ABSTRACT
Cardiovascular problems can cause poor performance and sudden cardiac death during high-intensity competitions in athletics both humans and horses. A cardiac examination is one of the most important parameters used to define cardiac performance and cardiovascular diseases in sports horses. Some studies look at the cardiac morphology of horses from racing, eventing, endurance, and dressage. However, there is no information directly comparing these sports on the cardiac response. The objective was to evaluate the cardiac response of horses to various types of sports. Forty-eight horses were enrolled in this study and divided into 4 groups according to the type of sport: 12 control nonathlete horses, 12 show-jumping horses, 12 dressage horses, and 12 endurance horses. Electrocardiography with modified precordial leads was used to obtain electrophysiological heart function, and transthoracic echocardiography was evaluated. There were no significant differences among the groups concerning mean age and body weight. The electrocardiography and echocardiography results suggested that left ventricular internal diameter was highly associated with long-duration high-intensity exercise, such as endurance sports, and left ventricular wall thickness was significantly associated with short-duration high-intensity sports, such as show jumping. This study demonstrated exercise-induced cardiac morphology changes in each type of sports training.
Introduction
It has been widely verified that exercise enhances health outcomes (Nightingale et al. Citation2018; Bowman et al. Citation2021). In human athletes, sudden cardiac death can occur in football and basketball players which suggests that high dynamic intensity players are at higher risk of sudden cardiac death (Chandra et al. Citation2013; D'Silva and Sharma Citation2014; Alberti et al. Citation2021). Non-human animal athletes, such as horses, are also at risk of sudden cardiac death. However, the underlying cardiac responses in sudden death are not well understood. The role of the cardiac response has been of interest in human and equine sports medicine. The horse is often considered to be the principal model of an athlete among mammals (Young Citation2003; Lightowler et al. Citation2004) The horse’s heart is larger than other large mammals and the stroke volume is considered to determine aerobic capacity for the horse. As a result of cardiac adaptation in exercise training, the equine heart provides the advantage in optimizing oxygen transport and stroke volume for the athlete horses (Li et al. Citation2018).
Many studies on animal models investigated the association between exercise and cardiovascular responses (Shave et al. Citation2017; Polyák et al. Citation2018; Navas de Solis Citation2019). Animal models, such as murine models, display limitations in high heart rate and different ionic currents for cardiomyocyte depolarization, in the murine heart, the L-type Ca2 + current (ICa,L) contributes less to the ventricular action potential (AP) than in humans and therefore the murine AP shows gradual repolarization rather than a distinct plateau phase. (Lindsey et al. Citation2018). The cardiac remodelling induced by sports training has only received limited attention in the veterinary literature. Thus, the equine athlete could be used as a model to represent a system for studying the cardiac response to competitive training.
The cardiac response to the demand for sports during competition and training for sports types may reflect the intensity and duration of the sports type. Equine sport can be classified into 2 main types: the aerobic and anaerobic exercise according to the intensity and duration of the exercise (Kiely et al. Citation2020). Intermittent anaerobic high-intensity exercise, such as jumping is associated with increased cardiac afterload. In contrast, aerobic exercise such as endurance produced diastolic load (Trachsel et al. Citation2016).
The objective of this study was to evaluate cardiac response associated with three types of sports in horses: show jumping, dressage, and endurance. We hypothesized that cardiac morphology and function would differ depending on the type of sport. The information of this study may be useful for evaluating the cardiac response to the type of sport to improve performance and monitor the adverse incidents associated with high-intensity types of sports in horses.
Materials & methods
Animals
Sixty-three horses were collectively housed, managed, and trained under almost identical conditions within each training establishment. The population was composed of horses of different breeds as shown in . Fifteen horses affected by arrhythmias or audible murmurs of valve regurgitation were excluded from the study, and the colour flow Doppler echocardiography was confirmed by only one cardiologist to test that the horses had no cardiovascular disease. Horses were classified into 4 groups depending on the type of sport the horse participates in: control nonathlete horses (n = 12), show jumping horses (n = 12), dressage horses (n = 12), and endurance horses (n = 12). The study conformed to the guidelines of the Ethical Committee for Animal Experiments, Kasetsart University, Thailand (ACKU62-VET-061). Equine weight tape was used to obtain an approximate weight of the horses by measuring the heart girth. All non-invasive electrocardiography (ECG) and echocardiography parameters were recorded continuously for further analysis using a blinded assessment.
Table 1. Characteristics of athlete horses.
Electrocardiography measurements
Electrocardiography (ECG) was recorded with six electrodes placed on the skin at rest using ECG gel pads to provide the single 1 min ECGs recordings to provide the sufficient ECG component for the analysis. Horses with rhythm abnormalities such as atrioventricular block or premature beat were excluded from the study. The ECGs were recorded at 25 mm/s paper speed with a sensitivity of 0.5 cm/mV. The precordial ECG lead systems were modified and used on each horse as previously described, ECG values from lead II of the modified precordial of at least 3 consecutive beats are used to calculate various variables. (da Costa et al. Citation2017; Cherdchutham et al. Citation2020). The ECG parameters, such as P-wave duration time and amplitude, PR interval, QRS duration and amplitude, QT interval, and mean electrical axis (MEA), were manually analyzed using the vector method.
Echocardiography evaluations
An echocardiogram using a 2.0 MHz frequency transducer (Mindray, China) was performed on forty-eight horses at rest in the standing position to evaluate the heart structure and functions as described previously (Zucca et al. Citation2008). Horses with valve structure abnormalities were excluded from the study. The echocardiography images were recorded and stored. The left ventricular internal diameter, valve structure, and wall thickness were calculated by measuring the stored images from a two-dimensional plane and the motion mode (M-mode). The two-dimension and the motion mode were obtained from the right thorax of the horses. Left ventricular wall structures were calculated by measuring the images from two-dimensional and M-mode planes. Ventricular wall thickness and dimensions were recorded during diastole and systole to obtain parameters such as interventricular septum thickness (IVS), left ventricular internal diameter (LVID), and left ventricular proximal wall thickness (LVPW).
Statistical analysis
All data are shown as mean ± standard deviation (mean ± SD). Statistical analysis was performed using one-way analysis of variance (ANOVA), the statistical comparisons were made following Bonferroni correction for multiple comparisons. For each type of sport, a correlation matrix was used to represent the pair correlation of all the variables, correlation coefficients were used to describe the association between variables, and to analyze the multiple linear regression models that contained several independent variables. (GraphPad Prism Software version 9.0, USA). A p-value of 0.05 or less was indicated for statistical significance.
Results
Forty-eight healthy horses (aged 14.98 ± 0.61 years and weighing 412.92 ± 9.06 kg) completed the study without complications. There were no significant differences among the groups concerning mean age, body weight, and training hours per week (). An ECG example in various types of sports is shown in , and the echocardiogram is shown in . The results of echocardiography and electrocardiography are shown in and respectively.
Figure 1. A section of modified precordial lead ECG recording. Control horse, Show Jumping horse, Dressage horse, and Endurance horse.
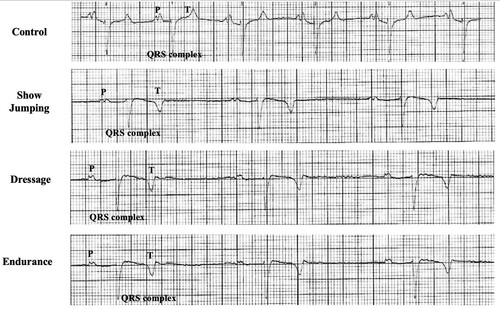
Figure 2. Echocardiogram of a horse. (A) Two dimensions of the echocardiogram were obtained from the right parasternal window. (B) M-mode echocardiogram, (C) The colour flow Doppler echocardiogram of the mitral valve (MV), (D) The colour flow Doppler echocardiogram of the tricuspid valve (TV)
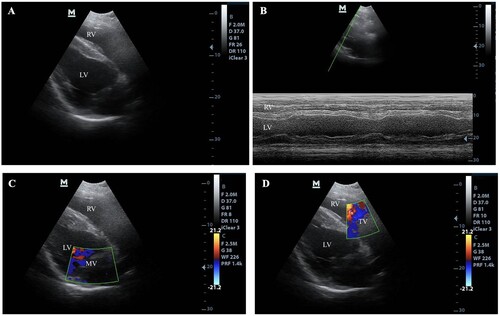
Table 2. Echocardiagraphic parameters.
Table 3. ECG measurements.
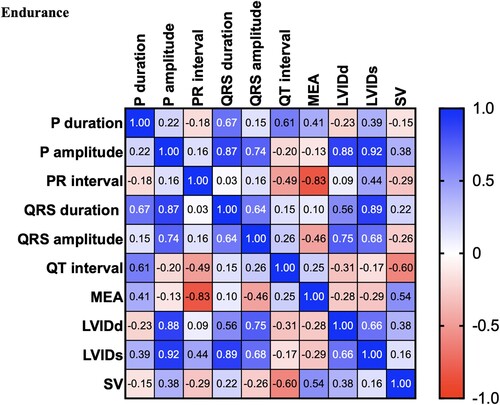
There were significant differences between the control and dressage horses in P wave duration obtained by modified precordial leads. However, there was no statistical difference among other ECG parameters. Several morphologies of the T wave were observed, and the biphasic configuration was generally presented in this study. In the present study, the R wave amplitude was lower in the showjumping group compared to the other group. Similar to the previous study, there was a slight difference in MEA among the groups when using the modified precordial lead system (da Costa et al. Citation2017; Cherdchutham et al. Citation2020). The correlation matrix of the cardiac response in show jumping sport (). The results showed a strong correlation between cardiac morphology and functional changes in low to moderate-intensity aerobic exercise such as dressage (). Interestingly, endurance exercise showed a strong relationship between the left ventricular internal diameter and the cardiac electrical activity response ().
Figure 3. Correlation matrix of all variables used in the cross-lagged models of horses participating in the Show Jumping exercise. The colour bar represents correlation coefficients from − 1 (red) to + 1 (blue). Blue squares represent significant positive correlations. Red squares represent significant negative correlations.
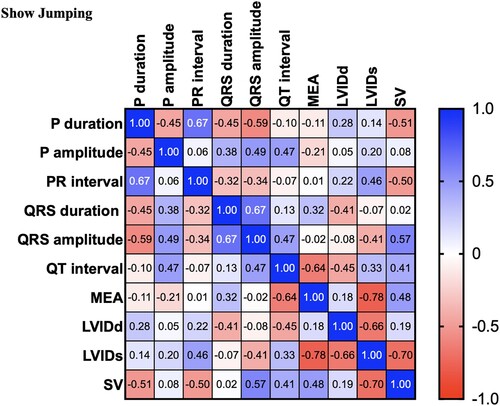
Figure 4. Correlation matrix of all variables used in the cross-lagged models of horses participating in Dressage exercise. The colour bar represents correlation coefficients from − 1 (red) to + 1 (blue). Blue squares represent significant positive correlations. Red squares represent significant negative correlations.
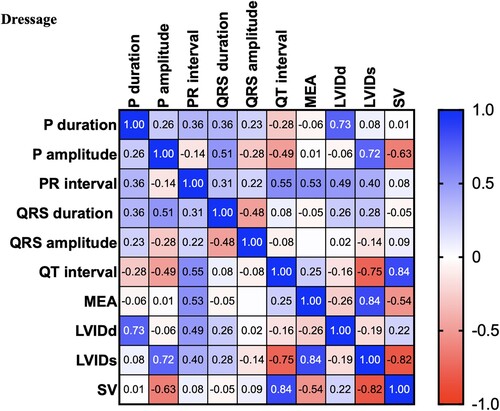
Figure 5. Correlation matrix of all variables used in the cross-lagged models of horses participating in Endurance exercise. The colour bar represents correlation coefficients from − 1 (red) to + 1 (blue). Blue squares represent significant positive correlations. Red squares represent significant negative correlations.
Discussion
The results from this study showed the difference in cardiac morphology and function in horses participating in different types of sports. Long-duration high-intensity exercise leads to adaptation to respond to diastolic demands. In contrast, the high-power short-duration sport led to an increase in the wall thickness in response to the high afterload demand.
Multivariable cardiac morphology and function were analyzed with various types of sports. The results from this study showed that cardiac morphology and function are affected by the type of sport and training duration. The cardiac response increased the stroke volume by increasing the afterload (). The results showed a strong correlation between the stroke volume and the left ventricular wall and septum thickness. However, cardiac morphology and functional changes in response to increases in both the diastolic load and afterload were visualized during low to moderate-intensity exercise such as dressage (). In addition, the electrical response also showed a strong positive correlation between the electrical response of the ventricle (QRS wave duration and amplitude) and the wall thickness (). Interestingly, aerobic exercise such as endurance showed a strong relationship between the left ventricular internal diameter and the cardiac electrical activity response. This study provides evidence that shows jumping exercise, which is a high-power intensity intermittent anaerobic sport, causes an increased cardiac wall thickness, which is similar to a previous report in human athletes that found that the left ventricular wall thickness was the most commonly associated with the participation in sports with a high-intensity demand (Pluim et al. Citation2000; Barbier et al. Citation2006).
As previously observed, increases in hematochemical parameters such as creatine kinase and glutamate oxaloacetate transaminases are found in response to stress-induced from the exercise, and renal function is also affected by prerenal factors (Piccione et al. Citation2009). In future studies, the blood collection should be taken to obtain the hematological parameters and may be used as reference data for various types of sports in horses.
The result of this study suggested that the horses with an abnormal cardiac function but need to attend the sports exercise may avoid sports that produce a high-pressure load on the left ventricle because horses participating in high-intensity sports, such as show jumping, adapt to training by increasing the wall thickness to support an increase in afterload. In a previous study, the electrocardiography parameters such as the P waves are not identical among the groups. At a slow heart rate in athletic horses, the P wave is bifid. Other echocardiography parameters such as the wall thickness and the chamber width were associated with low to moderate-intensity aerobic sports, such as dressage exercise. However, the results showed a moderate positive correlation among the cardiac variables. The result suggested that horses with light training adapt through increases in both left ventricular wall thickness and dimension. An increase in cardiac dimensions was consistently present in moderate-intensity aerobic sports, such as endurance exercise. The cardiac variables such as chamber dimensions were more positively correlated in endurance exercise than in dressage and show jumping exercises.
A cardiovascular assessment such as blood pressure, heart rate, and heart rate variability will help to identify and prevent significant factors influencing cardiovascular health. Therefore, a cardiovascular examination should be a part of the health monitoring in sports horses. Furthermore, the horse should not have any heart problems such as valves regurgitation or aortic valve aneurysm before starting the training.
In addition, these data suggested that moderate-intensity aerobic sports lead to the development of cardiac morphology that is appropriate to the endurance sport, which needs a more diastolic load. Previous studies have reported the association between cardiac size and performance in Thoroughbred racehorses (Buhl et al. Citation2005; Young et al. Citation2005). However, this information is less available in a population of horses participating in various types of sports. In this study, we were able to correlate cardiac function and performance in various types of sports. Additionally, we were unable to control the training environment and nutrition in these sports horse populations, which is a limitation of our study. In our pilot study, an insufficient number of horses in each group could affect the statistical analysis, which was a limitation of this present study. In addition, a longitudinal study with information before and after training of horses is needed to characterize the cardiac adaptation in response to exercise. However, the results from our study may provide insight into the association between cardiac function in various sports types and may help to define the role of heart morphology and function to evaluate and monitor the performance of sports horses.
Conclusions
The results from this study showed the difference in cardiac morphology and function in horses participating in different types of sports. Long-duration high-intensity exercise leads to adaptation to respond to diastolic demands. In contrast, the high-power short-duration sport led to an increase in the wall thickness in response to the high afterload demand. We suggest that further study aims to correlate cardiac function and performance in various types of sports. This would provide a complete cardiac adaptation and performance in response to each type of sports training.
Author contribution
Conceptualization: Soontaree Petchdee; Data curation: Soontaree Petchdee; Metha Chanda; Formal analysis: Soontaree Petchdee; Metha Chanda; Investigation: Soontaree Petchdee; Metha Chanda, Methodology: Soontaree Petchdee; Metha Chanda, Writing - original draft: Soontaree Petchdee; Writing - review & editing: Soontaree Petchdee; Metha Chanda.
Data availability
The following information was supplied regarding data availability: The comprehensive results are available in the supplemental files.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Alberti E, Stucchi L, Lo Feudo CM, Stancari G, Conturba B, Ferrucci F, Zucca E. 2021. Evaluation of cardiac arrhythmias before, during, and after treadmill exercise testing in poorly performing standardbred racehorses. Animals (Basel). 11(8):2413. doi:10.3390/ani11082413. PMID: 34438870; PMCID: PMC8388799.
- Barbier J, Ville N, Kervio G, Walther G, Carré F. 2006. Sports-specific features of athlete's heart and their relation to echocardiographic parameters. Herz Kardiovaskuläre Erkrankungen. 31(6):531–543. doi:10.1007/s00059-006-2862-2. PMID: 17036184.
- Bowman PRT, Smith GL, Gould GW. 2021. Run for your life: can exercise be used to effectively target GLUT4 in diabetic cardiac disease? Peer J. 9(9):e11485. doi:10.7717/peerj.11485. PMID: 34113491; PMCID: PMC8162245.
- Buhl R, Ersbøll AK, Eriksen L, Koch J. 2005. Changes over time in echocardiographic measurements in young standardbred racehorses undergoing training and racing and association with racing performance. J Am Vet Med Assoc. 226(11):1881–1887. doi:10.2460/javma.2005.226.1881. PMID: 15934256.
- Chandra N, Bastiaenen R, Papadakis M, Sharma S. 2013. Sudden cardiac death in Young athletes. J Am Coll Cardiol. 61(10):1027–1040. doi:10.1016/j.jacc.2012.08.1032. PMID: 23473408.
- Cherdchutham W, Koomgun K, Singtoniwet S, Wongsutthawart N, Nontakanun N, Wanmad W, Petchdee S. 2020. June-2020. Veterinary World. 13(6):1229–1233.
- da Costa CF, Samesima N, Pastore CA. 2017. Cardiac mean electrical axis in thoroughbreds—standardization by the dubois lead positioning system. PLoS One. 12(1):e0169619. doi:10.1371/journal.pone.0169619. PMID: 28095442; PMCID: PMC5241011.
- D'Silva A, Sharma S. 2014. Exercise, the athlete's heart, and sudden cardiac death. Phys Sportsmed. 42(2):100–113. doi:10.3810/psm.2014.05.2062. PMID: 24875977.
- Kiely M, Warrington GD, McGoldrick A, Pugh J, Cullen S. 2020. Physiological demands of professional flat and jump horse racing. J Strength Cond Res. 34(8):2173–2177. doi:10.1519/JSC.0000000000003677. PMID: 32735425.
- Li M, Chadda KR, Matthews GDK, Marr CM, Huang CL, Jeevaratnam K. 2018. Cardiac electrophysiological adaptations in the equine athlete—restitution analysis of electrocardiographic features. PLoS One. 13(3):e0194008. doi:10.1371/journal.pone.0194008.
- Lightowler C, Piccione G, Giudice E, del Olmo GR, Cattaneo ML. 2004. Echocardiography and electrocardiography as means to evaluate potential performance in horses. J Vet Sci. 5(3):259–262. PMID: 15365242.
- Lindsey ML, Kassiri Z, Virag JAI, de Castro Brás LE, Scherrer-Crosbie M. 2018. Guidelines for measuring cardiac physiology in mice. Am J Physiol-Heart Circulatory Physiol. 314(4):H733–H752. doi:10.1152/ajpheart.00339.2017.
- Navas de Solis C. 2019. Cardiovascular response to exercise and training, exercise testing in horses. Vet Clin N Am: Equine Pract. 35(1):159–173. doi:10.1016/j.cveq.2018.11.003. PMID: 30871829.
- Nightingale TE, Rouse PC, Walhin JP, Thompson D, Bilzon JLJ. 2018. Home-Based exercise enhances health-related quality of life in persons With spinal cord injury: A randomized controlled trial. Arch Phys Med Rehabil. 99(10):1998–2006.e1. doi:10.1016/j.apmr.2018.05.008. PMID: 29902472.
- Piccione G, Casella S, Giannetto C, Monteverde V, Ferrantelli V. 2009. Exercise-induced modifications on haematochemical and electrophoretic parameters during 1600 and 2000 meters trot races in standardbred horses. J Appl Anim Res. 35(2):131–135. doi:10.1080/09712119.2009.9707002.
- Pluim BM, Zwinderman AH, van der Laarse A, van der Wall EE. 2000. The athlete’s heart. Circulation. 101(3):336–344. doi:10.1161/01.CIR.101.3.336. PMID: 10645932.
- Polyák A, Kui P, Morvay N, Leprán I, Ágoston G, Varga A, Nagy N, Baczkó I, Farkas A, Papp JG, et al. 2018. Long-term endurance training-induced cardiac adaptation in new rabbit and dog animal models of the human athlete's heart. Rev Cardiovasc Med. 19(4):135–142. doi:10.31083/j.rcm.2018.04.4161. PMID: 31064165.
- Shave R, Howatson G, Dickson D, Young L. 2017. Exercise-Induced cardiac remodeling: lessons from humans, horses, and dogs. Vet Sci. 4(1):9. doi:10.3390/vetsci4010009. PMID: 29056668; PMCID: PMC5606617.
- Trachsel DS, Giraudet A, Maso D, Hervé G, Hauri DD, Barrey E, Robert C. 2016. Relationships between body dimensions, body weight, age, gender, breed, and echocardiographic dimensions in young endurance horses. BMC Vet Res. 12(1):226. doi:10.1186/s12917-016-0846-x. PMID: 27724944; PMCID: PMC5057441.
- Young LE. 2003. Physiological society symposium - the athlete's heart. Exp Physiol. 88(5):659–663. doi:10.1113/eph8802615. PMID: 12955166.
- Young LE, Rogers K, Wood JL. 2005. Left ventricular size and systolic function in Thoroughbred racehorses and their relationships to race performance. J Appl Physiol. 99(4):1278–1285. doi:10.1152/japplphysiol.01319.2004.
- Zucca E, Ferrucci F, Croci C, Di Fabio V, Zaninelli M, Ferro E. 2008. Echocardiographic measurements of cardiac dimensions in normal standardbred racehorses. J Vet Cardiol. 10(1):45–51. doi:10.1016/j.jvc.2008.04.002. Epub 2008 Jun 3. PMID: 18511359.
