ABSTRACT
Veterinarians and animal producers use non-essential compounds via either feed additives or implantation for a long history as growth promoters for farm animals. These compounds, such as anabolic hormones, bovine somatotropin, Beta-agonists, have multiple positive effects and improve animal performance in many meat-exporting countries due to their anabolic activity. However, some of them may cause carcinogenic effects to the consumer. Anabolic hormones are now permitted to be used as growth promoters on a global scale, but have not been permitted in the EU since 1988. Unlawfully, drugs are sometimes added to farm animal feeds to improve muscle growth, feed utilization efficiency, milk production, and nitrogen retention in order to obtain an economic benefit in a shorter period.. In this article, we review the current knowledge on the safety and hazard of an anabolic compound as endocrine disruptor, not only on food, animals and public health but also on the environment, to arrive at recommendations for risk management and avoidance. The U.S. Food and Drug Administration approves seven hormone drugs (testosterone propionate, trenbolone acetate, estradiol, zeranol, progesterone, melengestrol acetate, and bovine somatotropin) for use in food animals for many purposes. Synthetic hormones were higher stability and longer persistence in the environment.
1. Introduction
Growth promoters include any substances added to feeds as the addition or injection of drugs to promote feed efficiency and utilization and even to improve the growth of farm animals. Although the use of growth promoters is forbidden in the EU, and in most countries outside the EU, some countries use them in areas where increasing trends of meat and milk demand to meet the human needs. Hormones are extensively used agents in veterinary medicine. There are three brief purposes of their administration to farm animals. Firstly, in the cure of sick animals such as glucocorticoids. Secondly, in reproductive disorders, synchronization, and control of oestrus treated by sex hormones. Thirdly, in hormonal application to increase milk production and improve animals’ growth rate, they serve as growth promoters. Hormones are generally highly active compounds that affect the physiology of animals in small doses. The most extensively applied hormonal growth promoters are natural and synthetic hormones, anabolic implants (estrogenic and androgenic) and bovine somatotropin (bST) (Herago and Agonafir Citation2017). They refer to Beta-agonists, as repartitioning agents, are pharmacologically active substances. Many research studies have been conducted to assess the effects of hormonal growth promoters on performances and carcass characteristics of farm animals, aspects of an anabolic hormonal application. Anabolic implants and bST improve growth rate and feed efficiency by reducing fat deposition in farm animals (Ebarb et al. Citation2017). Treated animals with steroids increased the body weight gain through nitrogen retention and net protein accretion without any changes in the digestibility of nitrogen intake (Scarth et al. Citation2009).
Nachman and Smith (Citation2015) and Herago and Agonafir (Citation2017) reviewed that in the United States (U.S.), several active compound drugs approved by the Food and Drug Administration (FDA) for use in food animal production, such as beef cattle and sheep, are endogenous or steroid hormones, such as progesterone and estradiol (E2). In addition , non-endogenous or esters of steroid hormones, such as estradiol benzoate and testosterone propionate (TP). Also, compounds have a high affinity for human androgenic, estrogenic and progesterone hormone receptors, such as trenbolone acetate (TBA), zeranol, and melengestrol acetate (MGA), respectively. These hormones or drugs are approved as growth promotion for use in cattle and, in the case of zeranol, sheep to increase body weight gain and feed efficiency. In addition, progesterone, MGA, and E2 are approved to regulate estrus in sheep and beef cattle. In dairy cattle, bST is approved as a manner for increasing the production of milk. Hormones are not approved for use in swine or poultry (). The differences between the regulated use in the U.S.A. and the illegal use in the EU of anabolic growth promoters and their bann are explained in .
Figure 1. Timeline or history of anabolic growth promoters. This figure is for the EU below and above the line for the U.S.A. situation. Adapted from Ronquillo and Hernandez (Citation2017) and from Herago and Agonafir (Citation2017).
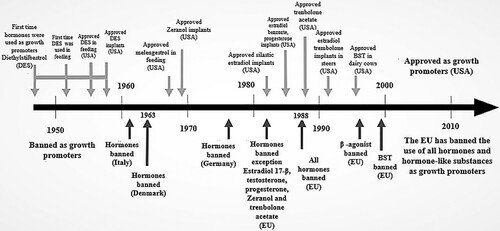
Table 1. FDA drug approvals by species, indication, and status adapted from Nachman and Smith (Citation2015).
Nevertheless, most growth promotes have not gained extensive consumer acceptability, particularly, the European Union (EU) has banned growth-promoting hormones. Subsequently, this study aimed to give an overview of the different types of natural and synthetic hormonal growth promoters and their importance in farm animal production, indicate the impact of residues of hormonal growth promotors on public health by their accumulation in human tissues. Besides reviewing how residue impact environment and risk avoidance, policies on growth promoters are used for farm animal production.
2. Hormonal growth promoters and repartitioning agents
Hormonal growth promoters increase the feed efficiency, gain, carcass quality and/or milk production of animals. The cattle growth promoters can be bifurcated into hormonal and non-hormonal groups. However, the non-hormonal growth promoters, such as antibiotics and probiotics, will not be discussed in this review. The hormonal growth promoters groups, such as hormonal implants, growth hormone (GH) or (Somatotropin) (ST), repartitioning agents (β-agonists) (Schiffer et al. Citation2001), genetics and nutritional factors are the most important factors affecting animal productivity. The amount of fed proteinconverted into muscle deposition is interested in meat animal producers (Moloney and McGee Citation2017). The amount of protein formation can be measured by comparing the amount of the fed nitrogen to the amount of nitrogen in the animals’ waste. However, most growth promoters can enhance the animals’ efficiency using nitrogen in the ratio to build amino acids and then build their protein. Thus, anabolic hormones, as growth promoters, accelerate the retention of nitrogen in the body (Wierup Citation2001). The safety and concerns of hormonal implants, bovine somatotropin, and repartitioning agents in farm animal production will be discussed in the following sections.
2.1. Hormonal implants as a growth promoter
Currently, in the animal industry, especially the beef cattle industry, of many non-EU countries, such as the U.S.A., Australia and Canada, , implanting hormonal growth promoters is widespread for the best gain and for improved feed efficiency. They are implanted behind the animals’ ear under the skin as depot capsules form that release a specific dose of hormones over a fixed period (Mader Citation1997). The implantation of these hormones may improve growth during all stages of meat production: suckling, growing and finishing stages (Platter et al. Citation2003). According to the American Association, 63% of estimated beef cattle in the U.S.A. are implanted with growth-promoting hormones (Lange et al. Citation2002).
The implanting hormones widely used in food animals are mostly only synthetic. In general, implanting hormones can be divided into two groups. (1) Endogenous or natural hormones are naturally produced by humans and animals. Those hormones are extracted from animals or manufactured using recombinant DNA or some other technology; (2) Synthetic or xenobiotic hormones are chemically synthesized compounds to mimic the effect of natural hormones and do not occur naturally in animals (Cepeda and Fente Citation2012). The natural hormones include estradiol, progesterone, and testosterone and synthetic ones include zeranol and TBA (Elmore Citation1992). The steroidal hormones have multiple actions either estrogenic (i.e. estradiol, responsible for female behaviour), androgenic (i.e. testosterone, responsible for male behaviour), and gestagenic (i.e. progesterone responsible for maintaining a pregnancy). The other synthetic hormones mimic the action of the natural hormones: zeranol mimics estradiol, TBA mimics the biological activity of testosterone, and MGA mimics progesterone (Passantino Citation2012). The growth promoted by estradiol by activating appetite and enhancing feed conversion efficiency (FCE) (Elmore Citation1992). The rate of body weight gain and feed efficiency improved by testosterone or TP, alone or incorporated with other hormonally active compounds by androgens’ anabolic action (Kefi Citation2014).
Progesterone serves as the precursor of all the major steroid hormones, such as corticosteroids, estrogens and androgens in the adrenals and gonads Besides it is converted to one or more metabolites by many body tissues to increase animals’ growth rate (Wiebe Citation2006). The TBA, as a synthetic steroid, may exceed testosterone in an anabolic potency. TBA, as a prodrug, converts into its major active form 17β-trenbolone in the muscle and then isomerizes into major metabolite 17α-trenbolone in the liver tissue and excreta, including bile. It has anabolic action via interaction with receptors of androgen and glucocorticoid (Elliott et al. Citation1993). Zeranol is derived from the naturally occurring myco estrogen-zearalenone, and has a potent estrogen receptor (Takemura et al. Citation2007). The actions of zeranol resemble those of estradiol (Leffers et al. Citation2001) and are used alone or in incorporation with TBA as a growth-promoting hormone in various livestock (Yuri et al. Citation2006).
The use of these hormone implants in cattle according to recommended procedures was avowedly safe and approved by the following groups. Food and Drug Administration (FDA), Food and Agriculture Organization/World Health Organization (FAO/WHO), Codex Committee on Residues of Veterinary Drugs in Foods, EEC Scientific Working Group, European Agriculture Commission Scientific Conference and Sub-Group of the Veterinary Products Committee (UK) on anabolic agents, growth promotion in meat production (Organization Citation2016). In the EU total ban is on the hormonal application for growth promotion. At the same time, other feed additives cannot be placed on the market unless they are authorized based on a scientific assessment of their efficacy, their effect on human and animal health and the environment.
2.2. Growth hormones as a growth promoter
Since the 1920s, ST or GH has been known as a long single natural polypeptide chain containing 191 amino acids that vary considerably within species (Eversole et al. Citation1989). It improves weight gain by motivating metabolism leading to protein accretion and reduced fat deposition (Courtheyn et al. Citation2002). BST is synthesized by cow’s pituitary gland and is called bGH. BST is considered a protein, like insulin, not considered a steroid hormone, such as cortisone or sex hormones. During lactation, BST not only mobilizes body fat for utilizing as energy but also diverts feed energy towards milk production rather than body tissue synthesis. Indeed, BST improved milk production efficiency by 10%–15% (Lee et al. Citation2010). The BST is used mainly to increase milk production, its effects on bovine meat increase growth rates, lower carcass fat, enhance feed conversion and lean carcass. BST effect on meat quality via reduced carcass fat reduced acceptability in respect of lower tenderness scores (Bidanel et al. Citation1991). Increasing long-chain fatty acids and somatic cell counts of the milk when BST is used in cows, while other milk components are not altered (Waltner-Toews and McEwen Citation1994). The EU, the U.S.A. and other countries approved rbST for commercial use. While some countries do not have yet approved rbST for commercial use due to various concerns such as animal welfare and safety, production quota-based marketing, and other concerns (Raymond et al. Citation2010).
There are side effects of the bST use on an animal’s welfare and health that was a reason for the ban in the EU. One of the most side effects of bST on animal welfare is mastitis or mammary gland inflammation due to increased productivity caused by bST. Mastitis causes pain in the adder, fever and depression, and can be fatal in severe cases. The rbST is associated with skeletal disorders, such as clinical lameness in dairy cows. Another animal welfare implication of rbST used in dairy cows is irritation and swelling at the injection site and/or other complications such as draining, hematoma, and lesion. The overall impact of cows treated with bST on animal welfare is more prone to heat stress under high temperatures and higher culling rates for cows treated with bST (Pell et al. Citation1992; Scahaw Citation1999).
Health issues such as lower pregnancy rate are attributed to failure to conceive, digestive disorders such as diarrhoea, indigestion, and bloating; increased frequency of multiple births; and lower body condition score at the end of the lactation period (Elvinger et al. Citation1992; Scahaw Citation1999).
2.3. Repartitioning agents (β-adrenergic agonists) as a growth promoter
The β-AA improves growth efficiency through stimulation receptors of β-adrenergic on a cell membrane. They act as repartitioning agents to increase muscle growth by up to 40% and lower fat deposition by up to 40% by modifying the composition of the carcass by altering nutrient partitioning. The increased protein content is mediated by the binding of the β-agonist to β1 and β2 receptors in the muscle, resulting in increased skeletal muscle protein synthesis (Manickam and Wahli Citation2016). β-agonists have primary mechanisms for promoting cell hypertrophy and protein synthesis in muscle tissue by inhibiting of proteolysis while promoting lipolysis in adipose tissue (Liu et al. Citation2016). The secondary mechanisms of β-agonists are mediated by other hormones by rising blood flow (Pearson and Hussain Citation2015).
Many compounds act as β-agonists, including clenbuterol, zilpaterol, ractopamine, cimaterol, fenoterol, salbutamol, isoprenaline, terbutaline, and mabuterol. In South Africa and Mexico livestock, zilpaterol, an active β2-agonist, is used for fattening. Zilpaterol hydrochloride is a powerful β-agonist and more effective than ractopamine and clenbuterol (Herago and Agonafir Citation2017). Zilpaterol supplementation benefits via enhanced growth performance and increased carcass yield of fattening steers in Mexican (Lee et al. Citation2010).
Two problems arise from using β-agonists to promote growth for a long period. Firstly, a falloff in effect over time due to down-regulation of the receptor (Herago and Agonafir Citation2017). Secondly, a ‘rebound’ when removed from the product, leading to promoting fat deposition and reducing muscle mass (Elliott et al. Citation1993). Thus, the most effective use of β-agonists or a repartitioning agent in the finishing period before slaughter, particularly in the one to two months. The legal or illegal uses of β-agonists and the countries are these compounds registered for use are mentioned in Section 3.2.3 The safety aspect of β-Adrenergic agonists. If necessary, illegally used β-Adrenergic agonists in farm animals must be strictly controlled.
3. Assess the burden and safety issues of hormones and their metabolite residues as endocrine disruptors on food, animal products and public health in the EU, the U.S.A., and other countries
In the EU, European food safety authority EFSA (Citation2007) reported the evidence for an association between some forms of hormone-dependent cancers, such as estradiol-17β and red meat consumption, and stated that all six hormones may cause adverse toxic actions on consumers’ health, such as endocrine, neurobiological, developmental, genotoxic, immunotoxic, and carcinogenic impacts, especially for susceptible risk groups (such as prepubertal children). The epidemiological and toxicological records studied by the Commission panels do not allow a quantitative risk estimate, leading to the panel’s indicated no threshold levels could be defined for any of the six steroid hormones (Passantino Citation2012).
For comprehension, the burden posed by exposure to anabolic hormones needed data on residue levels in food and animal products. To obtain the FDA approval for administrating hormones to farm animals, feeding studies by drug companies or sponsors of a new animal drug needed to know the persistence and depletion rates of these substances in the edible tissues and other animal products of dosed farm animals. These data are used to know recommended dosages and withdrawal periods to determine the days needed to stop using the drug before slaughter to ensure the remaining residue levels fell to levels the FDA considers ‘safe’ for the human. Feeding research studies conducted outside the new animal drug application (NADA) were uncommon (EFSA Citation2007).
Food animal feeding studies reviewed many techniques for residue determination of the hormone in animal products and retail market samples. Many studies focus on free/unconjugated hormone residues due to having the most biologically active forms (Farlow et al. Citation2009). Two French studies occur in French supermarkets to determine natural levels of total estradiol and testosterone in eggs and milk. In addition, these studies attempt to compress between exogenous dietary intake and daily endogenous production in pre-pubertal children as a basis for risk assessment. Compression between these two studies observed measurable levels of all compounds (Courant et al. Citation2007; Courant et al. Citation2008). A few researchers have tried to describe synthetic hormone residues in beef cattle products (Courant et al. Citation2008; Nachman and Smith Citation2015). Hormonal residues can negatively affect the immune system and then induce some common cancers.
Additionally, it influences human health, such as many human reproduction and development aspects in case high doses of hormones are injected into experimental animals. Currently, there is sufficient proof for estradiol carcinogenicity and limited estradiol for testosterone carcinogenicity in humans (Passantino Citation2012). Other compounds may cause side effects in experimental animals when used in implants (WHO Citation1997). Some carcass traits can be influenced by implants or lifetime implant protocols, with lower dressing percentages, reduced marbling, beef quality, palatability and a reduction in top-grade carcasses (Platter et al. Citation2003). Hormonally active substances used in animal production are shown in .
Table 2. Hormonally active substances used in animal production.
3.1. FDA hormonal residue tolerance levels
The FDA is responsible for setting levels of tolerance for residues of hormones that may remain in animal products as a result of their administration to food animals . These levels are set as residue limits in the specific tissues of a particular species (). Interestingly, if the tested edible tissues are below codified ‘safe concentrations’, the agency notes that a marker residue is not needed. No observed effects limits (NOELs) mean quantities of hormone below the no-hormonal effect levels (NHEL) do not result in a response in the target tissue.
3.2. Hormone residue testing under the USDA/FSIS/National residue program
Within the U.S., the national residue program (NRP) managed by the food safety and inspection service (FSIS) of the United States Department of Agriculture (USDA) is the only federal effort that routinely examines food animal products for drug residues. The testing examined only tissues not commonly consumed by people, such as the liver and kidney. In its entire history, the NRP has only tested zeranol, TBA, and MGA. The primary purpose of the NRP is to the remove of food animal products with violative residue concentrations from the food supply. European food safety authority EFSA (Citation2007) reported the evidence for an association between some forms of hormone-dependent cancers and red meat consumption, which may cause adverse toxic actions on consumers’ health.
Some hormones given to animals cannot be completely absorbed or metabolized in the bodies of the animals; thus, this increase concern about the potential effects of residues in meat, milk, and eggs. The concern about hormonal safety naturally includes the potential risks from consuming foodstuffs of animal origin (Ronquillo and Hernandez Citation2017). Croubels et al. (Citation2004) monitored foods of animal origin for the presence of a veterinary drug. Hormones, like other chemicals, exert toxic effects related to an excess of their normal endocrine (‘physiological’) action and excessive exposure to the substance. Doyle (Citation2000) reviewed eight studies that recommended hormonal residue levels in cattle meat that would not adversely affect human health. On the contrary, other studies expressed concern about the safety of implants (Ronquillo and Hernandez Citation2017; Yang et al. Citation2017). Much controversy about the use of these compounds in food animals is focused on two main points: (1) the effects of residues in foods on consumer health; (2) whether western society needs or wants to use these compounds. Widely disparate points of view on these matters are held (Squires Citation2011). Natural and/or synthetic surrogate hormones have been used in agricultural practice for several decades due to the ability to improve weight gain and feed efficiency in meat-producing animals (Akzira Citation2016).
3.2.1. Safety issues of natural anabolic steroids (oestradiol, progesterone, testosterone)
Since they were first licensed for use in the early of 1950s in North America (Squires Citation2011) and began their use in the 1970s in Turkey (Nazli et al. Citation2005), anabolic agents or compounds have had a very impact on beef production due to their effects in increasing the rate of body weight gain, meat quality, and improving the feed efficiency. Anabolic steroids enhance body protein accretion by increasing protein synthesis or decreasing protein degradation, and metabolizing fat stores, resulting in increased growth rates (Stolker et al. Citation2007; Smith Citation2014). When either natural or synthetic anabolic compounds are neutralized by the organism or not discharged, residues occur. Those residues in meat and meat products may be consumed by humans and cause severe health problems. This undesirable situation may cause severe health problems.
Anabolic steroid treatment will reduce cycling in mares and sperm production, testis size in stallions and hepatotoxic in human. High doses of anabolic steroids can decrease high-density lipoprotein (HDL) while increasing low-density lipoprotein (LDL), increase the risk of severe coronary heart disease, and reduce the immune response. Thus the use of anabolic agents in animal husbandry must be strictly controlled (Nazli et al. Citation2005). All natural anabolic steroids (progesterone, estradiol, and testosterone) appear to be safe, and only the human health risk associated with the actual ingestion of implant site of their use, especially administration in improper locations, and repeatedly treated or multiple implants of some animals (Reig and Toldra Citation2008; Cepeda and Fente Citation2012). Chemical structures of steroids and steroid analogues are used as anabolic agents ().
Figure 2. Chemical structures of steroids and steroid analogues used as anabolic agents (Squires Citation2011; Akzira Citation2016).
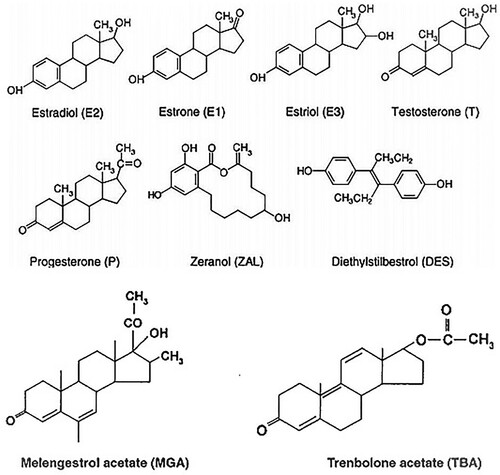
Estradiol (estrogen), testosterone and progesterone are sex hormones that occur naturally in mammals (including humans). Thus, these hormones are normally present in food from animals at different levels depending upon the age, physiological status and pregnancy status of the animal (Jeong et al. Citation2010; FAO/WHO Citation2012). Accordingly, ADI and withdrawal times, the human safety or hazards of residues of steroid hormones in edible tissues from animals have been established within normal ranges (Passantino Citation2012). These sex steroid hormones play a role in human and animal cancer (Millán et al. Citation2013).
The natural sex hormones are not genotoxic under therapeutic and physiological conditions (Joosten et al. Citation2004). Compounds of this type are not carcinogenic at concentrations less than that which induces a measurable hormonal effect. However, sex steroid hormones are capable of increasing the risk of cancer and therefore are classified as ‘carcinogens’ which promote tumours from those that have a genotoxic effect (Millán et al. Citation2013). Acceptance of this concept is the basis for the establishment of the so-called no-hormonal-effect-levels (NHEL) in animal models. These can be used to set safe, acceptable daily intakes (ADI) (Waltner-Toews and McEwen Citation1994). In contrast, many believe that the concept of safe levels does not apply to genotoxic carcinogens (Dorne and Fink-Gremmels Citation2013). However, in 1999 the (FAO/WHO)-(JECFA) mentioned the use of estradiol, progesterone and testosterone in meat production and declared that they were safe (FDA Citation2015). The estradiol benzoate/progesterone were the first approved hormones for use in cattle in 1956 by the U.S. Food and Drug Administration (FDA), then later, testosterone, zeranol, TBA, and combinations of these hormones by the FDA. One or more implants of five hormones (progesterone, testosterone, estradiol-17β, zeranol, and TBA) are approved and widely used for enhancing cattle’s growth (FDA Citation2015).
The ADI, NOELs, maximum residue levels (MRL) for oestradiol, progesterone, and testosterone are mentioned in . Theoretical (ADI) can be calculated from data on tissue levels of these compounds in implanted animals and consider that an individual would consume 300 g of muscle, 100 g of liver, 50 g of kidney and 50 g of fat per day (Squires Citation2011).
Table 3. FDA natural and synthetic steroidal hormone tolerance limits: the safe, acceptable daily intake using a safety factor of 100, maximum residue levels and the no observed effects limits.
Estradiol-17β (estrogen)
To date, studies of estradiol 1 and 2 levels in various milk products of dairy cattle were most common. The total estradiol 1 levels were 118.9, 54.1 pg g−1, and 20.4 pg mL−1 in butter, cream, and half-and-half, respectively (Pape-Zambito et al. Citation2010). An implant of Estradiol-17β hormone according to recommended procedures increased tissue levels of animals during late pregnancy by a factor of 2–5; however, excessive doses could be carcinogenic. The amounts of estradiol-17β produced daily by pregnant women and pre-pubertal boys greater than the amount of residue of estradiol hormone (Passantino Citation2012). The Joint FDA (Citation2015) does not recommend an acceptable residue level because levels of estradiol that would be present in edible tissues of implanted animals did not exert any toxic effect through hormonal action in humans. The FAO/WHO reported that the natural ADI for oestradiol 17β is higher levels in meat from animals in late pregnancy than that from steers with a hormonal implant, and from plants and plant products than from beef of treated animals. The high levels of natural estrogens in intact males and in the milk of pregnant cows (Henricks et al. Citation1983; Squires Citation2011). A few recent studies indicated that estradiol-17β has genotoxic potential, while others found that there is no dosage below the no-hormonal-effect level that cause adverse effects in animals or humans (Organization Citation2016). The conjugated estrogens raised concerns, but they can be deconjugated in the gastrointestinal tract, leading to the release of this substance as free forms that become available for absorption and then binding to receptors of hormone (Nachman and Smith Citation2015).
Progesterone
Progesterone (in incorporation with estradiol-17β) is used in s/c injection in calves and steers and is also used in the estrous cycle regulation in swine and ruminants (Sangsritavong et al. Citation2002). Progesterone does a carcinogenic effect on its hormonal activity (FAO/WHO Citation2012; FDA Citation2015). Consequently, it does not appear toxic at levels below those necessary for hormonal effect. Residue levels of progesterone in milk and tissues of administrated animals are under the daily production of the hormone, thus are considered not to pose a risk to human health (Seri Citation2013; FDA Citation2015). Progesterone increases the incidence of mammary, ovarian, and uterine tumours in experimental animals (Galbraith Citation2002).
Testosterone
Similar comments apply to testosterone (in combination with estradiol-17β), used in implants in heifers (Jeong et al. Citation2010; FDA Citation2015). The levels of α-testosterone and β-testosterone were 1.54–2.62 and 1.06–1.56 μg−1kg−1, respectively, and the other study found the range of total testosterone was 0.16–1.88 μg kg−1 (Nachman and Smith Citation2015) or was 27.46–94.86 ng L−1 α-in dairy products (Courant et al. Citation2007), concentrations of testosterone increased as a function of the fat content of the milk.
3.2.2. Xenobiotic anabolic agents
Xenobiotics used in some countries (not legal in all cases) are TBA, MGA, and zeranol (Ronquillo and Hernandez Citation2017). Zeranol, TBA, and MGA are xenobiotic anabolic agents (Schiffer et al. Citation2001). The biological activity of the xenobiotic hormonal substances was measured by toxicity, genotoxicity and hormonal effect assays in laboratory animals. For the TBA, MGA, zeranol compounds legally available, hormonal effects are the most significant. Quantities of hormone below the NHEL do not, by definition, result in a response in the target tissue/s of that hormone. Properly administered, these substances should not leave residues in the milk and edible tissues resulting in undesirable hormonal effects in humans. Theoretically, the risk of treated animals entering the food chain before the proper withdrawal times have been observed is probably most significant for zeranol. That is attributed to the wide use of zeranol for cattle to long withdrawal time and the most significant risk of animals culled by farmers for a medical reasons after implanted cattle (Waltner-Toews and McEwen Citation1994). Weight (Citation2003) suggested that xenobiotics MGA and zeranol compounds pose a minimal risk to Canadians by the relatively low toxicity of zeranol and MGA. The MRL for TBA, zeranol, and MGA is mentioned in . In the United States, two hormones: MGA for feedlot heifers and ractopamine for swine, are approved as feed additives. Other anabolic growth promoters are usually implanted in the animal’s ear so that the active substance is released slowly into the bloodstream and possessed to contain the problem (Khan et al. Citation2008).
Trenbolone acetate
The TBA, a kind of 19-nortestosterone, is a synthetic steroidal compound with anabolic properties(Nazli et al. Citation2005). Based on short-term genotoxicity assays and long-term feeding experiments in laboratory farm animals, TBA appears not to be a genotoxic substance (Weight Citation2003). TBA increases muscle protein rate when the rate of degradation is less than the rate of synthesis by decreasing the rate of synthesis and degradation of protein (Kamanga-Sollo et al. Citation2011). The permitted limit concentrate for trenbolone is 10 ppb in the liver and 2 ppb in muscle (Tsai et al. Citation2004). TBA is an implant licensed for use in beef cattle in the U.S.A., Europe, and even in Canada after not being licensed for tissues of food animals for hormonal substances, including TBA. Because the risk of exposure and toxicity to residues of TBA in animal tissues is probably very low and of no hormonal significance. Thus TBA approved anabolic agents (Kicman Citation2008).
Zeranol
Zeranol (α-zearalanol) as an anabolic agent is a nonsteroidal, oestrogenic mycotoxin produced by Fusarium spp. It is used in livestock as an implant for increasing body weight gain, and improving the feed efficiency and the meat quality. Zeranol has a rather long withdrawal time which is discharged 65 days after implantation by 96.3%, and zeranol concentration decreases in all organs and tissues below 2 ppb (µg kg−1) (Gençer et al. Citation2012). Residues of zeranol should not exceed 0.05, 10 and 2 ppb in daily human food, cattle liver, and cattle muscle. Zeranol residues can be found in the liver after implantation up to 120 days and are highest in edible tissues 14 days after administration (Nazli et al. Citation2005). These mycotoxin compounds share some toxic properties-particularly those of estrogenic effect (Cortinovis et al. Citation2013; Pizzo et al. Citation2016). The recommended ADI is 0–0.5 µg kg−1 body weight day-1 for humans (FDA Citation2015; Organization Citation2016). There is no masked evidence that zeranol is genotoxic (Metzler and Pfeiffer Citation2001). Zeranol activates estrogen receptors-dependent cell proliferation in mammary glands, resulting in breast cancer in humans (Leffers et al. Citation2001).
Melangestrol acetate (MGA)
The MGA, an orally active progestogen, is a synthetic steroid, used in heifers as a feed additive. An MRL of MGA 25 µg kg−1 (25 ppb) in edible tissues has been set (Pasquale Citation2015), and although a 48 h withholding time was mandatory, heifers fed MGA at determined doses have tissue levels less than MRL without feed withdrawal. Exposure assessment of MGA as xenobiotic anabolic agents requires only a brief withdrawal time (48 h) from the feed of animals before slaughter when used as a feed additive because it rapidly excreted. Waltner-Toews and McEwen (Citation1994) reviewed that 373 specimens of fat from heifers were tested for MGA, and none were violative.
3.2.3. Safety aspects of β-adrenergic agonists
β-agonists are synthetic analogues of adrenaline and noradrenaline; thus, act on adrenergic receptors resulting in anabolic effects in animals (Smith Citation2014). β-AAs have halogenated aromatic ring systems, such as clenbuterol, which are metabolized in the liver by oxidative and conjugative pathways and have a very long half-life leading to high concentrations that can accumulate in the liver and adipose tissue. The structures, pharmacokinetics, and metabolism of the different β-AAs differ between oral potencies of different β-AAs. The effective therapeutic dose for clenbuterol is 10–20 µg, with a NOEL of 2.5 µg day−1. While the effective dose for other β-AAs compounds is 2000–10,000 µg. These less potent β-AAs suggest that safe and effective use of β-AAs may be possible (Squires Citation2011). Contrary to other β-AAs, Ractopamine was approved for use in swine growth in December 1999 in the U.S.A. In contrast to ST and most anabolic steroids, β-AAs are orally active, heat stable and not destroyed by cooking. This increases concern about the potential effects of residues of β-AA in meat. Half-life estimates for plasma cimaterol in steers were 54 min, and for plasma clenbuterol, in veal calves, it was 18 h for the initial distribution phase and 55 h for the terminal half-life (Squires Citation2011). Al-Doski (Citation2015) mentioned that cimaterol administered to sheep increases glycolytic potential in muscles by increasing the synthesis of serine and other related metabolites required for growth. Nazli et al. (Citation2005) found that levels of β-agonist and steroidal anabolic higher than acceptable limits. They noted that meat and meat products with excessive levels of anabolic residues might harm the consumer.
The use of substances with hormonal or thyreostatic action as well as β-agonists are banned in the EU and other countries worldwide. However, sometimes restricted drugs may be added to feed illegally to promote increased muscle development or increased water retention or even reduce subcutaneous fat and thus obtain an economic benefit through an unsustainable diet (Shankar et al. Citation2010). The result is a fraudulent weight increase of meat with lower fat content, and the increased levels of residues that may remain in the tissue pose a great concern or real threat among consumers through either exposure to the residues or allergy risk (Reig and Toldra Citation2008; Bussche et al. Citation2014). For example, the ingestion of 160–291 ppb clenbuterol for bronchodilator in calves or tocolysis to prevent premature birth in cows or even used as a growth promoter, may cause food poisoning (intoxication) and linked epidemiologically symptoms include gross muscle tremors of the extremities, nervousness, tachycardia, headache, cardiac palpitation, dizziness, nausea, vomiting, fever, and chills (Barbosa et al. Citation2005).
β-agonists are mainly used for treating asthma in humans and animals (Olin and Wechsler Citation2014). In addition, they are used as growth promoters in livestock (Ronquillo and Hernandez Citation2017). The most widely used β-agonist is clenbuterol (Dervilly-Pinel et al. Citation2015). The anabolic effect of clenbuterol at levels in excess of 5–10-fold the recommended therapeutic dose in meat-producing animals (>1 µg kg−1 BW/day) (Nazli et al. Citation2005). He mentioned the maximum residue of 0.5 ppb in all edible tissues resulting in a harmful effect on humans. The highest accumulation of clenbuterol occurs in pigmented tissues, such as hair and the retina in the eye, due to binding with melanin. This finding can be used as a highly sensitive test for detecting clenbuterol residues in these tissues up to 60 days after treatment (Dervilly-Pinel et al. Citation2015).
Although the use of compounds of β-agonists, such as ractopamine more effective for farm animal, animal product consumption, including ractopamine residue, has increased the risk of residue toxicity on human health, such as cardiovascular disease (Testa et al. Citation2000). In the early years, the synthetic stilbenes, diethylstilbestrol, hexestrol, and dienestrol is used. These were subsequently banned in the EU, the U.S.A., and Canada because of their potential carcinogenic activity (Squires Citation2011).
3.2.4. Safety/quality aspects of somatotropin or growth hormone
The GH is cleaved into polypeptides and amino acids by the digestive process protein, destroyed by cooking and rapidly broken down in the gut. Therefore, the potential threat from residues should be small. ST has several effects on animals that could increase the milk production of cows. A slight reduction in meat tenderness in pigs treated with bST may be due to decreased intramuscular fat content (Squires Citation2011). ST is also an anabolic agent in swine and rats (Waltner-Toews and McEwen Citation1994).
3.2.5. Safety concerns of recombinant bovine somatotropin and insulin-like growth factor 1
The insulin-like growth factor 1 receptor is a protein found in the cell membranes belongs to the tyrosine kinase receptors class and is activated by the hormone of insulin-like growth factor 1 (IGF-1). IGF-1 plays a role in the growth and has anabolic effects in adult human and food animals to induce skeletal muscle hypertrophy and other target tissues (Davies et al. Citation2014). Bovine somatotropin (bST) or (in some cases, bovine growth hormone (bGH), recombinant bovine somatotropin (rbST), and recombinant bovine growth hormone (rbGH)) is approved by the FDA for use in dairy cattle to increase milk production. The rbST and rbGH in cattle are used interchangeably to describe the genetically engineered product. The rbST produced by the modern recombinant DNA technology is equivalent biologically to the natural pituitary-derived ST, and improves the productivity of lactating dairy cows by an average of approximately 15% (Raymond et al. Citation2010). The rBST as growth-promoting agent has an anabolic effect and is used in the meat industry to enhance milk production in the dairy industry. The increase in milk production resulting from the administration of rBST is approximately 15% under optimal management conditions (Meyer Citation2001).
BST normally occurs in the milk (at about 1 ppb) can cause an increase in the relative amount of long-chain fatty acids, and can increase the somatic cell counts of the milk when used in cows in a positive energy balance (Ahmad Citation2002). However, protein, fat and lactose concentrations of the milk are not appreciably altered (Hammond Citation2007). Thus, most scientists found that BST used in dairy cattle is safe and acceptable for humans and agricultural policies because the composition and nutritional value of animal milk are not altered and do not result public health but socio-economics (Reig and Toldra Citation2008). Other researchers, however, found that BST use has the importance of changes in IGF-1 and other hormones in the milk and meat from BST-treated cows, and the effect of the hormone on antibody responses, thus raising concerns for human safety (Gülay and Hatipoğlu Citation2005; Hammond Citation2007). Pasteurization reduces the amount of BST in the milk by 90%, but it does not reduce the quantity of insulin-like growth factor-1 (IGF-1; a mediator of GH action) (Melnik Citation2009).
In 1990, the use of BST in lactating dairy cattle was a paused in the state of Wisconsin (U.S.A.), because of many issues of milk production and the safety of milk products for human consumption resulting in public concern, but the decision was overturned later (Singer et al. Citation2017). bST only slightly alters milk composition and milk production by altering the nutrient requirements of the cow. An increased lameness in bST-treated cows might be due to the negative effects of bST on connective tissue and bone development. Cows treated with bST can take longer to enter oestrus after parturition than an untreated cow. However, levels of GnRH, FSH, and LH are unchanged by bST treatment. bST has no oral activity in adult humans and is destroyed by digestion. Bovine IGF-I has the same sequence as human IGF-I, and bST and IGF-I will be at least partially denatured by heat during pasteurization. The FAD of the U.S.A. reviewed more than 120 studies before the approval of bST (Squires Citation2011).
The published study by European Commission (EC) indicated the concerns that arise from the use of rBST are from two to five times greater IGF-1 levels in milk products. The levels of IGF-1 were affected by animal age, stage of lactation, and nutritional status (Nachman and Smith Citation2015). By European Council 1990 decision, Europe banned the supplementation of rBST in any method to dairy cattle for 10 years, except for scientific and technical purposes. After 1999, this bann was permanent, and an exception was removed. Because BST increased the risk of mastitis, foot and leg disorders in dairy cattle and increased the duration of necessary treatment for the affliction. By contrast, the US permits using rBST (Wiener and Rogers Citation2002).
4. Assess the burden of hormonal residues and their metabolites as endocrine disruptors in the environment
Simplified, endocrine disruptors are exogenous chemicals, or chemical mixtures, that interfere with normal hormone action and alter the functions of the endocrine system and consequently cause adverse health effects in an intact organism or its progeny or (sub) populations (Bergman et al. Citation2013). There are many known sources of endocrine-disrupting chemicals (EDCs) and other hazardous chemicals (Bornman et al. Citation2017).
4.1. Behaviour of endocrine-disrupting hormones in the environment
Endogenous or natural anabolic hormones of human or animal origin have been reaching the environment for a prolonged period, with a dramatically increasing extent due to the growing population and farming that is more intensive.
Among the first group (endogenous hormones of animal origin) are testosterone, estradiol, progesterone, and somatotropin released and deposited in the environment for thousands of years. However, recent years have seen a significant increase due to the increasing population and more intensive farming practices (Pasquale Citation2015). On the other hand, this natural recycling has not caused any severe adverse effect on wildlife in various ecosystems or the human endocrine system or fertility, but environmental EDCs has an important aspect. However, during the last decades, the synthetic or (semi)xenobiotic hormones originated from natural hormones and are made by humans and are rarely orally active due to rapid deactivation through biotransformation. Thus, synthetic hormones chemically a higher stability and lower deactivates via biotransformation. This higher stability of synthetic substances shows itself within living organisms, and affects the ecological balance due to their prolonged persistence in the environment (Ronquillo and Hernandez Citation2017). Synthetic chemicals may cause a risk to the reproductive health of humans. The effects of many synthetic endocrine-disruptor hormones can interfere with the normal regulation of reproductive processes by steroid hormones. Exogenous hormones bind to estrogen and androgen receptors in target tissues, sex hormone-binding globulin, and androgen-binding protein (Danzo Citation1998).
Residual hormones that reach soil and water via livestock faeces may cause problems to animal or human health. Thus, the quantity of sex steroids excreted by humans and animals seems equally important. Besides endogenous anabolic hormones, exogenous or synthetic sex steroids used in animals are excreted and reach the environment. The fate of steroids in the environment originating from farm animals’ excreta is strongly affected by the storage state and soil type of the fields wherever spreading dung. Particle size and organic substance robustly affect migration and adsorption to the soil due to chemical and microbiological degradation. EDCs may have a deep impact on the sexual differentiation and development of vertebrates (Lange et al. Citation2002). Endogenous hormones are excreted in varying amounts by all animal species depending on species, age, sex or reproductive stage as the highest levels of oestrogens are excreted and eliminated by females during late pregnancy and milk production. Intestinal and environmental microbes have steroidal transform activity in feces, but their activity is not enough for complete degradation and elimination of hormones, especially synthetic. The environmental concentrations, bioavailability, biodegradability, and bioconcentration are important factors in to complete analysis of hormonal excretion hazards originating from livestock production (Lange et al. Citation2002). They reviewed that hormone steroids are a group of endocrine disruptors are excreted by humans and food animals. Natural and synthetic estrogenic steroids have a solubility of around 13 mg L−1 and 0.3–4.8 mg L−1, respectively. These steroids have a mild binding on precipitates and degrade rapidly in water and soil. This groundwater pollution is connected with cattle manure waste and poultry litter applied to the soils (Ying et al. Citation2002).
The steroidal hormones have sensitive effects on aquatic ecosystems, such as the effects of oestrogens on fish and amphibians. Oestrogens induce egg yolk vitellogenin proteins in females or zona radiata proteins in juveniles, males, and females. Both proteins are produced in the liver of oviparous vertebrates by oestradiol-17β stimulating (Hoar et al. Citation1983). The annual rate of oestrogen excretion in cattle, pregnant cattle contributed by a large portion, reaches 33 and 49 t in the EU and U.S.A., respectively. The steroidal stability in excreta depends on matrix, structure, lighting, temperature conditions and oxygen supply. The problem of residual hormones found not only in meat, milk and other animal products, but also in water and soil originating from animal excreta may have negative effects on endocrine activity in aquatic fauna and terrestrial animals (Secundo et al. Citation2012).
Lange et al. (Citation2002) reported that in terrestrial vertebrates, including human signs of potential oral activity of hormonal residues in fish or drinking water through excrements. Recently, the presence of endocrine-disrupting environmental pollutants was reducing male reproductive health and increasing some endocrine cancers, such as testicular and breast cancer. Abortion occurs in cattle fed via broiler litter attributed to estrogens gained from poultry fed 150–250 mg kg−1 of dienestrol diacetate. The residues and degradation of anabolic hormones determine in solid dung, liquid manure, and soil for investigating endocrine-disrupting activity in agricultural ecosystems (Schiffer et al. Citation2001). The steroids TBA and MGA substances should have potential endocrine-disrupting activity in agricultural ecosystems, as they are very mobile in agricultural lands (Schiffer et al. Citation2001; Lange et al. Citation2002).
4.2. Plant toxicity
The influence of synthetic sex hormones of mammals on plant growth and generative development has been reviewed in various studies. Briefly, many scientific papers show the stimulation of growth and induced flowering. Yet, when the steroids are applied to higher-level plants, the plant growth is adversely affected. The action and effect of steroidal sex hormones in plants may be connected with receptors of that hormone (Janeczko and Skoczowski Citation2005). Brassinosteroids are plant-specific steroids, and they function to regulating of multiple physiological and developmental processes in plants, such as gene expression, cell division, differentiation, homeostasis, and apoptosis (Thummel and Chory Citation2002). Due to many similarities between animal and plant steroids in biosynthesis and function, the interference of animal hormones with the brassinosteroid signaling cascade was expected.
5. Risk a voidance via policies and legislation on hormonal growth promoters
In 1963, Denmark was among the first countries to ban the use of growth-promoting hormones in animals’ meat. In 1977, in Europe widespread concern about growth-promoting hormones after detecting many breast cancer in young boys in Italy. Researchers reported that estrogen in meat or poultry might be related to the incident (Yuri et al. Citation2006). The EU has banned using all hormones, whereas other countries allow using anabolic steroids and hormone-like compounds alone or combinations that aim to improve body weight gain and feed efficiency of farm animals. The biological active hormones implanted in a small amount into the subcutaneous of the ears are recommended for application. Because both ears are discharged at slaughter completely (Galbraith Citation2002; Cepeda and Fente Citation2012; Herago and Agonafir Citation2017).
In the United States, Australia, Canada, New Zealand, and in many non-EU countries in Asia, Africa, and South America, the 17ß-oestradiol, testosterone, and progesterone as the natural steroidal hormones, and zeranol, trenbolone, and MGA as the synthetic hormones can be used to promoting growth (Passantino Citation2012). Currently, seven anabolic hormones estradiol-17, testosterone, progesterone, zeranol, MGA, TBA and bST were approved for implants in beef and dairy cattle in the U.S.A. Conversely, these natural or synthetic implants have been prohibited officially in Europe since 1989 (Wiener and Rogers Citation2002; Nachman and Smith Citation2015).
Steroidal implanting hormones are widely used in Canada, the U.S.A., and Australia. Around 30 countries have confirmed one or more of these hormones for improving the growth rate of cattle (Herago and Agonafir Citation2017). Currently, β-agonists compounds are not registered for growth promoters in the EU cattle and are prohibited under EU Directive 96/22/EC. Zilpaterol is the only product recorded as a growth promoter in cattle in Mexico and South Africa, and ractopamine is the only product in pigs in the U.S.A., Brazil, Korea, Mexico, and Philippines (Marchant-Forde et al. Citation2003). The EU prohibition of these hormones adversely affected international exports of meat and meat products from the United States and Canada. In 1997, the World Trade Organization (WTO) panel decided that the ban did not agree with the agreement on the application of sanitary and phytosanitary measures. However, WTO reversed most of that decision after an appeal by the EU. Still, it indicated that the prohibition did not comply with measures based on an assessment of the risks to human health and that US animals and meat were ‘like’ EU animals and meat (Hayes et al. Citation2002).
A deceitful overweight of the meat resulted from banning anabolic agents, but the worst, residues of those substances may remain in the meat and may form a real threat to the consumer by exposure to the residues lead to a significant concern among consumers. The ADI, withdrawal times, bioavailability, and biological half-life of hormonal or its analogs must be considered, to reduce the potential effects of residues in meat and other products of the farm animals. Specially, before slaughter or marketing of farm animals or even their products, by giving enough time to remove residue of hormonal or their analogous and complete hormones kinetics: absorption, metabolism, and excretion by faeces or urine. These restricted measures and harder regulations must apply to prevent the misuse of drugs or anabolic hormones in farm animals, application withdrawal time and the inspection of animals for drug residues before marketing.
6. Methods for detection and risk avoidance of veterinary drugs residues in meat
The main veterinary drugs and substances having anabolic effects belonging to group A and group B according to Council Directive 96/23/EC are listed in (Reig and Toldra Citation2008), some of them have established maximum residue limits (MRL). The use of forbidden substances that possess hormonal or thyreostatic action, such as β-agonists in animal foods and feeds, is controlled by official inspection and analytical services followed by Commission Directive 96/23/EC in the EU on measures to monitor certain substances and residues in live animals and animal products. The risk assessment and risk management process for food additives specific steps food additives are shown in .
Table 4. Lists of main veterinary drugs and substances having anabolic effects belonging to group A and group B according to Council Directive 96/23/EC (Reig and Toldra Citation2008), some of them have established maximum residue limits (MRL).
Table 5. The risk assessment/risk management process for food additives specific steps for food additives.
Many available types of research dealing with urine and faece samples to measure sex hormones, faecal progesterone metabolite analysis to the monitoring of corpus luteum function, and faecal estrogen determination for pregnancy diagnosis of farm and wildlife animals that have the foeto-placental unit as the source of high amounts of estrogens (Schwarzenberger et al. Citation1996). For an example of a typical procedure for analysis of meat samples: blending and homogenization of samples. Then, solid-phase extraction. In addition, screening methods either compliant or non-compliant. After that, if the screening method is non-compliant, operate further purification/treatment. Finally, confirmatory methods result in either being compliant or non-compliant (Reig and Toldra Citation2008). For effective control must be the availability of simple and useful screening techniques. Recently, screening and confirmatory analytical methods have been used for hormone residue analysis. Shankar et al. (Citation2010) described the following rapid methods for detecting veterinary drug residues in meat: Analytical methods for rapid screening. Immunological techniques: ELISA test kits, radioimmunoassay. Multi-array biosensors. High-performance thin-layer chromatography (HPTLC). Confirmatory analytical methodologies: HPLC-electrospray ionization (ESI) tandem mass spectrometry (ESI ionization technique). Liquid chromatography-mass spectrometry with atmospheric pressure chemical ionization (APCI ionization).
7. Conclusions
A recent review on the use of veterinary natural and synthetic growth promoters in food-producing animals reveals a growing awareness of the risks these supplements can pose for animal and human health. Anabolic growth enhancers for farm animals or even for poultry can be particularly detrimental to not only humans but also farm animals. Anabolic hormones, especially synthetic, as endocrine disruptors, have a burden on the environment and food. The review indicated that estradiol, testosterone, and progesterone as natural anabolic steroids appear to be safe, and acceptable and only the human health risk associated with the actual ingestion of implant sites of their use. Illegal use of β-agonists such as clenbuterol have some concerns and limits, especially they possess heat stable and are orally active. For BST, the issue is not public health but socio-economics. Thus, meat and meat products with excessive levels of anabolics might be harmful to the consumer, and their use in farm animals must be strictly controlled.
Several growth-promoting hormones or those actions like hormones used in farm animals are anabolic steroid implants, β-agonists, and GHs. They have a different mechanism of action to improve farming animals’ production by increasing the average daily gain, feed conversion efficiency, carcass quality or milk production of animals. However, growth-promoting hormones have importance for animal production, and the final consumer can affect different side effects like carcinogenic effects. For this reason, growth-promoting hormones that are used in humans and generally illegal use of hormonal growth promoters should be terminated or at least controlled unless and until carried out risk assessments. Countries should develop strict policies on using different hormonal and other animal growth promoters. Future studies are needed to detect new drug applications as growth promotors and understand the risk or impact of endogenous and exogenous hormones on public health, farm animal and wildlife health, and the ecosystem.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ahmad T. 2002. Effect of bovine somatotropin on the lactational and reproductive performance of lactating dairy cows-a review. Commission on Science and Technology for Sustainable Development in the South. 8:36.
- Akzira SAAO. 2016. The effect of hormones on the quality of poultry meat. Int J Curr Res Biosci Plant Biol. 3:113–121.
- Al-Doski S. 2015. Effects of growth promoters on sheep metabolism and growth. Doctoral dissertation. Nottingham (UK): University of Nottingham.
- Barbosa J, Cruz C, Martins J, Manuel Silva J, Neves C, Alves C, Ramos F, Noronha Da Silveira MI. 2005. Food poisoning by clenbuterol in Portugal. Food Addit Contam. 22:563–566.
- Bergman Å, Heindel JJ, Jobling S, Kidd K, Zoeller TR, Organization WH. 2013. State of the science of endocrine disrupting chemicals 2012: summary for decision-makers.
- Bidanel J, Bonneau M, Pointillart A, Gruand J, Mourot J, Demade I. 1991. Effects of exogenous porcine somatotropin (pST) administration on growth performance, carcass traits, and pork meat quality of Meishan, Pietrain, and crossbred gilts. J Anim Sci. 69:3511–3522.
- Bornman MS, Aneck-Hahn NH, De Jager C, Wagenaar GM, Bouwman H, Barnhoorn IE, Patrick SM, Vandenberg LN, Kortenkamp A, Blumberg B. 2017. Endocrine disruptors and health effects in Africa: a call for action.
- Bussche JV, Decloedt A, Van Meulebroek L, De Clercq N, Lock S, Stahl-Zeng J, Vanhaecke L. 2014. A novel approach to the quantitative detection of anabolic steroids in bovine muscle tissue by means of a hybrid quadrupole time-of-flight-mass spectrometry instrument. J Chromatogr A. 1360:229–239.
- Cepeda A, Fente CA. 2012. Natural hormones in food-producing animals: legal measurements and analytical implications. In: Regal P, Cepeda A, Fente CA, editors. Food production-approaches, challenges and tasks. Fente University of Santiago de Compostela (Spain): InTech. 12:205-203.
- Cortinovis C, Pizzo F, Spicer LJ, Caloni F. 2013. Fusarium mycotoxins: effects on reproductive function in domestic animals – a review. Theriogenology. 80:557–564.
- Courant F, Antignac J-P, Laille J, Monteau F, Andre F, Le Bizec B. 2008. Exposure assessment of prepubertal children to steroid endocrine disruptors. 2. Determination of steroid hormones in milk, egg, and meat samples. J Agric Food Chem. 56:3176–3184.
- Courant F, Antignac J-P, Maume D, Monteau F, André F, Le Bizec B. 2007. Determination of naturally occurring oestrogens and androgens in retail samples of milk and eggs. Food Addit Contam. 24:1358–1366.
- Courtheyn D, Le Bizec B, Brambilla G, De Brabander H, Cobbaert E, Van de Wiele M, Vercammen J, De Wasch K. 2002. Recent developments in the use and abuse of growth promoters. Anal Chim Acta. 473:71–82.
- Croubels S, Daeseleire E, De Baere S, De Backer P, Courtheyn D. 2004. Residues in meat and meat products, feed and drug residues. Encyclop Meat Sci. 3:1172–1187.
- Danzo B. 1998. The effects of environmental hormones on reproduction. Cell Mol Life Sci. 54:1249–1264.
- Davies KT, Tsagkogeorga G, Bennett NC, Dávalos LM, Faulkes CG, Rossiter SJ. 2014. Molecular evolution of growth hormone and insulin-like growth factor 1 receptors in long-lived, small-bodied mammals. Gene. 549:228–236.
- Dervilly-Pinel G, Chereau S, Cesbron N, Monteau F, Le Bizec B. 2015. LC-HRMS based metabolomics screening model to detect various β-agonists treatments in bovines. Metabolomics. 11:403–411.
- Dorne J, Fink-Gremmels J. 2013. Human and animal health risk assessments of chemicals in the food chain: comparative aspects and future perspectives. Toxicol Appl Pharmacol. 270:187–195.
- Doyle ME. 2000. Human safety of hormone implants used to promote growth in cattle: a review of the scientific literature. Food Research Institute, Department Food Microbiology & Toxicology, University of Wisconsin – Madison.
- Ebarb S, Phelps K, Drouillard J, Maddock-Carlin K, Vaughn M, Burnett D, Noel J, Bibber-Krueger V, Paulk C, Grieger D. 2017. Effects of anabolic implants and ractopamine-HCl on muscle fiber morphometrics, collagen solubility, and tenderness of beef longissimus lumborum steaks. J Anim Sci. 95:1219–1231.
- EFSA. 2007. Opinion of the scientific panel on contaminants in the food chain on a request from the European commission related to hormone residues in bovine meat and meat products. EFSA J. 510:1–62.
- Elliott C, Crooks S, McEvoy J, McCaughey W, Hewitt S, Patterson D, Kilpatrick D. 1993. Observations on the effects of long-term withdrawal on carcass composition and residue concentrations in clenbuterol-medicated cattle. Vet Res Commun. 17:459–468.
- Elmore R. 1992. Focus on bovine reproductive disorders: managing cases of fetal mummification. Veterinary Medicine (USA).
- Elvinger F, Natzke RP, Hansen PJ. 1992. Interactions of heat stress and bovine somatotropin affecting physiology and immunology of lactating Cows1. J Dairy Sci. 75:449–462.
- Eversole D, Fontenot J, Kirk D. 1989. Implanting trenbolone acetate and estradiol in finishing beef steers. Nutrition Reports International (USA).
- FAO/WHO. 2012. Evaluation of certain veterinary drug residues in food. Food and Agriculture Organization/World Health Organization.
- Farlow DW, Xu X, Veenstra TD. 2009. Quantitative measurement of endogenous estrogen metabolites, risk-factors for development of breast cancer, in commercial milk products by LC–MS/MS. J Chromatogr B. 877:1327–1334.
- FDA. 2015. Steroid hormone implants used for growth in food-producing animals. Food and Drug Administration (FDA). 2015. 41:47–121.
- Galbraith H. 2002. Hormones in international meat production: biological, sociological and consumer issues. Nutr Res Rev. 15:293–314.
- Gençer N, Ergün A, Demir D. 2012. In vitro effects of some anabolic compounds on erythrocyte carbonic anhydrase I and II. J Enzyme Inhib Med Chem. 27:208–210.
- Gülay MŞ, Hatipoğlu FŞ. 2005. Use of bovine somatotropin in the management of transition dairy cows. Turkish J Vet Anim Sci. 29:571–580.
- Hammond B. 2007. The food safety assessment of bovine somatotropin (bST). Food Safety of Proteins in Agricultural Biotechnology. p. 167–208.
- Hayes DJ, Jensen HH, Fabiosa J. 2002. Technology choice and the economic effects of a ban on the use of antimicrobial feed additives in swine rations. Food Control. 13:97–101.
- Henricks D, Gray S, Hoover J. 1983. Residue levels of endogenous estrogens in beef tissues. J Anim Sci. 57:247–255.
- Herago T, Agonafir A. 2017. Growth promoters in cattle. Adv Biol Res (Rennes). 11:24–34.
- Hoar WS, Randall DJ, Donaldson EM. 1983. Reproduction: endocrine tissues and hormones. Academic Press.
- Janeczko A, Skoczowski A. 2005. Mammalian sex hormones in plants. Folia Histochem Cytobiol. 43:71–79.
- Jeong S-H, Kang D, Lim M-W, Kang CS, Sung HJ. 2010. Risk assessment of growth hormones and antimicrobial residues in meat. Toxicol Res. 26:301–313.
- Joosten H, Van Acker F, Van den Dobbelsteen D, Horbach G, Krajnc E. 2004. Genotoxicity of hormonal steroids. Toxicol Lett. 151:113–134.
- Kamanga-Sollo E, White M, Hathaway M, Weber W, Dayton W. 2011. Effect of trenbolone acetate on protein synthesis and degradation rates in fused bovine satellite cell cultures. Domest Anim Endocrinol. 40:60–66.
- Kefi AS. 2014. Influence of dietary soya beans (glycine max(l.) merr.) protei n and lipid combination, and androgen (17 α–methyl testosterone) levels on th e growth and reproduction of Oreochromis andersonii (Castelnau, 1861) [PhD thesis]. [Lilongwe, Malawi]: Bunda College of Agriculture, University of Malawi.
- Khan S, Roser D, Davies C, Peters G, Stuetz R, Tucker R, Ashbolt N. 2008. Chemical contaminants in feedlot wastes: concentrations, effects and attenuation. Environ Int. 34:839–859.
- Kicman A. 2008. Pharmacology of anabolic steroids. Br J Pharmacol. 154:502–521.
- Lange IG, Daxenberger A, Schiffer B, Witters H, Ibarreta D, Meyer HH. 2002. Sex hormones originating from different livestock production systems: fate and potential disrupting activity in the environment. Anal Chim Acta. 473:27–37.
- Lee J, Knowles S, Judson G. 2010. Sheep nutrition. Wallingford: CAB International. 285-312.
- Leffers H, Næsby M, Vendelbo B, Skakkebæk NE, Jørgensen M. 2001. Oestrogenic potencies of zeranol, oestradiol, diethylstilboestrol, bisphenol-A and genistein: implications for exposure assessment of potential endocrine disrupters. Hum Reprod. 16:1037–1045.
- Liu D, Bordicchia M, Zhang C, Fang H, Wei W, Li J-L, Guilherme A, Guntur K, Czech MP, Collins S. 2016. Activation of mTORC1 is essential for β-adrenergic stimulation of adipose browning. J Clin Invest. 126:1704–1716.
- Mader T.. 1997. Carryover and lifetime effects of growth promoting implants. In: Synposium: Impact of implants on performance and carcass value of beef cattle. Oklahoma Agric. Exp. Stn. P-957. Stillwater (OK): Oklahoma State Univ; p. 88.
- Manickam R, Wahli W. 2016. Roles of peroxisome proliferator-activated receptor β/δ in skeletal muscle physiology. Biochimie. 136:42–48.
- Marchant-Forde J, Lay D, Pajor E, Richert B, Schinckel A. 2003. The effects of ractopamine on the behavior and physiology of finishing pigs. J Anim Sci. 81:416–422.
- Melnik B. 2009. Milk consumption: aggravating factor of acne and promoter of chronic diseases of western societies. JDDG. 7:364–370.
- Metzler M, Pfeiffer E. 2001. Genotoxic potential of xenobiotic growth promoters and their metabolites. Apmis. 109:89–95.
- Meyer HH. 2001. Biochemistry and physiology of anabolic hormones used for improvement of meat production. Apmis. 109:S336–S344.
- Millán Y, Guil-Luna S, Reymundo C, Sánchez-Céspedes R, de las Mulas JMn. 2013. Sex steroid hormones and tumors in domestic animals. In: Insights from veterinary medicine. Vol. 27. InTech; p. 191–214.
- Moloney AP, McGee M. 2017. Factors influencing the growth of meat animals. In: Lawrie’s meat science. 8th ed. Woodhead Publishing Series in Food Science, Technology and Nutrition. Ireland: Elsevier; p. 19–47.
- Nachman KE, Smith TJ. 2015. Hormone use in food animal production: assessing potential dietary exposures and breast cancer risk. Curr Environ Health Rep. 2:1–14.
- Nazli B, Çolak H, Aydin A, Hampikyan H. 2005. The presence of some anabolic residues in meat and meat products sold in Istanbul. Turkish J Vet Anim Sci. 29:691–699.
- Olin JT, Wechsler ME. 2014. Asthma: pathogenesis and novel drugs for treatment. Br Med J. 349:g5517.
- Pape-Zambito D, Roberts R, Kensinger R. 2010. Estrone and 17β-estradiol concentrations in pasteurized-homogenized milk and commercial dairy products. J Dairy Sci. 93:2533–2540.
- Pasquale E. 2015. Residues from anabolic agents in food-producing animals: a focused evaluation of semi-natural occurrence of hormonal active compounds.
- Passantino A. 2012. Steroid hormones in food producing animals: regulatory situation in Europe: a bird’s-eye view of veterinary medicine. Annamaria Passantino Department of Veterinary Public Health, Faculty of Veterinary Medicine, University of Messina (Italy): InTech.
- Pearson SJ, Hussain SR. 2015. A review on the mechanisms of blood-flow restriction resistance training-induced muscle hypertrophy. Sports Med. 45:187–200.
- Pell A, Tsang D, Howlett B, Huyler M, Meserole V, Samuels W, Hartnell G, Hintz R. 1992. Effects of a prolonged-release formulation of sometribove (n-methionyl bovine somatotropin) on Jersey cows. J Dairy Sci. 75:3416–3431.
- Pizzo F, Caloni F, Schreiber NB, Cortinovis C, Spicer LJ. 2016. In vitro effects of deoxynivalenol and zearalenone major metabolites alone and combined, on cell proliferation, steroid production and gene expression in bovine small-follicle granulosa cells. Toxicon. 109:70–83.
- Platter W, Tatum J, Belk K, Scanga J, Smith G. 2003. Effects of repetitive use of hormonal implants on beef carcass quality, tenderness, and consumer ratings of beef palatability. J Anim Sci. 81:984–996.
- Raymond R, Bales CW, Bauman RDE, Clemmons D, Kleinman R, Lanna D, Nickerson S, Sejrsen K. 2010. Recombinant bovine somatotropin (rbST): A safety assessment. In: Joint annual meeting of the American Dairy science Association, Canadian Society of Animal Science, and American Society of Animal science. Montreal, Canada.
- Reig M, Toldra F. 2008. Veterinary drug residues in meat: concerns and rapid methods for detection. Meat Sci. 78:60–67.
- Ronquillo MG, Hernandez JCA. 2017. Antibiotic and synthetic growth promoters in animal diets: review of impact and analytical methods. Food Control. 72:255–267.
- Sangsritavong S, Combs D, Sartori R, Armentano L, Wiltbank M. 2002. High feed intake increases liver blood flow and metabolism of progesterone and estradiol-17β in dairy cattle. J Dairy Sci. 85:2831–2842.
- Scahaw. 1999. Report on animal welfare aspects of the use of bovine somatotrophin.
- Scarth J, Akre C, Van Ginkel L, Le Bizec B, De Brabander H, Korth W, Points J, Teale P, Kay J. 2009. Presence and metabolism of endogenous androgenic–anabolic steroid hormones in meat-producing animals: a review. Food Addit Contam. 26:640–671.
- Schiffer B, Daxenberger A, Meyer K, Meyer H. 2001. The fate of trenbolone acetate and melengestrol acetate after application as growth promoters in cattle: environmental studies. Environ Health Perspect. 109:1145–1151.
- Schwarzenberger F, Möstl E, Palme R, Bamberg E. 1996. Faecal steroid analysis for non-invasive monitoring of reproductive status in farm, wild and zoo animals. Anim Reprod Sci. 42:515–526.
- Secundo F, Bacigalupo MA, Scalera C, Quici S. 2012. Rapid time-resolved fluoroimmunoassay for diethylstilbestrol in cow milk samples with a highly luminescent Tb 3 + chelate. J Food Compos Anal. 25:221–225.
- Seri HI. 2013. Introduction to veterinary drug residues: hazards and risks. In: Workshop on veterinary drug residues in food derived from animals (our goal of protecting consumers). Organized by the National Medicinal and Poisons Board, Sudan. p. 1–7.
- Shankar B, Manjunatha Prabhu B, Chandan S, Ranjith D, Shivakumar V. 2010. Rapid methods for detection of veterinary drug residues in meat. Vet World. 3:241–246.
- Singer RS, Ruegg PL, Bauman DE. 2017. Quantitative risk assessment of antimicrobial-resistant foodborne infections in humans Due to recombinant bovine somatotropin usage in dairy cows. J Food Prot. 80:1099–1116.
- Smith M. 2014. Veterinary drugs residues: anabolics. In: Motarjemi Y, editor. Encyclopedia of food safety, Vol. 3. Waltham (MA): Academic. p. 55–62.
- Squires EJ. 2011. Applied animal endocrinology. In: Chapter 3: manipulation of growth and carcass composition. 2nd ed. University of Guelph, Cabi. (Canada); p. 89–155.
- Stolker A, Zuidema T, Nielen M. 2007. Residue analysis of veterinary drugs and growth-promoting agents. TrAC, Trends Anal Chem. 26:967–979.
- Takemura H, Shim J-Y, Sayama K, Tsubura A, Zhu BT, Shimoi K. 2007. Characterization of the estrogenic activities of zearalenone and zeranol in vivo and in vitro. J Steroid Biochem Mol Biol. 103:170–177.
- Testa C, Boatto G, Brambilla G, Neri B, Fiori M, Calaresu G, Pulina A, Rubattu N. 2000. Evaluation of conventional screening procedures for the detection of new Beta agonists. In: Euroresidue IV conference on residues of veterinay drugs in food. Veldhoven (The Netherlands); p. 1073–1076.
- Thummel CS, Chory J. 2002. Steroid signaling in plants and insects – common themes, different pathways. Genes Dev. 16:3113–3129.
- Tsai C-F, Chang M-H, Pan J-Q, Chou S-S. 2004. A method for the determination of trenbolone in bovine muscle and liver.
- Velle W. 1982. The use of hormones in animal production. FAP animal production and health papers, 31. p. 53.
- Waltner-Toews D, McEwen SA. 1994. Residues of hormonal substances in foods of animal origin: a risk assessment. Prev Vet Med. 20:235–247.
- Weight T. 2003. A review to update Australia’s position on the human safety of residues of hormone growth promotants (HGPS) used in cattle.
- WHO. 1997. The medical impact of the use of antimicrobials in food animals: report of a World Health Organization (WHO) meeting. Berlin, Germany, 13–17 October 1997.
- Wiebe JP. 2006. Progesterone metabolites in breast cancer. Endocr Relat Cancer. 13:717–738.
- Wiener JB, Rogers MD. 2002. Comparing precaution in the United States and Europe. J Risk Res. 5:317–349.
- Wierup M. 2001. The experience of reducing antibiotics used in animal production in the Nordic countries. Int J Antimicrob Agents. 18:287–290.
- World Health Organization. 2016. Evaluation of certain veterinary drug residues in food: eighty-first report of the joint FAO/WHO expert committee on food additives, Vol. 81. World Health Organization.
- Yang R, Raper KC, Lusk JL. 2017. The impact of hormone use perception on consumer meat preference. 2017 Annual meeting; Mobile, Alabama; 2017 February 4–7.
- Ying G-G, Kookana RS, Ru Y-J. 2002. Occurrence and fate of hormone steroids in the environment. Environ Int. 28:545–551.
- Yuri T, Tsukamoto R, Miki K, Uehara N, Matsuoka Y, Tsubura A. 2006. Biphasic effects of zeranol on the growth of estrogen receptor-positive human breast carcinoma cells. Oncol Rep. 16:1307–1312.
