?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Poultry semen cryopreservation technology is one of the most direct methods to protect poultry germplasm, and in this study, the back and abdomen massage method is used to collect the semen of 20 Huoyan male geese, which is on the basis of EK diluent adding different combinations of dimethyl sulfoxide (DMSO) and trehalose, in total 7 different combinations treatments were achieved: D1T0 (5%DMSO), D1T1 (5% DMSO + 50 mM trehalose), D1T2 (5%DMSO + 100 mM trehalose), D2T0 (10% DMSO), D2T1 (10% DMSO + 50 mM trehalose), D2T2 (10% DMSO + 100 mol trehalose) and no addition group D0T0 (0% DMSO + 0 mM trehalose). Diluted semen was frozen in granules in liquid nitrogen vapour. After thawing in 40°C water bath, determinate sperm livability, acrosome integrity, plasma membrane integrity, catalase (CAT) activity, superoxide dismutase (SOD) activity, glutathione peroxidase (GSH-Px) activity, malondialdehyde (MDA) content and reactive oxygen species (ROS) level, and the results showed that 10% DMSO and 0.05 mol/L trehalose could significantly improve the sperm motility, acrosome integrity rate and plasma membrane integrity rate after thawing, and significantly increase the levels of antioxidant enzymes CAT, SOD and GSH-Px in seminal plasma (P < .05), significantly decreased MDA and ROS levels (P < .05).
KEYWORDS:
1. Introduction
Huoyan goose is one of the main circulating species in Northeast of China, which is often used as a hybrid female parent by breeders because of its high egg production rate. However, due to the unplanned introduction of goose species from other areas by breeders, and the irregular outbreak of some poultry infectious diseases, the number of purebred geese has been excessively hybridized and the number has decreased sharply. At present, semen freezing technology is a commonly used method for in vitro preservation of genetic resources, which is widely used in mammalian production practice but has not been widely used in poultry production practice. This is due to the special morphology of poultry sperm, higher glutamate content and lower ATP content in semen, resulting in lower sperm motility and lower fertilization rate in semen after thawing. Geese have the characteristics of seasonal reproduction, and goose semen freezing technology can break this limitation and solve the disadvantages of low reproductive rate of geese (Lukaszewicz Citation2001; Akhtar et al. Citation2021).
Among the related research on poultry semen cryopreservation technology, chicken is the earliest. As early as the 1930s, Burrows et al. invented a method of rooster semen collection-abdominal massage, which was later widely used in the semen collection of other birds (Farooq et al. Citation2021). With the advancement of society, the need for longer-term sperm storage in the poultry industry has led to numerous studies on semen cryopreservation (Hezavehei et al. Citation2018). Polge et al. found that glycerol (Gly) can protect sperm from damage to a certain extent at low temperature, which is an important turning point in the field of sperm cryopreservation (Polge et al. Citation1949; Weissenberg et al. Citation2001). This was further supported by Lake et al., who subsequently cited glycerol as a cryoprotectant (Lake and Stewart Citation1978).
Glycerol is widely used in the cryopreservation of poultry semen. However, in recent years, studies have reported that glycerol can adversely affect the fluidity of the nuclear and plasma membranes of sperm, thereby reducing the quality of thawed semen and ultimately reducing the fertilizing capacity of sperm (Aboagla Eiman and Terada Citation2003; Mehdipour et al. Citation2020). Therefore, finding a low-toxicity and high-efficiency cryoprotectant as a Gly substitute is the key to cryopreservation of poultry sperm.
DMSO is a low-molecular-weight permeable cryoprotectant widely used in cryopreservation of cells and tissues (Chang et al. Citation2015), and in recent years, its potential to improve the cryopreservation of animal semen has been gradually discovered (Clulow et al. Citation2007; Kim et al. Citation2011).
Trehalose is a non-permeable cryoprotectant with antioxidant properties, which exhibits a unique ability to protect sperm plasma membrane (Bucak et al. Citation2007; Gläfke et al. Citation2012; Mosca et al. Citation2016). There have been numerous reports of positive effects of alginate in combination with other classes of protective agents in the cryopreservation of semen from different species (Bucak et al. Citation2007, Citation2020, Citation2022; Gale et al. Citation2015; Öztürk et al. Citation2017; Akhtarshenas et al. Citation2018; Hezavehei et al. Citation2018). At present, there is no report on the combined application of the two substances, DMSO and alginate, for the cryopreservation of goose semen.
In this study, we optimized the existing EK diluent with DMSO and trehalose as cryoprotectants, and then design a special diluent formula for goose semen freezing, so as to provide a theoretical basis for the subsequent establishment of goose sperm bank.
2. Materials and methods
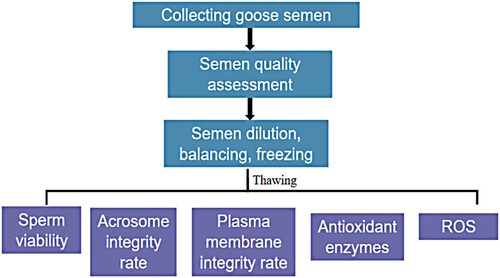
The overall idea of this experiment and the test method are shown in and . The experiment used EK diluent as the base diluent and continued to add different concentrations of cryoprotectants (DMSO, trehalose) for goose semen cryopreservation. After thawing, the sperm viability, acrosome integrity, plasma membrane integrity, antioxidant enzymes (CAT, SOD, GSH-Px), oxidation products (MDA), and ROS levels were measured to screen the goose semen diluent with DMSO and trehalose as cryoprotectants at the best-added concentrations.
Figure 1. Experiment in which EK diluent was used as the base diluent to which cryoprotectants (DMSO and trehalose) were continued to be added for goose semen cryopreservation, and all of the diluted semen was preserved in liquid nitrogen.
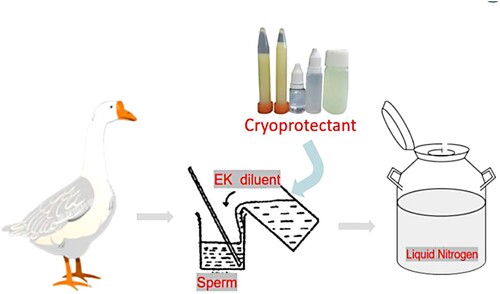
Figure 2. Best-added concentrations of DMSO and trehalose for goose semen cryopreservation were screened by detecting sperm viability, acrosome integrity, plasma membrane integrity, antioxidant enzymes, oxidation products, and ROS levels after thawing in the experiment, and combining all indicators.
2.1. Experimental animals
A total of 20 Huoyan male geese (about 3 years of age) with a tidy shiny coat, healthy and with excellent individual development, were used for semen collection. The animal experiment was approved by the Animal Health Care Committee of Animal Science and Technology College of Jilin Agricultural University (Approval No. GR (J) 18-003).
2.2. Experimental methods
2.2.1. Selection, training of male geese and semen collection
Ganders were trained for semen collection on through abdominal massage in order to make a specific reflex to an external stimulus. After two weeks of training, ganders with stable sexual reflexes will be artificially collected semen twice a week. During the experiment, male geese are fed regularly and quantitatively and drink freely. The dietary composition and nutritional composition of the test geese are formulated with reference to the basis of previous research in the laboratory and the feeding standards of geese.
2.2.2. Sperm quality assessment
Sperm viability: Take 10 µL fresh semen and place it on a slide (Leica, Germany). The number of sperm moving in a straight line was observed using a microscope (Leica, Germany) at 37°C. Record the percentage of sperm advancing rapidly in a straight line at 3 fields of view (Sacha et al. Citation2021).
Sperm density: Using the Blood Cells Counting Plate method (Sangon Biotech, Shanghai). A small amount of diluent was dropped into the blood cell counting board. After standing at room temperature for 5 min under appropriate humidity, put it under the microscope for observation. Count the number of sperm in five squares (generally the square with four corners and the middle) in the microscope.
pH: The volume of poultry semen is small, and the single ejaculation volume of goose is about 0.2 mL, which is difficult to be measured by ordinary pH meter. Therefore, precision pH test paper (Sangon Biotech, Shanghai) can be used for routine determination of semen pH of Huoyan Goose.
2.2.3. Extender preparation and processing
EK diluent (1.40 g L-sodium glutamate, 0.14 g potassium citrate H2O, 0.70 g glucose, 0.70 g inositol, 0.10 g polivinylpyrrolidone, 0.02 g protamine sulfate, 0.98 g sodium hydrogen phosphate, 0.21 g sodium dihydrogen phosphate 0.05 g streptomycin sulfate, 0.05 g ampicillin sodium were diluted to 100 mL with distilled water; pH 7.3, osmotic pressure 390 mOsmol/kg) was used as the base diluent for the entire experiment and as a control group. The dilutions for the test group were supplemented with the two cryoprotectants studied at different concentrations. The composition and dosage of EK solution used in this test are shown in .
Table 1. Main components of the test semen base dilutions.
To reduce the damage of sperm in the process of freezing and resuscitation to a greater extent, EK diluent was optimized in the experiment. Permeable cryoprotectant DMSO (5% and 10%) and impermeable cryoprotectant trehalose (0, 50 and 100 mM) were used in combination to freeze goose semen. The synergetic effect of the two can reduce the formation of ice crystals in cells, inhibit the formation of ice crystals outside cells, protect cell membranes, and thus reduce the damage of ice crystals to sperm. Six experimental groups (diluted 1:4): Group 1: (D1T0: 5%DMSO + 0 mM trehalose), Group 2: (D1T1: 5%DMSO + 50 mM trehalose), Group 3: (D1T2: 5%DMSO + 100 mM trehalose), Group 4: (D2T0: 10%DMSO + 0 mM trehalose), Group 5: (D2T1: 10%DMSO + 50 mM trehalose), Group 6: (D2T2: 10%DMSO + 100 mM trehalose). Control group: (D0T0: 0%DMSO + 0 mM trehalose). The biology experiment was repeated three times for all groups.
2.2.4. Semen dilution and balance
The semen with abnormal features (Contaminated, yellow, or flocculated samples) were discarded. The collected milky semen was mixed. Then semen(5 µL) was dropped into the preheated slide to examine under a microscope (400×). Sperm viability upper than 0.7 was diluted and all the preceding operations were completed within 30 min. The semen dilution was divided into two steps. Dilution 1 is the base dilution, without cryoprotectant, incubated at 40°C for 30 min in an electric thermostat, dilution II was a base dilution solution containing a cryoprotectant and was cooled to 4°C in the refrigerator. Dilution I was added to the fresh semen, shaken well and incubated in a 40°C incubator for 30 min. Diluent II was then added, mixed well and placed in a refrigerator (Haier) (4°C) for equilibration for 2 h for subsequent tests.
2.2.5. Semen freezing
The experiment uses the particle method to freeze geese semen. Liquid nitrogen was poured into the patented device (foam container) 5 min in advance and the liquid nitrogen depth was 10 cm. Then, drop the goose semen treated in Section 2.2.4 on the aluminium foil surface (about 50 µL per drop), fumigate it for 5 min, and then freeze it for 10 min to form semen particles. Collect it in a cryopreservation tube and store it in liquid nitrogen for subsequent tests.
2.2.6. Semen thawing
Cryopreserved sperm pellet were removed with forceps from cryopreservation tube to the beaker and quickly placed in a 40℃ water bath (Shanghai Bilon Instrument Manufacturing LLC), which was preheated in advance. After that, the beaker was gently shaken and the frozen sperm pellets were removed when it is melted 2/3 (about 10 s). In addition, using residual temperature to completely melt the frozen sperm pellets, and the semen tests were conducted after that.
2.2.7. Sperm viability after thawing in different treatment groups
Thawed semen of 10 µL was tacked out using a mechanical pipette, after using the same temperature base dilutions to dilute it 20 times, and using the method as same as in Section 2.2.2 to evaluate sperm viability. The percentage of linear motility sperm in the visual field was recorded and calculated.
2.2.8. Sperm acrosome integrity rate after thawing in different treatment groups
Sperm acrosome integrity rate was evaluated based on Giemsa Staining method. PBS (2 mL) was used to wash each frozen sperm pellet for three times. The resulting suspensions were centrifuged for 10 min at 1500 rpm (Shanghai Luxiangyi Centrifugal Machine Instrument LLC), then the supernatant was removed; however, this operation was replicated 2 times. A sperm smear is made by dropping 3–5 µL of the precipitate onto a slide and left at room temperature until the sperm film is naturally and completely dry. Thin smear was fixed in Paraformaldehyde (4%) for 15 min, and then washed with ultrapure water. The next step was staining with Giemsa Staining Solution (Sangon Biotech, Shanghai). The prepared slides were removed from the fixative and left in room temperature for 5 min to dry. The sperm were stained for 1.5 h, washed with ultrapure water and they were evaluated by an optical microscope (Shanghai Optical Instrument No.1 Factory) by observing at least three fields of visions. Sperm top body integrity rate was evaluated by counting at least 200 spermatozoa in each slide. The percentage of acrosome sperm in the visual field was recorded and calculated.
2.2.9. Sperm membrane integrity rate after thawing in different treatment groups
Hypoosmotic swelling test (HOST) was used to evaluate the sperm membrane integrity rate. Trisodium citrate dehydrate (0.5646 g) and Fructose (1.0377 g) were dissolved in distilled water (100 mOsm / kg) was kept in a 37°C incubator (Shanghai Yiheng Scientific Instruments Company) (Kumar et al. Citation2006; Ramu and Jeyendran Citation2013). PBS (2 ml) was added to each frozen sperm pellet after thawing and the mixture was well mixed. The resulting suspensions were centrifuged for 5 min at 1500 rpm (Shanghai Luxiangyi Centrifugal Machine Instrument LLC), then the supernatant was removed and this operation was replicated 2 times. Following, semen sediment (20 μL) was mixed with a low osmotic pressure solution (50 μL) and cultivated at 37°C for 30 min. Take 5 µL of the resulting solution on a preheated slide and cover it with a coverslip. The spermatozoa showing swollen heads, swollen and coiled tails were classified as normal spermatozoa having intact plasma membrane. A total of 200 spermatozoa were counted at four separate fields under a Phase contrast microscope (400×; Leica, Germany).
2.2.10. Levels of sperm antioxidant enzymes and oxidation products after thawing in different treatment groups
CAT, SOD, GSH-Px and MDA Elisa kit (Jining Industrial Co.Ltd, Shanghai) were utilized to determine catalase (CAT) activity, for the measurement of superoxide dismutase (SOD) activity, to measure the Malondialdehyde (MDA) of the semen and to determine the glutathione peroxidase (GSH-Px) activity. Strict instructions were followed in each experiment. The experiment was repeated three times for all activity measures. The operation steps are as follows:
Standard spiking: set up standard wells and sample wells, and add 50 µL of standards of different concentrations to each of the standard wells.
Sample addition: add 10 µL of the sample to be tested in the sample wells of the enzyme-coated plate, followed by 40 µL of the sample dilution; blank wells are not added.
Add antibody and incubate: add 100 µL of horseradish peroxidase (HRP)-labelled detection antibody to each well of the standard and sample wells, except for the blank wells, seal the reaction wells and incubate for 60 min in a 37°C thermostat.
Washing: remove the sealing membrane, discard the liquid, shake dry, fill each well with washing solution, let stand for 30 s and discard, repeat this 5 times and pat dry.
Colour development: add 50 µL each of substrate A and B to each well and incubate for 15 min at 37°C, protected from light.
Termination: termination solution (50 µL) was added to each well and the OD value of each well was measured at 450 nm within 15 min.
Calculation: using the concentration of the standard as the horizontal coordinate and the corresponding OD value as the vertical coordinate, draw a linear regression curve for the standard and calculate the concentration value of each sample according to the curve equation. Then multiply by the dilution multiple, which is the actual concentration of the sample.
2.2.11. Sperm ROS levels after thawing in different treatment groups
Sperm ROS level was tested by ROS Elisa Kit (geese) (Jining Industrial Co.Ltd, Shanghai). Strict instructions were followed and each experiment was replicated 3 times. The ROS detection procedure is similar to that described in Section 2.2.10.
2.2.12. Statistical analysis
The statistical tests were performed with SPSS 23.0 software (IBM, Armonk, NY, USA). The data were visualized using the GraphPad Prism 8 software (GraphPad, San Diego, CA, USA). The statistical significance was determined using one-way ANOVA by Duncan’s multiple-range test. The significant difference in the data was considered as p < .05. The results were expressed as mean ± SEM in the figures.
3. Results
3.1. Semen quality test
As shown in , the average sperm viability of Huoyan goose is 0.77, the average single ejaculation volume is 0.18 ml, the average Sperm density is 14.3 × 108/ml, average pH is 7.22. So it can see that goose semen is a kind of alkaline liquid with big density.
Table 2. Semen quality of Huoyan goose before freezing.
3.2. Sperm viability test
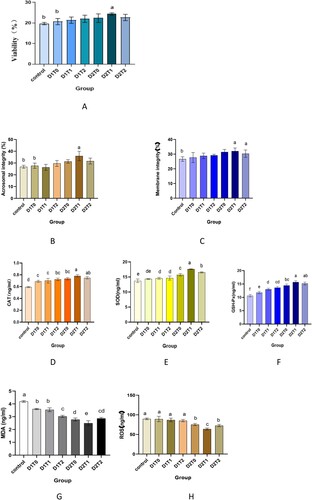
Sperm viability rate (%) of goose semen supplemented with different concentrations of DMSO and trehalose is shown in (A). The extender supplemented with 10% DMSO and 50 mM of trehalose led to higher sperm viability rate and it works best . By now the sperm viability after thawing is 0.24 (P < .05), 26.3% higher than the control group.
Figure 3. Sperm viabilityA, acrosome integrity rateB, membrane integrity rateC, antioxidant enzyme levelD–F and oxidation product levelD,H were detected after thawing in different treatment groups. abbreviation: CAT, catalase; SOD, superoxide dismutase; GSH Px, glutathione peroxidase; MDA, malondialdehyde; ROS, reactive oxygen species.
3.3. Acrosome integrity rate and membrane integrity rate test
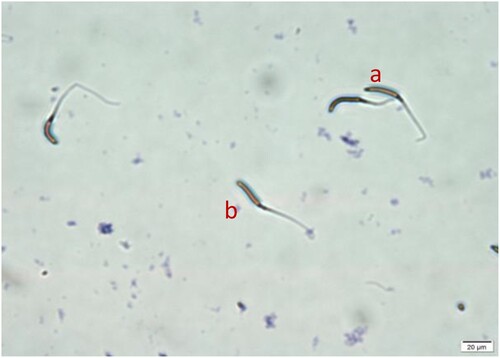
Sperm Acrosome integrity and membrane integrity rates (%) of goose semen supplemented with different concentrations of DMSO and trehalose are shown in (B,C). DMSO at 10% and trehalose at 50 mM gave higher (P < .05) sperm acrosome integrity (0.36 ± 0.03) compared to the control group (0.27 ± 0.01). In addition, it has been also found that when trehalose is added continuously fewer than 5% DMSO concentration, the acrosome integrity rate after thawing will be reduced, but not significantly (P > .05). Supplementing DMSO at 10% dose (D2T0) and 10% DMSO with 50 mM of trehalose (D2T1) let to significantly improved membrane integrity rate (0.31 ± 0.15, 0.30 ± 0.20, respectively), in comparison to the control group (0.26 ± 0.15) (P < .05). Also, there is some but not significant improvement effect in other treatment groups (P > .05). The morphology of goose sperm is shown in .
3.4. Measurement of enzymatic antioxidants activity and lipids peroxidation (MDA) level
The data on effect of DMSO and trehalose on antioxidant enzymes (catalase, superoxide dismutase, and glutathione peroxidase) profile of geese semen are presented in (D–F). Profiles of semen catalase (U/ml), superoxide dismutase (U/ml) and glutathione peroxidase (U/ml) were higher in extender containing 10% DMSO and 50 mM trehalose profile of goose semen DMSO and trehalose to the diluent can significantly increase CAT, SOD and GSH-PX levels after thawing (P < .05). Although, the lipid peroxidation state (U/ml) of goose semen with different concentrations of DMSO and trehalose are shown in (G). 10%DMSO and trehalose at 50 mM dose significantly decreased (P < .05) MDA levels (2.50 ± 0.19) compared to control (4.19 ± 0.07).
3.5. Sperm ROS levels
Shown in (H), the addition of different concentrations of trehalose with 10% DMSO dose (75.32 ± 2.73, 63.84 ± 2.49, 72.63 ± 2.99, respectively) decreased significantly (P < .05) the ROS levels in these groups compared to control (89.64 ± 2.14) and the higher negative effect was observed in group with 50 mM of trehalose (D2T1).
4. Discussion
Sperm with high viability are able to complete the process of linear forward motion and sperm penetration of the egg, which is highly correlated with other indicators and is a visual response to other functions (Mortimer and Mortimer Citation2013). In the present study, the addition of different concentrations of DMSO and trehalose to the dilution solution improved the viability of goose sperm after thawing to some extent compared to the control group, which is consistent with the findings of Iaffaldano, Balogun, et al. (Kim et al. Citation2017; Izanloo et al. Citation2021). Some studies have shown that the best protection of spermatozoa was achieved when the concentration of cryoprotectant in the dilution solution was between 8% and 10% (Balamurugan and Munuswamy Citation2017). Han et al. showed that the dilution solution containing 10% DMSO had the best protection of duck spermatozoa and sperm viability was significantly higher than other concentrations (Jung et al. Citation2020). The results of this experiment found that sperm viability increased by 15% and 10% when 10% DMSO was added to the dilution solution compared to the control and 5% DMSO groups, respectively, indicating that 10% DMSO was the best concentration to add to the cryoprotectant in the semen of the explanted goose. In contrast, Rakha found that the 8% DMSO group protected the rooster sperm better than the 10% DMSO group with the highest sperm viability, which is inconsistent with the results of this study because sperm of different species have a specific optimum concentration range for cryoprotectants, which is related to their species specificity, and this difference is caused by the initial cholesterol/phospholipid ratio of sperm of different species (Kowalczyk and Łukaszewicz Citation2015; Rakha et al. Citation2016). This may be due to its synergistic effect with DMSO, which can form a unique protective film on the cell surface under severe environmental conditions such as high temperature, high cold, high osmotic pressure and dry water loss, effectively protecting the protein molecules from inactivation, thus maintaining the life processes and biological characteristics of spermatozoa and improving their vitality (Hashemi et al. Citation2021). The unsaturated fatty acids in sperm membranes improve cell mobility and plasma membrane elasticity, but they are sensitive to the action of free radicals and are prone to oxidative stress (Lucio et al. Citation2017). Poultry sperm contains approximately 10 times more unsaturated fatty acids than mammals, which makes them more susceptible to ROS attack during the freeze–thaw process, severely reducing sperm viability after thawing (Knox Citation2015). In this study, the addition of 10% DMSO to the dilution solution improved the acrosome and plasma membrane integrity of goose spermatozoa, especially after the addition of 50 mM trehalose, the sperm of goose increased by more than 33% compared to the control group, because the antioxidant properties of trehalose scavenged excess free radicals to bring the semen to redox balance. This indicates that the synergistic addition of DMSO and trehalose can effectively improve the quality of goose semen after thawing. Michele Di Iorio et al. reported that the cryopreservation of turkey sperm was better when DMSO was added in combination with trehalose, sucrose and polysucrose, respectively, than when DMSO was added alone, indicating that the combination of the two cryoprotectants added significantly improved the plasma membrane integrity of sperm, a finding similar to that of the present study. However, the protective effect on sperm decreased when the concentration of trehalose was 100 mM (Di Iorio et al. Citation2020). This may be due to the fact that the addition of excess trehalose caused changes in the osmosis of the diluent and the redox balance of the semen, which in turn affected the acrosome and plasma membrane integrity of the sperm (Liu et al. Citation2020).
During freezing and thawing, sperm produce large amounts of ROS from their own respiratory metabolism, and the oxygen radicals (O2-, H2O2, OH-) they carry cause lipid peroxidation of unsaturated fatty acids in sperm cell membranes (Tselutin et al. Citation1999), interfering with normal sperm metabolism and causing damage to sperm cell membranes, oxidation of protein sulfhydryl groups, loss of plasma membrane selective permeability, and loss of channel regulation (Dupré and Carvajal Citation2019). In this study, it was found that the addition of trehalose to the diluent reduced ROS levels to some extent, and when added in combination with 10% DMSO, ROS levels were reduced by more than 23%, with the lowest ROS levels, highest plasma membrane integrity and highest sperm viability at an addition concentration of 50 mM. It is evident trehalose can slow down the level of oxidative stress in goose sperm, which is consistent with the findings of Izanloo et al. (Citation2021). It was found that trehalose inhibited ROS in equine sperm, increased GSH-Px activity in seminal plasma, reduced MDA levels in equine sperm, reduced oxidative stress and improved semen quality after thawing (Iaffaldano et al. Citation2018). Animal sperm have their own antioxidant defence system, but during freezing and thawing of semen, the enzymes produced by the sperm are over-utilized making them less active and unable to scavenge excess ROS (Sariözkan et al. Citation2009). In this study, we found that the addition of seaweed sugar to the dilution solution increased CAT, SOD and GSH-Px levels, which in turn improved the antioxidant capacity of goose sperm, which was similar to the results of Hu et al. (Citation2010) and Zhu et al. (Citation2017). In addition, it has been reported that trehalose as a non-osmotic protective agent can exert a protective effect by forming ice crystals on the outside of the cells, causing an increase in the extracellular osmotic pressure, resulting in water flowing out of the sperm interior and thus preventing sperm from forming ice crystals during freezing, a process that, in conjunction with the protective effect of DMSO on the sperm interior, can provide better protection for sperm (Ahmad Samar et al. Citation2020). This is because trehalose has antioxidant properties at low doses and mainly plays a role in reducing intracellular ice crystal formation at relatively high doses (Iqbal et al. Citation2016).
To our knowledge, this study was the first to demonstrate that certain concentrations of DMSO、trehalose, DMSO + trehalose have a positive effect on physiological characteristics and antioxidant enzymatic activities in Huoyan goose sperm freezing. However, the mechanism of the synergistic effect of DMSO and trehalose in the freezing of goose semen is not yet clear, and it can be assumed that the two cryoprotectants protect goose sperm in a ‘complementary’ manner, taking into account the findings of Tselutin K et al. and the results of this experiment, DMSO acts osmotically and trehalose acts non-osmotically to protect the membrane integrity of sperm, reduce ice crystal formation within sperm and reduce lipid peroxidation in sperm through different modes of action, thereby improving sperm viability after thawing (Tselutin et al. Citation1999).
5. Conclusion
The results obtained from this study show that supplementing of the dilute goose semen solution with 10% DMSO and 50 mM trehalose can significantly improve several parameters of geese sperm such as sperm viability, acrosome integrity rate, membrane integrity rate and antioxidant enzymes (the levels of CAT, SOD and GSH-Px in seminal plasma) and also reduce the levels of MDA and ROS in seminal plasma. At present, the synergistic mechanism of DMSO and trehalose is not clear, and it can be speculated that the two cryoprotectants may protect goose sperm in a ‘complementary’ way, with DMSO exerting an osmotic effect and trehalose exerting a non-osmotic effect, and protecting goose spermatozoa through different modes of action, in combination with the conclusion of Tselutin et al. (Citation1999). However, further research is needed to determine whether the combined application of the two agents can be applied in practical production.
Institutional review board statement
The animal experiment was approved by the Animal Health Care Committee of Animal Science and Technology College of Jilin Agricultural University (Approval No. GR (J) 18-003).
Acknowledgements
The authors appreciate the daily management of the goose farm at Jilin Agricultural University.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Aboagla Eiman ME, Terada T. 2003. Trehalose-enhanced fluidity of the goat sperm membrane and its protection during freezing. Biol Reprod. 69(4):1245–1250. doi:10.1095/biolreprod.103.017889
- Ahmad Samar Y, Frieland JK, Mackay DS. 2020. Effect of sucralose and aspartame on glucose metabolism and gut hormones. Nutr Rev. 78(9):725–746. doi:10.1093/nutrit/nuz099.
- Akttar MF, Shafiq M, Ali I. 2021. Improving gander reproductive efficacy in the context of globally sustainable goose production. Animals (basel). 12(1):44. doi:10.3390/ani12010044.
- Akhtarshenas B, Shabankareh HK, Hajarian H, Bucak MN, mohammadi ARA, Dashtizad M. 2018. The protease inhibitor antipain has a beneficial synergistic effect with trehalose for ram semen cryopreservation. Reprod Domest Anim. 53(6):1359–1366. doi:10.1111/rda.13253.
- Balamurugan R, Munuswamy N. 2017. Cryopreservation of sperm in Grey mullet Mugil cephalus (Linnaeus, 1758). Anim Reprod Sci. 185:205–213. doi:10.1016/j.anireprosci.2017.08.022.
- Bucak MN, Ateşşahin A, Varişli O, Yüce A, Tekin N, Akçay A. 2007. The influence of trehalose, taurine, cysteamine and hyaluronan on ram semen microscopic and oxidative stress parameters after freeze-thawing process. Theriogenology. 67(5):1060–1067. doi:10.1016/j.theriogenology.2006.12.004
- Bucak MN, Keskin N, Bodu M, et al. 2022. Combination of trehalose and low boron in presence of decreased glycerol improves post-thawed ram sperm parameters: A model study in boron research. Andrology. 10(3):585–594. doi:10.1111/andr.13130.
- Bucak MN, Keskin N, Ili P, et al. 2020. Decreasing glycerol content by co-supplementation of trehalose and taxifolin hydrate in ram semen extender: microscopic, oxidative stress, and gene expression analyses. Cryobiology. 96:19–29. doi:10.1016/j.cryobiol.2020.09.001.
- Chang Q, Chen Cheng C, Jing H, et al. 2015. Cryoprotective effect and optimal concentration of trehalose on aortic valve homografts. J Heart Valve Dis. 24(1):74–82.
- Clulow JR, Maxwell WMC, Evans G, Morris LHA. 2007. A comparison of duck and chicken egg yolk for cryopreservation of stallion sperm. Aust Vet J. 85(6):232–235. doi:10.1111/j.1751-0813.2007.00151.x
- Di lorio M, Rusco G, Lampietro R, et al. 2020. Finding an effective freezing protocol for Turkey semen: benefits of ficoll as non-permeant cryoprotectant and 1:4 as dilution rate. Animals (Basel). 10(3):421. doi:10.3390/ani10030421.
- Dupré E, Carvajal J. 2019. Cryopreservation of embryos and larvae of the edible sea urchin loxechinus albus (Molina, 1782). Cryobiology. 86:84–88. doi:10.1016/j.cryobiol.2018.11.005.
- Farooq U, Malecki IA, Martin GB, Mahmood M. 2021. Mobility of Japanese quail spermatozoa and its relationship to egg fertility. Reprod Domest Anim. 56(12):1543–1554. doi:10.1111/rda.14018.
- Gale SL, Buritt DJ, Robin Tervit H, McGowan LT, Adams SL. 2015. Can additives ameliorate oxidative stress and improve development of Greenshell mussel (Perna canaliculus) oocytes during cryopreservation? Cryo Lett. 36(1):37–44.
- Gläfke C, Akhoondi M, Oldenhof H, et al. 2012. Cryopreservation of platelets using trehalose: the role of membrane phase behavior during freezing. Biotechnol Prog. 28(5):1347–1354. doi:10.1002/btpr.1600.
- Hashemi M, Dastjerdi AM, Shakerardekaniet al A. 2021. Effect of alginate coating enriched with shirazi thyme essential oil on quality of the fresh pistachio (L.). J Food Sci Technol. 58(1):34–43. doi:10.1007/s13197-020-04510-6.
- Hezavehei M, Sharafi M, Kouchesfahani HM, et al. 2018. Sperm cryopreservation: A review on current molecular cryobiology and advanced approaches. Reprod Biomed Online. 37(3):327–339. doi:10.1016/j.rbmo.2018.05.012.
- Hu JH, Zan LS, Zhao XL, et al. 2010. Effects of trehalose supplementation on semen quality and oxidative stress variables in frozen-thawed bovine semen. J Anim Sci. 88(5):1657–1662. doi:10.2527/jas.2009-2335.
- Iaffaldano N, Di Iorio M, Mannina L, et al. 2018. Age-dependent changes in metabolic profile of Turkey spermatozoa as assessed by NMR analysis. PloS one. 13(3):e0194219. doi:10.1371/journal.pone.0194219.
- Iqbal S, Andrabi SMH, Riaz A, et al. 2016. Trehalose improves semen antioxidant enzymes activity, post-thaw quality, and fertility in Nili Ravi buffaloes (Bubalus bubalis). Theriogenology. 85(5):954–959. doi:10.1016/j.theriogenology.2015.11.004.
- Izanloo H, Soleimanzadeh A, Bucak MN, et al. 2021. The effects of varying concentrations of glutathione and trehalose in improving microscopic and oxidative stress parameters in Turkey semen during liquid storage at 5 °C. Cryobiology. 101:12–19. doi:10.1016/j.cryobiol.2021.07.002.
- Jung S-E, Kim M, Ahn JS, et al. 2020. Effect of equilibration time and temperature on murine spermatogonial stem cell cryopreservation. Biopreserv Biobank. 18(3):213–221. doi:10.1089/bio.2019.0116.
- Kim KM, Huh JY, Kim JJ, et al. 2017. Quality comparison of umbilical cord blood cryopreserved with conventional versus automated systems. Cryobiology. 78:65–69. doi:10.1016/j.cryobiol.2017.07.001.
- Kim S, Lee Y-J, Ji D-B, et al. 2011. Evaluation of different cryoprotectants (CPAs) in boar semen cryopreservation. J Vet Med Sci. 73(7):961–963. doi:10.1292/jvms.10-0345
- Knox RV. 2015. The fertility of frozen boar sperm when used for artificial insemination. Reprod Domest Anim. 50(Suppl 2):90–97. doi:10.1111/rda.12552.
- Kowalczyk A, Łukaszewicz E. 2015. Simple and effective methods of freezing capercaillie (Tetrao urogallus L.) semen. PLoS one. 10(1):e0116797. doi:10.1371/journal.pone.0116797.
- Kumar N, Verma RP, Singh LP, et al. 2006. Effect of different levels and sources of zinc supplementation on quantitative and qualitative semen attributes and serum testosterone level in crossbred cattle (Bos indicus x Bos Taurus) bulls. Reprod Nutr Dev. 46(6):663–675. doi:10.1051/rnd:2006041
- Lake PE, Stewart JM. 1978. Preservation of fowl semen in liquid nitrogen–an improved method. Br Poult Sci. 19(2):187–194. doi:10.1080/00071667808416462
- Liu G, Pan B, Li S, et al. 2020. Effect of bioactive peptide on ram semen cryopreservation. Cryobiology. 97:153–158. doi:10.1016/j.cryobiol.2020.08.007.
- Lucio CF, Brito MM, Angrimani D, et al. 2017. Lipid composition of the canine sperm plasma membrane as markers of sperm motility. Reprod Domest Anim. 52(Suppl 2):208–213. doi:10.1111/rda.12860.
- Lukaszewicz E. 2001. Effects of semen filtration and dilution rate on morphology and fertility of frozen gander spermatozoa. Theriogenology. 55(9):1819–1829. doi:10.1016/S0093-691X(01)00524-6
- Mehdipour M, Kia HD, Martínez-Pastor F. 2020. Poloxamer 188 exerts a cryoprotective effect on rooster sperm and allows decreasing glycerol concentration in the freezing extender. Poult Sci. 99(11):6212–6220. doi:10.1016/j.psj.2020.08.041.
- Mortimer D, Mortimer ST. 2013. Computer-Aided sperm analysis (CASA) of sperm motility and hyperactivation. Methods Mol Biol. 927:77–87. doi:10.1007/978-1-62703-038-0_8
- Mosca F, Madeddu M, Sayed AA, et al. 2016. Combined effect of permeant and non-permeant cryoprotectants on the quality of frozen/thawed chicken sperm. Cryobiology. 73(3):343–347. doi:10.1016/j.cryobiol.2016.10.001.
- Öztürk C, Güngör Ş, Ataman MB, et al. 2017. Effects of arginine and trehalose on post-thawed bovine sperm quality. Acta Vet Hung. 65(3):429–439. doi:10.1556/004.2017.040.
- Polge C, Smith AU, Parkes AS. 1949. Revival of spermatozoa after vitrification and dehydration at low temperatures. Nature. 164(4172):666. doi:10.1038/164666a0
- Rakha BA, Ansari MS, Akhter S, et al. 2016. Cryopreservation of Indian red jungle fowl (Gallus gallus murghi) semen. Anim Reprod Sci. 174:45–55. doi:10.1016/j.anireprosci.2016.09.004.
- Ramu S, Jeyendran RS. 2013. The hypo-osmotic swelling test for evaluation of sperm membrane integrity. Methods Mol Biol. 927:21–25. doi:10.1007/978-1-62703-038-0_3
- Sacha CR, Vagios S, Hammer K, et al. 2021. The effect of semen collection location and time to processing on sperm parameters and early IVF/ICSI outcomes. J Assist Reprod Genet. 38(6):1449–1457. doi:10.1007/s10815-021-02128-x.
- Sariözkan S, Bucak MN, Tuncer PB, et al. 2009. The influence of cysteine and taurine on microscopic-oxidative stress parameters and fertilizing ability of bull semen following cryopreservation. Cryobiology. 58(2):134–138. doi:10.1016/j.cryobiol.2008.11.006.
- Tselutin K, Seigneurin F, Blesbois E. 1999. Comparison of cryoprotectants and methods of cryopreservation of fowl spermatozoa. Poult Sci. 78(4):586–590. doi:10.1093/ps/78.4.586
- Weissenberg R, Menashe Y, Madgar I. 2001. Inception and five-year Run of a semen cryobank. clinical and behavioral aspects. Cell Tissue Bank. 2(4):235–239. doi:10.1023/A:1021118403063
- Zhu Z, Fan X, Pan Y, et al. 2017. Trehalose improves rabbit sperm quality during cryopreservation. Cryobiology. 75:45–51. doi:10.1016/j.cryobiol.2017.02.006.