ABSTRACT
This paper describes a field case of pig poisoning due to accidental contamination by alkaloid-rich lupin seeds of the grain legume mixtures used as protein sources in the feeds. The accident happened in Northern Italy in 2016, involved several farms and affected 2170 pigs of different categories (pregnant or lactating sows, gilts and fattening pigs). The observed clinical symptomatology was spanning from partial or total feed rejection to depression, recumbency, hypersalivation, vomiting and eventually death by necropsy by torsion of the stomach and gastro-enteric bloat. In feed formulations, the quinolizidine alkaloids (QAs), quantified by a GC-MS method, spanned from 0.051 to 1.245 mg/g. There was a relationship between the QAs content and the severity of clinical symptomatology: at a higher concentration, the outcomes were a larger incidence of clinically affected individuals (up to 50%) and a more severe clinical picture with mortality (up to 20%), which involved especially lactating sows.
KEYWORDS:
Highlights
Bitter lupin seeds contain relevant amounts of quinolizidine alkaloids
Quinolizidine alkaloids have a toxicological impact
A field case of pig poisoning happened in some Italian farms in 2016
The poisoning was caused by feeds containing alkaloid-rich lupin seeds
Introduction
The quality of protein ingredients is very relevant to the nutritional and safety characteristics of animal feeds (Sapkota et al. Citation2007). In the European Union, seeds are the main protein ingredients used in livestock feeding, since the inclusion of animal by-products, such as bones, feathers and blood, is forbidden by the current legislation, especially for monogastric animals (Jędrejek et al. Citation2016). Usually, non-ruminants are fed with a mixture of cereals and high-protein oilseed meals, mostly from soybean and rapeseed and sunflower (EFSA Citation2019). Recently, some alternatives have been proposed, such as lupin species (the main ones being Lupinus albus, Lupinus angustifolius, Lupinus luteus or Lupinus mutabilis) the most interesting ones among grain legumes, either for their nutritional or agricultural features (Lucas et al. Citation2015; Hanczakowska et al. Citation2017; Abraham et al. Citation2019; Zaworska-Zakrzewska et al. Citation2020). Lupin may be used as a whole seed or ground and incorporated into the feed (Abraham et al. Citation2019). In particular, lupin may be an important source of plant proteins in genetically modified (GM) organism-free food chains, where GM soybean is not usable (ACAF Citation2000).
One drawback of lupin is the possible presence of quinolizidine alkaloids (QAs) (Reinhard et al. Citation2006; Resta et al. Citation2008), secondary metabolites synthesized by lupin plants and other species of the Genisteae family as a defence mechanism against insects and herbivores (Pothier et al. Citation1998). QAs are biosynthesized in the green tissues of the plant, transported via the phloem and stored in all plant organs, including seeds (Boschin and Resta Citation2013; Frick et al. Citation2017). Each lupin species has its own QA fingerprint, considering the four main species, L. albus is characterized by numerous QAs, among which lupanine, 13α-isolupanine, 13α-hydroxylupanine, albine and multiflorine are the main ones, whereas L. angustifolius is characterized by angustifoline, 13α-isolupanine, lupanine, 13α-hydroxylupanine and L. luteus by the presence of lupinine and L. mutabilis seeds by sparteine, lupanine and 3β-hydroxylupanine (Wink et al. Citation1995; Boschin and Resta Citation2013).
Based on the bitter taste of these substances, lupin seeds with high QA contents are generally defined as ‘bitter’ or alkaloid-rich, whereas those with a low QA content are defined as ‘sweet’ or alkaloid-poor. In agricultural practice, a QA content of 500 mg/kg is historically considered the boundary between alkaloid-rich and alkaloid-poor lupin seeds (Aniszewski Citation1993). More recently, the catalogue of feed materials specifies that only sweet lupin seeds can be used for animal feeding (Regulation Citation2013) regarding ‘sweet’ those lupin varieties whose seeds contain less than 5% of bitter seeds (Regulation Citation2009).
Acute studies performed on rats with bitter lupin seeds and with pure lupanine or sparteine have displayed moderate acute toxicity due to an anticholinergic activity (Butler et al. Citation1996; Pothier et al. Citation1998; Panter et al. Citation1999). Specifically, neurological (weakness, mydriasis, confusion, loss of coordination and visual disturbances), cardiovascular (dysrhythmias) and gastrointestinal (nausea and vomiting) symptoms have been reported (Rotkiewicz et al. Citation2007; Kasprowicz-Potocka et al. Citation2013). The toxicity of QAs on pigs has been assessed in some trials using L. angustifolius or L. albus seeds (Godfrey et al. Citation1985; Rotkiewicz et al. Citation2007; Kasprowicz-Potocka et al. Citation2013), and a case report of lupin toxicosis in swine was described in 1991 (Casper et al. Citation1991). The QA composition of different lupin species may explain the different species-specific responses. Pigs do not tolerate more than 0.12 mg/g of total QAs of L. albus, whereas the limit is 0.2 mg/g for L. angustifolius (Godfrey et al. Citation1985) and 0.45 mg/g for L. luteus (Kim et al. Citation2007). For this reason, it is important to know both the qualitative and quantitative composition of the QAs content in feedstuffs, as either the total content or the different pattern, are important factors for pigs (Kim et al. Citation2007).
Risk assessments regarding human health have been published in many countries, such as the UK (ACNFP Citation1996), France (Bulletin Citation1998), Australia and New Zealand (ANZFA Citation2001) and North Europe countries such as Denmark, Finland, Iceland, Norway and Sweden (Pilegaard and Gry Citation2009). The regulations of the Health Authorities of these countries indicate a limit of 200 mg/kg of the total QA content in foods, with a provisional tolerable daily intake of 0.035 mg/kg body weight per day for humans (ANZFA Citation2001). Some poisoning cases have been related to the ingestion of lupin seeds after an inadequate or missing debittering process (ANZFA Citation2001; Di Grande et al. Citation2004; Litkey and Dailey Citation2007), whereas no case attributed to the consumption of industrial lupin foods has been reported (Reinhard et al. Citation2006; Resta et al. Citation2008).
In Citation2019, the European Food Safety Authority (EFSA) published a scientific opinion on the risk for both animal and human health related to the presence of QAs in feed and food, in particular in lupin seeds and derived products in Europe, summarizing experimental data published until now and underlying the importance of having more experimental data about this topic (EFSA Citation2019).
In this context, this paper has the objective of presenting some information about a field toxicosis case that happened in June–October 2016 in Northern Italy, involving more than 2170 pigs of different categories, i.e. pregnant or lactating sows, gilts and fattening pigs, accidentally poisoned with feeds containing alkaloid-rich lupin seeds. The paper reports physiological data on animals and qualitative and quantitative data on quinolizidine alkaloid (QA) content of the feeds, assessing a correlation between the QAs content and the severity of the symptoms.
Materials and methods
Animals
Six farms and 2170 pigs of different categories were involved: 1810 fattening pigs, 314 pregnant sows and 46 sows in the lactation phase (). Although the exact feed amount consumed by each animal is not available, the following average data are considered: the fattening pig’s diet was about 2.2 kg of feed/day for animals of about 80 kg, 3 kg/day for animals of about 120 kg, up to 3.5 kg/day for heavier animals, 2.7–3.3 kg/day for pregnant sows and 7–9 kg/day for sows in the lactation phase. These amounts were usually supplied to the animals in the involved farms, with no significant differences among them.
Table 1. Veterinary data: farm code, feed batch identification number, number and average weight of involved animals, number of dead pigs and symptomatology.
Reagents and solvents
All reagents and solvents for both QA extraction and analyses were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Sampling
Both feeds (15 samples, F1-F15) and legume mixtures (5 samples, LM1-LM5) were analysed. Feeds were mixtures of cereal flours added with variable percentages of legume mixtures, i.e. dry seeds of pea, soybean, beans, lupin and others in different amounts. Among these grains, only lupin seeds may contain QAs. F15 was the only feed prepared startingfrom a lupin-free legume mixture. Feeds F1-F7 were directly sampled from the troughs of animals in the farms where the poisoning episodes had happened (see details in ), whereas samples F8-F15 were collected from still unused bags given by the feed factory to the farmers. Three samples of about 100 g were collected for each feed.
The 5 legume mixtures (LM1-LM5) were directly taken from the transport truck at the feed factory using an automatic autosampler working on an unloading flow. This instrument assures that samples accurately represent the entire batch. LM1 and LM2 were used to prepare the feeds, as reported in detail in , whereas LM3-LM5 were discarded after analysis. About 500 g of each LM was collected and from each batch, a sample of 20 g was taken, ground to flour and processed as described in the ‘QAs Determination’ paragraph.
QA’s determination
Feeds were defatted and extracted, whereas legume mixtures were ground to flour, defatted and then extracted; extraction details were reported elsewhere (Boschin et al. Citation2008; Resta et al. Citation2008). Each sample was independently extracted three times. Analyses were performed on a GC-MS (Agilent Technologies) equipped with a GC 7890A and a mass spectrometer 5975C VL MSD with Triple-Axis Detector. Further analytical details were column, AT-1 ms (30 m × 0.25 mm i.d., 0.25 µm film, Alltech); helium at 0.8 mL/min flow; temperature programme, 150°C for 5 min, from 150°C to 300°C at 5°C/min and 300°C for 15 min; a split ratio of 1:25; an injection volume of 1 µL; an injection temperature of 250°C; an interface temperature of 300°C; an acquisition scan range of 50–450 m/z and the source operated in EI mode at 70 eV. Identification and quantification of QAs, main analytical features of the detected QAs, i.e. retention time (min), retention index and mass spectral data were reported elsewhere (Boschin et al. Citation2008; Resta et al. Citation2008). Each extracted sample was analysed at least three times and the total QA content and percentage of single QAs are shown in and as average value ± standard deviation.
Table 2. Feed composition and analysis. Added legume mixture with percentage, total QA’s content expressed as mean ± standard deviation and single QA’s percentages.
Table 3. Analysis of the legume mixtures. Total QA’s content is expressed as mean ± standard deviation and single QA’s percentages.
Statistical analysis
One-way analysis of variance and Fisher’s least significant difference (LSD) procedure were applied to QA content (Statgraphics Plus 2.1 for Windows); significantly different values (p < 0.05) are marked with different letters.
Results
Toxicosis description
In October 2016, six farms were involved in a case of pig toxicosis after the consumption of feeds F1-F7. reports all the relevant details of this event. A total of 2170 pigs were affected belonging to different categories, such as fattening pigs, gilts, pregnant or lactating sows. The observed symptomatology was similar in all farms: animals showed partial to severe feed rejection, depression, recumbency, mydriasis, vomiting and hypersalivation. Twenty-three animals died, nine of them were lactating sows. The autoptic examination showed that the deaths were associated with necropsy by torsion of the stomach or gastro-enteric bloat.
Spleen, liver and kidney of four dead animals were analysed and only parenchyma congestion was revealed, confirming the hypothesis that death was caused by intestinal fermentation. The hypothesis was that death was caused by the presence in the feeds of a substance having a cholinergic effect.
Feed analysis
After having verified that pesticide and mycotoxin residues were both negative (data not shown), the presence of lupin seeds in the feed formulation suggested quantifying the QAs, whose reported toxicity seemed to be compatible with the observed symptoms. Fifteen feed samples () were analysed and 5 legume mixtures (), i.e. the lupin-containing ingredients, which had been added in different percentages to prepare the feeds, as indicated in detail in .
Feeds F1-F7, containing LM1 or LM2, were directly involved in the toxicosis cases, whereas feeds F8-F15, containing LM1, were not given to animals after the first toxicological events and were then discarded. For the same reason, also feeds LM3-LM5 were not used to prepare feeds.
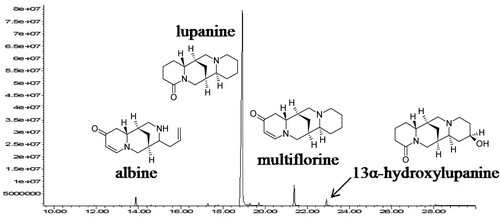
QAs were extracted from the feeds and legume mixtures and analysed by GC-MS (Boschin et al. Citation2008; Resta et al. Citation2008). shows an exemplary GC-MS chromatogram and and report the total amounts and relative percentages of the identified QAs in the feeds and legume mixtures, respectively. Four QAs were detected and quantified, i.e. albine, multiflorine, lupanine and 13α-hydroxylupanine (in elution time order).
In the samples, lupanine was always the most abundant QA, with a relative percentage spanning from 61% to 100%, multiflorine was the second one with an average value of 8% (interval 4–12%), followed by 13α-hydroxylupanine with an average value of 5.6% (interval 3–11%) and albine with an average value of 4.8% (interval 3–8%). These last three QAs have a greater intra-sample variability than lupanine, in agreement with the literature data (Wink et al. Citation1995; Boschin and Resta Citation2013).
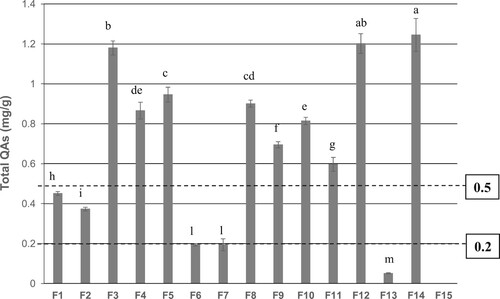
shows a comparative chart of the total QAs contents of all analysed feeds: only three (F6, F7 and F13) were below the limit of 0.2 mg/g suggested for human use (ANZFA Citation2001), two (F1 and F2) respected the limit of 0.5 mg/g fixed for feed, whereas nine exceeded this limit. The samples F1-F5, involved in the most serious toxicosis cases (see ), had a QA content ranging from 0.373 to –1.180 mg/g. They had been prepared by adding different percentages of the legume mixture LM1, which had the highest alkaloid content of 7.17 mg/g (). Samples F6 and F7, containing 0.194 mg/g of QAs, had been prepared by adding legume mixture LM2 having a QA content of 5.54 mg/g. Both samples caused only a partial feed rejection (see ). Samples F8-F14, again based on the legume mixture LM1 and discarded after the first toxicosis cases, had QA contents ranging from 0.051 to –1.245 mg/g. Sample F15, representative of a previous feed batch stored at the feed farm not containing lupin seeds, was devoid of any QA. LM3-LM5 had QA contents ranging from 0.536 to –5.314 mg/g that obliged their immediate disposal.
Discussion
Poisoning cases of domesticated animals are usually related to poor-quality or contaminated feedstuffs (i.e. with residues of pesticides, mycotoxins or toxic plants) or incorrect doses of feedstuff supplements or drugs (Modra and Svobodova Citation2009).
Feeds are usually mixtures of cereal flour, added with a source of plant protein, especially soybean. In searching for alternative protein sources for non-ruminants and GMO-free food chains, there is an increasing interest in lupin. In the past its use was limited because of its content of QAs; nowadays, the availability of alkaloid-poor varieties enables to overcome this problem (Abraham et al. Citation2019). Lupin seeds can be included in the feeds as flours or whole seeds, usually as a percentage of the total plant protein source (Lucas et al. Citation2015).
In the lupin genus the most dangerous phytochemicals are α-pyridone alkaloids, such as cytisine and anagyrine or piperidine alkaloids, such as ammodendrine (Panter et al. Citation1999). They all show high toxicity, mainly teratogenicity. In particular, anagyrine and ammodendrine are responsible for the so-called crooked calf disease (Lee et al. Citation2007) a set of congenital birth defects of calves (scoliosis, arthrogryposis, torticollis and cleft palate) detected in the late 1950s after the ingestion of wild lupin seeds. These alkaloids are not biosynthesized by L. albus, the lupin species whose seeds had been used for the preparation of the feeds analysed in this paper.
In the case described here, alkaloid-rich lupin seeds were accidentally present in the mixture of dry legume seeds added to the feeds. Possibly, they were the residue of previous cultivation, as lupin is often used as a green manure (Lucas et al. Citation2015) or they could be derived from alkaloid-poor cultivars that had become ‘bitter’ due to cross-pollination from alkaloid-rich plants. Moreover, pedoclimatic factors, such as rainfall amounts, low temperatures, the chemical characteristics of the soil or biotic aspects, such as the presence of herbivores or insects, can strongly affect the QA content of lupin seeds (Boschin et al. Citation2008; Magalhaes et al. Citation2017). Seed multiplication is exposed to the risk of a genetic shift towards higher QA content due to the pollen flow from bitter material, which increases over generations because of the higher advantage of greater QA content under natural selection (Huyghe Citation1997; Magalhaes et al. Citation2017). A recent paper suggests also a possible transfer of QAs to the soil (Hama and Strobel Citation2020). In this context, the need for careful monitoring of the QA content of seeds is of utmost importance.
Unfortunately, little information is available on the toxicity of each QA in farm animals and in particular on pigs. Literature reports just one in vivo study performed in pigs with pure lupanine (Wasilewko et al. Citation1997), whereas no experiment was performed on any other QA.
Both the total QA content and the amount of feed intake are important ( and ). In particular, all pigs fed with F3, which had the highest total QA content (1.18 mg/g), showed an almost total feed refusal, about one-half of the animals showed severe ill symptoms, such as depression, recumbency, mydriasis, vomiting, hypersalivation and about 20% died for a necropsy by torsion of the stomach and gastroenteric bloat. The animals poisoned in this site were sows in the lactation phase, possibly more sensitive than other animals. Furthermore, the feed intake of a lactating sow is about two-fold higher than that of other pigs.
In another farm, 200 fattening pigs were fed with F5 whose QA content was 0.946 mg/g. Its ingestion caused a severe feed refusal on all pigs, but only 7–10% of animals showed ill symptoms and about 2.5% died. The animals fed with F6 and F7, which were below the limit of 0.2 mg/g, showed only a partial feed rejection.
The QA’s bitter taste affects palatability causing feed to reject and a consequent decrease in feed intake and body weight gain (Magalhaes et al. Citation2017), in good agreement with our data. In a previous paper, a linear relationship between QAs of feed containing L. angustifolius and both the growth rate of animals and feed intake have been shown (Godfrey et al. Citation1985). No symptoms were detected when the total QA’s content was below 0.2 mg/g.
There are literature indications that pigs are more susceptible to QAs than other species and case reports describe feed refusal, poor growth rate, lethargy, enlarged abdomen and death as the main manifestations (Godfrey et al. Citation1985; Casper et al. Citation1991; Rotkiewicz et al. Citation2007; Kasprowicz-Potocka et al. Citation2017). A case report of lupin toxicosis was reported in the past (Casper et al. Citation1991), in which swine of different categories have been fed with a lupin-based meal on various farms. All animals have shown feed refusal, almost complete when the percentage of lupin was >10% of the ration at the beginning and poor growth rate. Moreover, dead animals have reached even 30% in some cases and an enlarged abdomen has been found at necropsy to be caused by colon distention (megacolon). All these data are in good agreement with our results.
Conclusions
Feed quality is strongly related to animal health. Moreover, animals’ age and particular physiological conditions, such as pregnancy and lactating, may affect the animal’s sensitivity to particular substances present in the diet. The pig sensitivity to the QAs of lupin seeds observed in the toxicosis case is certainly an example of these facts.
Utmost attention should be paid to bitter seeds that might be left on the field and infest the harvest (Hama and Strobel Citation2020). Moreover, breeding programmes aiming to improve lupin seed nutritional and agricultural features (Lucas et al. Citation2015; Abraham et al. Citation2019) have to assure the development of alkaloid-poor plants. This control is necessary for production lots, and across the various stages of seed multiplication also considering the different environmental conditions of their cultivation. Finally, there is a need for toxicological studies on a single QA, for more data on QAs in food and feed and for data on the possible transfer of QAs from feed to the food of animal origin to assure also consumers’ health (EFSA Citation2019).
Acknowledgements
G.B. thanks Donatella Resta for her precious help and interesting discussions.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Abraham EM, Parissi Z, Ganopoulos I, Mylona P, Madesis P, Mavromatis A, Nianiou-Obeidat I, Polidoros A, Tani E, Vlachostergios D. 2019. The use of lupin as a source of protein in animal feeding: genomic tools and breeding approaches. Int J Mol Sci. 20:851.
- ACAF. Advisory Committee on Animal Feedingstuffs. 2000. The use of sweet lupins in animal feed. Fifth ACAF Meeting. ACAF/00/26.
- ACNFP. 1996. ACNFP Report on seeds from narrow leafed lupin, Appendix IX. London: MAFF Publications. p. 107.
- Aniszewski T. 1993. Nutritive quality of the alkaloid-poor Washington lupin (Lupinus polyphyllus lindl var SF/TA) as a potential protein crop. J Sci Food Agric. 61:409–421.
- ANZFA. 2001. Lupin alkaloids in food. A toxicological review and risk assessment. Technical Report Series 3; p. 1–21.
- Boschin G, Annicchiarico P, Resta D, D'Agostina A, Arnoldi A. 2008. Quinolizidine alkaloids in seeds of lupin genotypes of different origins. J Agric Food Chem. 56:3657–3663.
- Boschin G, Resta D. 2013. Alkaloids derived from lysine: quinolizidine, a focus on lupin alkaloids. In: Ramawat KG, Merillon JM, Henry M, editors. Natural products – phytochemistry, botany and metabolism of alkaloids, phenolics and terpenes. Berlin Heidelberg: Springer-Verlag; p. 381–403.
- Bulletin. 1998. Bulletin Officiel 98/27 du Conseil superieur d’hygiene publique de France, 1998.
- Butler WH, Ford GP, Creasy DM. 1996. A 90-day feeding study of lupin (Lupinus angustifolius) flour spiked with lupin alkaloids in the rat. Food Chem Toxicol. 34:531–536.
- Casper HH, Berg IE, Crenshaw JD, Colville JL, Wass WM. 1991. Lupin bean meal toxicosis in swine. J Vet Diagn Invest. 3:172–173.
- Di Grande A, Paradiso R, Amico S, Fulco G, Fantauzza B, Noto P. 2004. Anticholinergic toxicity associated with lupin seed ingestion: case report. Eur J Emerg Med. 11:119–120.
- Frick KM, Kamphuis LG, Singh KB, Siddique KHM, Foley RC. 2017. Quinolizidine alkaloid biosynthesis in lupins and prospects for grain quality improvement. Front Plant Sci. 8:87.
- Godfrey NW, Mercy AR, Emms Y, Payne HG. 1985. Tolerance of growing pigs to lupin alkaloids. Aust J Exp Agric. 25:791–795.
- Hama JR, Strobel BW. 2020. Natural alkaloids from narrow-leaf and yellow lupins transfer to soil and soil solution in agricultural fields. Environ Sci Eur. 32:126.
- Hanczakowska E, Księżak J, Świątkiewicz M. 2017. Efficiency of lupine seed (Lupinus angustifolium and Lupinus luteus) in sow, piglet and fattener feeding. Agric Food Sci. 26:1–15.
- Huyghe C. 1997. White lupin (Lupinus albus L.). Field Crops Res. 53:147–160.
- Jędrejek D, Levic J, Wallace J, Oleszek W. 2016. Animal by-products for feed: characteristics, European regulatory framework, and potential impacts on human and animal health and the environment. J Anim Feed Sci. 25:189–202.
- Kasprowicz-Potocka M, Chilomer K, Zaworska A, Nowak W, Frankiewicz A. 2013. The effect of feeding raw and germinated Lupinus luteus and Lupinus angustifolius seeds on the growth performance of young pigs. J Anim Feed Sci. 22:116–121.
- Kasprowicz-Potocka M, Zaworska A, Kaczmarek S, Hejdysz M, Mikula R, Rutkowski A. 2017. The effect of Lupinus albus seeds on digestibility, performance and gastrointestinal tract indices in pigs. J Anim Physiol Anim Nutr. 101:e216–e224.
- Kim JC, Pluske JR, Mullan BP. 2007. Lupins as a protein source in pig diets. CAB Rev. 2. 3:1–12.
- Lee ST, Cook D, Panter KE, Gardner DR, Ralphs MH, Motteram ES, Pfister JA, Gay CC. 2007. Lupine induced “crooked calf disease” in Washington and Oregon: identification of the alkaloid profiles in Lupinus sulfureus, Lupinus leucophyllus, and Lupinus sericeus. J Agric Food Chem. 55:10649–10655.
- Litkey J, Dailey MW. 2007. Anticholinergic toxicity associated with the ingestion of lupini beans. Am J Emerg Med. 25:215–217.
- Lucas MM, Pueyo JJ, Stoddard FL, Annicchiarico P, Frias J, Martinez-Villaluenga C, Sussmann D, Duranti M, Seger A, Zander PM. 2015. The future of lupin as a protein crop in Europe. Front Plant Sci. 6:705.
- Magalhães SCQ, Fernandes F, Cabrita ARJ, Fonseca AJM, Valentao P, Andrade PB. 2017. Alkaloids in the valorization of European Lupinus spp. seeds crop. Ind Crops Prod. 95:286–295.
- Modra H, Svobodova Z. 2009. Incidence of animal poisoning cases in the Czech Republic: current situation. Interdiscip Toxicol. 2:48–51.
- Panter KE, James LF, Gardner DR. 1999. Lupines, poison-hemlock and Nicotiana spp: toxicity and teratogenicity in livestock. J Nat Toxins. 8:117–134.
- Pilegaard K, Gry J. 2009. Alkaloids in edible lupin seeds: a toxicological review and recommendations. Copenhagen: Nordic Council of Ministers. TemaNord. p. 605.
- Pothier J, Cheav SL, Galand N, Dormeau C, Viel C. 1998. A comparative study of the effects of sparteine, lupanine and lupine extract on the central nervous system of the mouse. J Pharm Pharmacol. 50:949–954.
- Regulation. 2009. Commission Regulation (EC) No 1121/2009; p. 27–64.
- Regulation. 2013. Commission Regulation (EU) No 68/2013 of the Catalogue of feed materials; p. 1–64.
- Reinhard H, Rupp H, Sager F, Streule M, Zoller O. 2006. Quinolizidine alkaloids and phomopsins in lupin seeds and lupin containing food. J Chromatogr A. 1112:353–360.
- Resta D, Boschin G, D'Agostina A, Arnoldi A. 2008. Evaluation of total quinolizidine alkaloids content in lupin flours, lupin-based ingredients, and foods. Mol Nutr Food Res. 52:490–495.
- Rotkiewicz T, Stanek M, Wisniewska M, Otrocka-Domagala L, Bogusz J, Purwin C, Bomba G. 2007. Pathomorphological and histochemical examinations of the digestive tracts and some internalorgans of pigs fed diets containing narrow-leaved lupin (Lupinus angustifolius) seeds. Ann Anim Sci. 7:83–88.
- Sapkota AR, Lefferts LY, McKenzie S, Walker P. 2007. What do we feed to food-production animals? A review of animal feed ingredients and their potential impacts on human health. Environ Health Perspect. 115:663–670.
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain) Schrenk D, Bodin L, Chipman JK, del Mazo J, Grasl-Kraupp B, Hogstrand C, Hoogenboom L, Leblanc JC, Nebbia CS, Nielsen E, et al. 2019. Scientific opinion on the risks for animal and human health related to the presence of quinolizidine alkaloids in feed and food, in particular in lupins and lupin-derived products. EFSA J. 17(11):05860. 113 pp.
- Wasilewko J, Buraczewska L, Lechowski R, Wysocka W. 1997. Effect of dietary lupanine on nutrient digestibility and on blood indices in pigs. EAAP Publ. 88:430–433.
- Wink M, Meißner C, Witte L. 1995. Patterns of quinolizidine alkaloids in 56 species of the genus Lupinus. Phytochemistry. 38:139–153.
- Zaworska-Zakrzewska A, Kasprowicz-Potocka M, Mikuła R, Frankiewicz A, Taciak M, Pruszyńska-Oszmałek E. 2020. Growth performance, gut environment and physiology of the gastrointestinal tract in weaned piglets fed a diet supplemented with raw and fermented narrow-leafed lupine seeds. Animals (Basel). 10:2084.