ABSTRACT
The aim of this study was to investigate the pattern of body growth and intestinal development of female Chinese native geese from 1 to 10 weeks of age. At weekly intervals, from hatch through 10 weeks of age, ten geese were sampled to measure the absolute and relative weight and length of the intestinal segments and overall intestine of geese. The body weight of Bertalanffy (R2 = 0.989), Gompertz (R2 = 0.990), Logistic (R2 = 0.980) and Richards (R2 = 0.982) models showed an increase in the asymptotes at 1–10 weeks old, while the inflection points were at 30.53, 28.56, and 25.59 d, respectively. The relative weight and length of the intestinal segments and overall intestine were decreased linearly (P < 0.01), while the absolute weight and length of the intestinal segments and overall intestine were increased linearly (P < 0.01) during 1–7 weeks old, but significantly decreased (P < 0.01) during 8–10 weeks old, suggesting that the intestinal system was developing rapidly at the starter period based on the intestinal weight and length, but was declining at the later period. The knowledge of growth curve and intestinal development pattern could provide some useful information on the optimum management practices and breeding strategies in geese production.
Introduction
To meet the requirement of animal protein due to the increase of population, the production of poultry other than chicken and ducks, such as geese, was increasing year by year in China (Kozák Citation2021). The size of the geese population in China increased to 639 million heads in 2020 (Hou and Liu Citation2021). In addition, the meat products of geese were regarded as one of the most valuable protein co-products and were increasingly popular in the Chinese market. Growth traits were important characteristics of both economic profitability and population dynamics in poultry production (Kenny et al. Citation2018). Growth curve models can be used for predicting body weight (BW) changes with age, which provide a visual assessment of growth as a function of time (Narinc et al. Citation2017). Therefore, knowledge about the growth pattern of meat goose was necessary to optimize the geese production system (e.g. by selection, feeding management and marketing strategies) (Boz et al. Citation2017). For example, it was unclear how much of an impact on growth production was the semi-intensive and intensive production systems, instead of the free-range backyard type (Boz et al. Citation2021). Therefore, the relationship between body weight and age depended on the different breeds and sexes (Uhlířová et al. Citation2018). To explain the growth curve of poultry, Logistic, Bertalanffy and Gompertz models were often used in male and female chicken, turkeys and ducks (Rogers Citation1987; Cigdem and Hulya Citation2001; Maruyama et al. Citation2001; Vitezica et al. Citation2010; Thinh et al. Citation2021). Moreover, precision feeding was separately performed for geese according to gender, in China. These models were applied to Chinese native geese of mixed gender (Zhao et al. Citation2007; Liu et al. Citation2017), but rarely to female geese. The intestinal system is the primary site to perform the functions of digestion, absorption, and protection (de Carvalho et al. Citation2021). The intestinal developmental patterns had positive effects on growth performance in poultry (González-Alvarado et al. Citation2008; Wang et al. Citation2014; Zhang et al. Citation2020). The knowledge of growth curve and intestinal development patterns could provide some useful information on the design of optimum management practices and breeding strategies in geese production. However, little information was available concerning the pattern of intestinal development in geese. Therefore, the purpose of this study was to investigate the developmental pattern of body weight and intestinal tract of female native Magang geese under the intensive production system, in favour of the breeding strategy to modify the trajectory of growth.
Methods and materials
Animals, management and housing
All procedures of this study were approved by the animal care and welfare committee institute of South China Agricultural University (SCAU-10564), and the study was performed following the Regulations for the Administration of Affairs Concerning Experimental Animals. The study was conducted at the South China Agricultural University Agricultural Faculty’s Experimental Farm.
A total of one hundred and eighty female Magang goslings were assigned randomly to ten pens and eighteen birds per pen were raised in the environmental chambers under an intensive production system. Pens were separated by a wire mesh. Each pen contained one round feeder and one round drinker. The temperature started at 30 ± 1°C and was reduced by 3 ± 1°C each week until 21.1°C was attained at week 10. All birds were fed a starter diet from 0 to 4 weeks, and a grower diet from 4 to 10 weeks that met or exceeded the nutrient recommendations for geese () by National Research Council (Citation1994). Feed and water were provided ad libitum and economic white bulbs were used for lighting. The feeding and lighting programme were performed according to the guideline of management for Magang goose. The birds were individually weighed at 08:00 h and body weight (BW) was recorded at weekly intervals for 10 weeks. At the end of each week, one bird from each pen was slaughtered and the average BW of the remaining birds was used as the data point for the growth curve model.
Table 1. Composition and nutrient levels of the experimental diets (as-fed basis).
Sample collections
One bird based on the average BW per pen was selected, weighed, and killed by carbon dioxide asphyxiation. The gastrointestinal tract and organs were carefully excised and determined with a modified method (Amerah et al. Citation2008). In brief, the empty weights of digestive tract segments from the duodenum to the caeca of each bird were determined. The length of each intestinal segment was determined with a flexible tape on a glass surface to prevent inadvertent stretching. After division and freeing of each intestinal segment, separating all connective tissue and fat, and removing the content with ice-cold saline flushing, empty weights (± 0.01 g) were determined. The relative values were calculated as a ratio of live body weight.
Statistical analyses
The non-linear regression models of Logistic, Gompert, Bertalanffy, Richard, Brody and Weibull were fitted using the NLIN models of SAS software (SAS Institute Citation2000). The forms of equations () for the Logistic, Gompert, Bertalanffy, Richard, Brody and Weibull models were: where W is the weight corresponding to age (t) with 3 parameters: A = asymptotic or maximum growth response, B = intercept, or weight when age (t) = 0 and K = rate constant. For digestive tract measurements, individual birds were considered as the experimental unit. All data were subjected to a one-way analysis of variance by using PROC GLM of SAS software. Orthogonal polynomials were applied for linear and quadratic effects on the parameters of intestinal development corresponding to age. Differences were considered to be significant at P < 0.05, and significant differences between means were separated by the least significant difference test.
Table 2. Growth curve parameters in different models for Magang geese (n = 10).
Results and discussion
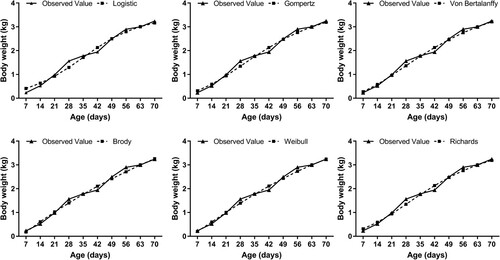
The development of the meat goose industry requires further knowledge of the overall growth patterns for different breeds and sexes (Uhlířová et al. Citation2018). However, there was limited information on the growth curve for some native geese lines (Nder et al. Citation2017). In the present study, the growth curve analysis for female Chinese native Magang goose showed an increase in the asymptotes during the selection period of 1–10 weeks of age, and the age to reach the inflection point was estimated at 30.53, 28.56, 25.59, and 29.2 d, using the Logistic, Gompertz, Bertalanffy and Richards models, respectively (). The ages to reach the inflection points provided the estimates of maturity of the growth processes. In the current study, estimation of the inflection points was close to that reported in Chinese Sichuang white geese with mixed gender using the Gompertz model (27.51 d) (Liu et al. Citation2017) and female Turkish native geese using the Logistic model (32.9 d) (Nder et al. Citation2017) and shorter than that reported in Lion-head geese with mixed gender using the Gompertz model (52.5 d) (Zhao et al. Citation2007). In the present study, the accuracy of Bertalanffy (R2 = 0.990) and Gompertz (R2 = 0.989) models fitted body weights was higher than the Logistic (R2 = 0.980) and Richards (R2 = 0.982) models, indicating that the Gompertz and Bertalanffy models with high coefficients of determination could be suitable models for female Chinese native Magang geese growth. Laird (Citation1966) reported that the Gompertz function has been preferred over the logistic function for fitting monophasic growth curves of chickens. When analysed by growth curves derived from the Bertalanffy model, the body weight of Magang geese during the periods of 2–4 weeks (521 g/week) and 7–8 weeks (483 g/week) of age was faster than the other growth period (). The greatest body weight gain appeared at 4 weeks of age (581 g/week), which was similar to the data obtained from female Bohemian and Italian White geese (Knizetova et al. Citation1994). As reported in ducks, the age of the inflection point that remained constant was 26 d for Pekin ducks, 21 d for Mallard ducks and 37 d for Muscovy ducks (Gille and Salomon Citation1994). It was inferred that the geese were characterized by an early maturing rate, nearly as early as ducks (Knizetova et al. Citation1991b) and earlier than chickens (Knizetova et al. Citation1991a). It was apparent that the selection programme, without a delay in maturity, reduced the time to reach the market weight to achieve more efficient geese production.
Figure 1. Predicted average body weight and observed growth curves for the Logistic, Gompert, Bertalanffy, Richard, Brody and Weibull models.
The intestinal system was the primary site of entry for any orally administered compound, including dietary ingredients. There was a growing interest on the influence of diet on the development of the gastrointestinal tract in poultry (González-Alvarado et al. Citation2008; Jiménez-Moreno et al. Citation2009; Wang et al. Citation2014; Zhang et al. Citation2020). In the present study, the relative weight and length of the intestinal segments and overall intestine decreased linearly with the increased week of age ( and ). The greatest relative weight and length of the intestine were observed during the first three weeks. It was suggested that the intestinal system of a hatchling must undergo tremendous change before it was capable of efficiently digesting the dietary nutrients. Previous studies demonstrated that the growth of the intestinal system may exceed that of the rest of the body by as much as five-fold in chickens during the first 5–7 days of post-hatch (Uni et al. Citation1999; Sklan Citation2001). A previous study reported that avian species with high growth rate capacities at the starter period were also characterized by a rapid development of the digestive organs (Lilja Citation1983), which was confirmed in geese of the current study. The absolute weight and length of the duodenum, jejunum and ileum segments and overall intestine were increased quadratically in response to the increased age ( and ). The absolute length of the intestinal segments and overall intestine was increased as observed over the 1–5 week period in geese, which was in agreement with those reported in broilers from post-hatching to 21 days old (Uni et al. Citation1999). No significant changes were observed in the absolute length of the intestine during 6–10 weeks of age. It was suggested that the intestines tend to be mature by 5–6 weeks of age. Some interesting patterns were observed in the absolute weight of the digestive system during the growth period. The absolute weight and length of the intestinal segments and overall intestine increased linearly during 1–7 weeks and the age to reach the inflection points was at 7 weeks of age. However, there was a significant decrease in the absolute weight of the duodenum, jejunum and ileum segments and overall intestine during 8–10 weeks old. It was implied that the shrinking of gastrointestinal system development occurred during the later growth period, which resulted in the lower growth rate capacity in geese during 8–10 weeks. The reduced weight of the small intestine was in line with the results that the absolute mass of the small intestine in domesticated ducks declined by 38% at 5 weeks post-hatching (Watkins et al. Citation2004). This reduction of the intestinal segments can be due more to the changes in the intestinal thickness rather than the intestinal length, which could be associated with the alteration of bioavailability and utilization of nutrients. There is no obvious explanation for this decline because it does not appear to optimize digestion.
Table 3. Effect of age on the relative weight of intestine of Magang geese from 1 to 10 weeks of age (g/kg)Table FootnoteA.
Table 4. Effect of age on the relative length of the intestine of Magang geese from 1 to 10 weeks of age (cm/kg)Table FootnoteA.
Table 5. Effect of age on the absolute weight of the intestine of Magang geese from 1 to 10 weeks of age (g/kg) A.
Table 6. Effect of age on the absolute length of the intestine of Magang geese from 1 to 10 weeks of age (cm/kg) A.
Conclusion
Considering that the R2 values of the Gompertz and Bertalanffy models were higher than for the Logistic and Richards models, Gompertz and Bertalanffy models with high coefficients of determination could be suitable models for female Chinese native Magang geese growth, and the age of the inflection points was estimated at 30.53 and 28.56 d, respectively. It was suggested that the intestinal system was well developed during 1–7 weeks old in terms of the intestinal weight and length, but displayed a decline in geese during 8–10 weeks old. The knowledge of growth curve models and intestinal development pattern could provide some useful information on the design of optimum management practices and breeding strategies in geese production.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability
The data that support this study are available in the article.
Additional information
Funding
References
- Amerah AM, Ravindran V, Lentle RG, Thomas DG. 2008. Influence of feed particle size on the performance, energy utilization, digestive tract development, and digesta parameters of broiler starters fed wheat- and corn-based diets. Poult Sci. 87(11):2320–2328.
- Boz M, Sarica M, Yamak U. 2017. Production traits of artificially and naturally hatched geese in intensive and free-range systems: I. Growth traits. Br Poult Sci. 58(2):132–138.
- Boz MA, Sarıca M, Yamak US, Erensoy K. 2021. Behavioral traits of artificially and naturally hatched geese in intensive and free-range production systems. Appl Anim Behav Sci. 236:105273.
- Cigdem Y, Hulya A. 2001. Comparison of growth curve models on broilers growth curve I: parameters estimation. J Biolog Sci. 1(7):682–684.
- de Carvalho NM, Oliveira DL, Saleh MAD, Pintado ME, Madureira AR. 2021. Importance of gastrointestinal in vitro models for the poultry industry and feed formulations. Anim Feed Sci Technol. 271:114730.
- Gille U, Salomon FV. 1994. Heart and body growth in ducks. Growth Dev Aging. 58:75–81.
- González-Alvarado J, Jiménez-Moreno E, Valencia D, Lázaro R, Mateos G. 2008. Effects of fiber source and heat processing of the cereal on the development and pH of the gastrointestinal tract of broilers fed diets based on corn or rice. Poult Sci. 87(9):1779–1795.
- Hou S, Liu L. 2021. The present situation, development prospect and suggestion of waterfowl industry in China in 2020. Chinese J Anim Sci. 57:235–239.
- Jiménez-Moreno E, González-Alvarado J, de Coca-Sinova A, Lázaro R, Mateos G. 2009. Effects of source of fibre on the development and pH of the gastrointestinal tract of broilers. Anim Feed Sci Technol. 154(1-2):93–101.
- Kenny D, Fitzsimons C, Waters S, McGee M. 2018. Invited review: improving feed efficiency of beef cattle–the current state of the art and future challenges. Animal. 12(9):1815–1826.
- Knizetova H, Hyanek J, Kniže B, Prochazkova H. 1991b. Analysis of growth curves of fowl. II. Ducks. British Poult Sci. 32(5):1039–1053.
- Knizetova H, Hyanek J, Kníže B, Roubíček J. 1991a. Analysis of growth curves of fowl. I. Chickens. British Poult Sci. 32(5):1027–1038.
- Knizetova H, Hyanek J, Veselsky A. 1994. Analysis of growth curves of fowl. III. Geese. British Poult Sci. 35(3):335–344.
- Kozák J. 2021. Goose production and goose products. Worlds Poult Sci J. 77(1):1–12.
- Laird AK. 1966. Postnatal growth of birds and mammals. Growth. 30:349–363.
- Lilja C. 1983. A comparative study of postnatal growth and organ development in some species of birds. Growth. 47:317–339.
- Liu Z, Huang Y, Wang Q, Peng X, Wang Y, Li J, Lan Y, Wang C. 2017. Fitting and analysis of the growth curve of body weight, muscle, and digestive tract in Sichuan white geese. Chinese J Anim Sci. 53:21–27.
- Maruyama K, Vinyard B, Akbar MK, Shafer DJ, Turk CM. 2001. Growth curve analyses in selected duck lines. Br Poult Sci. 42(5):574–582.
- Narinc D, Narinç NÖ, Aygün A. 2017. Growth curve analyses in poultry science. Worlds Poult Sci J. 73(2):395–408.
- National Research Council. 1994. Nutrient requirements of poultry., 9th ed. Washington, DC: Natl Acad Press.
- Nder H, Boz MA, Sarca M, Abac SH, Yamak US. 2017. Comparison of growth curve models in Turkish native geese. European Poult Sci. 81:1–8.
- Rogers S. 1987. Comparison of three non-linear regression models for describing broiler growth curves. Growth. 51:229–239.
- SAS Institute. 2000. SAS/STAT user’s guide (version 8). NC: Cary1.
- Sklan D. 2001. Development of the digestive tract of poultry. Worlds Poult Sci J. 57:415–428.
- Thinh N, Doan B, Dang P, Canh N, Giang N, Minh L, Do D. 2021. Modelling growth curve of eastern spot-billed ducks (anas zonorhyncha) raised in Vietnam. J Anim Feed Sci. 30(1):76–81.
- Uhlířová L, Tůmová E, Chodová D, Vlčková J, Ketta M, Volek Z, Skřivanová V. 2018. The effect of age, genotype and sex on carcass traits, meat quality and sensory attributes of geese. Asian-Australas J Anim Sci. 31(3):421–428.
- Uni Z, Noy Y, Sklan D. 1999. Posthatch development of small intestinal function in the poult. Poult Sci. 78:215–222.
- Vitezica ZG, Marie-Etancelin C, Bernadet M, Fernandez X, Robert-Granie C. 2010. Comparison of nonlinear and spline regression models for describing mule duck growth curves. Poult Sci. 89(8):1778–1784.
- Wang Z, Yang H, Lu J, Li W, Zou J. 2014. Influence of whole hulled rice and rice husk feeding on the performance, carcass yield and digestive tract development of geese. Anim Feed Sci Technol. 194:99–105.
- Watkins EJ, Butler PJ, Kenyon BP. 2004. Posthatch growth of the digestive system in wild and domesticated ducks. Br Poult Sci. 45(3):331–341.
- Zhang Q, Jie Y, Zhou C, Wang L, Huang L, Yang L, Zhu Y. 2020. Effect of oral spray with lactobacillus on growth performance, intestinal development and microflora population of ducklings. Asian-Australas J Anim Sci. 33(3):456–464.
- Zhao WM, Chen Q, Cheng JH, Lin QT, Xin-Sheng WU, Chen GH, Jiang QL. 2007. Optimum estimation of growth and development parameters about early bodyweight of local goose breeds. J Yangzhou Univ (Agri Life Sci Ed). 2007(03):47–50.
