ABSTRACT
This study investigated the biochemical and histological changes associated with administering a methanolic extract of Zingiber officinale (MEZO) in Wistar rats. Fifteen (15) female rats with weights ranging from 120 - 160 g were randomly divided into three (3) groups (A-C). Group A received no treatment and served as the control group. Groups B and C received 200 and 400 mg/kg of MEZO orally for 21 days, respectively. There were no significant changes in the SOD, GSH, and CAT levels in all the groups treated with MEZO compared to the control (p < 0.05). There was no difference in the expression of inflammatory cytokines in the duodenum of rats treated with MEZO compared to the control. Histopathological studies showed acute duodenitis with intense inflammatory cell infiltrations and an eroded villus. This study shows that MEZO causes no significant oxidative changes in the female rat duodenum. However, it could elicit transient and non-dose-dependent proinflammatory potentials in the female duodenum of Wistar rats at a low dose which could not be explained by the tissue antioxidants and expression of IL1α and IL4. There is a need for reevaluation of the safety of ginger extracts in the female rat duodenum.
Introduction
The use of herbal medicine is now up to 50% in western countries, with less than 10% being used to prevent or treat digestive disorders (Langmead and Rampton (Citation2001)). The menace of intestinal disorder and the low cost and availability of herbal medicine contributed to this increased consumption of medicinal plants, especially in resource-poor countries (Minaiyan et al. (Citation2006)). Ginger (Zingiber officinale) is a widely consumed plant with many culinary and medicinal applications. It has been used to treat different gastrointestinal tract (GIT) diseases, including vomiting, constipation, and indigestion (Shahrajaban et al. (Citation2019)). Ginger is considered one of the essential spices globally, with its extracts still being recommended for different digestive diseases in the pharmacopeias of different countries (Borrelli et al. (Citation2004)). Over forty natural compounds had been isolated from ginger, including paradols, dihydroparadols, gingerols, shogaols, 3-dihydroshogaols, gingerdiols, mono and diacetyl derivatives of gingerdiols, 1-dehydrogingerdiones, and diarylheptanoids (Jolad et al. (Citation2004)). Ginger has demonstrated many pharmacological properties including anti-bacterial (Islam et al. (Citation2014)), antioxidant (Bak et al. (Citation2012)), antiviral (Chang et al. (Citation2013)), anti-ulcer (Minaiyan et al. (Citation2006)), hypoglycemic, and hypolipidemic effects (Al-Qudah et al. (Citation2016)), and is considered beneficial for nausea relief (Nikkhah et al. (Citation2019)). Furthermore, ginger has shown anticancer potential in the GIT (Prasad and Tyagi (Citation2015)). The increased consumption of extracts of medicinal plants for gastrointestinal disorders has created a need for scientific inquiry on the claimed therapeutic effects and to scrutinize for possible toxicity. As noted by Nikkhah and colleagues (Nikkhah et al. (Citation2019)), there is a dearth in the number of available studies demonstrating ginger's efficacy as a gastroprotective agent. Ginger extracts (hydroalcoholic and ethanolic) have been used to study the effect of ginger on the male rat duodenum (Minaiyan et al. (Citation2006)), (Chatturong et al. (Citation2018)). However, there is a dearth of studies on the female duodenum for a comparative analysis of ginger extract's antioxidative and anti-inflammatory effects. This present study investigated the effect of methanolic ginger extract in the duodenal expression of inflammatory cytokines, antioxidants, and histopathology at the preliminary level.
Materials and methods
Study setting
This experimental study was carried out in the research laboratory of the Department of Anatomy, Faculty of Basic Medical Sciences, College of Health Science, Nnamdi Azikiwe University, Nnewi Campus, Anambra State, Nigeria, and lasted for about three months, including the study design, planning, ethical approval, experimental research, sample collection, and data analysis.
Plant collection, identification, and preparation
The aerial part of the plant was harvested from Nnewi, Anambra state. The plant calyces were shade-dried and ground, yielding a finely powdered sample of 1000g. The botanical identification and authentication were carried out in the Department of Pharmacognosy and Traditional Medicine, College of Pharmacy, Nnamdi Azikiwe University, Agulu Campus, Anambra State, Nigeria with identification number PCG/474/A/024.
Plant extraction
The methanolic extraction of ginger was done using the maceration method as described by Sharif and Benneth (Sharif and Bennett (Citation2016)). To maximize extraction yield while minimizing the extraction cost, 80% methanol was used (Michiels et al. (Citation2012)) in a sample-solvent ratio of 1g per 20 mL. 10g of ginger were weighed and placed into a Schott bottle, adding 200 mL of methanol and covering with an aluminum foil before placing on an orbital shaker at 200 rpm for 8 h. After solvent extraction, the solution was filtered through a cotton-wrapped muslin cloth with an additional 100 ml of the same fresh solvent into a conical flask before filtering through a filter paper (Whatman No.1). The methanol in the ginger extract was then evaporated using a rotary evaporator under reduced pressure at 40 °C, while the trace amounts of solvent were evaporated by blowing nitrogen gas over the extracts. The final extract was stored in the refrigerator at 4⁰C. Each solvent extraction yielded 23% of its starting sample. The extract was constituted to a solution of known concentration using distilled water and administered according to body weight and group treatment doses.
Animal procurement, care and handling
Fifteen (15) female Wistar rats were procured from the animal house of College of Health Sciences, Nnamdi Azikiwe University Nnewi Campus and acclimatized for two (2) weeks (to exclude any intercurrent infection) under standard housing condition (ventilated room with 12/12-hour light/dark cycle at 24 ± 2°C). The rats were fed ad libitum with water and standard rat chow throughout the experimental period. Animal health status was monitored throughout the experiment according to the federation of European Laboratory Animal Science Associations (FELASA) guidelines.
Experimental design
Fifteen (15) rats with weights ranging from 120 - 160 g were randomly divided into three (3) groups (A-C). Group A received no treatment asides from distilled water and served as the control group. Groups B and C received oral administration of 200 and 400 mg/kg of methanolic extract of Zingiber officinale (MEZO), respectively. The extract was administered once a day for 21 days. The dose and duration of MEZO used in this present study were determined in line with our previous studies (Okafor et al. (Citation2020a); Okafor et al. (Citation2020b)), which have found some notable effects on the body tissues with ginger extract administration and other studies on the rat female duodenum. Hence acute toxicity test was not conducted for this present study to determine the MEZO dosage.
Animal Sacrifice and Sample Collection
The animals were fasted overnight after the last day of MEZO administration and anesthetized using chloroform. 2 mL of blood was collected from the animals by ocular puncture using capillary tubes into a plain sample tube for the anti-inflammatory marker investigation. The animals were sacrificed after blood collection, and the duodenal tissues were harvested, weighed, and divided into two parts. One was fixed in a 10% formal saline for histological processing and analysis, and the second part was homogenized and used for oxidative status analysis.
Antioxidants quantification
The oxidant status was determined in the duodenal tissue by quantifying the Superoxide Dismutase (SOD), Glutathione (GSH), and Catalase (CAT) levels in the duodenal tissue samples using the tissue homogenate as described in our earlier studies (Okafor and Gbotolorun (Citation2018).
Tissue processing
The duodenal tissue samples were trimmed down to about 3mm×3 mm thick for an easy study of sections under the microscope and fixed in 10% formalin. After fixation, the fixed tissues were dehydrated in ascending grades of alcohol 50%, 70%, 95%, and 100%, and cleared in xylene. The cleared tissues were stained with hematoxylin and eosin (H&E) and mounted using DPX, after which the sections were viewed under a light microscope. Photomicrographs of these sections were obtained using the Leica DM 750 digital microscope computer software.
Assay procedure for inflammatory cytokines
This study assessed two inflammatory cytokines (Interleukin 1α and Interleukin 4) using the blood. The blood was allowed to clot for 10-20 min at room temperature and centrifuged at 2000–3000 revolutions per minute for 20 min. 40μl of sample was added to sample wells and then 10μl anti-pro-IL1α antibody (for IL1α assay) or anti-STX1A antibody (for IL4 assay) to sample wells, then 50μl streptavidin-HRP was added to sample wells and standard wells (not blank control well) and mixed well. The plate was covered with a sealer and incubated for 60 min at 37°C. The sealer was removed, and the plate was washed five times with wash buffer. 50μl of substrate solution A was added to each well, and 50μl of substrate solution B was added to each well. The plate covered with a new sealer was incubated for 10 min at 37°C in the dark. Stop Solution measuring 50μl was added to each well, leading to an immediate colour change to yellow. Each well's optical density (OD value) was determined immediately using a microplate reader set to 450 nm within 10 min after adding the stop solution. All the reagents were brought to room temperature before use.
Ethical Statement
This study was approved by the Research Ethics Committee of the Anatomy Department, Faculty of Basic Medical Sciences, Nnamdi Azikiwe University, Nnewi Campus. The experimental procedures of this study complied with ARRIVE guidelines, National Institutes of Health (NIH) guidelines, and National Health Research ethics committee of Nigeria (NHREC) guidelines for the care and use of laboratory animals. Animal health status was monitored throughout the experiment according to the federation of European Laboratory Animal Science Associations (FELASA) guidelines. No informed consent was required for this study.
Results
The effect of MEZO on the inflammatory cytokines and duodenal antioxidants
No significant changes were seen in the expression of inflammatory cytokines in all treated groups when compared to the control (p < 0.05) (). No significant changes were seen in the levels of SOD, GSH, and CAT across all the test groups when compared to the control (p < 0.05) ().
Table 1. The effect of MEZO on the expression of inflammatory cytokines in the duodenum of female Wistar rats.
Table 2. The oxidant status of the duodenum after the administration of MEZO in female Wistar rats.
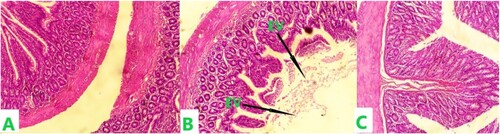
Histopathological findings
(A-C) shows the histological section of rat duodenum treated with different doses of MEZO. The plate labelled A represents the histological section of a rat duodenum that received only distilled water for 21 days. The histological section shows viable muscularis propria wall and mucosal lining cells. The plate labelled B represents the histological section of a rat duodenum administered 200 mg/kg MEZO. The section shows an intense inflammatory cell infiltrations with an eroded villus. Plate C represents the duodenum of a rat treated with 400 mg/kg MEZO. The histologic section of tissue shows viable muscularis propria wall and mucosal lining cells. No abnormalities were seen.
Figure 1. The histological section of the duodenum following the administration of MEZO in female Wistar rats.
Note: Plate A represents the control and was administered only distilled water for 21 days. Plate B received 200 mg/kg MEZO for 21 days. Plate C received 400 mg/kg MEZO 21 days. EV - Eroded villi. Staining for all sections was done using H&E and photomicrography was taken at x200.
Discussion
Ginger has historically been used as a folk remedy for ailments including rheumatism, piles, jaundice, ulcer, nausea, and diabetes (Babu et al. (Citation2008)), (Huang et al. (Citation2019)). Some of the traditionally assigned therapeutic properties of ginger, like anti-ulcer and anti-diabetic effects, have been validated by modern experimental studies (Minaiyan et al. (Citation2006)), (Anfenan (Citation2014)). Ginger has also been used to inhibit the production of oxygen species and lipid peroxidation (Hosseinzadeh et al. (Citation2017)), with a wide array of other biological effects attributed to it. In this present study, we investigated the effects of the methanolic extract of Zingiber officinale on the expression of inflammatory cytokines, oxidative profile, and histopathological status of the duodenum of female Wistar rats.
Drastic changes in the expression of inflammatory cytokines are often assoiated with inflammatory cell infiltration and tissue toxicity, as seen in diseases like inflammatory bowel diseases (IBD) (Muzes et al. (Citation2012)). Our result showed that methanolic ginger extract did not significantly affect the expression of inflammatory cytokines in the duodenum; the levels of IL1α and IL4 were not significantly different in the MEZO-treated groups when compared to the control (). Similarly, no significant change was seen in the evaluated antioxidants. SOD, GSH, and CAT were not significantly altered in the MEZO-treated groups when compared to the control (p < 0.05) (). This observed oxidative stress profile in the duodenum could explain the unchanged expression of inflammatory cytokines as inflammatory disruptions have been shown to induce oxidative stress (Chatterjee (Citation2016)). Unexpectedly, the histopathological studies revealed signs of acute duodenitis after administering a low dose (200 mg/kg) of MEZO. The histological section showed an intense inflammatory cell infiltrations, with a completely eroded villus in group B when compared to group A ().
This finding is unexpected and differs from a recent review study on the role of ginger in the treatment of IBD, which demonstrated the anti-inflammatory effects of ginger extracts (Lashgari et al. (Citation2022)). The unchanged level of inflammatory cytokines observed in this study may indicate an absence of proinflammatory induction through IL1α and IL4. We suspect that the infiltrates of immune cells observed in group B could be modulated by other inflammatory cytokines different from those that were evaluated in this present study. It is important to note that the histological response elicited by the low-dose MEZO was transient and could not be sustained at a higher dose. A chronic study with MEZO could unravel this mystery. Future studies should also be focused on other interleukins (for example, IL6, IL9, TNF-alpha, and others), which may explain the changes seen in this current study. Again, the dynamics of female hormones and their intermittent physiological interferences could be questioned. This study did not consider the different hormonal states of the female rats used for this study, and this limitation could be a factor to consider in understanding the context of this study. The above could explain why an apparent inflammatory cell infiltrates did not cause any change in the antioxidant levels and the expression levels of IL1α and IL4.
In summary, this study revealed that MEZO causes no significant oxidative changes in the female rat duodenum but a low-dose induced inflammatory response in the duodenum. This calls for a comparative study of the effects of MEZO on the rat duodenum to underscore the mechanisms behind the variations seen in earlier published studies on the male rat duodenum (Lashgari et al. (Citation2022); El-Abhar et al. (Citation2008); Rong et al. (Citation2009); Kim and Kim (Citation2018); Javid et al. (Citation2019)) This present finding questions the validity of the conclusion on the safety of ginger in rat (Morten and Katrin (Citation2000)).
Conclusion
This study gives preliminary evidence that MEZO could elicit transient and non-dose-dependent proinflammatory potentials in the female duodenum of Wistar rats at a low dose which could not be explained by the tissue antioxidants and expression of IL1α and IL4. Following this preliminary evidence, further studies are needed to confirm this inflammatory action of MEZO in the female rat duodenum and ascertain the mechanism of action and the phytochemical drivers of the observed effect.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Al-Qudah MMA, Haddad MA, El-Qudah JMF. 2016. The effects of aqueous ginger extract on pancreas histology and on blood glucose in normal and alloxan monohydrate-induced diabetic rats. Biomed Res - India. 27(2):350–356.
- Anfenan MLK. 2014. Evaluation of nutritional and anti-diabetic activity of different forms of ginger in rats. Middle - East Journal of Scientific Research. doi:10.5829/idosi.mejsr.2014.21.01.21154.
- Babu KN, Sabu M, Shiva KN, Divakaran M, Ravindran PN. 2008. Ginger. Reactions Weekly. doi:10.2165/00128415-200811880-00045.
- Bak MJ, Ok S, Jun M, Jeong WS. 2012. 6-shogaol-rich extract from ginger up-regulates the antioxidant defense systems in cells and mice. Molecules. doi:10.3390/molecules17078037.
- Borrelli F, Capasso R, Pinto A, Izzo AA. 2004. Inhibitory effect of ginger (zingiber officinale) on rat ileal motility in vitro. Life Sci. doi:10.1016/j.lfs.2003.10.023.
- Chang JS, Wang KC, Yeh CF, Shieh DE, Chiang LC. 2013. Fresh ginger (zingiber officinale) has anti-viral activity against human respiratory syncytial virus in human respiratory tract cell lines. J Ethnopharmacol. doi:10.1016/j.jep.2012.10.043.
- Chatterjee S. 2016. Oxidative stress and biomaterials. Oxidative Stress and Biomaterials. doi:10.1016/B978-0-12-803269-5.00002-4.
- Chatturong U, Kajsongkram T, Tunsophon S, Chanasong R, Chootip K. 2018. Ginger extract and [6]-gingerol inhibit contraction of Rat entire small intestine. Journal of Evidence-Based Integrative Medicine. doi:10.1177/2515690X18774273.
- El-Abhar HS, Hammad LN, Gawad HS. 2008. Modulating effect of ginger extract on rats with ulcerative colitis. J Ethnopharmacol. doi:10.1016/j.jep.2008.04.026.
- Hosseinzadeh A, Bahrampour JK, Fatemi MJ, Kamarul T, Bagheri A, Tekiyehmaroof N, Sharifi AM. 2017. Protective effect of ginger (zingiber officinale roscoe) extract against oxidative stress and mitochondrial apoptosis induced by interleukin-1β in cultured chondrocytes. Cells Tissues Organs. doi:10.1159/000479789.
- Huang FY, Deng T, Meng LX, Ma XL. 2019. Dietary ginger as a traditional therapy for blood sugar control in patients with type 2 diabetes mellitus. Medicine (Baltimore). doi:10.1097/MD.0000000000015054.
- Islam K, Rowsni AA, Khan M, Kabir S. 2014. Antimicrobal activity of ginger (zingiber officinale) extracts against food-borne pathogenic bacteria. International Journal of Science, Environment and Technology. 3(3):867–871.
- Javid MA, Abbas G, Waqas MY, Basit MA, Asif M, Akhtar MS, Masood S, Saleem MU, Qamar SH, Kiani FA. 2019. Evaluation of comparative effect of feed additive of allium sativum and zingeber officinale on bird growth and histomorphometric characteristics of small intestine in broilers. Brazilian Journal of Poultry Science. doi:10.1590/1806-9061-2019-0993.
- Jolad SD, Lantz RC, Solyom AM, Chen GJ, Bates RB, Timmermann BN. 2004. Fresh organically grown ginger (zingiber officinale): composition and effects on LPS-induced PGE2 production. Phytochemistry. doi:10.1016/j.phytochem.2004.06.008.
- Kim MS, Kim JY. 2018. Ginger attenuates inflammation in a mouse model of dextran sulfate sodium-induced colitis. Food Sci Biotechnol. doi:10.1007/s10068-018-0438-6.
- Langmead L, Rampton DS. 2001. Review article: herbal treatment in gastrointestinal and liver disease-benefits and dangers. Aliment Pharmacol Ther. doi:10.1046/j.1365-2036.2001.01053.x.
- Lashgari NA, Momeni Roudsari N, Khayatan D, Shayan M, Momtaz S, Roufogalis BD, Abdolghaffari AH, Sahebkar A. 2022. Ginger and its constituents: role in treatment of inflammatory bowel disease. BioFactors. doi:10.1002/biof.1808.
- Michiels JA, Kevers C, Pincemail J, Defraigne JO, Dommes J. 2012. Extraction conditions Can greatly influence antioxidant capacity assays in plant food matrices. Food Chem. 130(4):986–993.
- Minaiyan A, Ghannadi A, Karimzadeh AR. 2006. Anti-ulcerogenic effect of ginger (rhizome of zingiber officinale roscoe) on cystemine induced duodenal ulcer in rats. Daru Journal of Pharmaceutical Siences. 14(2):97–101.
- Morten SW, Katrin S. 2000. The safety of a ginger extract in the rat. J Ethnopharmacol. doi:10.1016/S0378-8741(00)00340-8.
- Muzes G, Molnár B, Tulassay Z, Sipos F. 2012. Changes of the cytokine profile in inflammatory bowel diseases. World J Gastroenterol. doi:10.3748/wjg.v18.i41.5848.
- Nikkhah M, Maleki I, Hekmatdoost A. 2019. Ginger in gastrointestinal disorders: A systematic review of clinical trials. Food Sci Nutr. doi:10.1002/fsn3.807.
- Okafor IA, Gbotolorun SC. 2018. Resveratrol prevents cisplatin-induced lipid peroxidation in the non-gravid uterus of sprague-dawley rats. Middle East Fertil Soc J. doi:10.1016/j.mefs.2017.12.003.
- Okafor IA, Nnamah US, Nweke JO, Nnaka JA, Ahiatrogah S, Okeke UV. 2020a. Zingiber officinale (ginger) extract has no effect on Kiss1 gene expression in the testis and blood but may cause inflammation-induced morphological sperm disruptions in wistar rats. Acta Scientific Medical Sciences. doi:10.31080/ASMS.2020.04.0789.
- Okafor IA, Nnamah US, Nweke JO, Nnaka JA, Ahiatrogah S, Okeke UV, Okoro CC. 2020b. The role of zingiber officinale (ginger) extract in the Kiss1 gene expression in the ovary and blood of wistar rats. Acta Scientific Medical Sciences. doi:10.31080/ASMS.2020.04.0777ING.
- Prasad S, Tyagi AK. 2015. Ginger and its constituents: role in prevention and treatment of gastrointestinal cancer. Gastroenterol Res Pract. doi:10.1155/2015/142979.
- Rong X, Peng G, Suzuki T, Yang Q, Yamahara J, Li Y. 2009. A 35-day gavage safety assessment of ginger in rats. Regul Toxicol Pharmacol. doi:10.1016/j.yrtph.2009.03.002.
- Shahrajaban MH, Sun W, Cheng Q. 2019. Pharmacological uses and health benefits of ginger (zingiber officinale) in traditional Asian and ancient Chinese medicine, and modern practice. Notulae Scientia Biologicae. doi:10.15835/nsb11310419.
- Sharif MF, Bennett MT. 2016. The effect of different methods and solvents on the extraction of polyphenols in ginger (Zingiber officinale). Jurnal Teknologi. doi:10.11113/jt.v78.9943.
