ABSTRACT
This study compared the effects of CIDR type and eCG treatment on ovarian follicular dynamics, estrus response and pregnancy per AI (P/AI) in Holstein heifers subjected to a 5-d estrus synchronization protocol. On Day 0, heifers (n = 133) received either a new or a once-used CIDR. On Day 5, CIDR was removed, 500 μg cloprostenol administered and heifers received or not 300 IU eCG (2 × 2 design). Heifers in estrus were inseminated 12 h after first observation. All heifers without estrus by 84 h after CIDR removal received 100 μg GnRH concurrently with AI. Ultrasonography was done in a subset of 77 heifers at CIDR insertion and removal, at AI and every 12 h after AI to determine follicular dynamics. Diameter of largest follicle at CIDR removal and AI was larger in heifers given a used CIDR (P < .05). The overall estrus response and ovulation (85.0 and 95.5%) did not differ between CIDR types, heifers given a new CIDR had greater P/AI (67.2 vs. 42.6%; P = .004). In summary, P/AI was reduced in dairy heifers treated with a used CIDR, despite having larger preovulatory follicles at AI. In addition, eCG treatment did not affect follicular dynamics, estrus response nor P/AI.
Introduction
GnRH-based protocols for synchronization of estrus and ovulation have been developed and used for the last decades, especially in commercial dairy herds. The most commonly used GnRH-based protocol in dairy heifers is the 7-d CO-Synch plus the addition of an intravaginal insert containing progesterone (P4; Colazo and Mapletoft Citation2014). Recent studies have looked at reducing the length of the GnRH-based protocol from 7 to 5 d, in order to decrease the period of follicular dominance and extend the proestrus period (Bridges et al. Citation2008), which provided for a better estradiol exposure due to the continuous gonadotropin support for the dominant follicle. As only 25–40% of heifers usually ovulate to the initial GnRH (Rivera et al. Citation2006; Colazo and Ambrose Citation2011), some studies removed this step reducing cost, but still obtaining acceptable P/AI in beef (Cruppe et al. Citation2014) and dairy heifers (Colazo and Mapletoft Citation2017) subjected to a 5-d GnRH-based protocol.
It is well known that circulating P4 regulates luteinizing hormone (LH) pulse frequency and can alter the size of the ovulatory follicle and its estradiol synthesis (Adams et al. Citation1992; Inskeep Citation2004). In this regard, an increase in circulating P4 reduced LH concentrations due to decreased GnRH pulses from the hypothalamus and decreased LH release from the anterior pituitary in response to GnRH (Roberson et al. Citation1989). On the contrary, low plasma P4 concentration resulted in elevated LH pulse-frequency (Rahe et al. Citation1980) and the development of persistent follicles (Kinder et al. Citation1996) with reduced fertility in cattle (Inskeep Citation2004). However, a short exposure to low circulating P4 concentrations during the ovulatory wave resulted in a larger ovulatory follicle and a larger corpus luteum that produced more P4, with no adverse effect on P/AI in pubertal beef heifers and suckled beef cows (Pfeifer et al. Citation2009). Therefore, the use of inserts with different P4 content could affect ovarian follicular growth and fertility in cattle subjected to a 5-d GnRH-based protocol.
The use of equine chorionic gonadotropin (eCG), through its action of follicle stimulating hormone (FSH) and LH in cattle, has been reported to increase follicle size and growth, ovulation rate and P/AI (Sá Filho et al. Citation2010), especially in a shortened estradiol-based protocol (Menchaca et al. Citation2007). In beef heifers subjected to a modified 5-d GnRH-based TAI protocol (without initial GnRH at CIDR insertion and a single PGF at CIDR removal) the occurrence of estrus before TAI was associated with high fertility (Colazo et al. Citation2018) and the addition of eCG at CIDR removal increased estrus response (Macmillan et al. Citation2020). Although several studies have examined the application of used P4 inserts in beef and dairy cattle (Colazo et al. Citation2004; Cerri et al. Citation2009), the comparison of new vs. once-used CIDR in combination with eCG in dairy heifers subjected to a shortened estrus synchronization protocol has not been previously reported.
Therefore, the objective of this study was to compare ovarian follicular dynamics, estrus response and P/AI in dairy heifers subjected to a 5-d estrus synchronization protocol given a new or a previously used CIDR insert, in combination with or without 300 IU of eCG at CIDR withdrawal. We hypothesized that: (1) the insertion of once-used CIDRs increases follicle growth, estrus response and P/AI, compared to new CIDRs; (2) eCG administration enhances follicle growth, estrus response and P/AI.
Materials and methods
This study was conducted on a commercial dairy farm located near Wetaskiwin, Alberta, Canada (53°02'54.0"N, 113°19'28.0"W) from July to December 2018.
Animals, housing and nutrition management
This study was carried out with 133 cyclic Holstein heifers between 14 and 15 mo of age. Heifers were housed in a bedded pack facility, bedded with chopped straw and had free access to fresh water and an outdoor area. During the experimental period, heifers were fed once daily (17 kg) a total mixed ration consisting of grass hay (7.44%), grass silage (17.85%), barley silage (47.58%), beer bagasse (17.85%), barley grain (5.95%), barley straw (2.97%), correctors, vitamins and minerals (0.36%). The chemical composition of the ration was 45% DM, 15.18% CP, 30.41% ADF, 42.69% NDF with a NE of 1.36 Mcal/kg.
Study design
Heifers were blocked by age and whether they were or not previously inseminated, and randomly assigned (Day 0) within block to treatments. On Day 0, heifers at random stages of the estrous cycle received either a new or a once-used (New vs. Used) autoclaved P4 insert (CIDR, 1.38 g of P4; Zoetis Animal Health, Florham Park, NJ, USA) without any further treatment (). On Day 5, CIDR was removed, 500 μg cloprostenol (PGF; Bioestrovet; Vetoquinol N.-A Inc., Lavaltrie, QC, Canada) administered im and heifers were further subdivided to receive (+eCG) or not (-eCG) 300 IU im of equine chorionic gonadotropin (eCG; Pregnecol® 6000; Vetoquinol N.-A Inc., Canada), in a 2×2 factorial design. The number of heifers assigned to each of the four treatments was as follows: New/-eCG (N = 29), New/+eCG (N = 29), Used/-eCG (N = 36), and Used/+eCG (N = 39). The number of heifers previously inseminated were 15, 15, 16 and 19 in the New/-eCG, New/+eCG, Used/-eCG, and Used/+eCG groups, respectively. After an initial use of 5 d, the once-used CIDR inserts were individually scrubbed with a brush, thoroughly rinsed with warm water, allowed top air-dry and autoclaved (at 120 °C and 15 psi for 30 min), fast-dried exhaust for 20 min, wrapped in aluminium paper, and stored at room temperature until use.
Figure 1. Illustration of the synchronization protocol used in this study. Holstein heifers (n = 133) at random stages of the estrous cycle were allocated to one of four treatments (2 × 2 design). Estrus detection (ED) patches were applied on Day 5. Transrectal ultrasonography (U/S) was done to determine cyclicity, ovulation, follicle diameter and pregnancy.
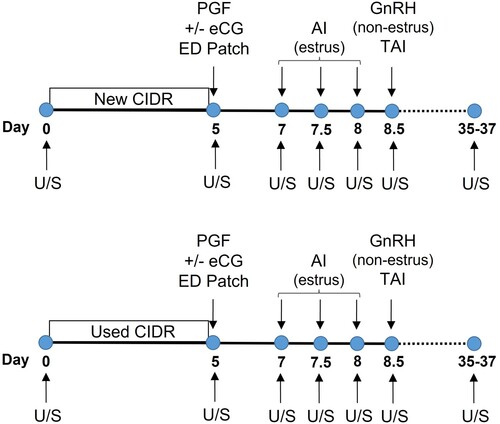
At CIDR removal (Day 5), estrus detection (ED) patches (EstrotectTM; Rockway Inc., Spring Valley, WI, USA) were applied about mid-way between the hip and tail head in all heifers. Estrus detection was performed twice daily (morning and evening) up to 84 h following CIDR removal. The ED patches were scored from 0 to 2, based on colour change from initial application (0 = unchanged, 1 = ≤ 50% colour change, 2 = > 50% colour change). Estrus was defined to have occurred when an ED patch was scored 2. Estrus response (%) was defined as the number of heifers with an ED patch scored 2 over the total number of heifers. Heifers with a patch scored 2 were inseminated approximately 12 h after the first observation using the AM-PM rule. All heifers failing to exhibit estrus (n = 20) received 100 μg im of gonadorelin acetate (GnRH; Fertiline; Vetoquinol N.-A Inc., Canada) and were TAI at 84 h after CIDR removal. All heifers were inseminated by the same technician with frozen-thawed sex-sorted (n = 73) or conventional (n = 60) semen from one of four commercially available sires equally distributed among treatments (Alta Genetics, Balzac, AB, Canada).
The number of heifers inseminated with sex-sorted semen in the New- and Used-CIDR groups were 31 and 42, respectively.
Determination of cyclicity, ovarian follicular dynamics and pregnancy
All 133 heifers were examined by transrectal ultrasonography (MicroMAXX, colour Doppler scanner equipped with a multifrequency 5–10 MHz linear transducer; Sonosite, Bothell, WS, USA) at the initiation of study (Day 0) to determine cyclicity (presence of luteal tissue) and normalcy of the reproductive tract. In all heifers ultrasonographic examinations were also done at CIDR removal and every 12 h after AI to confirm ovulation. Ovulation was confirmed by the disappearance of a large (>10 mm) follicle that had been detected at the previous examination.
In a subset of 77 heifers, ultrasonographic examinations were done at CIDR removal and at AI to measure the diameter of the largest follicle. A diagram of the relative position of the structures, number and diameter of follicles and corpus luteum (obtained by averaging the height and width of each structure) was sketched to examine the effect of treatments on follicular growth. Pregnancy diagnosis was performed by ultrasonography 28–30 d after AI. Presence of a viable embryo (positive heartbeat) was used as a determinant of pregnancy.
Statistical analyses
All statistical analyses were performed using SAS statistical package (Statistical Analysis System, Version 9.4 for Windows; SAS Institute Inc., Cary, NC, USA).
Largest follicle diameter at CIDR removal and at AI, and the largest follicle growth (from CIDR removal to AI) were analysed using the GLIMMIX procedure, considering as type of CIDR (new vs. used), eCG treatment (yes vs. no), pregnancy status (pregnant vs. non-pregnant) and their interactions as fixed effects, while the replicate was included as a random effect. As none of the interactions was significant, the final model only included the aforementioned fixed effects. Pregnancy status was treated as fixed effect in order to retrospectively determine whether there was a difference in largest follicle diameter at CIDR removal and at AI and the largest follicle growth between heifers consequently becoming pregnant or non-pregnant. The model specifications included a Poisson distribution and link log function. Differences in means were tested using Tukey–Kramer multiple means comparison test.
The interval from CIDR removal to estrus detection, estrus response rate or ovulation rate were analysed using the GLIMMIX procedure, considering type of CIDR (new vs. used), eCG treatment (yes vs. no) and their interactions as fixed effects. As the interaction was not significant, the final model only included the fixed effects. The model specifications included a binomial distribution and logit function, and an option to retrieve odds ratios and their confidence intervals for estrus response rate and ovulation rate. Differences in means were tested using Tukey–Kramer multiple means comparison test.
Pregnancy per AI was modelled against type of CIDR (new vs. used), eCG treatment (yes vs. no), type of semen (conventional vs. sex-sorted), GnRH + TAI (yes vs. no), previous AI (yes vs. no), and their interactions and analysed using the GLIMMIX procedure. As none of the interactions was significant, the final model only included the aforementioned fixed effects. The model specifications included a binomial distribution and link logit function, and an option to retrieve odds ratios and their confidence intervals. Differences in means were tested using Tukey–Kramer multiple means comparison test.
The association between pregnancy per AI and the interval from CIDR removal to estrus detection was analysed using a logistic regression model (LOGISTIC procedure). For all statistical analyses, P ≤ .05 was considered statistically significant, whereas differences between P > .05 and P ≤ .10 were considered a tendency.
Results
Ovarian follicle development
Six heifers were excluded from the analyses because they did not ovulate. Thus, data from 71 heifers were analysed, and the number of heifers by treatment group was as follows: New/-eCG (N = 15), New/+eCG (N = 16), Used/-eCG (N = 19), Used/+eCG (N = 21). The results of ovarian follicular dynamics are summarized in . The diameter of the largest follicle at CIDR removal was larger in heifers that received a used CIDR than in those receiving a new CIDR (P = .05). Similarly, heifers given a used CIDR had larger follicles at AI than heifers given a new CIDR (P = .04). Follicle diameter at CIDR removal and at AI were not associated with pregnancy status (P = .65). The growth rate of the preovulatory follicle was not significantly affected by the type of CIDR (1.29 ± 0.2 vs. 1.15 ± 0.2 mm/d for New and Used CIDR; P = .42) nor by the administration of eCG (1.11 ± 0.2 vs. 1.33 ± 0.2 mm/d for -eCG and + eCG; P = .20). However, follicle growth rate tended to be slower in heifers that became pregnant compared to heifers that were non-pregnant (1.06 ± 0.1 vs. 1.38 ± 0.1 mm/d; P = .08). Finally, the interval that elapsed from CIDR removal to AI was not associated with type of CIDR (82.0 vs. 77.7 h, for New and Used CIDR, respectively; P = .33), eCG treatment (77.3 vs. 82.3 h, for -eCG and + eCG, respectively; P = .26) or pregnancy status (77.5 vs. 82.1 h, for pregnant and non-pregnant, respectively; P = .30).
Table 1. Diameter (LSM ± SE; mm) of the largest follicle at CIDR removal and at AI, regarding to the type of CIDR (New vs. Used) and the administration (+) or not (-) of 300 IU of equine chorionic gonadotropin (eCG).
Estrus response
The overall estrus response rate was 85.0% (113/133) and did not differ among treatment groups (). The interval from CIDR removal to detected estrus was not influenced by the type of CIDR (62.6 vs. 65.0 h, for New and Used CIDR, respectively; P = .45) nor by the administration of eCG (63.4 vs. 64.2 h, for -eCG and + eCG, respectively; P = .81).
Table 2. Estrus response, ovulation rate and pregnancy per AI (P/AI).
Ovulation rate
The overall ovulation rate determined by transrectal ultrasonography was 95.5% (127/133) and did not differ among treatment groups ().
Pregnancy per AI
The overall P/AI was 53.4% (71/133). The interaction between the type of CIDR and eCG treatment on P/AI was not significant (20/29 (69.0%), 19/29 (65.5%), 17/36 (47.2%) and 15/39 (38.5%) for New/-eCG, New/+eCG, Used/-eCG and Used/+eCG, respectively; P = .58). Pregnancy per AI was not affected by the inclusion of eCG (P = .57; ), type of semen (52.3 vs. 57.1%, for conventional and sex-sorted semen, respectively; P = .39) or the number of AI (59.3 vs. 48.6%, with and without a previous AI, respectively; P = .33). However, P/AI was associated with the type of CIDR used (P = .004; ). In this regard, heifers that received a new CIDR had 24.6 percentage-points greater P/AI compared to heifers receiving a used CIDR. In addition, heifers given a new CIDR had 3.3 greater odds of becoming pregnant than those given a used CIDR.
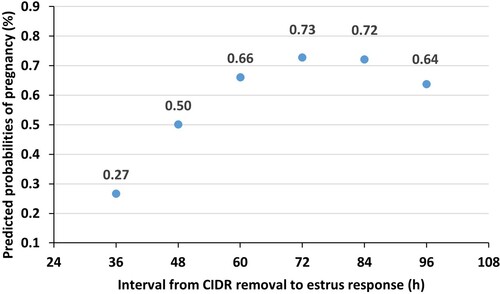
Only one out of the 20 heifers (5.0%) that did not express estrus and received GnRH and were TAI became pregnant. Conversely, 61.9% (70/113) of heifers that did express estrus became pregnant (P = .002). The predicted probability of pregnancy ranged from 0.27 to 0.73 (at 36 or 72 h after CIDR removal, respectively). The association between the interval from CIDR removal to estrus response and P/AI was quadratic and heifers exhibiting estrus 72 and 84 h after CIDR removal tended to have the highest probability of pregnancy (P = .06; ).
Figure 2. Relationship between the interval from CIDR removal to estrus response and estimated probability of pregnancy in dairy heifers subjected to a 5-d estrus synchronization protocol with or without the administration of 300 IU of equine chorionic gonadotropin at CIDR removal. Heifers exhibiting estrus at 72 and 84 h after CIDR removal tended (P = .06) to have the highest probability of pregnancy.
Discussion
The present study investigated ovarian follicular dynamics, estrus response and P/AI in dairy heifers subjected to a 5-d estrus synchronization protocol given a new or once-used CIDR, in combination with or without 300 IU of eCG at CIDR removal. Although several studies have examined the application of used P4 inserts on cattle fertility, the efficacy of a once-used CIDR and the administration of eCG in dairy heifers subjected to a 5-d estrus synchronization protocol has not been previously reported.
In the current study, heifers given a used CIDR had larger follicles at CIDR removal and at AI compared to heifers that received a new CIDR. Therefore, our hypothesis that reducing circulating P4 concentrations (with reused CIDRs) during ovarian follicle development will enhance follicle diameter compared with the use of new CIDRs was supported. Previous studies have shown that there were negative correlations between circulating P4 concentrations and follicle size in Bos indicus heifers (Butler et al. Citation2011), and Bos taurus heifers (Dadarwal et al. Citation2013; Núñez-Olivera et al. Citation2019) and cows (Dadarwal et al. Citation2013). Recently, the reuse of CIDRs (originally containing 1.38 g of P4) has been described as an effective strategy to reduce circulating P4, increase ovulatory follicle size and increase post-ovulatory CL diameter in dairy heifers subjected to a 5-d fixed-time embryo transfer protocol (Sala et al. Citation2020).
The residual P4 concentrations for CIDR inserts containing 1.38 g of P4 were 0.7 g, after 7 d of insertion, suggesting that CIDR inserts could be reused (upon cleaning and disinfecting) for at least 7 more days (Rathbone et al. Citation2002). Zuluaga and Williams (Citation2008) compared serum P4 concentration in ovariectomized cows receiving a new (1.38 g of P4), a reused chemically disinfected or a reused autoclaved CIDR. Mean serum P4 was greater for new or autoclaved inserts than for chemically disinfected inserts (3.7 and 3.4 vs. 2.8 ng/mL, respectively) during the 7-d period of insertion. In the study by Cerri et al. (Citation2009) cyclic lactating dairy cows received a new CIDR (1.38 g of P4) or a 7-d used autoclaved CIDR for 7 d. Plasma concentration of P4 tended to increase faster in cows receiving a used autoclaved CIDR, but cows receiving a new CIDR had greater concentrations of P4 during the 7-d treatment. Recently, Sala et al. (Citation2020) have reported that heifers subjected to a 5-d GnRH based protocol and treated with a new CIDR (1.38 g of P4) had greater P4 than second use, which was greater than third use, which was greater than fourth use, with CIDRs washed and chemically disinfected prior to each use. Our experiment was performed with autoclaved once-used CIDRs (1.38 g of P4) previously used for 5 d in Holstein heifers. Unfortunately, circulating P4 was not determined in the current study, but our findings regarding ovarian follicular dynamics and P/AI and results discussed above regarding circulating P4 are inconclusive and do not support the re-use of CIDR devices originally containing 1.38 g of P4.
An increase in the size of the preovulatory follicle has been correlated positively with improved fertility in some studies conducted with beef cattle (Perry and Perry Citation2008; Dadarwal et al. Citation2013), but not in other studies (Pfeifer et al. Citation2009; Butler et al. Citation2011; Núñez-Olivera et al. Citation2019). In the present study, the larger size of the preovulatory follicle observed in dairy heifers receiving a used CIDR was negatively associated with fertility. Contrary to our hypothesis, in the current study application of a once-used CIDR insert reduced P/AI by 24.6 percentage-points compared to the use of a new CIDR. In support, P/AI did not differ between cattle synchronized with an estradiol-based protocol and a new (containing 1.9 g of P4) or once-used CIDR, but P/AI was lower in cattle synchronized with a twice-used CIDR, which could have contained similar residual P4 than the once-used CIDR utilized in the current study (Colazo et al. Citation2004). Low circulating P4 concentrations, and consequently high LH pulse-frequency, during the ovulatory wave were associated with the development of persistent follicles (Kinder et al. Citation1996) with reduced fertility attributed to a premature oocyte maturation (Revah and Butler Citation1996; Mihm et al. Citation1999). Similarly, Denicol et al. (Citation2012) reported reduced fertility in induced ovulations from the first-wave dominant follicle (growth mostly in low P4 environment) compared to second-wave dominant follicle in lactating dairy cows. One of the proposed benefits of reducing the synchronization protocol from 7 to 5 d is fewer persistent follicles. However, as previous studies have reported reduced ovulatory response to the initial GnRH in heifers (Rivera et al. Citation2006; Colazo and Ambrose Citation2011), GnRH was not administered at the initiation of the 5-d GnRH-based protocol. This could have also favoured a higher incidence of persistent follicles, especially in heifers given a used CIDR, added to their insufficient progesterone activity to maintain a strong negative feedback on LH pulse frequency in a manner comparable to that of heifers given a new CIDR. Some larger preovulatory follicles in heifers given a used CIDR could correspond to persistent follicles, which could explain their reduced P/AI.
In the present study, the overall follicle growth and P/AI (1.22 mm/d and 53.4%, respectively) were similar to those reported in a previous study conducted with dairy heifers synchronized with a similar protocol (1.25 mm/d and 58.8%) (Colazo and Ambrose Citation2011). The growth rate of the preovulatory follicle was not affected by the type of CIDR nor eCG administration, although it tended to be lower in heifers that became pregnant compared to that in non-pregnant heifers. It has been documented that much of the variation in the response observed in estrus synchronization protocols is due to the follicular status at the time of treatments (Adams and Singh Citation2015). In this regard, the interval from PGF administration to ovulation depended on the stage of dominant follicle development at the time of treatment (Kastelic et al. Citation1990). Regardless of follicle development or growth, length of proestrus could affect fertility (Bridges et al. Citation2010). The intervals lapsed from CIDR removal to estrus response (overall 64 h) and to AI (overall 80 h) were not influenced by CIDR type nor eCG administration. However, heifers with an interval from CIDR removal to estrus response of 72 or 84 h tended to have the highest probability of pregnancy, which again could be related to the ovulation of persistent follicles that tend to ovulate earlier or due to the lack of gonadotrophin support. In this regard, Bridges et al. (Citation2008) reported an increase of 10 percentage-points in P/AI when preovulatory follicles had 72 h of gonadotropin support (proestrus period) compared to 60 h of gonadotropin support. Moreover, the relationship between length of proestrus and predicted probability of pregnancy at 28 d after TAI in Holstein heifers subjected to a 5-d GnRH-based protocol was quadratic indicating a maximum predicted probability of pregnancy of 80.1% when the length of proestrus was 3 d (Colazo and Ambrose Citation2011).
The overall 85.0% estrus response in the present study was higher than the estrus response reported in previous studies in dairy (69%; Colazo and Mapletoft Citation2017) and beef heifers (64%; Colazo et al. Citation2018) subjected to a similar synchronization protocol, probably because these previous studies reported the estrus expression up to 72 h after CIDR removal (12 hours less than in the present study). However, the estrus response was not associated with the type of CIDR nor to the administration of eCG. In a previous study, the application of once-used PRID-Delta devices resulted in 10 percentage-points higher estrus response (60 vs. 50%) compared with new devices containing 1.55 g of P4 in lactating dairy cows synchronized with a shortened P4-based protocol (López-Helguera et al. Citation2017), probably associated with larger follicles that produced more estradiol (Cerri et al. Citation2009). However, the increased estrus response had no significant effect on P/AI in the study by López-Helguera et al. (Citation2017). In the current study, only one heifer from those that did not express estrus by the time of TAI (84 h after CIDR removal) became pregnant (5.0%), compared to 61.9% of heifers that did express estrus and became pregnant following AI. In the study by Colazo et al. (Citation2018), in beef heifers treated with the modified 5-d GnRH-based TAI protocol, the occurrence of estrus was described to increase fertility (59.9 vs. 32.5%), regardless of semen type, as occurred in the present study.
The administration of eCG treatment did not affect follicular dynamics, estrus response nor fertility in the present study. Therefore, findings did not support our second hypothesis that the inclusion of eCG would increase follicle growth, estrus response and P/AI. A recent study has also reported no difference in preovulatory follicle size in beef heifers subjected to a shortened estradiol-based protocol (i.e. J-synch) with or without administration of eCG (Núñez-Olivera et al. Citation2020). Interestingly, same authors had previously reported an increase in follicle size and P/AI in beef heifers submitted to the J-synch protocol when eCG was added (Menchaca et al. Citation2015). In a recent study from our group, administration of eCG increased estrus response only in beef heifers without a CL at the start of the modified 5-d GnRH-based protocol and has shown a tendency to increase overall P/AI (Macmillan et al. Citation2020). The discrepancies of results regarding the administration of eCG could be related to the cyclicity status or body condition score of animals involved in the study (Menchaca et al. Citation2007). Cattle with low body condition score, as beef primiparous cows (Small et al. Citation2009), anestrus suckled cows (Baruselli et al. Citation2004) or anestrus dairy cows (Bryan et al. Citation2013), have also improved P/AI with the inclusion of eCG in their ovulation synchronization protocols. Other studies with Bos taurus heifers (Small et al. Citation2010), and Bos indicus heifers (Butler et al. Citation2011) and cows (Alvarez et al. Citation2018) in good body condition score have reported no effect of eCG on P/AI, in line with our finding. Dairy heifers are usually in good body condition score and cycling, which would explain the lack of eCG effect in our study.
From a practical point of view, the use of a 5-d estrus synchronization protocol combined with AI after a short period of estrus detection and TAI for heifers that did not express estrus was an efficacious approach to obtain an acceptable P/AI (53.4%) in cyclic Holstein heifers using either conventional or sex-sorted semen. However, the proposed strategies for increasing estrus rate and growth rate of the preovulatory follicle, with the utilization of used CIDRs or the administration of eCG, cannot be recommended for shortened estrus synchronization protocols in cyclic Holstein heifers. It should be noted that the number of experimental units per group used in the current study was based on previously published results that were moderately different than our results. Therefore, further studies with larger populations are necessary in order to validate our findings.
Conclusion
In summary, P/AI was reduced in dairy heifers treated with a used CIDR in a 5-d estrus synchronization protocol, despite having larger preovulatory follicles at CIDR removal and at AI. In addition, administration of eCG did not affect follicular dynamics, estrus response nor pregnancy risk. Therefore, the use of once-used CIDRs or the inclusion of eCG are not recommended in shortened estrus synchronization protocols for cyclic Holstein heifers.
Acknowledgements
Authors thank Vetoquinol N.-A Inc. (Lavaltrie, QC, Canada) and Rockway Inc. (Spring Valley, WI, USA) for their in-kind support and Breevliet Ltd (Wetaskiwin, AB, Canada) for cooperation during the study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Additional information
Funding
References
- Adams GP, Matteri RL, Ginther OJ. 1992. Effect of progesterone on ovarian follicles, emergence of follicular waves and circulating follicle-stimulating hormone in heifers. J Reprod Fert. 96:627–640. doi:10.1530/jrf.0.0960627.
- Adams GP, Singh J. 2015. Ovarian follicular and luteal dynamics in cattle. In: Richard McRae Hopper, editor. Bovine reproduction. Starkville: John Wiley & Sons, Inc; p. 237–262.
- Alvarez RH, Pugliesi G, Natal FLN, Rocha CC, Júnior GAA, Melo AJF, et al. 2018. Reproductive performance of Bos indicus beef cows treated with different doses of equine chorionic gonadotropin at the end of a progesterone-estrogen based protocol for fixed-time artificial insemination. Theriogenology. 118:150–156. doi:10.1016/j.theriogenology.2018.06.003.
- Baruselli PS, Reis EL, Marques MO, Nasser LF, Bó GA. 2004. The use of hormonal treatments to improve reproductive performance of anestrous beef cattle in tropical climates. Anim Reprod Sci. 82–83:479–486. doi:10.1016/j.anireprosci.2004.04.025.
- Bridges GA, Helser LA, Grum DE, Mussard ML, Gasser CL, Day ML. 2008. Decreasing the interval between GnRH and PGF2α from 7 to 5 days and lengthening proestrus increases timed-AI pregnancy rates beef cows. Theriogenology. 69:843–851. doi:10.1016/j.theriogenology.2007.12.011.
- Bridges GA, Mussard ML, Burke CR, Day ML. 2010. Influence of the length of proestrus on fertility and endocrine function in female cattle. Anim Reprod Sci. 117:208–215. doi:10.1016/j.anireprosci.2009.05.002.
- Bryan MA, Bó GA, Mapletoft RJ, Emslie FR. 2013. The use of equine chorionic gonadotropin in the treatment of anestrous dairy cows in gonadotropin-releasing hormone/progesterone protocols of 6 or 7 days. J Dairy Sci. 96:122–131. doi:10.3168/jds.2012-5452.
- Butler SAA, Atkinson PC, Boe-Hansen GB, Burns BM, Dawson K, Bó GA, et al. 2011. Pregnancy rates after fixed-time artificial insemination of brahman heifers treated to synchronize ovulation with low-dose intravaginal progesterone releasing devices, with or without eCG. Theriogenology. 76:1416–1423. doi:10.1016/j.theriogenology.2011.06.010.
- Cerri RLA, Rutigliano HM, Bruno RGS, Santos JEP. 2009. Progesterone concentration, follicular development and induction of cyclicity in dairy cows receiving intravaginal progesterone inserts. Anim Reprod Sci. 110:56–70. doi:10.1016/j.anireprosci.2007.12.005.
- Colazo MG, Kastelic JP, Whittaker PR, Gavaga QA, Wilde R, Mapletoft RJ. 2004. Fertility in beef cattle receiving a new or previously used CIDR device and estradiol, with or without progesterone. Anim Reprod Sci. 81:25–34. doi:10.1016/j.anireprosci.2003.09.003.
- Colazo MG, Ambrose DJ. 2011. Neither duration of progesterone insert nor initial GnRH treatment affect pregnancy per timed-insemination in dairy heifers subjected to a Co-synch protocol. Theriogenology. 76:578–588. doi:10.1016/j.theriogenology.2011.03.013.
- Colazo MG, Mapletoft RJ. 2014. A review of current timed-AI (TAI) programs for beef and dairy cattle. Can Vet J. 55:772–780. PMID: 25082993; PMCID: PMC4095965.
- Colazo MG, Mapletoft RJ. 2017. Pregnancy per AI in Holstein heifers inseminated with sex selected or conventional semen after estrus detection or timed-AI. Can Vet J. 58:365–370. PMID: 28373728; PMCID: PMC5347326.
- Colazo MG, Whittaker P, Macmillan K, Bignell D, Boender G, de Carvalho Guimaraes R, et al. 2018. Evaluation of a modified GnRH-based timed-AI protocol associated with estrus detection in beef heifers inseminated with sex-selected of conventional semen. Theriogenology. 118:90–95. doi:10.1016/j.theriogenology.2018.05.037.
- Cruppe LH, Day ML, Abreu FM, Kruse S, Lake SL, Biehl MV, et al. 2014. The requirement of GnRH at the beginning of the five-day CO-synch + controlled internal drug release protocol in beef heifers. J Anim Sci. 92:4198–4203. 14-7772.
- Dadarwal D, Mapletoft RJ, Adams GP, Pfeifer LFM, Creelman C, Singh J. 2013. Effect of progesterone concentration and duration of proestrus on fertility in beef cattle after fixed-time artificial insemination. Theriogenology. 79:859–866. doi:10.1016/j.theriogenology.2013.01.003.
- Denicol AC, Lopes Jr G, Mendonca LG, Rivera FA, Guagnini F, Perez RV, et al. 2012. Low progesterone concentration during the development of the first follicular wave reduces pregnancy per insemination of lactating dairy cows. J Dairy Sci. 95:1794–1806. doi:10.3168/jds.2011-4650.
- Inskeep EK. 2004. Preovulatory, postovulatory, and postmaternal recognition effects of concentrations of progesterone on embryonic survival in the cow. J Anim Sci. 82:E24–E39. doi:10.2527/2004.8213_supplE24x.
- Kastelic J, Knopf L, Ginther O. 1990. Effect of day of prostaglandin F2a treatment on selection and development of the ovulatory follicle in heifers. Anim Reprod Sci. 23:169–180. doi:10.1016/0378-4320(90)90001-V.
- Kinder JE, Kojima FN, Bergfeld EGM, Wehrman ME, Fike KE. 1996. Progestin and estrogen regulation of pulsatile LH release and development of persistent ovarian follicles in cattle. J Anim Sci. 74:1424–1440. doi:10.2527/1996.7461424x.
- López-Helguera I, López-Gatius F, Garcia-Ispierto I, Serrano-Perez B, Colazo MG. 2017. Effect of PRID-delta devices associated with shortened estrus synchronization protocols on estrous response and fertility in dairy cows. Annals Anim Sci. 17:757–770. doi:10.1515/aoas-2016-0083.
- Macmillan K, Gobikrushanth M, Sanz A, Bignell D, Boender G, Macrae L, et al. 2020. Comparison of the effects of two shortened timed-AI protocols on pregnancy per AI in beef cattle. Theriogenology. 142:85–91. doi:10.1016/j.theriogenology.2019.09.038.
- Menchaca A, Vilarino M, Ibarra D. 2007. GnRH and eCG associated with a progesterone treatment increased pregnancy rate after FTAI in prepubertal heifers. Proceedings of the Seventh International Symposium on Reproduction in Domestic Ruminants, Wellington, New Zealand, August 2006. Nottingham, UK: Nottingham University Press, p. 514.
- Menchaca A, Cuadro F, Núñez R, Bó GA. 2015. Pregnancy rates in beef heifers synchronized with shortened estradiol-based treatment that provides for a prolonged proestrus. Reprod Fertil Dev. 27:96 (Abstract).
- Mihm M, Curran N, Hyttel P, Knight PG, Boland MP, Roche JF. 1999. Effect of dominant follicle persistence on follicular fluid oestradiol and inhibin and on oocyte maturation in heifers. J Reprod Fert. 116:293–304. doi:10.1530/jrf.0.1160293.
- Núñez-Olivera R, Cuadro F, Menchaca A. 2019. Is prostaglandin F2α administration at the beginning of a progesterone and estradiol-based treatment for FTAI an effective strategy in Bos Taurus heifers? Anim Reprod Sci. 210:106201. doi:10.1016/j.anireprosci.2019.106201.
- Núñez-Olivera R, Cuadro F, Bosolasco D, de Brun V, de la Mata J, Brochado C, et al. 2020. Effect of equine chorionic gonadotropin (eCG) administration and proestrus length on ovarian response, uterine functionality and pregnancy rate in beef heifers inseminated at a fixed-time. Theriogenology. 151:16–27. doi:10.1016/j.theriogenology.2020.03.031.
- Perry GA, Perry BL. 2008. Effect of preovulatory concentrations of estradiol and initiation of standing estrus on uterine pH in beef cows. Domest Anim Endocrinol. 34:333–338. doi:10.1016/j.domaniend.2007.09.003.
- Pfeifer LFM, Mapletoft RJ, Kastelic JP, Small JA, Adams GP, Dionello NJ, et al. 2009. Effects of low versus physiologic plasma progesterone concentrations on ovarian follicular development and fertility in beef cattle. Theriogenology. 72:1237–1250. doi:10.1016/j.theriogenology.2009.07.019.
- Rahe CH, Owens RE, Fleeger JL, Newton HJ, Harms PG. 1980. Pattern of plasma luteinizing hormone in the cyclic cow: dependence upon the period of the cycle. Endocrinology. 107:498–503. doi:10.1210/endo-107-2-498.
- Rathbone MJ, Bunt CR, Ogle CR, Burggraaf S, Macmillan KL, Burke CR, et al. 2002. Reengineering of a commercially available bovine intravaginal insert (CIDR insert) containing progesterone. J Control Release. 85:105–115. doi:10.1016/s0168-3659(02)00288-2.
- Revah I, Butler WR. 1996. Prolonged dominance of follicles and reduced viability of bovine oocytes. J Reprod Fert. 106:39–47. doi:10.1530/jrf.0.1060039.
- Rivera H, Sterry RA, Fricke PM. 2006. Presynchronization with gonadotropin-releasing hormone does not improve fertility in Holstein heifers. J Dairy Sci. 89:3810–3816. doi:10.3168/jds.S0022-0302(06)72422-5.
- Roberson MS, Wolfe MW, Stumpf TT, Kittok RJ, Kinder JE. 1989. Luteinizing-hormone secretion and corpus-luteum function in cows receiving 2 levels of progesterone. Biol Reprod. 41:997–1003. doi:10.1095/biolreprod41.6.997.
- Sá Filho MF, Crespilho AM, Santos JE, Perry GA, Baruselli PS. 2010. Ovarian follicle diameter at timed insemination and estrous response influence likelihood of ovulation and pregnancy after estrous synchronization with progesterone or progestin-based protocols in suckled Bos indicus cows. Anim Reprod Sci. 120:23–30. doi:10.1016/j.anireprosci.2010.03.007.
- Sala RV, Melo LF, Motta JCL, Leffers-Neto L, Carrenho-Sala LC, Fosado M, et al. 2020. Optimization of a 5-day fixed-time embryo transfer (FTET) protocol in heifers I. manipulation of circulating progesterone through reutilization of intravaginal progesterone devices during FTET. Theriogenology. 156:171–180. doi:10.1016/j.theriogenology.2020.06.002.
- Small JA, Colazo MG, Kastelic JP, Mapletoft RJ. 2009. Effects of progesterone presynchronization and eCG on pregnancy rates to GnRH-based, timed-AI in beef cattle. Theriogenology. 71:698–706. doi:10.1016/j.theriogenology.2008.09.045.
- Small JA, Colazo MG, Kastelic JP, Erickson NE, Mapletoft RJ. 2010. Effects of presynchronization and eCG on pregnancy rates to GnRH-based, fixed-time artificial insemination in beef heifers. Can J Anim Sci. 90:23–34. doi:10.4141/CJAS09058.
- Zuluaga JF, Williams GL. 2008. High-pressure steam sterilization of previously used CIDR inserts enhances the magnitude of the acute increase in circulating progesterone after insertion in cows. Anim Reprod Sci. 107:30–35. doi:10.1016/j.anireprosci.2007.06.006.
