ABSTRACT
Several European countries, but Greece, reported data of Enzootic Pneumonia (EP)-like lesions at slaughter. In the present study, frequency and severity of EP-like lesions were evaluated at slaughter and their association with different vaccination protocols for Mycoplasma hyopneumoniae, the primary pathogen of EP, was investigated, in Greek swine herds. In total, 7047 lungs from 53 farrow-to-finish herds were scored at slaughter by using the Ceva Lung ProgramTM tool, from January 2016 to December 2019. The frequency of EP-like lesions was 47.6% and the median (range) of the total lung lesions score was 0 (0–25). Pigs, which were vaccinated with a monovalent vaccine for M. hyopneumoniae either once or twice and those which received an extra shot, were less likely to have EP-like lesions, compared to pigs that received a bivalent vaccine containing both M. hyopneumoniae and PCV2 antigens. For the pigs which received two shots of a monovalent vaccine, the odds of higher versus lower lung lesion score(s) were lower compared to pigs that received a bivalent vaccine. Almost half of the lungs were affected, although 98.3% of the pigs were vaccinated. Poor housing and management, commonly seen in Greek herds, may hamper the efficient reduction of M. hyopneumoniae spread.
1. Introduction
Mycoplasma hyopneumoniae (M. hyopneumoniae), a worldwide distributed bacterium, is the primary etiologic pathogen of Enzootic Pneumonia (EP), a respiratory disease characterized by chronic non-productive cough, deterioration of feed conversion ratio and enzootic pneumonia-like lesions (EP-like lesions) (Sibila et al. Citation2007; Citation2009; Fablet et al. Citation2012; Brewster et al. Citation2017; Li et al. Citation2019; Fitzgerald et al. Citation2020; Rodrigues da Costa et al. Citation2020). EP-like lesions are usually found bilaterally in the apical, cardiac, and intermediate lobes and less often in the anterior parts of the diaphragmatic lobes. The affected tissue is pink or plum-coloured and well demarcated from the normal tissue (Rodríguez et al. Citation2016). The cut surface is moist, and the consistency of the affected tissue is moderately firm. These lesions are suggestive of M. hyopneumoniae infection but not pathognomonic since other pathogens like swine influenza virus or the synergy of P. multocida and Aujeszky’s disease virus infection may cause similar lesions (Fuentes and Pijoan Citation1987; Done Citation1991; Pieters and Maes Citation2019). EP-like lesions are linked with reduced growth performance, resulting in severe economic losses to pig farmers along with the requirement for antibiotic treatment to prevent them (Maes et al. Citation1996; Henninger et al. Citation2014; Brewster et al. Citation2017).
The examination of lung lesions of fattening pigs at slaughter is a useful tool to estimate the frequency and severity of the respiratory disease in a farm and to evaluate the effect of disease control interventions. Therefore, the recording of lung lesions at slaughter with standardized scoring systems for EP-like lesions (Madec and Kobisch Citation1982; Cvjetković et al. Citation2018) has become a common practice. The parameters that are usually measured in lung scoring at the abattoir include the frequency of EP-like lesions and the percentage of affected surface per lobe (Cvjetković et al. Citation2018). The prevalence of lungs with pneumonia lesions was found to range from 20% to 80% in different countries (Leneveu et al. Citation2005; Ostanello et al. Citation2007; Marois et al. Citation2008; Fraile et al. Citation2010; Meyns et al. Citation2011; Fablet et al. Citation2012; Merialdi et al. Citation2012). However, frequency and severity data of EP-like lesions in the Greek swine herds are missing from the literature.
Antibiotics such as macrolides and tetracyclines are commonly used to reduce the clinical signs of EP and prevent the related lung lesions. However, antibiotics’ use increases the risk of antimicrobial resistance (Maes Citation2010). Since 1994, commercial vaccines against M. hyopneumoniae are routinely used in pig farming worldwide to prevent clinical disease and mitigate the risk of antimicrobial resistance. It has been shown that vaccination can reduce clinical signs, lung lesions and performance losses associated with EP (Maes et al. Citation1998; Citation1999; Jensen et al. Citation2002; Wilson et al. Citation2012; Del Pozo Sacristán et al. Citation2014). Nowadays, several vaccination protocols against M. hyopneumoniae are available with the most commonly applied being, single shot vaccination with a monovalent vaccine, single shot vaccination with a bivalent vaccine containing both M. hyopneumoniae and porcine circovirus type 2 (PCV2) antigens and double shot vaccination with a monovalent vaccine, which, in contrast with most European countries, is still preferred in Greece. The results of several studies comparing the efficacy of different vaccination protocols against M. hyopneumoniae were not consistent. Furthermore, the majority of them focused on the comparison of specific commercial vaccines and not on the vaccination strategies employed (Baccaro et al. Citation2006; Kim et al. Citation2011; Hillen et al. Citation2014; Cvjetković et al. Citation2018; Yang et al. Citation2020).
The aims of this study were two-fold: first, to estimate the frequency and severity of EP-like lesions at the abattoir in finishers raised in commercial farrow-to-finish Greek herds, and second, to evaluate the potential associations between the frequency and severity of EP-like lesions and different vaccination protocols against M. hyopneumoniae.
2. Materials and methods
2.1. Study population and sampling
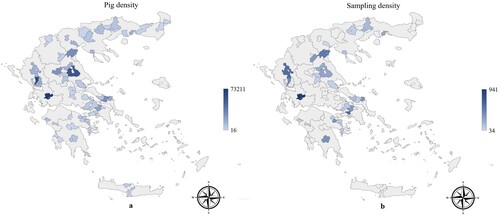
All Greek registered commercial farrow-to-finish herds with a capacity equal to or over 100 sows (Annual Porcine Census Citation2021) were invited to participate in the study. Fifty-three farmers responded positively and voluntarily participated. Therefore, the study population comprised 53 farrow-to-finish herds, with conventional health status. All the herds are located on the Greek mainland and the majority operate in regions with high pig density (). The lung inspection data were accumulated from January 2016 to December 2019. The selection of batches for slaughtering was performed by the herd’s manager or owner according to routinely applied commercial needs and criteria. All slaughter pigs in a batch were inspected but the size of the batch was decided by the herd manager, as well. In order to approximate real on-field conditions, we did not intervene in any way in the selection of the batches, thus no exclusion criteria were applied for any reason. Additionally, the potential application of any antibiotic treatment in the inspected batches of pigs, in any stage of their production, was not recorded. Ιf animals had received medication, they were slaughtered after the recommended withdrawal period.
Figure 1. Pig density (number of pigs/region) in Greece (Annual Porcine Census Citation2021) and sampling density (number of pigs sampled/region) are presented with blue colour in maps (a) and (b), respectively. The higher the density, the darker the blue colour of the regions.
The number of lungs per batch ranged from 15 to 180 with a median of 55 and an interquartile range (IQR) equal to 121. The number of batches examined per herd ranged from 1 to 9 (median = 2, IQR = 1). Per herd, the median number of inspected slaughter pigs ranged from 34 to 389 (median = 97, IQR = 97). Thirty-two herds offered more than one batch of slaughter pigs. For those herds, the number of batches examined ranged from 2 to 9 (median = 2, IQR = 1) and the number of pigs inspected per herd ranged from 52 to 389 (median = 125.5, IQR = 124). Seven out of 32 (21.9%) herds that contributed with more than one batch applied a different vaccination protocol from batch to batch. Specifically, 6/7 herds applied two different vaccination protocols since they participated with two batches each, whereas one herd offered five batches, of which three were vaccinated with the same protocol and the other two with a different one. Moreover, for 23/32 (71.9%) herds with more than one batch, lung scoring was performed in different slaughtering seasons.
The present study did not include any invasive procedures or treatments to pigs; therefore, animal ethics committee approval was not applicable.
2.2. Lung lesion scoring and data collection on vaccination against M. hyopneumoniae
The lungs of all slaughter pigs were examined at the abattoirs by a member of the research team. Training to perform lung scoring for EP-like lesions was performed by Ceva Santé Animale staff. The methodology applied was that presented by Cvjetković et al. (Citation2018). In brief, each lobe, namely the left and the right apical, the left and the right cardiac, the accessory and the left and the right diaphragmatic lobe, was scored individually on a severity scale from 0 to 4 according to the percentage of surface affected per lobe. Therefore, a lobe with 0% affected surface was scored with 0, a lobe with 1–25% affected surface was scored with 1 whereas a lobe was scored with 2 when its surface was affected by 26–50%. Scores 3 and 4 were assigned for lobes having 51–75% and 76–100% affected surface, respectively. The total lesion score per lung was obtained by adding the scores of lung’s lobes. Consequently, the total lesion score for each lung could range between 0 and 28, with values >0 being considered a bronchopneumonic lung. Before inspection and scoring, we were unaware of whether the slaughter pigs were vaccinated against M. hyopneumoniae and the vaccination protocol which was applied. All scores were transferred into the Ceva Lung ProgramTM database.
Subsequently, we obtained information on the vaccination against M. hyopneumoniae history of the slaughter pigs in each batch. The herd managers recorded whether the slaughter pigs were vaccinated against M. hyopneumoniae or not and recorded whether they applied a monovalent M. hyopneumoniae vaccine or a bivalent M. hyopneumoniae and porcine circovirus type 2 (PCV2) vaccine. Furthermore, when they recorded the use of a monovalent vaccine, they were asked whether the pigs were vaccinated with a single or a double-shot protocol, either using a double-shot vaccine or using a single-shot vaccine but with half dose per injection (which is a commonly applied off-label protocol by the Greek pig producers who are accustomed to boost M. hyopneumoniae vaccination with a second injection). Some managers recorded the use of an extra shot of a monovalent vaccine after vaccinating either with a bivalent or with a monovalent vaccine. All pigs which received an extra shot of a monovalent vaccine were considered as they were vaccinated with the same protocol because the percentage of pigs which received either a bivalent or a monovalent vaccine, separately, was very low. To represent all vaccination approaches that are routinely applied in the Greek swine herds the off-label protocols that were recorded were included in our study, as well. Lastly, for each batch of slaughter pigs, we recorded the season of the year at which they were slaughtered and the herd size, expressed as a number of sows.
2.3. Statistical analysis
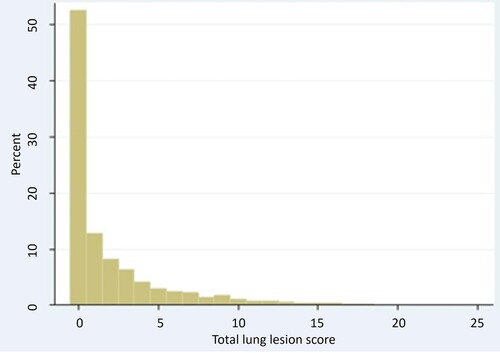
All statistical analyses were performed using Stata 13.1 (Stata Statistical Software. College Station, TX, 2013) and evaluated for significance at the 5% level. The total lung lesion score was obtained by adding the scores of the lobes and could range from 0 to 28. The distribution of the total lung lesion score was zero-inflated and skewed to the right (). Therefore, the medians of the total lung lesion score were calculated.
The possible association between the frequency of EP-like lesions and the vaccination protocols at the slaughter pig level was evaluated in a mixed-effect logistic regression model. The dependent variable was the dichotomized total lesion score with one category, including slaughter pigs with a total lesion score of 0 and the other category those with a total lesion score >0. The independent variables in the model were: (i) the vaccination protocol, with four categories namely, single-shot vaccination with any bivalent vaccine containing both M. hyopneumoniae and PCV2 antigens, single-shot vaccination with a monovalent vaccine against M. hyopneumoniae, double-shot vaccination with a monovalent vaccine against M. hyopneumoniae, including also the use of single-shot vaccines injected twice with half dose per injection, and vaccination with an extra shot of a monovalent vaccine either in addition to vaccination with a bivalent vaccine or to vaccination with a monovalent vaccine, either in a single or in a double shot protocol, (ii) the size of the herd where the slaughter pig was reared, categorized in three groups namely, 100–300, 301–600 and >600 sows, (iii) a dummy variable coding for the lung inspector with four categories, and (iv) a dummy variable coding for the slaughter season, distinguished between ‘cold’ extending from November to April and ‘warm’ extending from May to October, for each year of data collection, resulting in a variable with eight categories. The latter three variables were forced into the model to control for their likely confounding effects (Aalund et al. Citation1976; Elbers et al. Citation1992; Gardner et al. Citation2002; Li et al. Citation2019). The potential confounding effect of the aforementioned factors was verified with the use of a Mantel Haenszel test of homogeneity. The tests resulted in statistically significant values, for each examined factor (all p < .001), suggesting the rejection of the null hypothesis of no association of our outcome and the investigated exposure across strata of the confounders and providing evidence that the association of the frequency of EP-like lesions with the applied vaccination protocol differs, depending on ‘herd size’, ‘slaughter season’ or ‘lung inspector’. Adjustment of confounding effects was based on the applied statistical methods since it would have been impractical and infeasible to address by use of randomization, restriction, or matching during the study design (Jager et al. Citation2008). The applied analytical approach of multivariable regression modelling served as a standardized method of choice for controlling confounding with statistical analysis (Pourhoseingholi et al. Citation2012; Kahlert et al. Citation2017). Finally, the extent of confounding was evaluated by comparing the regression coefficients of the above-described multivariable final model with a corresponding simple one that did not account for the confounding factors and by estimating the resulted change in the respective measures of association. Moreover, the model included a random-effect term for herd and a random-effect term for batch nested within the herd to account for the anticipated heterogeneity of lesion frequency among batches. After an initial fit of the model to the data, all meaningful two-way interactions between the fixed effects were created, included one-by-one in the initial model and evaluated for significance at the 5% level.
A generalized linear latent mixed-effect model for ordinal data (Rabe-Hesketh et al. Citation2005) was used to examine the possible association between the total lung lesion score and the different vaccination protocols. Initially, the total lung lesion score was assigned in one of the four following categories (lung lesion score category – LLSC): LLSC 1 for lungs with total lesion score = 0, LLSC 2 for lungs with total lesion score ranging from 1 to 4, LLSC 3 for lungs with total lesion score ranging from 5 to 9 and LLSC 4 for lungs with total lesion score ≥10 (Wilson et al. Citation2012). However, due to the low percentage of lungs belonging to the fourth category (5.2%), categories 3 and 4 were merged and, thus, LLSC 3 included all lungs having a total lesion score >5. Except for the ‘vaccination protocol’, which was the primary variable of interest, the following variables: ‘herd size’, ‘investigator’ and ‘cold and warm period of each year of observation’ were forced into the model to control for their likely confounding effects. The procedure for confirming, controlling for and evaluating the potential confounding effect of these variables was the same as described in the previous model. The results of the Mantel Haenszel tests of homogeneity suggested that the association of the total lung lesion score with the applied vaccination protocol differs, depending on ‘herd size’, ‘slaughter season’ or ‘lung inspector’ (all p < .001). Similarly, the model included a random-effect term for herd and a random-effect term for batch nested within the herd to account for clustering within batches and herds sampled. After an initial fit of the model to the data all meaningful two-way interactions between the fixed effects were created, were then included one-by-one in the initial model and evaluated for significance at the 5% level. One of the assumptions underlying ordinal logistic regression is that the effects of any explanatory variables are consistent or proportional across the different thresholds. To examine if the assumption of proportionality in the odds was valid for all predictors, the thresh command was used to relax the parallel line’s assumption. Subsequently, a set of test postestimation commands was used to examine if the coefficients of the predictors differed significantly across equations (Rabe-Hesketh and Skrondal Citation2008). The difference was not significant for any of the predictors and, therefore, thresh command was not included in the final model.
3. Results
The number of herds and batches that participated in the study and the number of lungs which were scored for each year separately are shown in . In total, 7047 slaughter pigs from 114 batches were evaluated for EP-like lesions. Only 117/7047 (1.7%) animals in 3 batches from 2 herds were not vaccinated against M. hyopneumoniae. Among vaccinated pigs, 897/6930 (12.9%), in 13 batches from 9 herds, were vaccinated with a bivalent vaccine. Twenty-six batches from 20 herds, including 1643/6930 (23.7%) slaughter pigs, were vaccinated with a monovalent vaccine in a single-shot protocol. A monovalent vaccine in a double-shot protocol was administered in 3821/6930 (55.2%) slaughter pigs belonging to 62 batches from 28 herds. Lastly, seven herds were using an extra shot of a monovalent vaccine either in addition to vaccination with a bivalent or a monovalent vaccine, either in a single or in a double shot protocol. These herds contributed to the study 10 batches totalling 569/6930 (8.2%) slaughter pigs, of which 306/6930 (4.4%) received a bivalent vaccine and 263/6930 (3.8%) received a monovalent vaccine. The herd size ranged from 120 to 1200 sows (median = 350, IQR = 400 sows). Regarding the distribution of the animals participated in the study to the ‘herd size’ categories, 3040 out of 7047 (43.1%) pigs originated from herds with 100 to 300 sows, 2276 out of 7047 (32.3%) pigs originated from herds with 301 to 600 sows and 1731 out of 7047 (24.6%) pigs originated from herds with >600 sows.
Table 1. The number of herds, batches and slaughter pigs that were inspected each year of observation.
3.1. Frequency of EP-like lesions and associations with vaccination protocols
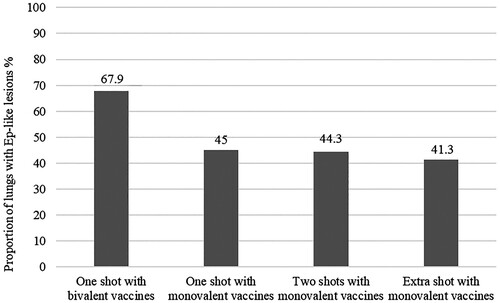
In total, 3354/7047 (47.6%) slaughter pigs were affected with EP-like lesions. The proportion of lungs affected with EP-like lesions by vaccination protocol is presented in . The possible association between the frequency of EP-like lesions and the vaccination protocols was investigated only for the 6930 animals that received an M. hyopneumoniae vaccine. In the final mixed-effect logistic regression model, the frequency of affected slaughter pigs was associated with the vaccination protocols. Specifically, pigs which were vaccinated with a monovalent vaccine in a single shot protocol were 2 [95% Confidence Interval (CI): 1.02, 3.85; p = .043] times less likely to have EP-like lesions at slaughter compared to pigs that were vaccinated with a bivalent vaccine. Similarly, pigs that were vaccinated with a monovalent vaccine in a double-shot protocol or received an extra shot of a monovalent vaccine were 2.3 (1.12, 4.76; p = .022) and 2.5 (1.04, 5.88; p = .040) times, respectively, less likely to have EP-like lesions at slaughter compared to pigs which were vaccinated with a bivalent vaccine. However, we found non-significant association (p = .557) between the frequency of EP-like lesions and the single-shot compared to the double-shot monovalent vaccine protocol. Similarly, the frequency of EP-like lesions was not significantly associated with the use of an extra dose of a monovalent vaccine for M. hyopneumoniae compared to the use of a monovalent vaccine either injected once (p = .560) or twice (p = .855). The variables ‘herd size’, ‘investigator’ and ‘cold and warm period of each year of observation’ were not significantly associated with the frequency of EP-like lesions (). Further, the comparison of the multiple logistic regression models, examining the association of our primary risk factor (vaccination protocol) and the given outcome of frequency of EP-like lesions, with and without including potential confounding factors (), resulted in a change of the estimated odds ratios that can be considered important in the context of our study (Dohoo et al. Citation2003). This finding, besides further validating the presence of confounding, suggests that part of the association between vaccination protocol and frequency of EP-like lesions was ascribed to the examined confounders.
Table 2. Odds ratios and 95% Confidence Interval (CI) for the associations between the frequency of EP-like lesions and the dependent variables of the mixed effect logistic regression model accounting (a) and without accounting (b) for confounding effects of ‘Herd size’, ‘Investigator’ and ‘Cold and warm period of each year of observation’.
3.2. Severity of EP-like lesions and associations with vaccination protocols
The total lung lesion score ranged from 0 to 25 (median = 0, IQR = 3) (). In total, 52.4%, 31.6% and 16% of the lungs belonged to LLSC 1, LLSC 2 and LLSC 3, respectively. The median, range, as well as 25th and 75th percentiles of the total lung lesion score for each vaccination protocol are in .
Table 3. Minimum (min.), maximum (max.), 25th, 50th and 75th percentiles (Q1, Q2 and Q3, respectively) of the total lung lesion score by vaccination protocol against M. hyopneumoniae.
The possible association between the severity of EP-like lesions and the vaccination protocols was investigated only for the 6930 animals that received an M. hyopneumoniae vaccine. In the final generalized linear latent and mixed effect model, the severity of EP-like lesions was significantly (p = .027) associated only with the use of a two-shot monovalent vaccine protocol versus the use of a bivalent vaccine. Specifically, for the animals which received two shots of a monovalent vaccine, the odds of LLSC 3 versus 2 or 1 and LLSC 3 or 2 versus 1 [higher versus lower score(s)], were 2.3 (1.1, 4.55; p = .027) times lower compared to animals which received a bivalent vaccine. The variables ‘herd size’, ‘investigator’ and ‘cold and warm period of each year of observation’ were not significantly associated with the severity of EP-like lesions (). Additionally, after comparing the multiple ordinal logistic regression models, examining the association of vaccination protocol and the total lung lesion score, with and without including potential confounding factors (), a change of the estimated odds ratios was observed, that can be considered important in the context of our study (Dohoo et al. Citation2003). This finding, besides further validating the presence of confounding, suggests that part of the association between vaccination protocol and the total lung lesion score was ascribed to the examined confounders.
Table 4. Odds ratios and 95% Confidence Interval (CI) for the associations between the severity of EP-like lesions and the dependent variables of the generalized linear latent and mixed effect model for ordinal data, accounting (a) and without accounting (b) for confounding effects of ‘Herd size’, ‘Investigator’ and ‘Cold and warm period of each year of observation’.
4. Discussion
This cross-sectional study reports an extensive countrywide field investigation of the frequency and severity of EP-like lesions in slaughter pigs from 53 Greek swine herds with conventional health status. Furthermore, the appreciable number of inspected slaughter pigs provides useful insights, which were lacking from the literature, into the frequency and severity of EP in Greek swine herds, although respective data are available for several other European countries (Fablet et al. Citation2012; Merialdi et al. Citation2012; Hillen et al. Citation2014; Brewster et al. Citation2017; Del Carmen et al. Citation2018; Rodrigues da Costa et al. Citation2020). We found that 47.6% of the inspected slaughter pigs had EP-like lesions to any extent. In 2012, Merialdi et al. (Citation2012) recorded that 46.3% of the inspected lungs were affected with EP-like lesions in Italy. In Spain, during a two-year long study by Del Carmen et al. (Citation2018), the average annual percentage of slaughter pigs affected with EP-like lesions was 47.3%. In contrast to the frequency estimated in Greece, Italy and Spain those calculated from data of nineteen European countries during 2017 were lower, with a median frequency reported at 41.2% (Krejci et al. Citation2018). Interestingly, in a study conducted by Hillen et al. (Citation2014) involving 20 German pig herds, 55.9% of the inspected slaughter pigs had lesions at slaughter, whereas an even higher frequency of EP-like lesions (69.3%) was reported from France (Fablet et al. Citation2012). Studies from Belgium, Ireland and the Czech Republic reported a lower frequency of EP-like lesions compared to this study and those previously cited (Meyns et al. Citation2011; Vanhara et al. Citation2018; Rodrigues da Costa et al. Citation2020).
Despite the fact that different scoring systems were used in the aforementioned studies, Garcia-Morante et al. (Citation2016) reported a good correlation among scoring systems used to estimate the severity of EP-like lesions in slaughter pigs. In most of the studies conducted in European countries, the percentage of affected lung surface per batch of slaughter pigs ranged from 1 to 5.8 (Meyns et al. Citation2011; Fablet et al. Citation2012; Merialdi et al. Citation2012; Del Carmen et al. Citation2018; Krejci et al. Citation2018; Vanhara et al. Citation2018; Rodrigues da Costa et al. Citation2020). In the present study, the median of the total lung lesion score on lung level was 0. Likewise, Hillen et al. (Citation2014), found a relatively low median value (1) of total EP-like lesion score on lung level.
EP-like lesions, although not pathognomonic, are highly suggestive of M. hyopneumoniae infection (Amanfu et al. Citation1984; Moorkamp et al. Citation2008; Merialdi et al. Citation2012; Hillen et al. Citation2014; Cvjetković et al. Citation2018; Li et al. Citation2019). Vaccination is a common measure to control M. hyopneumoniae infection in pig farming (Maes et al. Citation2008). Although 98.3% of the animals that were included in the present study were vaccinated against M. hyopneumoniae, inspection at slaughter revealed that almost half of them were affected with EP-like lesions. However, the severity of those lesions was low. Vaccination reduces the severity of pneumonia lesions at slaughter (Maes et al. Citation1999) but it can neither prevent M. hyopneumoniae colonization of the respiratory tract (Thacker et al. Citation1998) nor significantly decrease the transmission of the pathogen (Meyns et al. Citation2006; Villarreal et al. Citation2011). Therefore, in addition to vaccination, other management practices and improved housing conditions are of high importance in mitigating the risk of M. hyopneumoniae infections (Maes Citation2010).
We found that pigs which were vaccinated with a monovalent vaccine against M. hyopneumoniae either in a single- or in a double-shot protocol or those which were vaccinated with a bivalent or a monovalent vaccine but received an extra shot of a monovalent vaccine were less likely to have any EP-like lesions compared to pigs which were vaccinated with a bivalent vaccine containing both M. hyopneumoniae and PCV2 antigens. Also, pigs which were vaccinated with a monovalent vaccine in a double-shot protocol were less likely to belong to higher versus lower LLSC(s) compared to pigs that were vaccinated with a bivalent vaccine. Similarly, in 2018, Pallarés et al. (Citation2018) reported a significantly lower score of EP-like lesions in animals vaccinated with a monovalent vaccine compared to a bivalent vaccine. Pujols et al. (Citation2016) found that a combined vaccine against PCV2 and M. hyopneumoniae could support a long-term antibody response against PCV2 and moderate but weaker antibody response against M. hyopneumoniae. However, the correlation between seroconversion and protection from M. hyopneumoniae infection is poor (Thacker et al. Citation1998).
The frequency and severity of EP-like lesions did not differ significantly between the single- and the double-shot protocol with a monovalent vaccine. On the contrary, other studies reported significant differences between single- and double-shot vaccines, but with inconsistent results. Specifically, Cvjetković et al. (Citation2018) reported lower prevalence and severity of EP-like lesions for the pigs which were vaccinated with a single shot vaccine compared to a double shot vaccine. On the contrary, Hillen et al. (Citation2014) found that lung lesion scores differed significantly among the vaccination groups, with the most severe cases occurring with a single shot vaccine. Moreover, in 2007, Sibila et al. reported a significant reduction of EP-compatible gross lung lesions compared to non-vaccinated slaughter pigs, only when two doses of the same vaccine were administered. In active immunization, fundamentally, a second boosting dose of the antigen (reimmunization) results in a secondary immune response, which greatly enhances the host response (Tizard Citation2017). However, modern technology has developed innovative adjuvants which can permit reductions in antigen amount or lessen the doses applied by enhancing the speed or the magnitude of the host response to the vaccine (Tizard Citation2017). Therefore, new single-shot vaccines with innovative adjuvants have been proved to provide better protection compared to old double-shot vaccines granting less workload and stress to the animals (Cvjetković et al. Citation2018).
We found that the likelihood of EP-like lesions and higher compared to lower LLSC(s) did not differ significantly between pigs that were injected with an extra dose of a monovalent vaccine for M. hyopneumoniae compared to those which received a monovalent vaccine, regardless of the vaccination scheme. Moreover, pigs which were injected with an extra dose of a monovalent vaccine were not less likely to have higher compared to lower LLSC(s) compared to those which received a bivalent vaccine. However, due to the low percentage (8.2%) of pigs that received an extra shot of a monovalent vaccine, this result should be interpreted with caution. Furthermore, associations between the frequency and severity of EP-like lesions and the use of different vaccination protocols against M. hyopneumoniae are limited to the extent that M. hyopneumoniae infection of inspected lungs was not confirmed with laboratory diagnostics.
5. Conclusions
In our view, the results of the present study represent the first on EP-like lesions in pigs from Greece. Regardless of the high vaccination rate, almost half of the lungs that were examined were affected with EP-like lesions, but to a low extent. Although vaccination has improved the respiratory health status of commercial herds, wrong management practices and poor housing conditions, commonly seen in Greek swine herds, may hamper the efficient reduction of M. hyopneumoniae spread. Associations among different vaccination protocols for M. hyopneumoniae used in routine in Greek herds showed that frequency and severity of EP-like lesions did not differ significantly between one and two shots of monovalent vaccines, whereas bivalent vaccines presented higher values of EP-like lesions. Randomized clinical trials, which are better suited than cross-sectional studies to assess vaccine efficacy, together with laboratory confirmation, would be of value to conclude the most effective vaccination protocols to control M. hyopneumoniae infection in pigs, which is a field with a continuous need for research.
Acknowledgements
The authors wish to thank all farm owners and herd managers for their consent to participate in the study.
Disclosure statement
The authors M.L. and L.K. are employees of the sponsor company; A.I.K. was an employee of the sponsor company at the time of the project.
Additional information
Funding
References
- Aalund O, Willeberg P, Mandrup M, Riemann H. 1976. Lung lesions at slaughter: associations to factors in the pig herd. Nord Vet Med. 28:487–495.
- Amanfu W, Weng CN, Ross RF, Barnes HJ. 1984. Diagnosis of mycoplasmal pneumonia of swine – sequential study by direct immunofluorescence. Am J Vet Res. 45:1349–1352.
- Annual Porcine Census. 2021. [accessed 2021 Jun 4]. http://www.minagric.gr/index.php/el/eservisesmenu-2/e-services-minagric-anual-pig-census-gr.
- Baccaro MR, Hirose F, Umehara O, Gonçalves LC, Doto DS, Paixão R, Shinya LT, Moreno AM. 2006. Comparative efficacy of two single-dose bacterins in the control of Mycoplasma hyopneumoniae in swine raised under commercial conditions in Brazil. Vet J. 172:526–531.
- Brewster VR, Maiti HC, Tucker AW, Nevel A. 2017. Associations between EP-like lesions and pleuritis and post trimming carcass weights of finishing pigs in England. Livest Sci. 201:1–4.
- Cvjetković V, Sipos S, Szabó I, Sipos W. 2018. Clinical efficacy of two vaccination strategies against Mycoplasma hyopneumoniae in a pig herd suffering from respiratory disease. Porc Health Manag. 4:19.
- Del Carmen P, Carmona M, Lasierra M, Cárceles S, Espigares D. 2018. Assessment of the current situation of the porcine enzootic pneumonia and porcine pleuropneumonia in Spain using slaughterhouse lung evaluation. In: Proceedings of the 10th European Symposium of Porcine Health Management; May 9–11; Barcelona, Spain; pp. 190.
- Del Pozo Sacristán R, Sierens A, Marchioro SB, Vangroenweghe F, Jourquin J, Labarque G, Haesebrouck F, Maes D. 2014. Efficacy of early Mycoplasma hyopneumoniae vaccination against mixed respiratory disease in older fattening pigs. Vet Rec. 174:197.
- Dohoo I, Martin W, Strynh H. 2003. Veterinary epidemiologic research. Charlottetown (PEI): University of Prince Edward Island; p. 235–250.
- Done S. 1991. Environmental factors affecting the severity of pneumonia in pigs. Vet Rec. 128:582–586.
- Elbers ARW, Tielen MJM, Snijders JMA, Cromwijk WAJ, Hunneman WA. 1992. Epidemiological studies on lesions in finishing pigs in the Netherlands. I. Prevalence, seasonality and interrelationship. Prev Vet Med. 14:217–231.
- Fablet C, Marois C, Dorenlor V, Eono F, Eveno E, Jolly JP, Le Devendec L, Kobisch M, Madec F, Rose N. 2012. Bacterial pathogens associated with lung lesions in slaughter pigs from 125 herds. Res Vet Sci. 93:627–630.
- Fitzgerald RM, O’Shea H, Manzanilla EG, Moriarty J, McGlynn H, Calderón Díaz JA. 2020. Associations between animal and herd management factors, serological response to three respiratory pathogens and pluck lesions in finisher pigs on a farrow-to-finish farm. Porc Health Manag. 6:34.
- Fraile L, Alegre A, López-Jiménez R, Nofrarías M, Segalés J. 2010. Risk factors associated with pleuritis and cranio-ventral pulmonary consolidation in slaughter-aged pigs. Vet J. 184:326–333.
- Fuentes M, Pijoan C. 1987. Pneumonia in pigs induced by intranasal challenge exposure with pseudorabies virus and Pasteurella multocida. Am J Vet Res. 48:1446–1448.
- Garcia-Morante B, Segales J, Fraile L, Perez de Rozas A, Maiti H, Coll T, Sibila M. 2016. Assessment of Mycoplasma hyopneumoniae-induced pneumonia using different lung lesion scoring systems: a comparative review. J Comp Pathol. 154:125–134.
- Gardner IA, Willeberg P, Mousing J. 2002. Empirical and theoretical evidence for herd size as a risk factor for swine diseases. Anim Health Res Rev. 3:43–55.
- Henninger M, Labarque G, Fily B, Auvigne V. 2014. Quantification of the relation between lung lesions at slaughter and growth performance from birth to slaughter – proposal for a new synthetic indicator. Journ Rech Porc. 46:183–184.
- Hillen S, Bergc S, Köhler K, Reinacher M, Willems H, Reiner G. 2014. Occurrence and severity of lung lesions in slaughter pigs vaccinated against Mycoplasma hyopneumoniae with different strategies. Prev Vet Med. 113:580–588.
- Jager KJ, Zoccali C, MacLeod A, Dekker FW. 2008. Confounding: what it is and how to deal with it. Kidney Int. 73:256–260.
- Jensen CS, Ersbøll AK, Nielsen JP. 2002. A meta-analysis comparing the effect of vaccines against Mycoplasma hyopneumoniae on daily weight gain in pigs. Prev Vet Med. 54:265–278.
- Kahlert J, Gribsholt SB, Gammelager H, Dekkers OM, Luta G. 2017. Control of confounding in the analysis phase – an overview for clinicians. Clin Epidemiol. 9:195–204.
- Kim D, Kim CH, Han K, Seo HW, Oh Y, Park C, Kang I, Chae C. 2011. Comparative efficacy of commercial Mycoplasma hyopneumoniae and porcine circovirus 2 (PCV2) vaccines in pigs experimentally infected with M. hyopneumoniae and PCV2. Vaccine. 29:3206–3212.
- Krejci R, Mazerolles P, Mortier M. 2018. Lung scoring survey in European countries in 2017. In: Proceedings of the 10th European Symposium of Porcine Health Management; May 9–11; Barcelona, Spain; pp. 288.
- Leneveu P, Robert N, Keïta A, Pagot E, Pommier P, Tessier P. 2005. Lung lesions in pigs at slaughter: a 2-year epidemiological study in France. Int J Appl Res Vet Med. 3:259–265.
- Li R, Hu Y, Ge M, Zhao D, Yang T, Qing R, Yu X. 2019. Analysis of correlation between the detection rate of Mycoplasma hyopneumoniae in slaughter pigs and season, climate change, and presence of lung lesions. Med Weter. 75:175–178.
- Madec F, Kobisch M. 1982. Bilan lésionnel des poumons de porcs charcutiers à l‘abattoir. Journ Rech Porc Fr. 14:405–412.
- Maes D. 2010. Mycoplasma hyopneumoniae in pigs: update on epidemiology and control. In: D’Allaire S, Friendship R, editors. Proceedings of the 21st International Pig Veterinary Society Congress; Jul 18–21; Vancouver, Canada; p. 30–35.
- Maes D, Deluyker H, Verdonck M, Castryck F, Miry C, Lein A, Vrijens B, de Kruif A. 1998. Effect of vaccination against Mycoplasma hyopneumoniae in pig herds with a continuous production system. J Vet Med. 45:495–505.
- Maes D, Deluyker H, Verdonck M, Castryck F, Miry C, Vrijens B, Verbeke W, Viaene J, de Kruif A. 1999. Effect of vaccination against Mycoplasma hyopneumoniae in pig herds with an all-in/all-out production system. Vaccine. 17:1024–1034.
- Maes D, Segales J, Meyns T, Sibila M, Pieters M, Haesebrouck F. 2008. Control of Mycoplasma hyopneumoniae infections in pigs. Vet Microbiol. 126:297–309.
- Maes D, Verdonck M, Deluyker H, de Kruif A. 1996. Enzootic pneumonia in pigs. Vet Q. 18:104–109.
- Marois C, Cariolet R, Morvan H, Kobisch M. 2008. Transmission of pathogenic respiratory bacteria to specific pathogen free pigs at slaughter. Vet Microbiol. 129:325–332.
- Merialdi G, Dottori M, Bonilauri P, Luppi A, Gozio S, Pozzi P, Spaggiari B, Martelli P. 2012. Survey of pleuritis and pulmonary lesions in pigs at abattoir with a focus on the extent of the condition and herd risk factors. Vet J. 193:234–239.
- Meyns T, Dewulf J, de Kruif A, Calus D, Haesebrouck F, Maes D. 2006. Comparison of transmission of Mycoplasma hyopneumoniae in vaccinated and non-vaccinated populations. Vaccine. 24:7081–7086.
- Meyns T, Van Steelant J, Rolly E, Dewulf J, Haesebrouck F, Maes D. 2011. A cross sectional study of risk factors associated with pulmonary lesions in pigs at slaughter. Vet J. 187:388–392.
- Moorkamp L, Nathues H, Spergser J, Tegeler R, Beilage EG. 2008. Detection of respiratory pathogens in porcine lung tissue and lavage fluid. Vet J. 175:273–275.
- Ostanello F, Dottori M, Gusmara C, Leotti G, Sala V. 2007. Pneumonia disease assessment using a slaughterhouse lung-scoring method. J Vet Med. 5:70–75.
- Pallarés FJ, Espigares D, Cano LD, Del Carmen P, Ramis G. 2018. Vaccination against Mycoplasma hyopneumoniae with Hyogen®: prevalence and severity of lung lesions. In: Proceedings of the 10th European Symposium of Porcine Health Management; May 9–11; Barcelona, Spain; pp. 231.
- Pieters MG, Maes D. 2019. Mycoplasmosis. In: Zimmerman JJ, Karriker LA, Ramirez A, Schwartz KJ, Stevenson GW, Zhang J, editors. Diseases of swine. 11th ed. Hoboken (NJ): Wiley-Blackwell; p. 863–883.
- Pourhoseingholi MA, Baghestani AR, Vahedi M. 2012. How to control confounding effects by statistical analysis. Gastroenterol Hepatol Bed Bench. 5:79–83.
- Pujols J, Segalés J, Polo J, Rodríguez C, Campbell J, Crenshaw J. 2016. Influence of spray dried porcine plasma in starter diets associated with a conventional vaccination program on wean to finish performance. Porc Health Manag. 2:4.
- Rabe-Hesketh S, Skrondal A. 2008. Multilevel and longitudinal modeling using stata. 2nd ed. College Station (TX): Stata Press; p. 317–322.
- Rabe-Hesketh S, Skrondal A, Pickles A. 2005. Maximum likelihood estimation of limited and discrete dependent variable models with nested random effects. J Econom. 128:301–323.
- Rodríguez F, Batista M, Hernández J, Afonso AM, Poveda JB. 2016. Relationship between expression of interleukin-5 and interleukin-13 by epithelial cells and bronchiolar changes in pigs infected with Mycoplasma hyopneumoniae. J Comp Pathol. 154:165–168.
- Rodrigues da Costa M, Fitzgerald RM, Manzanilla ED, O’Shea H, Moriarty J, McElroy MC, Leonard FC. 2020. A cross-sectional survey on respiratory disease in a cohort of Irish pig farms. Ir Vet J. 73:24.
- Sibila M, Nofrarías M, López-Soria S, Segalés J, Valero O, Espinalm A, Calsamiglia Μ. 2007. Chronological study of Mycoplasma hyopneumoniae infection seroconversion and associated lung lesions in vaccinated and non-vaccinated pigs. Vet Microbiol. 122:97–107.
- Sibila M, Pieters M, Molitor T, Maes D, Haesebrouck F, Segalés J. 2009. Current perspectives on the diagnosis and epidemiology of Mycoplasma hyopneumoniae infection. Vet J. 181:221–231.
- Thacker EL, Thacker BJ, Boettcher TB, Jayappa H. 1998. Comparison of antibody production, lymphocyte stimulation, and protection induced by four commercial Mycoplasma hyopneumoniae bacterins. J Swine Health Prod. 6:107–112.
- Tizard I.R. 2017. Vaccines and their production. In: Tizard I.R., editor. Veterinary immunology. 10th ed. St. Louis (MO): Elsevier/Saunders; p. 258–271.
- Vanhara J, Jirasek T, Krejci R, Sperling D. 2018. Evaluation of the prevalence and severity of enzootic pneumonia and pleuropneumonia on Czech pig farms based on lung lesion scoring in the years of 2015–2017. In: Proceedings of the 10th European Symposium of Porcine Health Management; May 9–11; Barcelona, Spain; pp. 332.
- Villarreal I, Meyns T, Dewulf J, Vranckx K, Calus D, Pasmans F, Haesebrouck F, Maes D. 2011. The effect of vaccination on the transmission of Mycoplasma hyopneumoniae in pigs under field conditions. Vet J. 188:48–52.
- Wilson S, Van Brussel L, Saunders G, Taylor L, Zimmermman L, Heinritzi K, Ritzmann M, Banholzer E, Eddicks M. 2012. Vaccination of piglets at 1 week of age with an inactivated Mycoplasma hyopneumoniae vaccine reduces lung lesions and improves average daily gain in body weight. Vaccine. 30:7625–7629.
- Yang S, Park SJ, Oh T, Cho H, Chae C. 2020. Efficacy comparison of commercial porcine circovirus type 2 (PCV2) and Mycoplasma hyopneumoniae monovalent and bivalent vaccines against a dual challenge. Can J Vet Res. 84:272–282.