ABSTRACT
The house crow (Corvus splendens) is globally recognised as a pest. It is an invasive species that can populate Malaysia's urban landscape and urban coastal areas. C. splendens was imported from Sri Lanka to Klang, Malaysia, in the 1890s to control caterpillars on coffee plantations. After a few decades, crows are able to adapt and co-exist with humans as a result of significant growth in the human population and urbanization. These urban pests are important due to the large volume of faecal droppings on buildings and near human dwellings. In other parts of the world, house crows can transmit pathogens and diseases such as chlamydiosis, salmonellosis, colibacillosis, and avian tuberculosis. They also carry human pathogens such as Salmonella spp., Shigella serotypes, Vibrionaceae spp., Newcastle disease virus (NDV), avian influenza virus (AIV) and West Nile virus (WNV) that can be transmitted to humans through their faeces. The first AIV transmission from birds to humans that caused a pandemic was alarming because of the risk of AIV and other avian virus transmission from birds. This prompted investigations into crow populations in urban centres. This review details the potential of C. splendens to spread AIV, NDV and WNV in Malaysia's highly urbanized areas.
Introduction
In Malaysia, there are two distinct species of crows which are house crow (C. splendens) and jungle crow (Corvus macrorhynchos). C. splendens preferred to live in urban areas (Kurosawa et al. Citation2003) while C. macrorhynchos preferred to live in forest or rural areas for roosting and breeding (Shanbhag et al. Citation2012). C. splendens is also known with several names such as the Indian, grey-necked, Ceylon and Colombo crow (Krzemińska et al. Citation2016). Globally, they are recognized as pests and are one of the most invasive species that can establish a sizable population in the urban landscape and coastal areas in Malaysia (Wilson et al. Citation2015). This is due to the affinity of C. splendens toward open rubbish tips, food scraps and urban food sources (Csurhes Citation2016). Despite being unhygienic and noisy, C. splendens is perhaps best known for its aggressiveness. House crows have been reported to steal people's food and kill the chicks of native birds since this species is not afraid of people or other birds (Feare and Mungroo Citation1990; Kamel Citation2014; Csurhes Citation2016). C. splendens have also been reported to harbour numerous pathogens, including avian influenza virus (AIV) (Fraser et al. Citation2015), West Nile virus (WNV) (Kamel Citation2014) and Newcastle disease virus (NDV) (Cooper Citation1996).
Influenza A virus (IAV) is a global concern for both animal and public health. Except for the new H17N10 and H18N11, isolated from bats (Wu et al. Citation2014), all virus subtypes from H1-H16 and N1-N9 can be found in aquatic birds (Kombiah et al. Citation2020). AIVs can be transmitted to humans by exposure to contaminated environments, directly from infected birds or an intermediate host (Fadel and Afifi Citation2017). Highly pathogenic avian influenza (HPAI) H5N1 virus has been reported to circulate in India among different species of birds, including the C. splendens, resulting in die-off due to H5N1 and H9N2 in 2015 (Kombiah et al. Citation2020). In early 2004, the first human case of H5N1 was reported in Thailand, this strain may have mortality rates up 60% mortality rate (CNN Citation2004), which is concerning because the 1918 pandemic that killed 3% of the human population had only a 20% mortality rate (Vora Citation2012). In a 2017 study, it was found that 39 out of 44 house crow samples tested positive for AIV, which corresponds to 88.6% of the population (Fadel and Afifi Citation2017).
In the United States of America, the Corvus spp. play a crucial role in transmitting WNV because they are highly susceptible to the virus and act as amplifying hosts (Hinton et al. Citation2015). WNV spread among American crows (C. brachyrhynchos) likely by a mosquito vector (Hinton et al. Citation2015) or via bird-to-bird transmission (fecal-oral) (Dawson et al. Citation2007). In 2004–2013, out of 1513 WNV-positive bird carcases were collected in Orange County, South California (Liao et al. Citation2014), 82.7% of the WNV-positive birds were C. brachyrhynchos. Several reports in the past stated that the C. splendens plays a vital role in transmitting the virulent strains of NDV to domestic poultry (Rehan et al. Citation2019). The transmission of NDV from C. splendens to the poultry industry poses a serious threat to several countries, including Ukraine, Russia and Kazakhstan (Korotetskiĭ et al. Citation2010). NDV is also a real concern to Pakistan (Rehan et al. Citation2019), Egypt (Dewidar et al. Citation2021) and Bangladesh (Khatun et al. Citation2018), as it is a major cause of losses among poultry producers. In the region of Punjab, Pakistan, 16% (4 out of 25) of Corvus spp. tested positive for NDV (Munir et al. Citation2015).
Malaysia is one of the highest poultry consumers with an estimate of 1.44 million tons of poultry meat production in 2014 (Wahab and Rittgers Citation2014). Poultry consumption among Malaysians was approximately 49.3 kg per capita in 2020, 48.7 kg per capita in 2021 (Hirschmann Citation2021a) and expected to increase up to 51.28 kg per capita by 2025 (Hirschmann Citation2021b). The increasing poultry demand extends the potential of disease transmission from an infected Corvus spp. to poultry, posing major economic consequences, especially in the local poultry industry (Rehan et al. Citation2019). The diseases have the potential to be spread to humans via poultry or direct contact with the C. splendens (Islam and Ahmed Citation2019). The majority of AIV, NDV and WNV-infected humans were reported to visit live poultry markets or had a history of direct poultry handling (Uyeki and Peiris Citation2019). Food preparation from infected poultry and raw product consumption (such as drinking blood or eating uncooked embryonated eggs) have all been linked to an increased risk of disease transmission from poultry (Harder et al. Citation2016). Alternatively, the spread of harmful pathogens from C. splendens to humans can happen through direct and indirect contact with nasal secretions, bodily fluids and faeces shed by the infected C. splendens (Summa et al. Citation2018).
In this review, we investigate the potential of C. splendens to transmit pathogens, especially those that cause AIV, NDV and WNV, among Malaysia's local poultry and in highly urbanized areas. We also summarize the current state of our understanding of how C. splendens can affect the transmission of diseases in poultry and humans. Knowledge about C. splendens as a carrier of pathogenic viruses can potentially help prepare for and mitigate potential disease outbreaks into poultry, and subsequent transmission to humans.
Corvus splendens in Malaysia
C. splendens is an invasive species found worldwide except for South America and Antarctica (Krzemińska et al. Citation2016). This species has ecological plasticity (Montgomery Citation2019), which explains the presence of C. splendens in small breeding populations in Netherlands and South Africa (Krzemińska et al. Citation2016). According to Ryall (Citation2016), C. splendens migration to new countries is attributable to several factors including increased global trade and faster ship travel between countries. Inter-country migration can happen when C. splendens acts as a ‘hitchhiker’, travelling on ships to nearby islands and mainland sites. Due to this, there were reported cases of singles or pairs of C. splendens in certain places such as Mungab Island of South Korea and Nokomis Beach of Florida, United States of America. C. splendens can also migrate via human facilitated introduction of this species into its non-native range (for example the crow introduction to Zanzibar from India in 1890s) to combat the caterpillar plagues in a coffee plantation and handle anthropogenic wastes (Krzemińska et al. Citation2016).
In Malaysia, C. splendens was first introduced when the birds hitchhiked on incoming Indonesian ships in the 1800s (Wilson et al. Citation2015). Importation of C. splendens during the British occupation from Sri Lanka to Port Klang and Penang to resolve the caterpillar plague on the coffee plantation in the twentieth century further aided the spread of C. splendens across Peninsular Malaysia. C. splendens are associated with human habitations and are often seen in urban areas while C. macrorhynchos are usually found in human habitations and landscapes at higher altitudes (Mahesh and Suseela Citation2021). In , the differences in appearance between C. splendens and C. macrorhynchos are highlighted. C. splendens has broad, dull greyish feathers covering the nape, upper mantle, rear ear-coverts, neck and breast, while C. macrorhynchos is all black with purplish to dark bluish gloss feathers (Robson Citation2019). Both species posed a threat to native birds by pecking at them and stealing their eggs and food, however C. macrorhynchos tend to be more aggressive, especially when food sources are scarce (Shanbhag et al. Citation2012). Even so, C. splendens is still one of the world's worst invasive species as C. splendens has a higher population (Shanbhag et al. Citation2012), excellent adaptability to various environments (Fraser et al. Citation2015), and negative impacts on native birds, vertebrates and invertebrates (Yap and Sodhi Citation2004; Kamel Citation2014).
Figure 1. Comparison between house crows and jungle crows. (A) House crow (C. splendens) has a brighter colour on its neck, thus also called grey necked crow. (B) Large-billed crow species (C. macrorhynchos) or the jungle crow with full black feathers and a distinct big beak. Photo (a) was taken at Johor Bahru, Johor while photo (b) was taken by a fellow research officer in FRIM, Aina Amira Mahyudin at Ulu Muda Reserved Forest, Kedah.

C. splendens also posed a threat to humans as there were cases of bird strikes on airplanes, snatching food and swooping at people walking in parks and streets (Suliman et al. Citation2011; Shimba and Jonah Citation2017). C. splendens can become a disease carrier for humans and domestic animals (Suliman et al. Citation2011) as they can also be infected by the same pathogens such as Campylobacter spp., Escherichia coli and Salmonella spp. (Ryall and Meier Citation2008). The rapid increase in C. splendens population has also caused several problems in urban areas, such as damage to buildings and tourist attraction sites due to faecal droppings, annoying noises, acting as a crop pest, competing with native birds, and replacing native biodiversity (Alias and Hashim Citation2016).
C. splendens have never been observed to live independently of humans. It can be found abundantly in sites with poor food waste and garbage management (Alias and Hashim Citation2016). This is because C. splendens is an opportunistic feeder known to scavenge on carcases, stealing human food, invade a wide range of food crops, and kill livestock which includes poultry, young sheep and goats (Fraser et al. Citation2015). Therefore, C. splendens acts as an indicator of poor cleanliness and health. It has also become a threat to the indigenous bird species, as C. splendens can result in species displacement by attacking, killing and destroying the nests and eggs of birds (Fraser et al. Citation2015). It is also the culprit for reducing the number of small reptiles and amphibians (Kamel Citation2014). Furthermore, the close proximity between C. splendens and other birds and humans may contribute to spreading harmful pathogens through direct and indirect contact with the nasal secretions, bodily fluids and faecal droppings (Summa et al. Citation2018).
Corvus splendens as a disease carrier
Pathogen spillover between backyard chickens and wild birds has become more commonly reported in poultry agriculture (Ayala et al. Citation2020). Emerging and re-emerging avian infectious diseases have become a threat to birds all around the globe and may contribute to the extinction of species when coupled with invasive species displacement (Smith et al. Citation2006). Repeated disease transmission of multi-host pathogens into recipient species, such as the spillover of pathogens from wild birds to domestic chickens, can cause pathogen establishment and spillback from backyard poultries (Cross et al. Citation2019), for example, the H5N1 goose/Guangdong strain (Haider et al. Citation2017). NDV is also one of the poultry-wild bird transmission concerns as all bird species are susceptible to the virus replication, shedding and transmission (Snoeck et al. Citation2013). Free-ranging backyard flocks are potential sources of pathogens spillover into resident and endemic countries for many kinds of infectious diseases, including NDV (Gottdenker et al. Citation2005; Hernandez-Divers et al. Citation2008). Previously dead crows were found near poultry farms only after the outbreak, such as the isolation of H5N1 from two C. macrorhynchos after poultry outbreaks in the country (Nagarajan et al. Citation2010). The same virus strain was also isolated from C. corone and C. macrorhynchos near outbreak sites in Bangladesh (Khan et al. Citation2014). Studies on the infection dynamics are still limited. However, Corvus spp. are terrestrial birds that find good accessibility to food from backyard poultry waste products, making them a good candidate in disease transmission as their natural residence is located near human environments and close to poultry habitats (Fadel and Afifi Citation2017). Chickens can be infected by WNV, but they do not develop clinical symptoms, do not re-infect mosquitoes and are considered dead-end hosts for WNV (Langevin et al. Citation2001), thus they are often used as sentinels to monitor for WNV transmission (Holcomb et al. Citation2022). Humans are also considered dead-end hosts for WNV but may manifest symptoms such as encephalitis and meningitis which can be fatal in humans (Papa et al. Citation2010).
Humans are susceptible to avian infectious diseases that have been circulating in a variety of species, including in C. splendens (Summa et al. Citation2018). Chlamydiosis, salmonellosis, colibacillosis, arizonosis, avian tuberculosis, histoplasmosis and allergic alveolitis are among the avian diseases infecting humans (Jacob et al. Citation2011). In addition, C. splendens also carry human pathogens such as Salmonella spp., Shigella serotypes, Proteus spp., Vibrionaceae spp., Pseudomonas spp., Escherichia coli, Campylobacter spp., NDV, AIV and WNV (Cooper Citation1996; Kamel Citation2014; Fraser et al. Citation2015). These diseases can be transmitted to humans in many ways, as the birds shed these pathogens in their mucous, saliva and faeces (Uyeki and Peiris Citation2019), as demonstrated by the detection of pathogens in cloacal swab samples (Alias and Hashim Citation2016) and bird droppings (Prathipa et al. Citation2015).
Influenza A virus
Influenza is a negative-sense RNA, classified into influenza types A, B, C and D (Ahmed et al. Citation2018). Influenza A virus (IAV) is a virus that most commonly affects birds and humans (Du et al. Citation2019). Older adults and children are among the high-risk groups who are susceptible to IAV. Older adults (aged 75 and over) significantly have lower body temperatures (compared to younger adult) when infected with influenza-associated pneumonia, which may cause misdiagnosis and delay in treatment because it does not meet the fever threshold for influenza, making older adults susceptible to IAV with asymptomatic infections, probably because of their weakened innate and adaptive immune responses (Wong et al. Citation2020). Meanwhile, children (those under the age of six) are more vulnerable to IAV, possibly due to a lack of immune history and a narrow immune response that targets the IAV haemagglutinin of the strain that caused the infection only, as opposed to adults, who have a much broader response, including protection against the majority of group 1 subtype haemagglutinin (Meade et al. Citation2020). Each year, influenza infection is estimated to cause around 3–5 million cases of severe illness and 250,000–500,000 deaths in developing countries (Parash et al. Citation2019).
Influenza A virus in history
The pandemic potential of Influenza A virus outbreak has been recognized since the Spanish flu outbreak, also known as The Great Pandemic of 1918–1919 (Beveridge Citation1991). Spanish flu, which started in late August in 1918 (Reid et al. Citation2001), eventually helped to end World War I since thousands of soldiers were infected. It caused at least 50 million deaths worldwide (Taubenberger et al. Citation2019). The 1918 H1N1 virus underwent genetic reassortment and caused several more outbreaks, which are 1957 H2N2, 1968 H3N2 and 2009 H1N1pdm. The 2009 H1N1pdm is genetically similar to the 1918 Spanish flu, with additional gene swapping between avian, swine and human origin, where pigs are considered as ‘mixing vessels’ for the generation of the hybrid (Taubenberger et al. Citation2019). Thus, it gained its name, the swine flu. The ability of IAVs to mutate and infect a broad host range is concerning, as it has the potential to mutate into a highly pathogenic virus in the future. In Malaysia, influenza cases are usually seen without seasonal trends, with 22.3% seroprevalence of seasonal H1N1 and 14.7% for H3N2. The 2009 swine flu outbreak in Malaysia resulted in 12,307 reported cases, with 77 reported deaths (Jamal and Sam Citation2015).
Avian influenza virus
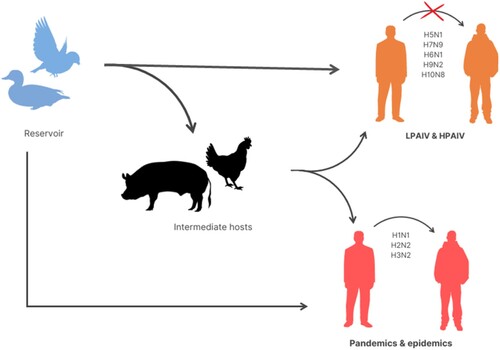
Avian influenza A viruses (AIVs) are mostly transmitted to humans through the reservoir, which is mainly waterfowl, either directly or indirectly through intermediate hosts such as swine and poultry (Ahmed et al. Citation2019). AIVs (H1N1, H2N2 and H3N2) with stable human-to-human transmissions have previously caused pandemics and are currently responsible for epidemics. The AIV in wild aquatic birds evolved slower than the AIV in poultries and humans (Wright et al. Citation2013). AIVs can be classified into two types which are the low pathogenic AIV (LPAIV) (H9N2 and H10N8) and highly pathogenic AIV (HPAIV) (H5N1 and H7N9). The subtypes of AIV from birds that have crossed the species barrier and infected humans are H5N1, H5N6, H6N1, H7N2, H7N3, H7N4, H7N7, H7N9, H9N2, H10N7 and H10N8, all of which can lead to sporadic infections or fatalities in humans (Ahmed et al. Citation2018). shows how AIVs are transmitted from natural reservoirs to humans either directly or indirectly via the intermediate hosts such as swine and poultry. Previously, there were several reports of H5N1 isolation from Corvus sp. For example, in 2011, H5N1 was isolated from C. splendens in Bangladesh (Khan et al. Citation2014) and it was also isolated from C. macrorhynchos in Japan around 2004 (Tanimura et al. Citation2006). Even though existing LPAIVs and HPAIVs do not cause stable human-to-human transmission, they nonetheless constitute a threat to future pandemics (Wright et al. Citation2013). Thus, surveillance studies of AIVs are strongly encouraged since the data is lacking, especially in developing countries.
Figure 2. Transmission of avian influenza viruses (AIVs). AIVs are mostly transmitted to humans through the reservoir, which is generally waterfowl, either directly or indirectly through intermediate hosts (such as swine and poultry). AIVs (H1N1, H2N2 and H3N2) with stable human-to-human transmissions have previously caused pandemics and are currently responsible for epidemics. Despite the fact that existing LPAIVs and HPAIVs (like H5N1, H7N9 and H10N8) do not cause stable human-to-human transmission, they nonetheless constitute a threat to future pandemics.
LPAIV and HPAIV outbreaks in domestic poultry
LPAIV was detected in Malaysia in 1989, and strains such as H4N3, H4N6 and H3N6 were detected from passerines and domestic birds (Ibrahim et al. Citation1990). H4N3 isolates were found from a dead magpie robin (Copsychus saularis) at Selangor transit aviary and from a sick, yellow-vented bulbul (Pycnonotus goiavier) that could not fly. H2N9 and H6N5 were isolated in 1992 (Verhagen Citation2016), H7N1 was detected in 1994; H10N5 in 1998; H3N2 in 2002 (Alexander Citation2007); H3N8 in 2004 (Ng et al. Citation2006); H9N2 in 1998 (He et al. Citation2013); and H3N2 in 2010 (Mohidem et al. Citation2017). There are multiple reasons for the spread of LPAIV, including chicken smuggling, migration of the infected wild birds, cross-country poultry trade, producers and trader's business activities, and commercial movements of birds and tourism activities in which infected humans may help spread the diseases (Wan and Tariq Citation2018).
As for HPAIV in Malaysia, there were four waves of the H5N1 outbreak from 2004 to 2017, that did not cause any human deaths (Wan and Tariq Citation2018). The H5N1 outbreak was first detected in Kelantan in 2004 (Mohidem et al. Citation2017). The HPAIV outbreak was restricted to around 12 localities in Kelantan near the Thailand border. H5N1 that caused the outbreak in Kelantan belonged to the 1.2.3 and 2.3.4 clades of the Vietnam/Thailand/Malaysia (VTM) strain. In poultry farms around the vicinity, the viruses were detected in ducks and chickens (Sharifah et al. Citation2005). The viruses were also detected in Kuala Lumpur and Perak in the same year (Rafidah and Asiah Citation2004). It was also noted that the second outbreak detected in Kuala Lumpur, Perak and Penang in 2006 had genetic similarity with the Fujian-like sublineage (clade 2.3.4) outbreak in 2004. The HPAIV H5N1 variant also shared similarities with both Indonesia and China strains, but the HA gene of H5N1 in 2006 does not group with the VTM sub lineage as in 2004. The next outbreak was in 2007 and was detected in Selangor, Kuala Lumpur and Kelantan in 2007 (Wan and Tariq Citation2018). The outbreak was due to the import of domestic fowl from neighbouring countries and tourism activities (WHO Citation2007). Kalimantan also had the same H5N1 outbreak in the same year. However, there were no reported cases of the H5N1 outbreak in Sabah and Sarawak. In 2017, another outbreak occurred at 43 locations and six districts in Kelantan. At the moment, no human cases of H5N1 infections have been reported in Malaysia, although it has gradually affected the neighbouring countries since the first death was reported in Thailand (Tee et al. Citation2009).
West Nile virus
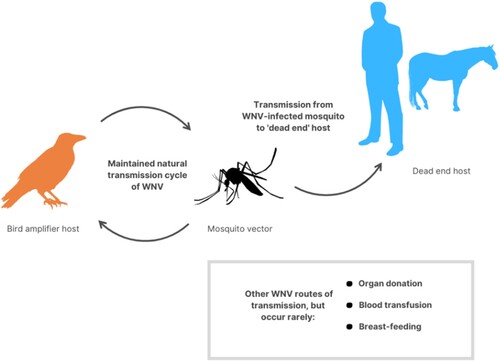
West Nile virus (WNV) is also one of the pathogens that Corvus spp. can harbour (Kamel Citation2014). It is a positive-sense single-stranded RNA virus that is a member of the flavivirus genus and belongs to the Japanese encephalitis antigenic complex of the family Flaviviridae. WNV is an arbovirus and is maintained in a cycle involving transmission between birds and mosquitoes (Culex spp.) (Pierson and Diamond Citation2013). shows the transmission of this virus between the vector, amplifying host and mammals, as well as via other possible routes of transmission. Humans and other mammals are considered incidental and dead-end hosts. WNV primarily consists of two lineages. Lineage 1 is associated with human clinical encephalitis, while lineage 2 is not associated with human clinical encephalitis (Bakonyi et al. Citation2006). Since its first discovery in Uganda, WNV has been a prevalent mosquito-borne disease associated with sporadic outbreaks of moderate sickness in humans in Africa, the Middle East and Europe until the mid-1990s (Hayes et al. Citation2005). WNV has been a common mosquito-borne disease in the continental United States of America since first introduced in 1999 on the East Coast. In the United States of America, WNV resulted in 29,000 human cases and more than 1100 deaths from 1999 to 2008 (Sugumaran et al. Citation2009). This indicates that WNV infections have high morbidity and mortality rates in humans and equines (Thomas-Bachli et al. Citation2020). Rarely, WNV also can be transmitted via organ donation, blood transfusion and breast-feeding (Rossi et al. Citation2010).
Figure 3. Transmission of WNV. WNV is naturally maintained between birds and mosquitos (especially Culex spp.), before being transmitted by infected mosquitoes to dead-end hosts such as humans, horses and other mammals. Rarely, WNV also can be transmitted via organ donation, blood transfusion and breast-feeding.
Some Passerine species, including Corvids like C. brachyrynchos and blue jays (Cyanocitta cristata), are susceptible to WNV infection and have the potential to produce high levels of viremia and mortality. According to Komar et al. (Citation2003), the most fatal WNV infections in birds occurred in Corvus brachyrynchos, which is related to the nature of Corvids carrion-feeding behaviour, which may expose the bird to the virus by eating infected bird carcases. The same rule might apply to C. cristata as they also feed on carcases. Until 2014, there was no data on WNV cases in wild birds in Malaysia, but several studies showed low prevalence (1.21%) (9/742) among Malaysia's Orang Asli (Marlina et al. Citation2014) and 4.41% (3/68) in companion bird populations in Selangor (Rais et al. Citation2011). The study is important since Malaysia's Orang Asli lives in rural areas, the frontier of the jungle and in the centre of the jungle where Culex pipiens mosquitoes can be abundantly found (Marlina et al. Citation2014). However, in 2020, the first reported study on the prevalence of WNV found a WNV seropositive rate of 18.71% and a molecular prevalence of 15.2% in migratory and resident wild birds found on the west coast of Malaysia. Phylogenetic analyses of the isolates revealed 99% similarity to the strains from South Africa and were clustered under lineage 2 (Yuseri et al. Citation2019). This finding indicates that WNV is circulating among the local wild birds and can be attributed to several key factors; the presence of migratory birds which may harbour the virus, the presence of breeding sites for mosquitoes especially a wide variety of Culex mosquito abundance in Malaysia that mainly serve as vectors, and the ability of the virus to infect a wide range of susceptible hosts. Active surveillance of WNV in birds is crucial in order to understand WNV transmission cycles. Thus, this may help in preventing the chain of transmission towards humans especially those with low antibody levels that are likely to develop severe symptoms.
Newcastle disease virus
Newcastle disease virus (NDV) is panzootic in racing pigeons, and it is still a significant concern in commercial poultry breeding because it can cause death even among vaccinated poultry (Seal et al. Citation1999). NDV are enveloped viruses with negative single-stranded RNA belonging to the genus Orthoavulavirus in the family Paramyxoviridae (Miller and Koch Citation2013). NDV can be transmitted by inhaling or ingesting the virus shed in faeces and respiratory secretions by diseased or carrier birds. The paramyxovirus strains can be categorized by the avian species it infects. Avian paramyxovirus-1 is highly susceptible to chickens; avian paramyxovirus-2 and avian paramyxovirus-3 cause disease in poultry, while avian paramyxovirus-6 and avian paramyxovirus-7 cause disease in turkeys (Alexander Citation2000). NDV is divided into three pathotypes: namely velogenic, mesogenic and lentogenic. NDV is believed to be spread by migratory birds of the Nearctic (mild respiratory waterfowl type) or the jungle bird of the tropics (severe viscerotropic type) (Hanson Citation1976).
In Malaysia, NDV with genotypes VI and VII are commonly found in imported birds. For genotype VI, VIa viruses are the most common ones. Although the genotype VIa has not led to outbreaks in Malaysia, it should not be ignored, as it has been reported to cause two outbreaks in South Africa. Conversely, genotype VII is usually associated with an NDV outbreak, especially in the Middle East and Asia. In Malaysia, genotype VII caused numerous outbreaks based on the data from 2000 until 2012. Genotype VIIi has been isolated from poultry, which is the same virus that caused the NDV outbreak in Pakistan and Indonesia (Leow et al. Citation2019; Dewidar et al. Citation2021). According to Geetha et al. (Citation2011), NDV was found in C. splendens droppings, thus suggesting the role of C. splendens in the epidemiology of NDV. We hypothesized that C. splendens might be infected with NDV from migrating birds and thus served as an intermediate host for NDV transmission in poultry.
Since NDV is a highly fatal disease, it is considered a global threat to the poultry industry, as it is still infective despite intensive vaccination. A major outbreak in the USA resulted in 4 million bird (backyard poultry flocks and most birds) deaths and losses of USD 162 million during the Exotic Newcastle disease outbreak (Rehan et al. Citation2019). In Pakistan, the poultry industry is one of the country's most significant sources of income, which contributes USD 2 billion of profit per year. The outbreak in 2012 caused the death of approximately 45 million poultry in the Jallo Wildlife Park, Punjab, Pakistan. NDV kills millions of village backyard poultry in Bangladesh yearly and impacts the household income of those in poor rural areas (Khatun et al. Citation2018). Every year, NDV causes losses of approximately USD 289 million in Bangladesh. These outbreak incidents prove that active NDV surveillance programmes should be carried out to prevent future outbreaks, leading to enormous losses for poultry producers.
Corvus splendens control programme in Malaysia
C. splendens is classified as a bird pest in Malaysia due to its role as a public nuisance and health threat. Among the suggested ways to reduce the bird pest problem is the periodic shooting of the C. splendens that the City Councils conduct in hot-spot areas like Klang, Kepong, Cyberjaya, Cheras, Kajang, and other states as an initiative to cut down the population of C. splendens. Other control methods practiced by Southeast Asian countries to address the overpopulation of bird pests like C. splendens include direct control, sterilization of birds, scaring and bio-acoustics technique and habitat modification (Yap and Sodhi Citation2004).
Direct control of birds includes baits and explosives; however, it is ineffective as the birds are quickly replaced by the number of juveniles either by reproduction or migration. The sterilization technique involved using chemosterilants, which can be laborious due to the efficiency of the chemicals towards different species of birds. Scarecrows are able to frighten away some bird species, but not C. splendens, as the species is very comfortable living among humans. As for habitat modification for C. splendens, there are several initiatives to prevent habitation of C. splendens, such as changes in the rubbish bin design and planting less suitable trees for house crows nesting, which will reduce the house crow population, but this may take a long time before becoming effective.
Additionally, a lack of precaution taken by the authorities during shooting exercises imposed high risks during and after these events. For decades, ongoing management has failed to eradicate the species, while they have successfully established a sizable population in several cities in Malaysia. Therefore, we call for the proper management of these birds to be implemented, with a better understanding of the species for efficient control of their population to avoid human health concerns and detrimental effects on the environment, economy and livestock. Two types of control measures have been shown to successfully eradicate C. splendens: physical control and chemical control (Shivambu et al. Citation2020). Successful physical control of C. splendens can be seen in Yemen in which they collect and kill the young birds followed by shooting of adults. In Australia, C. splendens were shot at the point of entry, thus stopping the establishment of C. splendens in the country (Ryall Citation2002). Mauritius's chemical control includes poisoning the house crows with starlicide (Puttoo and Archer Citation2004) and α-chloralose poison (Feare and Mungroo Citation1990).
In Malaysia, shooting adult C. splendens is a common practice aimed at reducing their number. Chemical control can also be employed, however, this requires proper handling practice, such as by mixing chemicals with food waste in the crow's territory, to avoid the non-target bird species (Dolbeer and Linz Citation2016). Other than that, public support plays a vital role in ensuring the success of the eradication programme. This can be done by creating awareness of how C. splendens can spread harmful pathogens towards humans and the importance of proper garbage management in reducing the number of C. splendens.
Conclusion
C. splendens is well-known to cause bacterial infection in humans (Fraser et al. Citation2015). However, the possibility of C. splendens transmitting viruses is still a neglected topic in Malaysia since there have never been abundant bird deaths reported due to viral infection in Malaysia. An NDV surveillance study is also crucial since Malaysia is one of the highest poultry consumers in the world, and the poultry industry is an essential source of supply protein to Malaysians. It has also been proven that NDV can cause significant loss in a country's economy due to the sacrifice of poultry to stop the spread of the disease. In the past, the influenza virus was once ignored due to the difficulty in diagnosing the symptoms in humans. Presently, the seroprevalence and surveillance data of AIVs are still lacking due to socio-economic costs and diagnostics facilities. WNV is also neglected due to the absence of reported cases in Malaysia. However, Malaysia is a suitable habitat for the WNV vector, Culex pipiens, and the climate is preferable for migrating birds during the Northern and Southern winter periods. Hence, the possibility of WNV infection in the future should be studied, and surveillance must be taken as a preventive measure. Furthermore, the transmission of viruses such as AIV, NDV and WNV has been reported from the C. splendens to the local flocks in other countries through surveillance studies. Thus, an active surveillance study may help prevent the spread of these RNA viruses carried by the ubiquitous C. splendens presence in the city.
Ethical approval
This article does not contain any studies involving animals performed by any of the authors.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ahmed M, Elsayed MA, Mettenleiter TC, Pleschka S. 2018. Zoonotic potential of influenza A viruses: a comprehensive overview. Viruses. 10(9):497.
- Ahmed SK, Ahmed K, Mokhtar RG, Rabeh ES, Sara M, Nabil H, Mohamed F, Basma S, Pamela PM, Richard JW, Ghazi K. 2019. Surveillance for avian influenza viruses in wild birds at live bird markets, Egypt, 2014-2016. Influenza Other Respir Viruses. 4:407–414.
- Alexander DJ. 2000. Newcastle disease and other avian paramyxoviruses. Rev Sci Tech Off Int Epizoot. 2:443–455.
- Alexander DJ. 2007. Summary of avian influenza activity in Europe, Asia, Africa, and Australasia, 2002—2006. Avian Dis. S1:161–166.
- Alias NA, Hashim HS. 2016. House crow presence as unsustainable urban indicator? Int J Malay World Civil (Iman). 4:59–65.
- Ayala AJ, Yabsley MJ, Hernandez SM. 2020. A review of pathogen transmission at the backyard chicken–wild bird interface. Front Vet Sci. 7:662.
- Bakonyi T, Ivanics É, Erdélyi K, Ursu K, Ferenczi E, Weissenböck H, Nowotny N. 2006. Lineage 1 and 2 strains of encephalitic West Nile virus, Central Europe. Emerg Infect Dis. 4:618–623.
- Beveridge WI. 1991. The chronicle of influenza epidemics. Hist Philos Life Sci. 2:223–234.
- CNN. 2004. Bird flu claims first Thai victim; [accessed 2021 Aug 6]. http://edition.cnn.com/2004/WORLD/asiapcf/01/25/bird.flu/.
- Cooper JE. 1996. Health studies on the Indian house crow (Corvus splendens). Avian Pathol. 2:381–386.
- Cross PC, Prosser DJ, Ramey AM, Hanks EM, Pepin KM. 2019. Confronting models with data: the challenges of estimating disease spillover. Philos Trans Royal Soc B. 1782:20180435.
- Csurhes S. 2016. Biology and ecology in Indian house crow invasive animal risk assessment; [accessed 2020 Feb 26]. https://www.daf.qld.gov.au/data/assets/pdffile/0007/74986/IPA-Indian-House-Crow-Risk-Assessment.pdf.
- Dawson JR, Stone WB, Ebel GD, Young DS, Galinski DS, Pensabene JP, Franke MA, Eidson M, Kramer LD. 2007. Crow deaths caused by West Nile virus during winter. Emerg Infect Dis. 12:1912–1914.
- Dewidar AAA, El-Sawah AA, Shany SAS, Dahshan AM, Ali A. 2021. Genetic characterization of genotype VII.1.1 Newcastle disease viruses from commercial and backyard broiler chickens in Egypt. German J Vet Res. 1:11–17.
- Dolbeer RA, Linz GM. 2016. Blackbirds. Wildlife Damage Management Technical Series, U.S. Department of Agriculture, Animal & Plant Health Inspection Service, Wildlife Service.https://digitalcommons.unl.edu/nwrcwdmts/1/.
- Du R, Cui Q, Rong L. 2019. Competitive cooperation of hemagglutinin and neuraminidase during influenza A virus entry. Viruses. 11:458.
- Fadel MH, Afifi R. 2017. Investigation of avian influenza infection in wild birds in Ismailia and Damietta cities, Egypt. Veterinary World. 10:695–701.
- Feare CJ, Mungroo Y. 1990. The status and management of the house crow Corvus splendens (Vieillot) in Mauritius. Biol Conserv. 51:63–70.
- Fraser DL, Aguilar G, Nagle W, Galbraith M, Ryall C. 2015. The house crow (Corvus splendens): a threat to New Zealand? ISPRS Int J Geoinf. 4:725–740.
- Geetha M, Gunaseelan L, Ganesan PI, Kumanan K, Selvaraju G. 2011. Role of Indian house crows (Corvus splendens) in the epidemiology of Newcastle disease. Indian J Comp Microbiol Immunol Infect Dis. 32:50–51.
- Gottdenker NL, Walsh T, Vargas H, Merkel J, Jiménez GU, Miller RE, Dailey M, Parker PG. 2005. Assessing the risks of introduced chickens and their pathogens to native birds in the Galápagos Archipelago. Biol Conserv. 126:429–439.
- Haider N, Sturm-Ramirez K, Khan SU, Rahman MZ, Sarkar S, Poh MK, Shivaprasad HL, Kalam MA, Paul SK, Karmakar PC, Balish A. 2017. Unusually high mortality in waterfowl caused by highly pathogenic avian influenza A (H5N1) in Bangladesh. Transbound Emerg Dis. 64:144–156.
- Hanson RP. 1976. Avian reservoirs of Newcastle disease. In: Page L.A., editor. Wildlife diseases. Boston, MA: Springer; p. 185–195.
- Harder TC, Buda S, Hengel H, Beer M, Mettenleiter TC. 2016. Poultry food products—a source of avian influenza virus transmission to humans? Clin Microbiol Infect. 22:141–146.
- Hayes EB, Komar N, Nasci RS, Montgomery SP, O’Leary DR, Campbell GL. 2005. Epidemiology and transmission dynamics of West Nile virus disease. Emerg Infect Dis. 11:1167–1173.
- He F, Prabakaran M, Tan Y, Indira K, Kumar SR, Kwang J. 2013. Development of dual function ELISA for effective antigen and antibody detection against H7 avian influenza virus. BMC Microbiol. 13:1–9.
- Hernandez-Divers SM, Villegas P, Jimenez C, Hernandez-Divers SJ, Garcia M, Riblet SM, Carroll CR, O’Connor BM, Webb JL, Yabsley MJ, Williams SM. 2008. Backyard chicken flocks pose a disease risk for neotropical birds in Costa Rica. Avian Diseases Digest. 52:558–566.
- Hinton MG, Reisen WK, Wheeler SS, Townsend AK. 2015. West Nile virus activity in a winter roost of American crows (Corvus brachyrhynchos): is bird-to-bird transmission important in persistence and amplification? J Med Entomol. 1:683–692.
- Hirschmann R. 2021a. Per capita meat consumption in Malaysia 2021, by type; [accessed 2021 Sep 7]. https://www.statista.com/statistics/756920/malaysia-meat-consumption-per-capita-by-type/.
- Hirschmann R. 2021b. Per capita poultry consumption in Malaysia 2006-2025; [accessed 2021 Sep 7]. https://www.statista.com/statistics/757983/malaysia-poultry-consumption-per-capita/.
- Holcomb KM, Nguyen C, Foy BD, Ahn M, Cramer K, Lonstrup ET, Mete A, Tell LA, Barker CM. 2022. Effects of ivermectin treatment of backyard chickens on mosquito dynamics and West Nile virus transmission. PLoS Negl Trop Dis. 16:e0010260.
- Ibrahim HM, Awang IPR, Alexander DJ, Manvell RJ, Aini I, Ibrahim AL. 1990. Isolation of influenza A viruses from passerine birds in Malaysia. Vet Record. 127:528.
- Islam S, Ahmed MS. 2019. Knowledge, attitude, and practice toward zoonotic diseases among different professionals at selected coastal areas in Bargunadistrict, Bangladesh. J Adv Vet Anim Res. 6:284–289.
- Jacob JP, Gaskin JM, Wilson HR, Mather FB. 2011. Avian diseases transmissible to humans. Lexington: Cooperative Extension Services, University of Kentucky.
- Jamal I, Sam C. 2015. The burden of human influenza in Malaysia. Med J Malaysia. 70:127–130.
- Kamel AM. 2014. Potential impacts of invasive house crows (Corvus splendens) bird species in Ismailia Governorate, Egypt; ecology, control and risk management. J Life Sci Technol. 2:86–89.
- Khan SU, Berman L, Haider N, Gerloff N, Rahman MZ, Shu B, Rahman M, Dey TK, Davis TC, Das BC, Balish A. 2014. Investigating a crow die-off in January–February 2011 during the introduction of a new clade of highly pathogenic avian influenza virus H5N1 into Bangladesh. Arch Virol. 159:509–518.
- Khatun M, Islam I, Ershaduzzaman M, Islam HM, Yasmin S, Hossen A, Hasan M. 2018. Economic impact of Newcastle disease on village chickens – a case of Bangladesh. J Econ Bus. 1:358–367.
- Komar N, Langevin S, Hinten S, Nemeth N, Edwards E, Hettler D, Davis B, Bowen R, Bunning M. 2003. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg Infect Dis. 9:311–322.
- Kombiah S, Kumar M, Murugkar HV, Nagarajan S, Tosh C, Kumar DS, Rajukumar K, Gautam S, Singh R, Karikalan M, Sharma AK. 2020. Experimental pathology of two highly pathogenic H5N1 viruses isolated from crows in BALB/c mice. Microb Pathog. 141:103984.
- Korotetskiĭ IS, Bogoiavlenskiĭ AP, Prilipov AG, Usachev EV, Usacheva OV, Turgambetova AS, Zaitseva IA, Kydyrmanov A, Shakhvorostova LI, Saiatov MK, et al. 2010. Molecular genetic characteristics of the Newcastle disease virus velogenic strains isolated in Russia, Ukraine, Kazakhstan, and Kirghizia. Probl Virol. 4:29–32.
- Krzemińska U, Wilson R, Song BK, Seneviratne S, Akhteruzzaman S, Gruszczyńska J, Swiderek W, Huy TS, Austin CM, Rahman S. 2016. Genetic diversity of native and introduced populations of the invasive house crow (Corvus splendens) in Asia and Africa. Biol Invasions. 7:1867–1881.
- Kurosawa R, Kanai Y, Matsuda M, Okuyama M. 2003. Conflict between humans and crows in greater Tokyo – garbage management as a possible solution. Glob Environ Res. 7:139–148.
- Langevin SA, Bunning M, Davis B, Komar N. 2001. Experimental infection of chickens as candidate sentinels for West Nile virus. Emerg Infect Dis. 7:726–729.
- Leow BL, Shohaimi SA, Mohd-Yusop FF, Sidik MR, Ong GH. 2019. Isolation and molecular characterization of Newcastle disease virus from imported birds at an animal quarantine station in Malaysia. Pertanika J Trop Agric Sci. 42:569–584.
- Liao Z, Nguyen K, Newton J, Nelson K, Cummings R. 2014. Developing a predictive risk model for West Nile virus activity based on mosquito breeding sources, environmental, and socioeconomic factors in Orange County, California. In Proceedings of the Mosquito and Vector Control Association of California Conference. 82:30–36.
- Mahesh V, Suseela L. 2021. Roosting behaviour and roosting interactions between house crow Corvus splendens and large-billed crow Corvus macrorhynchos at Machilipatnam, India. Int J Zoo Invest. 7:414–420.
- Marlina S, Radzi SF, Lani R, Sieng KC, Rahim NF, Hassan H, Li-Yen C, Abu Bakar S, Zandi K. 2014. Seroprevalence screening for the West Nile virus in Malaysia’s Orang Asli population. Parasit Vectors. 7:1–7.
- Meade P, Kuan G, Strohmeier S, Maier HE, Amanat F, Balmaseda A, Ito K, Kirkpatrick E, Javier A, Gresh L, et al. 2020. Influenza virus infection induces a narrow antibody response in children but a broad recall response in adults. mBio. 11:e03243–19.
- Miller PJ, Koch G. 2013. Newcastle disease. Dise Poul. 13:89–138.
- Mohidem NA, Hashim Z, Arshad SS. 2017. Avian influenza outbreaks in Malaysia, 1980–2017. Asia Pac Environ Occup Health J. 3:1–14.
- Montgomery S. 2019. Crow: Encyclopedia Britannica, Inc.; [accessed 2019 Oct 12]. https://www.britannica.com/animal/crow-bird.
- Munir T, Aslam A, Zahid B, Ahmed I, Imran MS, Ijaz M. 2015. Potential of commonly resident wild birds towards Newcastle disease virus transmission. Pak Vet J. 35:106–107.
- Nagarajan S, Tosh C, Murugkar HV, Venkatesh G, Katare M, Jain R, Behera P, Khandia R, Tripathi S, Kulkarni DD, Dubey SC. 2010. Isolation and molecular characterization of a H5N1 virus isolated from a Jungle crow (Corvus macrohynchos) in India. Virus Genes. 41:30–36.
- Ng WF, To KF, Lam WW, Ng TK, Lee KC. 2006. The comparative pathology of severe acute respiratory syndrome and avian influenza A subtype H5N1—a review. Hum Pathol. 37:381–390.
- Papa A, Danis K, Baka A, Bakas A, Dougas G, Lytras T, Theocharopoulos G, Chrysagis D, Vassiliadou E, Kamaria F, Liona A. 2010. Ongoing outbreak of West Nile virus infections in humans in Greece, July–August 2010. Eurosurveillance. 15:19644.
- Parash MT, Shimmi SC, Jeffree MS, Ibrahim MY, Ahmed K. 2019. Influenza!! A hidden burden of disease in Malaysia. Borneo J Med Sci. 13:1–2.
- Pierson TC, Diamond SM. 2013. Flaviviruses. In: D. M. Knipe, P. M. Howley, editor. Fields virology. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; p. 747–790.
- Prathipa A, Gomathinayagam S, Senthilkumar K, Palanivelrajan M, Niranjana C, Jayathangaraj MG. 2015. Endoparasitic infection in Indian house crow (Corvus splendens). Zoos’ Print. 2:16–17.
- Puttoo M, Archer T. 2004. Control and/or eradication of Indian crows (Corvus splendens) in Mauritius. Rev Agric Sucr L’île Maurice. 83:77.
- Rafidah A, Asiah N. 2004. Molecular identification of an avian influenza virus (AIV) subtype H6 isolated from cloacal swabs of ducks. In The 11th International Conference of The Association of Institutions for Tropical Veterinary Medicine. 255.
- Rais MN, Omar AR, Abu J, Omar MH. 2011. Prevalence of West Nile virus antibody in captive bird populations in selected areas in Selangor, Malaysia. In 6th Seminar on Veterinary Sciences: 11–14 January 2011; Faculty of Veterinary Medicine, Universiti Putra Malaysia 2011 Jan (Vol. 127).
- Rehan M, Aslam A, Khan MR, Abid M, Hussain S, Amber J, Hussain A. 2019. Potential economic impact of Newcastle disease virus isolated from wild birds on commercial poultry industry of Pakistan: A review. Hosts Viruses. 6:1–5.
- Reid AH, Taubenberger JK, Fanning TG. 2001. The 1918 Spanish influenza: integrating history and biology. Microbes Infect. 1:81–87.
- Robson C. 2019. Birds of South-East Asia: concise edition. Sydney, NSW: Bloomsbury Publishing Plc.; p. 413–414.
- Rossi SL, Ross TM, Evans JD. 2010. West Nile virus. Clin Lab Med. 30:47–65.
- Ryall C. 2002. Further records of range extension in the house crow Corvus splendens. Bull Br Ornithol Club. 122:231–240.
- Ryall C. 2016. Further records and updates of range expansion in house crow Corvus splendens. Bull BOC. 136:39–45.
- Ryall C, Meier G. 2008. House crow in the Middle East. Wildlife Middle East News. 3:7–8.
- Seal BS, King DJ, Sellers HS. 1999. The avian response to Newcastle disease virus. Dev Comp Immunol. 3:257–268.
- Shanbhag AP, Ishita G, Umakanth B. 2012. Interspecific behavioral studies of house crows (Corvus splendens protegatus) and jungle crows (Corvus macrorhynchos culminatus) on mutual foraging sites. Glob J Environ Res. 6:11–16.
- Sharifah SH, Suriani MN, Hassuzana K, Omar AR, Aini I. 2005. Avian influenza: managing risk and responses in Malaysia. In Kuala Lumpur, Malaysia. Proceedings of the 17th Congress of Veterinary Association Malaysia (published by Veterinary Association Malaysia).
- Shimba MJ, Jonah FE. 2017. Nest success of the Indian house crow Corvus splendens: an urban invasive bird species in Dar es Salaam, Tanzania. Ostrich. 88:27–31.
- Shivambu CT, Shivambu N, Downs CT. 2020. House Crows (Corvus splendens Vieillot, 1817). In: Downs CT, Hart LA, editors. Invasive Birds: Global Trends & Impacts. Wallingford: CAB International; p. 175–182.
- Smith KF, Sax DF, Lafferty KD. 2006. Evidence for the role of infectious disease in species extinction and endangerment. Conserv Biol. 20:1349–1357.
- Snoeck CJ, Owoade AA, Couacy-Hymann E, Alkali BR, Okwen MP, Adeyanju AT, Komoyo GF, Nakouné E, Le Faou A, Muller CP. 2013. High genetic diversity of Newcastle disease virus in poultry in West and Central Africa: cocirculation of genotype XIV and newly defined genotypes XVII and XVIII. J Clin Microbiol. 51:2250–2260.
- Sugumaran R, Larson SR, DeGroote JP. 2009. Spatio-temporal cluster analysis of county-based human West Nile virus incidence in the continental United States. Int J Health Geogr. 8:1–9.
- Suliman AS, Meier GG, Haverson PJ. 2011. Eradication of the house crow from Socotra Island, Yemen. Island invasives: eradication and management. Gland: IUCN; p. 361–363.
- Summa M, Henttonen H, Maunula L. 2018. Human noroviruses in the faeces of wild birds and rodents-new potential transmission routes. Zoonoses Public Health. 5:512–518.
- Tanimura N, Tsukamoto K, Okamatsu M, Mase M, Imada T, Nakamura K, Kubo M, Yamaguchi S, Irishio W, Hayashi M, Nakai T. 2006. Pathology of fatal highly pathogenic H5N1 avian influenza virus infection in large-billed crows (Corvus macrorhynchos) during the 2004 outbreak in Japan. Vet Pathol. 43:500–509.
- Taubenberger JK, Kash JC, Morens DM. 2019. The 1918 influenza pandemic: 100 years of questions answered and unanswered. Sci Transl Med. 11:eaau5485.
- Tee KK, Takebe Y, Kamarulzaman A. 2009. Emerging and reemerging viruses in Malaysia, 1997–2007. Int J Infect Dis. 3:307–318.
- Thomas-Bachli AL, Pearl DL, Parmley EJ, Berke O. 2020. The influence of sociodemographic factors on the engagement of citizens in the detection of dead Corvids during the emergence of West Nile virus in Ontario, Canada. Front Vet Sci. 6:483.
- Uyeki TM, Peiris M. 2019. Novel avian influenza A virus infections of humans. Infect Dis Clin North Am. 4:907–932.
- Verhagen J. 2016. Influenza A viruses in migratory birds: ecology, evolution and the wild-domestic interface. Rotterdam, NL: Erasmus University Rotterdam; p. 66-67.
- Vora N. 2012. The viral storm: the dawn of a new pandemic age. Lancet Infect Dis. 12:190.
- Wahab AG, Rittgers C. 2014. Broiler. Meat Sector, Malaysia. USDA foreign agriculturalservice; [accessed 2021 Sep 7]. http://www.thefarmsite.com/reports/contents/MalaysiaPoultry14March2014.pdf.
- Wan NWA, Tariq J. 2018. An overview of highly pathogenic avian influenza (H5N1) outbreak cases in Kelantan, West Malaysia in year 2017. Malays J Vet Res. 9:102–108.
- Wilson RF, Sarim D, Rahman S. 2015. Factors influencing the distribution of the invasive house crow (Corvus splendens) in rural and urban landscapes. Urban Ecosyst. 4:1389–1400.
- Wong PL, Sii HL, P’ng CK, Ee SS, Oong XY, Ng KT, Tan MP. 2020. The effects of age on clinical characteristics, hospitalization and mortality of patients with influenza-related illness at a tertiary care centre in Malaysia. Influenza Other Respir Viruses. 3:286–293.
- World Health Organization (WHO). 2007. Recommended composition of influenza virus vaccines for use in the 2007-08 influenza season; [accessed 2021 Aug 20]. http://www.who.int/wer/2007/ wer8209.pdf.
- Wright PF, Neumann G, Kawaoka Y. 2013. Orthomyxoviruses. In: Knipe DM, Howley PM, editors. Fields virology. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; p. 1186–1239.
- Wu Y, Wu Y, Tefsen B, Shi Y, Gao GF. 2014. Bat-derived influenza-like viruses H17N10 and H18N11. Trends Microbiol. 22:183–191.
- Yap CA, Sodhi NS. 2004. Southeast Asian invasive birds: ecology, impact and management. Ornithol Sci. 1:57–67.
- Yuseri NA, Rahaman NY, Omar AR. 2019. West Nile virus infection in human and animals: potential risks in Malaysia. Sains Malays. 48:2727–2735.
