ABSTRACT
This study was conducted to investigate the effect of different estrus synchronization protocols followed by artificial insemination on estrus response and the conception rate of Abergelle goats. Three estrus synchronization protocols: (i) the standard protocol associating progestogens, gonadotropins and prostaglandins (P4 + eCG + PGF2α), (ii) single injection of prostaglandin (PGFS), (iii) double injection of prostaglandin (PGFD) were evaluated and compared to a control group that did not receive a hormonal treatment. Estrus behaviour was monitored in all does and those in estrus were inseminated. The estrous response was significantly higher in P4 + eCG + PGF2α does than in counterparts in PGFS and PGFD groups (p < 0.001). Results also revealed an earlier onset of estrus in the PGFD-treated group, a longer duration of estrus for second- and third- parity -does and an earlier onset of estrus in animals having a 3.5 body condition score. Even if the conception rate (CR) was highest when using the PGFD protocol, the lowest kidding rate (KR) was obtained when using this same treatment. The P4 + eCG + PGF protocol yielded the highest litter size. Farmers’ perception surveys revealed that a higher proportion of goat keepers used a controlled mating system and had culling practices and farmers selected PGFD as a preferred protocol.
Introduction
Reproductive technologies are applied to accelerate genetic progress and enhance the reproductive performance of various livestock species (Gizaw et al. Citation2016). In goats, synchronization of estrus is usually performed using the standard protocol based on the use of progestogen impregnated intravaginal devices for 11 days followed by an intramuscular injection of eCG combined with prostaglandins, or their analogues, 2 days before the withdrawal of progestogen releasing device (Lopez-Sebastian et al. Citation2007; Holtz et al. Citation2008). Such a treatment could be used in cycling does, as well as in seasonally anestrus does, to induce or synchronize estrus (Amoah and Gelaye Citation1990; Wildeus Citation2000). Although the use of the standard protocol induces generally good fertility in and out of the breeding season (up to 60–75% kidding rate when combined with fixed time insemination; Corteel et al. Citation1988; Freitas et al. Citation1997; Leboeuf et al. Citation2003), the use of alternative synchronization strategies has been investigated (Holtz et al. Citation2008). In fact, studies have shown that prostaglandins alone and their analogues (as with cattle and sheep) can be used to synchronize cycling females (Whitley and Jackson Citation2004). Such protocols are easy to use and cheaper than the conventional progestogen-eCG-based treatments which are expensive and non-available in many countries (Flores-Najera et al. Citation2010). In addition, the intravaginal hormone-releasing device causes vaginal problems, such as vaginitis, the development of anti-eCG antibodies in repeatedly treated animals, which reduces fertility (Baril et al. Citation1996; Roy et al. Citation1999) and hampers sperm transport and viability (Hawk and Conley Citation1975). Coupled with the negative implications of progestogen residues in milk, the development of alternative methods of synchronizing estrus is of great importance (Lopez-Sebastian et al. Citation2007).
Under inter-tropical and equatorial African conditions, where changes in photoperiod and latitude are limited, local sheep and goat breeds have a non-pronounced seasonality and can reproduce throughout the year or may just experience a transient reduction in fertility (Derquaoui and Khaledi Citation1994; Chemineau et al. Citation1995). Therefore, the use of alternative estrus synchronizing options is possible.
The Abergelle goat is one of the Ethiopian goat breeds, under the rift valley family, having an estimated population of over 300,000 heads, reared mainly along the Tekeze River in Southern Tigray (Tembien and Inderta), Waghimra, Raya Azebo, North Gondar (Simien) and kept by the Agew and Tigray ethnic groups (FARM AFRICA Citation1996). Along with other livestock species, Abergelle goats may contribute up to 75% to the income of the smallholder farmers in the mid and lowland areas of Waghemira (Wondim et al. Citation2019). Under the traditional management conditions, the breed features a low body weight, long kidding intervals and low prolificacy. The breed is also known for the good quality of its meat and calm temperament that is very much appreciated by farmers (Alemu Citation2015).
The current study aimed to investigate in Abergelle goats the efficiency of three estrus synchronization protocols using the standard progestogen impregnated intravaginal devices coupled with an intramuscular injection of eCG and prostaglandin compared to two prostaglandin-based protocols (single and double injection) and a control group. Trials were set up in both ‘on-station’ and ‘on-farm’ conditions of Waghemira and measured traits were related to estrous response and to reproductive performance after artificial insemination (AI) performed on small cohorts of animals 24–30 h after estrus is detected. Keeper’s perception of estrus synchronization and AI technologies at the Community-based breeding programmes (CBBP) villages were also compiled and analyzed.
Material and methods
Study area
The experiment took place between mid-June to mid-July, which corresponds to the optimal period of the breeding season of Abergelle goats. Two management conditions were involved. The ‘on-station’ experiment was conducted at Aybra research station of Sekota Dryland Agricultural Research Center in the Eastern Amhara region, Ethiopia. The ‘on-farm’ experiment was conducted in Workadivno and Bilaqu villages, where community-based Abergelle goat genetic improvement programmes were implemented for the last five years ().
Table 1. Description of the study sites.
Experimental animals and management
In this trial, 335 does (i) 92 for the ‘on-station’ experiment and (ii) 243 for the ‘on-farm’ experiment were used. All the selected does were non-pregnant, aged between 2 and 6 years and had a parity of at least one. They were in a normal health condition, non-suckling and had a body condition score (BCS) from 2.5 to 3.5, scored by palpation of the lumbar region according to Koyuncu and Altıncekic (Citation2013) (scale 1 = emaciated and 5 = very fat). In the ‘on-station’ conditions, does were allowed to graze and browse daily for 8 h abundant acacia and other browse species around the research site. One month before the beginning of the experiment, each doe was supplemented with a mixture of 300 g of cowpea hay and wheat bran per day. In the ‘on-farm’ conditions, does were isolated from farmers’ flocks and kept by separate attendants according to the treatment group. They were allowed to graze/browse in the communal pasture area and received the same supplementation as the ‘on-station’ females. Animals were returned to farmers after 10 days of insemination date.
Bucks used for semen collection were selected based on their estimated breeding value (Ebv) ranking. They were aged between 3 and 6 years, with normal health conditions, and fit for different morphological criteria. Five and 18 Abergele bucks were used respectively for the ‘on-station’ and the ‘on-farm’ trials. For both management conditions, bucks were kept around the research station to graze/browse and exercise for about 4 h daily separately from does. Animals were daily flushed for about one and a half months before the beginning of the experiment with a mixture of 300 g cowpea hay (Gross energy content of 3910 kcal/kg and crude protein content of 28%) and wheat bran (Gross energy content of 4520 kcal/kg and crude protein content of about 15.3%). Before the experiment, animals under both management conditions were quarantined and vaccinated against anthrax, which was common in the study area. The manuscript does not contain clinical studies or patient data and the approval of the ethics committee was provided.
Experimental procedures
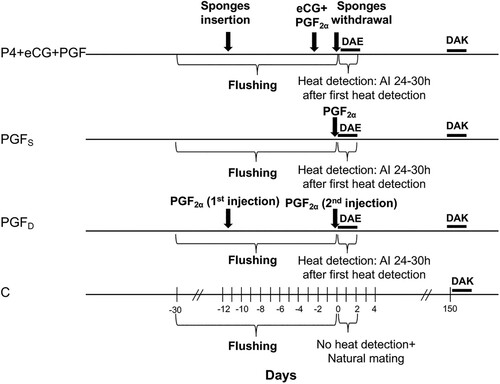
For both experimental conditions, does were assigned randomly to four groups with different synchronization protocols. The first group (P4 + eCG + PGF, n = 25 and n = 73 respectively for the ‘on-station’ and the ‘on-farm’ locations) received the standard protocol using intravaginal polyurethane sponges impregnated with 30 mg of fluorogestone (Syncro-part®; CEVA laboratories, Libourne, France) that were placed in the vagina for 11 days. All does were checked (morning and evening) to ensure that sponges remained in place during the treatment period. Forty-eight hours before sponge removal, each doe received an i.m. injection of 1 ml of PGF2α analogue dinoprost (1 ml Enzaprost®; CEVA Laboratories, Libourne, France) followed by 300 I.U. of equine chorionic gonadotropin (eCG) (Syncro-part PMSG®; CEVA laboratories, Libourne, France) as separate injections. Does in the second group (PGFS, n = 25 and n = 58, respectively for the ‘on-station’ and the ‘on-farm’ locations) received a singular i.m. of 5 mg of the PGF2α analogue dinoprost (1 ml Enzaprost®; CEVA Laboratories, Libourne, France). Does in the third group (PGFD, n = 25 and n = 72, respectively for the ‘on-station’ and the ‘on-farm’ management) received 2 i.m. of 5 mg of the PGF2α analogue dinoprost (1 ml Enzaprost®; CEVA laboratories, Libourne, France) administered 11 days apart. Does in the last group (C, n = 17 and n = 40 respectively for the ‘on-station’ and the ‘on-farm’ managements) were considered the control group, did not receive any synchronization and were naturally mated.
For does in all treatment groups, pregnancy was checked very carefully using an ultrasound scanner immediately before hormone administration.
Heat detection and artificial insemination
For all treatment groups, following sponge withdrawal or PGF2α injection, and for the consecutive 48 h, 6 aproned bucks were used to detect females in estrus and to assess the onset and duration of estrus. Every 4 h, goats were individually teased with an aproned male for up to 5 min at a time. Estrous behaviour, as evidenced by the immobility reflex (the most reliable indicator of estrus), tail flagging (the most conspicuous characteristic of estrus goats), mucus discharge and restlessness were recorded. For the control group estrus was not checked and only data after conception and kidding following natural mating were recorded. Time for the onset of estrus, (when the does started to show estrus) and duration of estrus, (period when the does remain in estrus) after treatment administration were also calculated as reported in paragraph 2.6 (Measured reproductive traits) and recorded. Semen from five proven Abergelle bucks from the research station and 18 bucks from the CBBP villages (trained to serve with artificial vagina, selected on the basis of their estimated breeding value and checked for normal breeding soundness) were collected using an artificial vagina and estrous-induced does. Ten successful semen ejaculates were collected at the station and similarly, 33 collections were obtained from the CBBP bucks.
Ejaculates that were retained for artificial insemination (AI) had a mean concentration of 3.17 109 ± 0.23 and 3.14 109 ± 0.27 sperm ml−1, respectively for the ‘on-station’ and the ‘on-farm’ managements and determined by spectrophotometry (Accucell®, Instruments de Médecine Vétérinaire, IMV Paris, France). The mean of individual motility was 3.46 ± 0.85 and 3.91 ± 0.57 on a scale of 5, respectively for the ‘on-station’ and the ‘on-farm’ management conditions using a contrast-phase microscope. Immediately after collection, semen was diluted with a commercial sheep and goat-specific diluent (OVIXcell®, Instruments de Médecine Vétérinaire, IMV Paris, France), and does were inseminated cervically with 0.25 ml of diluted semen containing an average 150 × 106 sperm. Cervical AI was performed by two well-trained technicians using a speculum equipped with a light source and an insemination gun (Instruments de Médecine Vétérinaire, IMV Paris, France) as described by Evans and Maxwell (Citation1987). Does in all treatment groups were inseminated at 24–30 h after first heat expression and grouped in small cohorts. The control groups were allowed to be mated naturally. The schedule of implementation of the 4 protocols is presented in .
Farmers’ perception surveys
In each of the two CBBP villages, 46 randomly selected goat keepers owning goats that were included in this study, were approached and interviewed. Twenty-nine had their flocks assigned to one of the synchronization protocols and 17 had their flocks in the control group.
Individual semi-structured questionnaires on the issues of the overall outlooks and feedback for reproductive technologies, goat rearing experience, desired goat reproductive characteristics, mating season, reproductive management practices of goats, buck usage and sharing modalities, estrous synchronization and AI technologies were used. The questionnaire was pretested to ensure that questions are socially appropriate, and responses are within the expected bounds.
Measured reproductive traits
The time of onset of estrus was calculated as the time halfway between the last rejection and the first toleration of a mounting male, while the end of estrus was set halfway between the last toleration and the first rejection (Holtz et al. Citation2008). Estrus duration was defined as the time elapsed between the first and last accepted mount within the same estrus period. Estrus response was calculated as [ER = 100 × (number of does expressing estrus/total number of does)]. Conception rate (CR) was calculated according to the formula [CR = 100 × (number of does that conceived/number of inseminated does)]. When lambing occurred, the number of lambs born was recorded and litter size was calculated as [LS = 100 × (number of does having twins or triplets/number of does giving birth)]. The kidding rate was calculated as [KR = number of kids born alive/number of does that conceived].
Statistical analyses
The time for the onset of estrus, duration of estrus, conception rate, kidding rate, litter size, birth weight of kids and farmer’s perception data were collected. The collected data were coded and entered into Microsoft excel 2007 software program for further analysis. Gen mode procedure of Statistical Analysis System SAS (9.0) and GLM procedure of the statistical analysis system SAS (9.0) were used for the analysis. Means were separated with Tukey’s HSD test and significant differences were tested at α = 0.05. The index technique was used to rank the different parameters of goat breeding and management practices of farmers using SPSS (version 20) and the Likert scale were used to compare farmers’ perception of reproductive technologies.
Results
Estrus response, onset and duration
Does of the ‘on-station’ trial show estrus earlier than the ‘on-farm’ animals (). Estrous response for P4 + eCG + PGF2α does was significantly higher than in PGFS and PGFD (p < 0.001) groups. However, the onset of estrus was earlier in the PGFD does followed by the P4 + eCG + PGF2α and finally the PGFS goats (). In general, the estrous response was not affected by parity or BCS except for does in their second and third parity which had a longer duration of estrus and there was a much earlier onset of estrus for female goats having a body condition score (BCS) of 3.5 ().
Table 2. The effect of the hormone, management condition, parity and BCS on estrus response (%), onset of estrus (LSM ± SE) and estrus duration (LSM ± SE) of Abergelle does.
Conception rate, kidding rate, litter size and birth weight of kids
While CR was significantly higher when using the PGFD protocol than the 2 other protocols and the control group (p < 0.05), the same protocol yielded the lowest kidding rate. Litter size was highest (p < 0.001) in does receiving the P4 + eCG + PGF protocol (). This study revealed also a higher litter birth weight when using PGFS and PGFD protocols than the P4 + eCG + PGF which is due to a higher litter size when using the latter protocol. Kids of does in the ‘on-farm’ treatment management condition had a higher birth weight than their counterparts in the ‘on-station’ conditions. Parity did not influence the different studied parameters ().
Table 3. The effect of the hormone, management condition and parity on conception rate (%), kidding rate (%), litter size (LSM ± SE) and birth weight (LSM ± SE) of Abergelle does.
Goat breeding practices and farmer’s perceptions of reproductive technologies
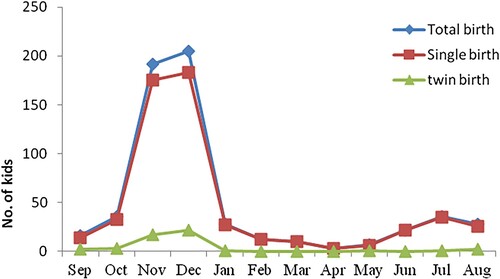
A very high proportion of the interviewed goat keepers in this study used a controlled mating system and 84.7% of them had culling practices (). The higher intensity of culling was obtained on male kids and yearling bucks than weaned females and does (). From the current result, 78.5% of the does gave birth from October to January, as indicated in . Amongst the tested estrus synchronization protocols and AI technologies, farmers selected PGFD, as indicated in .
Table 4. Different goat breeding and management practices used at the study areas.
Table 5. The Likert score for the perception of goat keepers for different reproductive technologies.
Discussion
In this study, does of the ‘on-station’ experiment showed estrus earlier than the ‘on-farm’ animals. Differences in nutrition and management conditions could account for the differences in response between the two types of management. In fact, nutrition can modulate breeding activity (Zarazaga et al. Citation2011a, Citation2011b, Citation2011c). This may also be partly explained by the better conditions to watch the animals during estrous control.
When comparing the three treatment groups, the estrus response was higher with the use of the standard method associating exogenous progestogens, eCG and PGF2α than the 2 prostaglandin-based protocols (PGFS and PGFD). Such a result is not surprising since the universality of this protocol in sheep and goats AI programs is well established and the added effect of progesterone priming in exhibiting estrous response is well-documented in sheep and goats. Our finding is consistent with the study of Hashemi and Safdarian (Citation2017) in which the lower estrus response in PGF-based groups than of progesterone-based groups in Persian downy does in South Iran was reported. Beyond the universal efficiency of the standard protocol, the onset of estrus was earlier using the PGFD treatment than P4 + eCG + PGF2α and PGFS protocols even if the use of eCG is known to reduce the time to onset of estrus (Omontese et al. Citation2012). This could be explained by the fact that 2 injections of prostaglandins were more efficient on the corpus luteum than a single injection or that the does were not cycling and a single injection of prostaglandins did not result in luteolysis of the corpus luteum and onset of estrus. The onset of estrus is important in predicting the pre-ovulatory events and in scheduling the appropriate timing of fixed-time artificial insemination (Hashemi and Safdarian Citation2017). Our result is different from that of Ishwar and Pandey (Citation1990) who reported in Black Bengal goats that the time interval between progesterone or prostaglandin administration and the onset of estrus was nearly the same. Differences between the studies could be related to the nature and way of administration of the different hormones. For the 3 synchronization protocols used in the current work, the time of onset of estrus was not within the range reported by other researchers and was less than that reported in other studies (Pendleton et al. Citation1992; Romano Citation1993, Citation1994, Citation1998; Romano and Fernandez Abella Citation1997; Bitaraf et al. Citation2007; Holtz et al. Citation2008). This could be related to breed specificity and to differences in the type of hormones used when synchronizing animals.
Although the PGFD protocol was effective in synchronizing estrus, giving higher CR than the 2 other protocols and the control group, P4 + eCG + PGF2α and PGFS protocols allowed a higher KR. In addition, the highest LS was obtained when using the standard protocol compared to the 2 other treatments and the control group resulting in a lower litter BW. This is predictable since the administration of eCG has a boosting effect on fertility and prolificacy. Our finding was different from the work of Alli (Citation2000) who found that different hormonal treatments using a controlled internal drugs release device alone or combined with eCG injection and double injection of prostaglandin analogue, did not show any significant difference in litter size.
There are conflicting reports on fertility following natural mating or AI when estrus was synchronized in does. Authors reported normal, reduced or improved fertility when prostaglandins are used. For instance, Amarantidis et al. (Citation2004) found that the long-term FGA-treatment, the combination of short-term FGA-treatment and PGF2α injection (with or without injection of eCG), and the double injection of PGF2α, 11 days apart had no significant difference on fertility, prolificacy and the birth type (single, twins, triplets) in 120 cycling and non-lactating indigenous Greece does (Capra prisca). Similarly, reproductive performance was the same in Sudanese Nubian does when synchronized by intravaginal sponges or cloprostenol (Ahmed et al. Citation1998) and in Nadooshani does synchronized with CIDR, fluogestone acetate sponges and cloprostenol during the breeding season (Bitaraf et al. Citation2007). In our study, the kidding rate was 91.8, 90.9, 85.3 and 100%, respectively for conventional, prostaglandins (single and double) and the control group. Our figures were much higher than the KR obtained in other studies concerning cycling does (Evans and Maxwell Citation1987; Bitaraf et al. Citation2007) and this could be explained by the fact that the experiment was carried out from mid-June to mid-July, which corresponds to the optimal period of the breeding season of Abergelle goats.
Our study revealed that kids of does in the ‘on-farm’ treatment management condition had a higher birth weight than their counterparts in the ‘on-station’ conditions this could be related to the management conditions (feed, health, initial body weight at mating and BCS).
In the current study most of the does gave birth between October and January which indicates that the Abergelle goat is almost a seasonal breeder. In fact, under tropical conditions and in the absence of a seasonality of reproduction that is driven by changes in photoperiod, reproductive activity decreases at certain periods of the year, especially in dry and very harsh environments. This reduced reproductive activity could be simply due to very long cycles in some animals or to an arrest of cyclicity as induced by poor nutrition (Gallego-Calvo et al. Citation2014). The bucks being also under nutritional stress may not fulfil their role in stimulating sociosexual interactions that contribute to the resumption of reproductive activity in anoestrus goats (Delgadillo et al. Citation2015). In Ethiopia, where the nutritional constraints are exacerbated by the seasonal availability of forages and the recurrent and prolonged drought in the arid and semiarid lowlands of the country (Yami Citation2008), several studies reported conception peaks of does corresponding to feed flushes and availability of crop residues (Mukasa-Mugerwa et al. Citation2002). This information concerning the peak of kidding and the corresponding mating season is crucial to optimize breeding management and enhance reproductive performance in the community-based genetic improvement program (CBBP). The other result emerging from this perception survey is the high proportion of farmers using controlled mating and culling practices which is due to their involvement in the CBBP. In fact, they use only bucks selected in accordance with the designed buck usage modality implemented in the CBBP. Finally, goat keepers in this study preferred the prostaglandin-based protocols for estrus synchronization. This result could be associated with the simplicity, the easiness and the lower cost of the prostaglandin-based methods than protocols using progestogen and eCG. The conception rates with prostaglandin-based protocols may have motivated the responses of the farmers. Furthermore, the farmers’ ranking for double injection of prostaglandin fits and supports the results of the obtained biological data.
Conclusion
In the present study, the reproductive performance of the Abergelle goats was slightly affected by estrous synchronization methods by an improvement in the CR using the PGFD and in LS and BW using the P4 + eCG + PGF2α treatment. Taking into consideration fertility rate, costs and the simplicity of methods, double injection of prostaglandins could be a more efficient method for estrus synchronization in fixed-time artificial insemination programs during the breeding season.
Acknowledgements
The authors thank farmers who kindly accepted to allow their flocks to be included in this study. We wish to extend our thanks to the Amhara Agriculture Research Institute for the study leave of the first author.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ahmed MMM, Makawi SE, Jubara AS. 1998. Synchronization of estrus in Nubian goats. Small Rumin Res. 30:113–120.
- Alemu A. 2015. On-farm phenotypic characterization and performance evaluation of Abergelle and central highland goat breeds as an input for designing community-based breeding program [MSc Thesis. Haramaya University. Thesis Submitted to the School of Animal and Range Sciences, School of Graduate Studies]. Ethiopia. 128 p.
- Alli M. 2000. Reproductive performance of desert goats under semi-intensive system in the Sudan [A thesis Submitted in partial Fulfillment of the Requirements of University of Khartoum for M.Sc Degree]. University of Nyala. Sudan. 102 p.
- Amarantidis I, Karagiannidis A, Saratsis PH, Brikas P. 2004. Efficiency of methods used for estrous synchronization in indigenous Greek goats. Small Rumin Res. 52:247–252.
- Amoah EA, Gelaye S. 1990. Superovulation, synchronization and breeding of does. Small Rumin Res. 3:63–72.
- Baril G, Remy B, Leboeuf B, Beckers JF, Saumande J. 1996. Synchronization of estrus in goats: the relationship between eCG binding in plasma, time of occurrence of estrus and fertility following artificial insemination. Theriogenology. 45:1553–1559.
- Bitaraf A, Zamiri MJ, Kafi M, Izadifard J. 2007. Efficacy of CIDR, fluogestone acetate sponges and cloprostenol for estrous synchronization of Nadooshani goats during the breeding season. Iran J Vet Res. 8:218–224.
- Chemineau P, Malpaux B, Thiery JC, Vigne C, Morello H, Zarazaga L, Pelletier J. 1995. The control of seasonality: A challenge to small ruminant breeding. In: Enne G., Greppi F., Lauria A., editor. Proc. International Symposium of “Società Italiana per il progresso della zootecnica”, milan, Italy, 11–13 Sept. 1995. Paris: Elsevier-Biofutur; p. 225–250.
- Corteel JN, Leboeuf B, Baril G. 1988. Artificial breeding of adult goats and kids induced with hormones to ovulate outside the breeding season. Small Rumin Res. 1:19–35.
- Delgadillo JA, Flores JA, Hernández H, Poindron P, Keller M, Fitz-Rodríguez G, Duarte G, Vielma J, Fernández IG, Chemineau P. 2015. Sexually active males prevent the display of seasonal anestrus in female goats. Horm Behav. 69:8–15.
- Derquaoui L, Khaledi OEL. 1994. Evaluation de l’activité sexuelle pendant la saison de baisse de fertilité chez la chèvre de race D’Man. 2e conférence african Small Ruminant Research Network, arusha, Tanzania, 7–11 déc. 1992; Addis-Abeba, Ethiopie, Cipea, p. 49–51.
- Evans G, Maxwell WMC. 1987. Salamon’s artificial insemination of sheep and goats. Sydney: Butterworths. p. 55–170.
- FARM-Africa. 1996. Goat Types of Ethiopia and Eritrea. Physical description and management systems. Published jointly by FARM-Africa, London, UK and International Livestock Research Institute, Nairobi, Kenya. 76 pp.
- Flores-Najera MJ, Meza-Herrera CA, Echavarrıa FG, Villagomez E, Iniguez L, Salinas H, Gonzalez-Bulnes A. 2010. Influence of nutritional and socio-sexual cues upon reproductive efficiency of goats exposed to the male effect under extensive conditions. Anim Prod Sci. 50:897–901.
- Freitas VJF, Baril G, Saumande J. 1997. Estrus synchronization in dairy goats: use of fluorogestone acetate vaginal sponges or norgestomet ear implants. Anim Reprod Sci. 46:237–244.
- Gallego-Calvo L, Gatica MC, Guzmán JL, Zarazaga LA. 2014. Role of body condition score and body weight in the control of seasonal reproduction in blanca andaluza goats. Anim Reprod Sci. 151:157–163.
- Gizaw S, Tesfaye Y, Mekuriaw Z, Tadesse M, Hoekstra D, Gebremedhin B, Tegegne A. 2016. Oestrus synchronization for accelerated delivery of improved dairy genetics in Ethiopia: results from action research and development interventions. LIVES Working Paper 12. Nairobi: International Livestock Research Institute (ILRI).
- Hashemi M, Safdarian M. 2017. Efficiency of different methods of estrus synchronization followed by fixed time artificial insemination in Persian downy does. Anim Reprod. 14:413–417.
- Hawk HW, Conley HH. 1975. Investigations on spermicidal activity in the sheep vagina. Theriogenology. 3:65–75.
- Holtz W, Sohnrey B, Gerland M, Driancourt MA. 2008. Ovsynch synchronization and fixed-time insemination in goats. Theriogenology. 69:785–792.
- Ishwar AK, Pandey JN. 1990. Estrus synchronization and fertility behavior in Black bengal goats following either progesterone or prostaglandin treatment. Theriogenology. 34:1015–1024.
- Koyuncu M, Altıncekic S. 2013. Importance of body condition score in dairy goats. Maced J Anim Sci. 3:167–173.
- Leboeuf B, Forgerit Y, Bernelas D, Pougnard E, Senty E, Driancourt MA. 2003. Efficacy of two types of vaginal sponges to control onset of oestrus, time of preovulatory LH peak and kidding rate in goats inseminated with variable numbers of spermatozoa. Theriogenology. 60:1371–1378.
- Lopez-Sebastian A, Gonzalez-Bulnes A, Carrizosa JA, Urrutia B, Diaz-Delfa C, Santiago-Moreno J, Gomez-Brunet A. 2007. New estrus synchronization and artificial insemination protocol for goats based on male exposure, progesterone and cloprostenol during the non-breeding season. Theriogenology. 68:1081–1087.
- Mukasa-Mugerwa E, Anindo D, Sovani S, Lahlou-Kassi A, Tebely S, Rege JEO, Baker RL. 2002. Reproductive performance and productivity of Menz and Horro sheep lambing in the wet and dry seasons in the highlands of Ethiopia. Small Rumin Res. 45:261–271.
- Omontese BO, Rekwot PI, Makun HJ, Ate IU, Rwuaan JS. 2012. Induction of estrus in Sahel goats using fluorogestone acetate (FGA) sponges and equine chorionic gonadotrophin (ECG). Sokoto J Vet Sci. 10:21–25.
- Pendleton RJ, Young CR, Rorie RW, Pool SH, Memon MA, Godke RA. 1992. Comparison of fluorogestone acetate sponges with norgestomet implants for induction of estrus and ovulation in anoestrous dairy goats. Small Rumin Res. 8:269–273.
- Romano JE. 1993. Effect of service on estrus duration in dairy goats. Theriogenology. 40:77–84.
- Romano JE. 1994. Effect of service number on estrus duration in dairy goats. Theriogenology. 41:1273–1277.
- Romano JE, Fernandez Abella D. 1997. Effect of service on duration of oestrus and ovulation in dairy goats. Anim Reprod Sci. 47:107–112.
- Romano JE. 1998. The effect of continuous presence of bucks on hastening the onset of estrus in synchronized does during the breeding season. Small Rumin Res. 30:99–103.
- Roy F, Maurel MC, Combes B, Vaiman D, Cribiu EP, Lantier I, Pobel T, Delétang F, Combarnous Y, Guillou F. 1999. The negative effect of repeated equine chorionic gonadotrophin treatment on subsequent fertility in alpine goats is due to a humoral immune response involving the major histocompatibility complex. Biol Reprod. 60:805–813.
- Whitley NC, Jackson DJ. 2004. An update on estrus synchronization in goats: a minor species. J Anim Sci. 82:E270–E276.
- Wildeus S. 2000. Current concepts in synchronization of estrus: sheep and goats. Proc Am Soc Anim. 77:1–14. doi:10.2527/jas2000.00218812007700ES0040x.
- Wondim B, Mulatu G, Baye B. 2019. Feed resource availability, livestock migration pattern and synthesis of feeding calendar at Wag-Lasta, Ethiopia Daagu International. J Basic Appl Res. 1:26–40.
- Yami A. 2008. Nutrition and feeding of sheep and goats. In: A. Yami, R.C. Merkel, editor. Sheep and goat production handbook for Ethiopia. Addis Ababa: Ethiopia Sheep and Goat Productivity Improvement Program (ESGPIP); p. 103–159.
- Zarazaga LA, Celi I, Guzmán JL, Malpaux B. 2011a. The effect of nutrition on the neural mechanisms potentially involved in melatonin-stimulated LH secretion in female Mediterranean goats. J Endocrinol. 211:263–272.
- Zarazaga LA, Celi I, Guzmán JL, Malpaux B. 2011b. The response of luteinizing hormone secretion to photoperiod is modified by the level of nutrition in female Mediterranean goats. Anim Reprod Sci. 126:83–90.
- Zarazaga LA, Celi I, Guzmán JL, Malpaux B. 2011c. The role of nutrition in the regulation of luteinizing hormone secretion by the opioidergic, dopaminergic and serotoninergic system in female Mediterranean goat. Biol Reprod. 84:447–454.