility was reduced to 50% in rats treated with 10% PSP. Keshri et al. (Citation1993) reported only 30% pregnancy in rats administered hexane extract of papaya seeds administered at 1 g/kg bwt daily. The number of pups delivered by female B. bengalensis treated with 5 and 10% papaya seed powder in present study was significantly lower than that delivered by control rats. Similar to the present results, Raji et al. (Citation2005) also reported a reduced number of pups in females rats orally treated with chloroform extract of papaya seeds. The decrease in estradiol level acts to down-regulate the brain’s hypothalamic mechanisms that reduce the release of LH and hence the ovulation. Reduced ovulation may further lead to a fewer implantation sites.
3β-HSD is directly involved in the conversion of pregnenolone into progesterone, which is responsible for the maintenance of pregnancy, while 17β-HSD is involved in the major pathway of the formation of androstenedione and testosterone, which are subsequently aromatized, leading to the formation of estradiol. Suppression in activities of 17β-HSD and 3β-HSD found in the present study, also observed by Uche-Nwachi et al. (Citation2011), may be the cause of the reduced level of estradiol. A decreased level of ACP andALP was also observed in the present study, which indicates cellular damage (Muthuviveganandavel et al. Citation2008).
5. Conclusion
The present study is the first record on the effect of cereal bait formulated with papaya seed powder as an ingredient on reproductive functions of female B. bengalensis to find a field applicable approach to controlling the rodent pest population. The findings revealed significant anti-reproductive effects of 10% papaya seed powder, which were found reversed after 30 days of treatment withdrawal. The study suggests a need to conduct further studies using higher concentrations of papaya seed powder or its extract to attain long-term irreversible effects.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Adebiyi A, Adaikan PG, Prasad RN. 2002. Papaya (Carica papaya) consumption is unsafe in pregnancy: fact or fable? scientific evaluation of a common belief in some parts of Asia using a rat model. Brit J Nutr. 88:199–203.
- Adebiyi A, Adaikan PG, Prasad RN. 2004. Effect of benzyl isothiocyanate on spontaneous and induced force of rat uterine contraction. Pharmacol Res. 49(5):415–422.
- Adebiyi A, Ganesan Adaikan P, Prasad RN. 2003. Tocolytic and toxic activity of papaya seed extract on isolated rat uterus. Life Sci 74:581–592.
- Agular BM, Vinggaar AM, Vind C. 1992. Regulation by dexamethasone of the 3β-hydroxy steroid dehydrogenase activity in adult rat Leydig cells. J Steroid BiochemMol Biol. 43:565–571.
- Aritonang TR, Rahayu S, Sirait LI, Karo BR, Simanjuntak TP, Natzir R, Sinrang AW, Massi NM, Hatta M, Kamelia E. 2017. The role of FSH, LH, estradiol and progesterone hormone on estrous cycle of female rats. Int J Sci Basic Appl Res. 35:92–100.
- Bessey OA, Lowry OH, Bruck MJ. 1946. A method for the rapid determination of alkaline phosphatase with five millimeters of serum. J Biol Chem. 164:321–329.
- Biswal S. 2014. Phytochemical analysis and a study on the antiestrogenic antifertility effect of leaves of Piper betel in female albino rat. Anc Sci Life. 34:16–22.
- Chinoy NJ, Dilip T, Harsha J. 1995. Effect of Carica papaya seed extract on female rat ovaries and uteri. Phytother Res. 9:169–175.
- Chinoy NJ, Dilip T, Harsha J. 2006. Effect of Carica papaya seed extract on female rat ovaries and uteri. Phytother Res. 9:169–175. doi:10.1002/ptr.2650090303.
- Chinoy NJ, Joshi H, Ghosh S. 1997. Antifertility investigations of alcoholic papaya seed extract in female rats. J Med Arom Plant Sci. 19:422–426.
- Cora MC, Kooistra L, Travlos G. 2015. Vaginal cytology of the laboratory rat and mouse: review and criteria for the staging of the estrous cycle using stained vaginal smears. Toxicol Pathol. 43:776–793.
- Dhar P, Singla N. 2014. Effect of triptolide on reproduction of female lesser bandicoot rat, Bandicota bengalensis. Drug Chem Toxicol. 37:448–458.
- Dosumu O, Akinola B, Oremosu A, Noronha C, Okanlawon A. 2008. Antifertility effects of the aqueous extract of Carica papaya seeds on estrous cycle and ovulation of adult cyclic Sprague–Dawley rats. Niger J Health Sci. 27:31–33.
- Ekhator CN, Shelu JO. 2015. An experimental study on the abortifacient potentials of unripe seed extract of Carica papaya in adult female Wistar rats. Open Sci J Pharm Pharmacol. 3:61–65.
- Estella OE, Ogoamaka OP, Emmanuel EF. 2020. Evaluation of the oxytocic and haematological effects of leaves of Carica papaya Linn (Caricaceae). World J Adv Res Rev. 6(2):212–226.
- Goldman JM, Murr AS, Cooper RL. 2007. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res Part B: Dev Reprod Toxicol. 80:84–97.
- Gunnannavar T, Prakash DP, Hipparagi K, Satish D, Gandolkar K, Sagar BS, Ryavalad S. 2017. Performance of papaya varieties for growth parameters under northern dry zone of Karnataka, India. Int J CurrMicrobiol App Sci. 6:2398–2404.
- Hakameri CS T, Usman E. 2020. Effect of giving young papaya (Carica papaya L.) fruit extract on endometrial histology of female rats (Rattus norvegicus). Sci Midwifery. 9:181–186.
- Julaeha E, Permatasari Y, Mayanti T, Diantini A. 2015. Antifertility compound from the seeds of Carica papaya. Procedia Chem. 17:66–69.
- Kaur H, Singla N, Mahal AK. 2021. Antifertility effect of cereal based bait containing papaya (Carica papaya L.) seed powder in male lesser bandicoot rat, Bandicota bengalensis and its reversibility. Indian J Exp Biol. 59:448–456.
- Keshri G, Singh MM, Vijai Lakshmi Gupta DN, Kamboj VP. 1993. Post-coital antifertility activity of the seeds of Carica papaya Linn. in female rats. Indian Drugs. 30:453–457.
- Kumari S, Kumar R, Singh VN. 2017. Contraceptive effects of aqueous extract of Carica papaya(Linn.) seed on seminal profile of Swiss albino mice. Int J Sci Res. 6:1963–1965.
- Lowry OH, Rosebrough NJ, Farr AL, Randall AJ. 1951. Protein measurement with Folin phenol reagent. J Biol Chem. 193:265–275.
- Memudu AE, Oluwole TJ. 2021. The contraceptive potential of Carica papaya seed on oestrus cycle, progesterone, and histomorphology of the Utero-ovarian tissue of adult Wistar rats. JBRA Assisted Reprod. 25(1):34–43.
- Mukhtar Y, Abdu K, Abdulkadir AI, Galalain AM, Maigari AK, Yunusa UM. 2018. Sustainable use of botanical products in plant protection as a promising panacea to integrated pests management and control: issues, challenges, benefits and future prospects. Int J Adv Acad Res. 4(4):65–77.
- Muthuviveganandavel V, Muthuraman P, Muthu S, Srikumar K. 2008. Study on low dose cypermethrin induced histopathology, lipid peroxidation and marker enzyme changes in male rat. Pestic Biochem Phys. 91:12–16.
- Naik NR, Malleswari D, Indira P. 2015. Effect of Mesuaferrea flower and Carica papaya seed extracts on estrous cycle of female albino rats for fertility and antifertility activity. Biolife. 3:730–734.
- Nattrass N, Stephens J, Loubser JJ. 2019. Animal welfare and ecology in the contested ethics of rodent control in Cape Town. J Urban Ecol. 1–10. https://doi.org/10.1093/jue/juz008.
- Nayaka HB, Londonkar RL, Andumesh MK. 2014. Evaluation of Portulaca oleracea for anti-fertility effect in female albino rats. Int J Pharm Pharm Sci. 6:86–89.
- Novitasari D, Triutomo DH, Arifah FH, Ivanawati A, Ulum Z, Murwanti R. 2018. Estrogenic activity of ethanolic extract of papaya peels (Carica papaya L.) on uterine weight and mammae gland proliferation onovariectomy rats. Indonesian J Cancer Chemoprevention. 9:86–91.
- Nwaehujor CO, Ode JO, Ekwere MR, Udegbunam RI. 2014. Anti-fertility effects of fractions from Carica papaya (Pawpaw) Linn. methanol root extract in male Wistar rats. Arabian J Chem. 12:1563–1568.
- Odirichukwu EO. 2015. Postcoitalanticonceptive activities of the aqueous methanolic extract of unripe Carica papaya fruits in rats. IOSR-JAVS. 8:17–21.
- Odirichukwu EO, Uchechukwu NVS, Ogwu D. 2016. The aqueous methanolic extract of unripe Carica papaya (Pawpaw) fruit disrupts cycle in albino rats. IOSR-JAVS. 9:57–62.
- Punitha N, Shettu N, Saravanan R. 2015. Effect of semi-ripe Carica papaya fruit extracts on the reproductive structures in female albino rats – a histological study. Int J Curr Res Life Sci. 4:241–245.
- Raji Y, Morakinyo AO, Oloyo A, Stephen A. 2005. Impact of the chloroform extract of Carica papaya seed on cycle and fertility in female albino rats. J Med Sci. 5:337–343.
- Sahu A, Maiti BR. 1978. Estrous cycle of the bandicoot rat – a rodent pest. Zool J Linn Soc. 63:309–314.
- Sandhu KK, Singla N. 2019. Growth and breeding biology of female Indian gerbil (Tatera indica): reproductive, biochemical and histological evaluation. Indian J Anim Res. 54(5):534–542.
- Satriyasa BK, Mahendra AN, Arijana IGK, Ruspawan DM. 2018. Unripe papaya seed ethanol extract (Carica papaya, Linn.) inhibits FSH and LH of male mice (Mus musculus). Biomed Pharmacol J. 11:979–984.
- Sidhu A, Singla N, Lonare M, Mahal AK. 2020. Effect of quinestrol on body weight, vital organs, biochemicals and genotoxicity in adult male lesser bandicoot rat, Bandicota bengalensis. Pestic Biochem Physiol. 165:104544.
- Sindhu G, Ragini G, Sreehari L, Gayathri G, Thejaswee A, Inthiyaz S, Usha Kiran Reddy T, Thyagaraju K. 2019. Pharmacognostical and pharmacological profile of Carica papaya – A review. World J Pharm Pharm Sci. 8:675–694.
- Singla LD, Singla N, Parshad VR, Juyal PD, Sood NK. 2008. Rodents as reservoir of parasites in India. Integr Zool. 3:21–26.
- Singla N. 2015. Anti-reproductive effects of total glycosides of Tripterygium wilfordii in male house rat, Rattus rattus. Appl Biol Res. 17(3):302–306.
- Singla N, Babbar BK. 2010. Rodent damage and infestation in wheat and rice crop fields: District wise analysis in Punjab state. Indian J Ecol. 37(2):184–188.
- Singla N, Dhar P, Singla LD, Gupta K. 2016. Patho-physiological observations in natural concurrent infections of helminth parasites of zoonotic importance in the wild rodents, Bandicota bengalensis. J Parasit Dis. 40(4):1435–1442.
- Singla N, Parshad VR. 2002. Acceptance and efficacy of ready-to-use coumatetralyl paste and freshly prepared cereal bait based on coumatetralyl against Rattus rattus. Int Pest Contr. 44(4):178–181.
- Smith R, Shore RF. 2015. Environmental impacts of rodenticides. In: Buckle A, Smith R, editors. Rodent pests and their control. 2nd ed. CAB International Publishers; p. 330–345. https://doi.org/10.1079/9781845938178.0330.
- Tilly JL. 2003. Ovarian follicle counts – not as simple as 1, 2, 3. Reprod Biol Endocrinol. 1:11.
- Uche-Nwachi EO, Mitchell CV, McEwen C. 2011. Steroidogenic enzyme histochemistry in the testis of Sprague Dawley rats following the administration of the water extracts from Carica papaya seed. Afr J Tradit Complement Alter Med. 8:69–78.
- Udoh FV, Uodh PB, Umoh EE. 2005. Activity of alkaloid extract of Carica papaya seeds on reproductive functions in male Wistar rats. J Pharm Biol. 43(6):563–567.
