ABSTRACT
In Ethiopia, the dairy cows’ major feed resources are natural pasture and crop residues, both of which are nutritionally low and do not even satisfy the maintenance requirement. Consequently, dairy productivity per head is low in the country. This paper reviews the existing knowledge of negative energy balance (NEB) and summarizes its implication on production and reproduction parameters, as well as the monitoring tools as a means of intervention in minimizing the effect on dairy cows. The presence of NEB in late gestation and early lactation of dairy cows could contribute to both short and long-term negative effects on production and reproduction ability. Lower body condition, reduced milk production, a change in the milk fat to protein ratio, an increasing incidence of health problems, a late time to become estrus, delayed ovarian cycle, and a lower conception rate are the common implications. Through the knowledge created so far, it can be concluded that NEB is almost a common phenomenon in transitional high milking dairy animals, but there is a possibility to shorten its persistence through strategic supplementation using the NEB monitoring tools.
1. Introduction
In Ethiopia, dairy is a fast growing (i.e. 8–10% annually for the last 10 years) industry and it bears considerable potential for enhancing incomes of farmers and other players along the different segments of the value chain (Tesfaye et al. Citation2020). The dairy sector in Ethiopia in many contexts has the potential to contribute significantly to providing income and food security for households and employment opportunities in local labour markets. Dairy has recently been identified by the federal government of Ethiopia as a priority commodity with promising growth and business opportunities, as the dairy sector might be a major engine of farm development for rural smallholders, urban and peri-urban areas (Shapiro et al. Citation2015).
In Ethiopia, major emphasis has been given to the genetic improvement of milk producing cattle as a development intervention. The total volume of milk produced in Ethiopia has increased over the last 15 years from less than 1 billion litres to 4.69 billion litres in 2020/2021 (CSA Citation2021), with an average daily milk yield of 1.42 litres/cow. However, due to the higher number of dairy cows and the long experience of crossbreeding programme, production is very low; as a result, the average per capita consumption of milk is about 19 kg per year, which is lower than the per capita consumption of East Africa (ATA Citation2016; CSA Citation2021). During the years 2009–2018, the country spent more than 2.65 billion Ethiopian birr on 24 million litres of imported dairy products (Tesfaye et al. Citation2019). Within the frame of the Ethiopian Growth and Transformation Plan (GTP) II period (2015–2020), the Ethiopian government designed an ambitious livestock master plan to increase the number of crossbred cows by about 800% from 2015 to 2020 and to increase the volume of milk production to 9,418 million litres of milk (Shapiro et al. Citation2015).
The idea is that by introducing crossbred dairy cows, we will be able to meet the ever increasing demand for higher quality meat and milk, but their genetic merit is typically expressed best in low-constrained environments. Roschinsky et al. (Citation2015) reported that when the conditions are favourable, crossbreeding scheme will produce healthy and productive dairy cows. As a result, the provision of quality and quantity of feed (supplement) for high yielding crossbred dairy cows, along with other husbandry practices, is critical, particularly during high-demanding physiological periods such as parturition and the beginning of the lactation, or the ‘transition period’ (Rhodes et al. Citation2003; Pedernera et al. Citation2008). This type of feeding programme will help the dairy cows adapt to the physiological change with the minimum negative effect of negative energy balance (NEB).
In Ethiopia’s existing situation, however, it is not possible to satisfy the nutrient requirements of the transitioning dairy animals with natural pasture and crop residues, which are the major (85%) livestock feed resources in the country (CSA Citation2021) but poor in quality. FAO (Citation2018) reported that feed supply and feed balance in the country showed a negative value on feed dry matter, feed metabolizable energy, and feed crude protein supply. As a result, the country’s ambitious crossbreeding programme, as well as GTP-II for the purpose of high milk production, is highly constrained by critical nutrients such as protein and energy nutrients, leading to the prevalence of NEB unless intervention is made (Shapiro et al. Citation2015).
Energy balance is the difference between energy consumed and energy used for both maintenance and production (milk, meat, reproduction, etc.) and consequently enters a state of NEB when energy expenditure exceeds intake (Baumgard et al. Citation2006). Energy availability, or more specifically a lack of available energy, frequently limits milk or milk component synthesis, reduce reproductive performance, and prevents body condition replacement in cows (Folnožić et al. Citation2019; Đuričić et al. Citation2020). When NEB is severe and persists for long time, it also alters global gene expression and immune responses in the uterus of postpartum dairy cows (Wathes et al. Citation2009; Soares et al. Citation2021). Then NEB is substantially universal among high yielding dairy cows in early lactation (Opsomer Citation2015). Factors such as feed type (total mixed ration and pasture-based systems), periods of poor feed quality, and adverse environmental situations such as heat stress and drought could also determine the severity and duration of NEB in dairy cows. The severity, magnitude, and day of negative energy balance are closely associated with metabolic disorders and reproductive failures (Rhoads et al. Citation2005).
In attempts to improve or alleviate NEB, more energy dense diets are provided as supplemental feed (Grummer et al. Citation2004; Pedernera et al. Citation2008). However, the effectiveness of these dietary strategies is frequently inconsistent and is associated with potential drawbacks (i.e. acidosis and reduced intake) (Hayirli and Grummer Citation2004). The good thing is that knowledge about on-farm dairy cows’ NEB monitoring tools has been developed. Body measurement, milk yield, milk chemical composition (fat to protein ratio), plasma metabolites such as non-esterified fatty acids (NEFA) and β-hydroxybutyric acid (BHBA), and endocrine changes are all commonly used NEB tools that affect the productive and reproductive performance of dairy cows (Folnožić et al. Citation2016; Folnožić et al. Citation2019; Đuričić et al. Citation2020). These two metabolites in milk, blood and urine are indicative of lipid mobilization and fatty acid oxidation in transition dairy cows (Wathes et al. Citation2007; Duffield et al. Citation2009; Esposito et al. Citation2014; Turk et al. Citation2016). Due to scientific advancement, the metabolic biomarkers could be determined easily, and based on their threshold levels, effective dietary recommendations could be made to minimize the negative effect of NEB in dairy cows (Delosière et al. Citation2019; Đuričić et al. Citation2020; Churakov et al. Citation2021).
However, the NEB concept and the monitoring tools are not well known in Ethiopia in the dairy sector, but the country is very sensitive to this nutritional challenge because of the poor feeds and feeding systems in the country. Although there is no specific report about NEB in dairy cows in Ethiopia, the prevalence of clinical or subclinical ketosis is high (Tadesse et al. Citation2012; Asrat et al. Citation2013; Ayele et al. Citation2014; Fasil and Juta Citation2016); dairy cows in a NEB have an associated increased risk of developing clinical or subclinical ketosis. Still, the country has an ambitious plan for crossbreeding the local with high yielding dairy cows, so knowledge about NEB is critical. Having and implementing the NEB’s existing monitoring and management practices or tools could help ensure the country’s crossbreeding programme’s success. Therefore, this review paper is initiated to review on the implication of NEB on productive and reproductive performance and health and minimize this negative implication using developed monitoring tools and implementing appropriate nutritional management practices in the dairy cows’ nutrition.
2. Concept of negative energy balance in dairy cows
Energy balance is defined as the difference between energy intake from feed and energy required for animal performance (maintenance, growth and fertility) (Leslie et al. Citation2003). Dairy cattle undergo tremendous metabolic and physiological changes three weeks before and three weeks after calving (Wisnieski et al. Citation2019). This change is to prepare for milk synthesis and secretion in the dairy cows, fetus development, and calf birth. In early lactation, the elevated energy requirements for milk production can result in NEB in dairy cows (Vries and Veerkamp Citation2000). High yielding dairy cows are commonly vulnerable to the NEB, though with better management practices. Although there is high energy demand during the transition period (late pregnancy to early lactation), dry matter intake (DMI) of the dairy cows is usually reduced because of rapid fetus growth; which takes up rumen space. Consequently, cows consume less energy than they require, resulting in a NEB and the associated loss of body weight. When animals are in the NEB, they are exposed to extreme metabolic changes and oxidative stress, which are critical for the health, production, and welfare of dairy cows (Leslie et al. Citation2003). The severity and duration of NEB after calving (postpartum) depends on body condition score at calving, the degree of the reduction of DMI, and the quality of the diet. To adapt this challenge, dairy cows could mobilize their tissue (adipose tissue and muscle protein) during parturition and the early postpartum period as an alternative source of energy needed in response to high milk production; as the result, there is a higher susceptibility to diseases and metabolic disorders (Lu et al. Citation2013). This subsequently leads to alterations in blood metabolite and hormone profiles, which in turn influence milk yield, quality, health, and fertility of the dairy cows. Kokkonen et al. (Citation2005) reported that lipid, glycogen, and protein reserves are mobilized to compensate for this energy deficit. Hence, using the metabolites, it is possible to determine the prevalence of NEB in the dairy cows.
Parity difference were observed in NEB, where the primiparous cows are more susceptible to metabolic stress during the transition period than multiparous cows (Berry et al. Citation2006; Wathes et al. Citation2007; Folnožić et al. Citation2016). The metabolic and endocrine profiles of the primiparous are more unbalanced as compared to the multiparous, which results in a more severe and prolonged recovery from NEB. There are differences in the control of tissue mobilization between primiparous and multiparous cows, which may promote nutrient partitioning into growth as well as milk during the first lactation. Contrarily, Friggens et al. (Citation2007) reported that Parity 1 cows have less sever NEB than parity 2 and 3 cows. The lactation stage is also considered as a factor for severity of NEB in dairy cows; early lactation is common but less severe in the later lactation stage (Gross et al. Citation2015). Feeding and other husbandry practices and breed difference could cause dairy cows to differ in NEB status. Friggens et al. (Citation2007) reported that Holsteins mobilized significantly more body energy in early lactation than the Danish Red and Jersey breeds. So, the duration and severity of NEB in the dairy cows depend on how the cows are managed. Though NEB is common in high yielding dairy cows with effects ranging from less severe to severe, its magnitude and severity could be minimized with proper nutrition and supplementation of feed additives like clinoptilolite (Folnožić et al. Citation2019) and by minimizing the rumen degradable protein to 10% of the dry matter (Tamminga Citation2006), and cows fed higher energy diet had a better energy balance (Friggens et al. Citation2007). Overall, NEB has been associated with harmful effects on cow health, milk production, and fertility, which in turn the profitability of the farm.
3. Negative energy balance impact on cow milk yield, quality and milk metabolimics
After the cow calves, the magnitude of NEB increases because her DMI lags behind the energy required for the rapid increase in milk yield. There is a significant decrease in DMI of over 30 percent in the last 3 weeks of gestation, limiting the availability of energy sources during the time of increased energy demand (Hayirli et al. Citation2002). Milk yield in fresh dairy cows is physiologically increased, but overall productivity is lower, and it is also associated with declining body condition (Roche et al. Citation2009). Cows in the NEB produced more milk with increased milk fat yield and higher concentrations of milk metabolomics such as lactose, citrate, cis-aconitate, and creatinine (Xu et al. Citation2020); metabolic changes occur in mammary gland cells.
Milk metabolomics, such as fat to protein ratio and fatty acids, are very important monitoring tools used by the scholars to monitor the prevalence of NEB in dairy cows (Lu et al. Citation2013; Xu et al. Citation2020, Citation2018). Milk fat has increased when the NEB is prevalent in the dairy animals, and the milk fatty acid profiles have also changed, especially in the early lactation, but remain unchanged after 12 weeks of calving (Josef et al. Citation2011). So, the milk metabolic profiles of dairy cows in early lactation have been changed physiologically to make things homeostatic (Xu et al. Citation2020) and could be used as milk indicators for NEB in high lactating dairy cows (Delosière et al. Citation2019). Milk fat yield, glycine, choline, and carnitine were important variables to estimate energy balance (Xu et al. Citation2018, Citation2020; Rocchetti and O’Callaghan Citation2021). The relationship of these milk metabolites with energy balance is proposed to be related to their roles in cell renewal (Josef et al. Citation2011). Frequently in a cows life cycle, there are instances when energy availability, or more specifically a lack of available energy, may limit milk or milk component synthesis.
4. Negative energy balance effect on dairy cows’ body condition and health
Transition dairy cows naturally try to adapt the NEB via metabolic physiology, but due to fewer and delayed interventions, or poor adaptation, dairy cows will enter the NEB. Dairy cows in the NEB mobilize adipose fat and muscle protein, which results in a loss of body condition. The reduced DMI and subsequently reduced body condition will aggravate the dairy cows’ reduced immune status (Roche et al. Citation2009). Equally important, higher prepartum body conditions could negatively affect dairy cows after calving (Kadivar et al. Citation2014). Both low and high body condition score (BCS) at calving increase the risk of disease: cows in the low group are more susceptible to reproductive problems, and fatter cows have an increased risk of metabolic diseases (Roche et al. Citation2013). The animal health problem reflects the magnitude and duration of NEB on dairy cows (Becker et al. Citation2021). Metabolic disorders are a key problem in the dairy cow’s transition period. Metabolic diseases develop as a result of an imbalance in the input and output of nutrients in transition dairy cows. Scholars discovered a link between NEB and metabolic disease, health, and well-being (Roche et al. Citation2009; LeBlanc Citation2012; Esposito et al. Citation2014). Metabolic disorders of dairy cows such as hypocalcaemia, hypomagnesaemia, and ketosis, and production disease like retained placenta, displacement of the abomasum, and laminitis (Ingvartsen Citation2006; Mulligan and Doherty Citation2008; Randhawa et al. Citation2014; Sundrum Citation2015) could be reflected in NEB.
The most economically important production diseases of high yielding dairy animals that occur as a result of NEB are ketosis and fat cow syndrome. Production diseases constitute a major proportion of the common health problems encountered on dairy farms, and because they predispose cows to infectious diseases, infertility, production losses, and lameness, they compromise the health and welfare of dairy cows and reduce farmer profitability (Randhawa et al. Citation2014). According to Esposito et al. (Citation2014), dairy cows in the NEB state have disproportional energy metabolism (fatty liver, ketosis, subacute, acute ruminal acidosis), disturbed mineral utilization (milk fever, sub-clinical hypocalcemia), and perturbed immune function (retained placenta, metritis, mastitis). After calving, uterine infection and inflammation are common in dairy cattle with NEB (Esposito et al. Citation2014). Consequently, NEB and reduced DMI are aggravating. The combined effects of all these challenges are reduced fertility, milk production, milk quality, and diminishing profits.
The transition physiology from the non-lactating pregnant state to non-pregnant lactating state requires the high producing dairy cow to drastically adjust its metabolism so that nutrients can be partitioned to support milk synthesis, a process referred to as homeorrhesis (Bauman Citation2000). A sharp increase in nutrient requirements generally occurs when feed intake is depressed in early lactation, which causes extensive mobilization of body tissues, particularly body fat but also amino acids, minerals, and vitamins (Bauman Citation2000). Despite tight homeostatic controls and homeorrhetic adjustments to cope with the changes in metabolism caused by milk production, 45–60% of dairy cows across different levels of milk production, breeds, and management systems develop metabolic and infectious diseases in the first months of lactation (Ribeiro et al. Citation2014). Extensive and prolonged mobilization of body fat, as reflected by increased NEFA concentrations and loss of body condition, generally leads to fat accumulation in the liver (hepatic lipidosis or fatty liver). Earlier monitoring about the underlying cause of periparturient health disorders may facilitate the design of nutritional regimens that will meet the energy requirements of cows during early lactation and reduce the susceptibility to disease as a function of compromised inflammatory responses (Sordillo et al. Citation2009).
5. Negative energy balance effect on dairy cow’s fertility
The subsequent NEB effects, such as loss of body condition and the associated alterations in blood metabolites and hormone profiles, influence the fertility of cows (Wathes et al. Citation2007; Roche et al. Citation2018). It has been reported that severity and duration of NEB have a significant effect on the fertility of dairy cows in terms of delaying ovulation, quality of oocytes ovulated 80–100 days later, ovarian functions, and reducing conception rate (Butler Citation2003; Knop and Cernescu Citation2009; Chandra et al. Citation2011). It has also been reported that luteal activity delayed in postpartum (Vries and Veerkamp Citation2000). Therefore, it is better to minimize the duration and level of NEB during the transition period to reduce the risk of fertility problems in dairy cows. The targeted breeding period in dairy cows is 60 to120 days postpartum (Harrison et al. Citation1990). At the same time, precaution should be taken in managing the dairy animals during the prepartum period; this is important to control because high BCS at calving leads to a greater loss after calving, which leads to a lower body condition, resulting in a fertility problem (Drennan and Berry Citation2006; Pires et al. Citation2013). There is a direct positive relationship between body condition deterioration and conception rates in dairy cows (Bewley and Schutz Citation2008). NEB and BCS loss are related to reduced serum progesterone concentrations during the breeding period and to lower pregnancy rates; in addition, the conception rate decreases about 10% per 0.5 unit BCS loss from calving to insemination (Butler Citation2003).
The reproductive efficiency of the lactating herd is a major component of profitability on dairy farms. Reproduction determines when primiparous cows become multiparous, leading to increments in milk yield; alters the average milk yield per day of calving; affects the number of replacement animals available and the risk of culling; and influences the rate of genetic progress. Improving the fertility of dairy cows is not simple, and it has been influenced by many factors (Wathes et al. Citation2008). The establishment and maintenance of a pregnancy are affected by several genetic, physiological, and environmental factors that can be manipulated in order to sustain high fertility (Tamminga Citation2006). It is well explained that poor nutritional status and metabolic health negatively influence reproduction in dairy cows (Bisinotto et al. Citation2018). The energetic status of a cow modulates the secretion of hormones that play key roles in growth of ovarian follicles, ovulation, corpus luteum formation, and oocyte competence (Bisinotto et al. Citation2018). Furthermore, extensive lipolysis and products from fat metabolism may be detrimental to oocyte competence and subsequent embryo development (Bisinotto et al. Citation2018).
During lactation, most of the glucose produced by the liver is used for synthesis of lactose to support milk production (Aschenbach et al. Citation2010). Bisinotto et al. (Citation2018) reported that a transient insulin resistance early postpartum diminishes the utilization of glucose by peripheral tissues to secure its availability for the mammary gland; as means of adaptation. Although the follicle is capable of controlling fluctuations in glucose availability, which generally results in concentrations in the follicular fluid greater than those observed in blood, intrafollicular glucose concentrations also decline around parturition (Leroy et al. Citation2004). It has been shown that glucose is critical for adequate oocyte maturation, affecting cumulus expansion, nuclear maturation, cleavage, and subsequent blastocyst development (Bach Citation2019). In fact, glucose concentrations compatible with those observed in cows suffering from clinical ketosis (1.4 mM) reduced cleavage and the proportion of embryos developing to blastocysts (Leroy et al. Citation2006). Although the oocyte does not directly use glucose as an energy source, it must be readily available to cumulus cells for glycolysis in order to provide pyruvate and lactate, oocyte’s preferred substrates for Adenosine triphosphate production (Collado-Fernandez et al. Citation2013). Therefore, it is possible that hypoglycemia in early lactation might compromise oocyte competence in dairy cows.
The stage set by the NEB modulates the activity of the hypothalamic-pituitary-ovarian axis because of insulin action (Wathes et al. Citation2007). Under nutrition has been linked to the inability of the hypothalamus to sustain high frequency luteinizing hormone (LH) pulses by the pituitary gland (Kadokawa et al. Citation2006; Bisinotto et al. Citation2018). Indeed, LH pulse frequency was shown to be positively correlated with energy balance and negatively correlated with blood NEFA concentration (Kadokawa et al. Citation2006). The underlying mechanism by which NEB reduces LH release is likely to involve the supply of oxidizable fuels to neurons and hormonal modulation of hypothalamic and pituitary cells (Bisinotto et al. Citation2018). Glucose is a preferred substrate for neuron energy metabolism and inadequate supply of glucose inhibits the gonadotropin-releasing hormone (GnRH) pulse generator (Dupont and Scaramuzzi Citation2016). Under a favourable nutritional status, the hormonal milieu to which the hypothalamus and pituitary gland are exposed favours the release of GnRH and gonadotropins (Bisinotto et al. Citation2018).
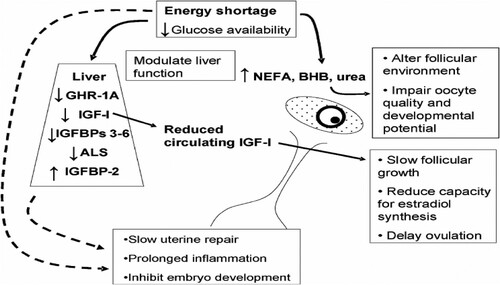
Cows with severe NEB have a longer interval from calving to the onset of ovarian cyclicity than cows without NEB (Opsomer et al. Citation2000; Shin et al. Citation2015), indicating that cows simply postpone their reproductive functions until energy balance does not compromise embryo or foetus survival. It has been found that an early resumption of ovarian activity leads to a subsequent improvement in fertility. Furthermore, cows in severe NEB may have suppressed LH pulse frequencies and reduced ovarian sensitivity to LH. A follicle developed under such conditions is more likely to become non-ovulatory and hence delay cyclicity than a follicle developed under normal conditions (Kadokawa et al. Citation2006; Bisinotto et al. Citation2018). Plasma hormones and metabolites related to NEB have been found to be correlated with the concentrations of metabolites and hormones in the follicular fluid of the dominant follicle (Leroy et al. Citation2004); e.g. oocytes cultivated in media with NEFA concentrations resembling those of cows in NEB or in low glucose concentrations together with BHBA concentrations resembling clinical or subclinical ketosis, show delayed maturation, fertilization, and oocyte cleavage (Leroy et al. Citation2006, Citation2005). Furthermore, in vitro studies have shown that NEFA can modulate proliferation and steroidogenesis in oocyte granulosa cells (Vanholder et al. Citation2005). depicts the detailed effects of negative energy balance on cow’s fertility.
Figure 1. Summary diagram showing how negative energy balance may influence fertility through effects on the liver, ovary and uterus and adapted from Wathes et al.(Citation2007).
6. Monitoring tools of dairy cattle in negative energy balance
The BCS, blood, milk and urine metabolites are commonly used as NEB monitoring tools for dairy cows (LeBlanc Citation2010; Wang et al. Citation2019). These biomarkers could allow for early and accurate detection of NEB in dairy cows. The lower BCS is expected in the early lactating animals because the body reserves mobilize in response to satisfying the nutrient requirement for milk production. This loss of body condition is associated with alterations in blood metabolite and hormone profiles, which in turn influence fertility (Carvalho et al. Citation2014). Body condition monitoring is a key indicator for the prevalence of NEB (), and as the same time, it is an important tool to manage NEB in dairy cows at an earlier time to avoid the short and long term consequences. Pushpakumara et al. (Citation2003) and Kadivar et al. (Citation2014) reported that cows that lose more body condition (>1.0 condition score) during the first month post-partum experience a longer interval to their first ovulation than cows that lose <1.0 body weight.
Table 1. The BCS loss and the subsequent effect on ovulation delay.
The other NEB monitoring tools are metabolites from blood, milk and urine of dairy cows (Adewuyi et al. Citation2005). Metabolite levels like NEFA are increased in the plasma because cows rely on mobilization of the reserved fat (60%) and because it is being used as monitoring tool for energy balance in transition dairy cows (Knop and Cernescu Citation2009). Circulating concentrations of NEFA and BHBA measure the success of adaptation to NEB (LeBlanc Citation2006; Churakov et al. Citation2021), but excessive elevation of NEFA or BHBA can indicate poor adaptation to NEB (Herdt Citation2000). The NEFA concentration reflects the magnitude of mobilization of fat stored in the adipose tissue, while the BHBA concentration reflects the completeness of oxidization of fat in the liver. Ketone bodies are the intermediate metabolites of oxidation of fatty acids, specifically resulting from the incomplete oxidation of fatty acids. The BHBA is considered by most to be a molecule that is essentially worthless to the dairy cow and is only present in the bloodstream, urine, and milk at higher than normal levels when there is an excessive amount of fat mobilized as a mechanism by which the animal tries to adapt to the NEB.
Levels above 1.2 mmol/liter of BHBA are considered to be abnormal, and the general recommendation is to treat animals that fall into this category with intravenous dextrose and/or oral glucose precursors (LeBlanc Citation2006; Churakov et al. Citation2021). The higher amounts of BHBA that are produced during the conversion of NEFA to energy are the underlying cause of ketosis, and this problem is reported in Ethiopian dairy farms (Tadesse et al. Citation2012; Asrat et al. Citation2013). As the supply of NEFA to the liver exceeds the ability of the liver to completely oxidize the fatty acids to supply energy, the amount of ketone production increases (). Ketone bodies can be used by muscle as an alternative fuel source to glucose, sparing glucose for milk production (LeBlanc Citation2006; Zarrin et al. Citation2013). However, ketone production does not result in as much net energy release as fatty acid oxidation. Additionally, increasing concentrations of ketones are thought to suppress feed intake (LeBlanc Citation2006).
High NEFA (> 0.4 mmol/l) in the last 7–10 days before expected calving is associated with increased risk of displaced abomasum, retained placenta, culling before 60 days in milk, and less milk production in the first 4 months of lactation (LeBlanc Citation2006). Subclinical ketosis (serum BHBA >1200–1400 micromol/l) in the first or second week after calving is associated with increased risk of displaced abomasum, metritis, clinical ketosis, endometritis, prolonged postpartum anovulation, increased severity of mastitis, and lower milk production in early lactation (Ghanem Citation2017). Glucose, insulin, and leptin are other plasma metabolites that can be used as tools for dairy cows’ NEB monitoring (Block et al. Citation2001). Furthermore, fat to protein ratio in milk is an important NEB indicator for dairy cows and could be used as a dairy farm monitoring tool to optimize herd management (Churakov et al. Citation2021).
7. Pasture-based dairy production and negative energy balance
Mixed dairy farming is popularly adopted in Ethiopia (Birendra et al. Citation2020). The majority is from the highlands, where mixed crop and livestock production systems are dominant, followed by pastoral and agro-pastoral systems. The majority of milk production (95%) in Ethiopia is coming from these production systems dominated by local breeds (CSA Citation2021). Commercial intensive dairy production systems are emerging in cities and peri-urban areas.
Feed resources in traditional mixed dairy farming systems are quite different from those in urban and peri-urban dairy production systems. The main supplement source in dairy nutrition for the later production system is concentrate or individual agro-industrial by products. However, the majority of dairy production system in Ethiopia is a natural pasture based production system, and its national contribution is 54%, followed by crop residues (31%), and for improved feed and by-products, they are 0.57% and 2%, respectively (CSA Citation2021). However, the quality of these basal roughage feeds is generally low and needs strategic supplementation with protein and energy rich feeds to help dairy cows maintain their body weight and produce milk (Chalchissa et al. Citation2014); otherwise, they could not allow dairy cows to adapt the NEB in the transition period. The pasture in Ethiopia is dominantly natural grazing land where there are no management practices such as fertilization and over seeding of quality forage (CSA Citation2021). So, the natural pasture in Ethiopia is characterized as poor in botanical composition, low in biomass yield, and low in nutritional value. In line with this idea, Pedernera et al. (Citation2008) reported that dairy cows fed on a predominantly grazed pasture diet mobilized their body reserves more than dairy cows fed supplements. And this was resulted in higher concentrations of NEFA and BHBA and lower concentrations of insulin-like growth factor-I. Overall, poor nutrition during the prepartum period can make a cow at calving to make more susceptible to metabolic disorders, body condition loss, and a more severe NEB at calving. LeBlanc (Citation2010) reported that inappropriate management of high milk producing dairy cows may significantly contribute to the cause of poor fertility rather than direct genetic effects.
8. Nutritional strategies to improve energy balance, health and fertility
Though NEB is common in transition high yielding dairy cows, prepartum and postpartum nutritional strategies could reduce its severity and duration, potentially impacting the animal’s health and fertility (Overton and Waldron Citation2004; Janovick et al. Citation2011; Drackley and Cardoso Citation2014). Gilmore et al. (Citation2011) reported that dairy cows fed a high-starch/high-fat diet become more fertile by decreasing delayed ovulation. Thus, to achieve one calving per cow per year, measures to prevent NEB should focus on the transition period. The dry period nutritional management of dairy cows can have a significant positive effect on the subsequent lactation. Feeding a high-energy diet throughout the entire dry period can be detrimental to cow health; e.g. Swedish cows fed a high energy diet for at least 8 weeks prepartum had a larger decrease in DMI and a larger increase in postpartum NEFA concentrations than cows fed a low energy diet (Holtenius et al. Citation2003).
In large herds, for example, in the United States, a common approach is to group the dry cows to allow for a low-energy diet during the early dry period and increasing energy content during the last 2–3 weeks before parturition, thereby minimizing the NEB effect (Overton and Waldron Citation2004). Scholars’ findings generally support the notion that feeding a higher energy diet for two to three weeks prior to parturition is beneficial in reducing NEB in dairy cows (Contreras et al. Citation2004; Janovick et al. Citation2011). Increasing the energy and protein (rumen undegradable protein) density of prepartum cow diets increased DMI and decreased NEB (Zhou et al. Citation2016). However, the availability of rumen degradable and metabolizable proteins may worsen the severity of NEB and its impact on dairy cow fertility (Tamminga Citation2006). Supplementation of nutrients such as dietary choline, specific fatty acids, and essential amino acids such as methionine to transition dairy cows may improve energy balance (Rulquin and Delaby Citation1997; Overton and Waldron Citation2004; Folnožić et al. Citation2019; Đuričić et al. Citation2020). Block (Citation1984) reported that dairy cows fed an anionic diet had no milk fever. Another important nutritional strategy is the optimum physically effective fibre length and roughage to concentrate ratio considered in minimizing NEB in dairy cows’ transition period (Zebeli et al. Citation2010).
The transition phase can also be eased by employing feed additives (Leslie et al. Citation2000), protected nutrients such as fat (Shelke et al. Citation2012; Zenobi et al. Citation2018), glucogenic precursors (Patton et al. Citation2004), and direct-fed microbials/probiotics (Sretenović et al. Citation2008); a phased feeding programme could avoid the NEB stress of dairy cows and enhance milk production, health, and reproductive performance (Bakshi et al. Citation2017). Starch-rich diets given postpartum, inducing a high concentration of insulin, have been shown to increase the proportion of cows ovulating within 50 days after calving (Gong Citation2002). However, if this type of insulinogenic diet is fed for longer periods, the high plasma insulin concentration may lead to negative effects on oocyte and embryo quality (Leroy et al. Citation2008). Garnsworthy et al. (Citation2009) therefore combined an insulinogenic diet to allow for early cycling, followed by a ‘mating diet’ rich in fat (which generated lower insulin concentrations) fed from the first rise in progesterone until 120 days in milk. This combination doubled the proportion of cows pregnant at 120 days compared with cows fed only the insulinogenic diet for the entire period (Garnsworthy et al. Citation2009).
The prophylactic use of additives that can increase ruminal propionate concentration, such as propylene glycol, has been associated with insulin stimulation, a decrease in lipolysis, and inhibiting the synthesis of ketone bodies, resulting in a better energetic status in dairy cows (Zhang et al. Citation2020). Feed additives have been studied in order to alleviate the NEB experienced by dairy cattle through increased energy density of the diet. Some of these additives are known as ‘protected fat’ or ‘by-pass’ fat (Pickett et al. Citation2003). This nutritional strategy consists in the utilization of fatty acids, originated from the diet that could positively influence the energetic status in the early post-partum by offering high energy in a period where depression of DMI is eminent. The fatty acids originated from the diet are utilized by extra-hepatic tissues, differently than NEFA, which are metabolized in the liver (Bertics and andGrummer Citation1999; Pickett et al. Citation2003). Therefore, the lipid mobilization would be less intense and would have a less negative effect on liver metabolism (Pickett et al. Citation2003). It is a common practice to utilize liver protectors during the early postpartum period in dairy cows (García et al. Citation2011), but the number of studies evaluating liver protectors are few. The potential use of silymarin, i.e. human liver protector, in dairy cows during the transition period has been reported by Tedesco et al. (Citation2004). The use of silymarin may aid in the prevention of liver disorders buy reducing lipid peroxidation and the destruction of hepatic cell membranes.
9. Conclusions
A shortage of dietary energy nutrients that are less than what is required during early lactation or transition physiology is almost natural for the high yielding dairy cows. So, the implications of NEB on dairy cows are poor in production, unhealthy and poor in fertility; this makes the dairy farm unprofitable. Early monitoring and implementation of nutritional strategies could minimize these negative effects of NEB on dairy cows. Therefore, regular monitoring of the nutritional status of the cattle in a herd and modifying the feeding management accordingly can be very helpful in the prevention of production diseases. Recently, it has been shown that nutritional management in early dry period, i.e. after cessation of milking, is important for maintaining the health and productivity of cows in transition. Otherwise, the short and long-term negative effects of NEB in the dairy industry pose big challenges in terms of milk quantity and quality, as well as economic profitability. Fortunately, its severity can be reduced by employing the on-farm monitoring tools which have been developed by scholars, and it is possible to rescue the high yielding dairy cows through the implementation of appropriate nutrition.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Adewuyi AA, Gruys E, Van Eerdenburg FJCM. 2005. Non esterified fatty acids (NEFA) in dairy cattle. A review. Vet Quart. 27(3):117–126.
- Aschenbach JR, Kristensen NB, Donkin SS, Hammon HM, Penner GB. 2010. Gluconeogenesis in dairy cows: the secret of making sweet milk from sour dough. IUBMB Life. 62(12):869–877.
- Asrat M, Tadesse GH, Gounder RV, Nagappan R. 2013. Prevalence and treatment of ketosis in dairy cows in and around Addis Ababa, Ethiopia. British J Dairy Sciences. 3(3):26–30.
- ATA (Agricultural Transformation Agency). 2016. Promising investment opportunities in Ethiopian agribusiness. WEF Grow Africa, Ethiopia.
- Ayele G, Mekibib B, Sheferaw D. 2014. Major postpartum problems of dairy cows managed in small and medium scale production systems in Wolaita Sodo, Ethiopia. African J Agri Res. 9(36):2775–2780.
- Bach À. 2019. Effects of nutrition and genetics on fertility in dairy cows. Reprod, Fert Develop. 31(1):40–54.
- Bakshi MPS, Wadhwa M, Makkar HP. 2017. Feeding of high-yielding bovines during transition phase. CAB Rev. 12(006):1–8.
- Bauman DE. 2000. Regulation of nutrient partitioning during lactation: homeostasis and homeorhesis. Ruminant Phys: Digestion, Metab, Growth, Reprod. 311–328.
- Baumgard LH, Odens LJ, Kay JK, Rhoads RP, VanBaale MJ, Collier RJ. 2006. Does negative energy balance (NEBAL) limit milk synthesis in early lactation? PP.181-187. In Proc. Southwest Nutr. Conf, http://animal.cals.arizona.edu/swnmc/2006/proceedings.php.
- Becker VAE, Stamer E, Spiekers H, Thaller G. 2021. Residual energy intake, energy balance, and liability to diseases: genetic parameters and relationships in German Holstein dairy cows. J Dairy Sci. 104(10):10970–10978.
- Berry DP, Veerkamp RF, Dillon P. 2006. Phenotypic profiles for body weight, body condition score, energy intake, and energy balance across different parities and concentrate feeding levels. Livestock Sci. 104(1-2):1–12.
- Bertics SJ, andGrummer RR. 1999. Effects of fat and methionine hydroxy analog on prevention or alleviation of fatty liver induced by feed restriction. J Dairy Sci. 82(12):2731–2736.
- Bewley JM, Schutz MM. 2008. An interdisciplinary review of body condition scoring for dairy cattle. The Prof Anim Sci. 24(6):507–529.
- Birendra KC, Schultz B, McIndoe I, Rutter H, Dark A, Prasad K, Sijapati S, Paudel K. 2020. Impacts of dairy farming systems on water quantity and quality in Brazil, Ethiopia, Nepal, New Zealand and the USA. Irrig Drain. 69(4):944–955.
- Bisinotto RS, Greco LF, Ribeiro ES, Martinez N, Lima FS, Staples CR, Thatcher WW, Santos JEP. 2018. Influences of nutrition and metabolism on fertility of dairy cows. Anim Reprod. 9(3):260–272.
- Block E. 1984. Manipulating dietary anions and cations for prepartum dairy cows to reduce incidence of milk fever. J Dairy Sci. 67(12):2939–2948.
- Block SS, Butler WR, Ehrhardt RA, Bell AW, Van Amburgh ME, Boisclair YR. 2001. Decreased concentration of plasma leptin in periparturient dairy cows is caused by negative energy balance. J Endocrinol. 171(2):339–348.
- Butler WR. 2003. Energy balance relationships with follicular development, ovulation and fertility in postpartum dairy cows. Livestock Prod Sci. 83(2-3):211–218.
- Carvalho PD, Souza AH, Amundson MC, Hackbart KS, Fuenzalida MJ, Herlihy MM, Shave RD, Wiltbank MC. 2014. Relationships between fertility and postpartum changes in body condition and body weight in lactating dairy cows. J Dairy Sci. 97(6):3666–3683.
- Central Statistical Agency (CSA). 2021. Agricultural sample survey. report on livestock and livestock characteristics (Private Peasant Holdings), Vol. II, Central Statistical Agency (CSA), Addis Ababa, Ethiopia.
- Chalchissa G, Mekasha Y, Urge M. 2014. Feed resources quality and feeding practices in urban and peri-urban dairy production of southern Ethiopia. Trop Subtropical Agroecosys. 17(3):539–546.
- Chandra G, Aggarwal A, Singh AK, Kumar M, Kushwaha R, Singh A, Singh YK. 2011. Negative energy balance and reproduction: A review. Agricultural Rev. 32(4):246–254.
- Churakov M, Karlsson J, Rasmussen AE, Holtenius K. 2021. Milk fatty acids as indicators of negative energy balance of dairy cows in early lactation. Animal. 15(7):100253.
- Collado-Fernandez E, Picton HM, Dumollard R. 2013. Metabolism throughout follicle and oocyte development in mammals. Int J Dev Biol. 56(10-11-12):799–808.
- Contreras LL, Ryan CM, Overton TR. 2004. Effects of dry cow grouping strategy and prepartum body condition score on performance and health of transition dairy cows. J Dairy Sci. 87(2):517–523.
- Delosière M, Pires J, Bernard L, Cassar-Malek I, Bonnet M. 2019. Milk proteome from in silico data aggregation allows the identification of putative biomarkers of negative energy balance in dairy cows. Sci Rep. 9(1):1–11.
- Drackley JK, Cardoso FC. 2014. Prepartum and postpartum nutritional management to optimize fertility in high-yielding dairy cows in confined TMR systems. Animal. 8(s1):5–14.
- Drennan MJ, Berry DP. 2006. Factors affecting body condition score, live weight and reproductive performance in spring-calving suckler cows. Irish J. Agric. Food Res. 25–38.
- Duffield TF, Lissemore KD, McBride BW, Leslie KE. 2009. Impact of hyperketonemia in early lactation dairy cows on health and production. J Dairy Sci. 92(2):571–580.
- Dupont J, Scaramuzzi RJ. 2016. Insulin signalling and glucose transport in the ovary and ovarian function during the ovarian cycle. Biochem J. 473(11):1483–1501.
- Đuričić D, Vince S, Lojkić M, Jelušić S, Turk R, Valpotić H, Gračner D, Maćešić N, Folnožić I, Šostar Z, Samardžija M. 2020. Effects of dietary clinoptilolite on reproductive performance, serum progesterone and insulin-like growth factor-1 concentrations in dairy cows during pregnancy and lactation. Pol J Vet Sci. 23:69–75.
- Esposito G, Irons PC, Webb EC, Chapwanya A. 2014. Interactions between negative energy balance, metabolic diseases, uterine health and immune response in transition dairy cows. Anim Reprod Sci. 144(3-4):60–71.
- FAO (Food and Agricultural Organization). 2018. Gateway to dairy production and products, feedresources.http://www.fao.org/dairy-productionproducts/production/feed-resources/en/.
- Fasil N, Juta TS. 2016. Major health challenges of dairy cattle in Hawassa town, SNNPRS, Ethiopia. J Vet Sci Technol. 7:367.
- Folnožić I, Samardžija M, Đuričić D, Vince S, Perkov S, Jelušić S, Valpotić H, Beer Ljubić B, Lojkić M, Gračner M, et al. 2019. Effects of in-feed clinoptilolite treatment on serum metabolic and antioxidative biomarkers and acute phase response in dairy cows during pregnancy and early lactation. Res. Vet. Sci. 127:57–64.
- Folnožić I, Turk R, Đuričić D, Vince S, Flegar-Meštrić Z, Sobiech P, Lojkić M, Valpotić H, Samardžija M. 2016. The effect of parity on metabolic profile and resumption of ovarian cyclicity in dairy cows. Veterinarski Arhiv. 86(5):641–653.
- Friggens NC, Berg P, Theilgaard P, Korsgaard IR, Ingvartsen KL, Løvendahl P, Jensen J. 2007. Breed and parity effects on energy balance profiles through lactation: evidence of genetically driven body energy change. J Dairy Sci. 90(11):5291–5305.
- García A, Cardoso FC, Campos R, Thedy DX, González FH. 2011. Metabolic evaluation of dairy cows submitted to three different strategies to decrease the effects of negative energy balance in early postpartum. Pesqui Vet Bras. 31:11–17.
- Garnsworthy PC, Gong JG, Armstrong DG, Mann GE, Sinclair KD, Webb R. 2009. Effect of site of starch digestion on metabolic hormones and ovarian function in dairy cows. Livestock Science. 125(2-3):161–168.
- Ghanem PM. 2017. Clinico-biochemical, oxidative markers and trace elements changes in cows with ketosis. Benha Vet Med J. 33(2):224–236.
- Gilmore HS, Young FJ, Patterson DC, Wylie ARG, Law RA, Kilpatrick DJ, Elliott CT, Mayne CS. 2011. An evaluation of the effect of altering nutrition and nutritional strategies in early lactation on reproductive performance and estrous behavior of high-yielding Holstein-Friesian dairy cows. J Dairy Sci. 94(7):3510–3526.
- Gong JG. 2002. Influence of metabolic hormones and nutrition on ovarian follicle development in cattle: practical implications. Domest Anim Endocrin. 23(1-2):229–241.
- Gross JJ, Kessler EC, Albrecht C, Bruckmaier RM. 2015. Response of the cholesterol metabolism to a negative energy balance in dairy cows depends on the lactational stage. PloS one. 10(6):e0121956.
- Grummer RR, Mashek DG, Hayirli A. 2004. Dry matter intake and energy balance in the transition period. Vet Clin: Food A. 20(3):447–470.
- Harrison RO, Ford SP, Young JW, Conley AJ, Freeman AE. 1990. Increased milk production versus reproductive and energy status of high producing dairy cows. J Dairy Sci. 73(10):2749–2758.
- Hayirli A, Grummer RR. 2004. Factors affecting dry matter intake prepartum in relationship to etiology of peripartum lipid-related metabolic disorders: A review. Can. J. Anim. Sci. 84(3):337–347.
- Hayirli A, Grummer RR, Nordheim EV, Crump PM. 2002. Animal and dietary factors affecting feed intake during the prefresh transition period in holsteins. J. Dairy Sci. 85:3430–3443.
- Herdt TH. 2000. Ruminant adaptation to negative energy balance: influences on the etiology of ketosis and fatty liver. Vet Clin North America: Food A. 16(2):215–230.
- Holtenius K, Agenas S, Delavaud C, Chilliard Y. 2003. Effects of feeding intensity during the dry period. 2. Metabolic and Hormonal Responses. J. Dairy Sci. 86:883–891.
- Ingvartsen KL. 2006. Feeding-and management-related diseases in the transition cow: physiological adaptations around calving and strategies to reduce feeding-related diseases. Anim Feed Sci Technol. 126(3-4):175–213.
- Janovick NA, Boisclair YR, Drackley JK. 2011. Prepartum dietary energy intake affects metabolism and health during the periparturient period in primiparous and multiparous Holstein cows. J Dairy Sci. 94(3):1385–1400.
- Josef G, Hendrika A, van Dorland R, Bruckmaier M, Frieder JS. 2011. Milk fatty acid profile related to energy balance in dairy cows. J Dairy Res. 78(4):479–488.
- Kadivar A, Ahmadi MR, Vatankhah M. 2014. Associations of prepartum body condition score with occurrence of clinical endometritis and resumption of postpartum ovarian activity in dairy cattle. Trop Anim Health Prod. 46(1):121–126.
- Kadokawa H, Blache D, Martin GB. 2006. Plasma leptin concentrations correlate with luteinizing hormone secretion in early postpartum Holstein cows. J Dairy Sci. 89:3020–3027.
- Knop R, Cernescu H. 2009. Effects of negative energy balance on reproduction in dairy cows. Lucrări Stiinłifice Medicină Veterinară. 42(2):198–205.
- Kokkonen T, Taponen J, Anttila T, Syrjälä-Qvist L, Delavaud C, Chilliard Y, Tuori M, Tesfa AT. 2005. Effect of body fatness and glucogenic supplement on lipid and protein mobilization and plasma leptin in dairy cows. J Dairy Sci. 88(3):1127–1141.
- Leblanc S. 2010. Monitoring metabolic health of dairy cattle in the transition period. J Reprod Develop. 56(S):S29–S35.
- LeBlanc SJ. 2006. Monitoring programs for transition dairy cows. In Proceedings of the 26th World Biuatrics Congress, Nice, 460-472.
- LeBlanc SJ. 2012. Interactions of metabolism, inflammation, and reproductive tract health in the postpartum period in dairy cattle. Reprod Domest Anim. 47:18–30.
- Leroy JLMR, Vanholder T, Delanghe JR, Opsomer G, Van Soom A, Bols PEJ, de Kruif A. 2004. Metabolite and ionic composition of follicular fluid from different-sized follicles and their relationship to serum concentrations in dairy cows. Anim Reprod Sci. 80(3-4):201–211.
- Leroy JLMR, Vanholder T, Mateusen B, Christophe A, Opsomer G, de Kruif A, Genicot G, Van Soom A. 2005. Non-esterified fatty acids in follicular fluid of dairy cows and their effect on developmental capacity of bovine oocytes in vitro. Reproduction. 130(4):485–495.
- Leroy JLMR, Vanholder T, Opsomer G, Van Soom A, de Kruif A. 2006. The in vitro development of bovine oocytes after maturation in glucose and β-hydroxybutyrate concentrations associated with negative energy balance in dairy cows. Reprod Domest Anim. 41(2):119–123.
- Leroy JLMR, Van Soom A, Opsomer G, Goovaerts IGF, Bols PEJ. 2008. Reduced fertility in high-yielding dairy cows: are the oocyte and embryo in danger? part II mechanisms linking nutrition and reduced oocyte and embryo quality in high-yielding dairy cows. Reprod Domest Anim. 43(5):623–632.
- Leslie K, Duffield T, Stephen L. 2003. Monitoring and managing energy balance in the transition dairy Cow. J Dairy Sci. 86:101–107.
- Leslie KE, Duffield TF, Schukken YH, LeBlanc SJ. 2000. The influence of negative energy balance on udder health. In: National mastitis council regional meeting proceedings. Madison, WI: Omnipress; p. 25–33.
- Lu J, Antunes Fernandes E, Páez Cano AE, Vinitwatanakhun J, Boeren S, van Hooijdonk T, … Hettinga KA. 2013. Changes in milk proteome and metabolome associated with dry period length, energy balance, and lactation stage in postparturient dairy cows. J Proteome Res. 12(7):3288–3296.
- Mulligan FJ, Doherty ML. 2008. Production diseases of the transition cow. Vet J. 176(1):3–9.
- Opsomer G. 2015. Interaction between metabolic challenges and productivity in high yielding dairy cows. Jpn J Vet Res. 63(Supplement 1):S1–S14.
- Opsomer G, Gröhn YT, Hertl J, Coryn M, Deluyker H, De Kruif A. 2000. Risk factors for post partum ovarian dysfunction in high producing dairy cows in Belgium: a field study. Theriogenology. 53(4):841–857.
- Overton TR, Waldron MR. 2004. Nutritional management of transition dairy cows: strategies to optimize metabolic health. J Dairy Sci. 87:E105–E119.
- Patton RS, Sorenson CE, Hippen AR. 2004. Effects of dietary glucogenic precursors and fat on feed intake and carbohydrate status of transition dairy cows. J Dairy Sci. 87(7):2122–2129.
- Pedernera M, Garcia SC, Horagadoga A, Barchia I, Fulkerson WJ. 2008. Energy balance and reproduction on dairy cows fed to achieve low or high milk production on a pasture-based system. J Dairy Sci. 91(10):3896–3907.
- Pickett MM, Piepenbrink MS, Overton TR. 2003. Effects of propylene glycol or fat drench on plasma metabolites, liver composition, and production of dairy cows during the periparturient period. J Dairy Sci. 86(6):2113–2121.
- Pires JA, Delavaud C, Faulconnier Y, Pomies D, Chilliard Y. 2013. Effects of body condition score at calving on indicators of fat and protein mobilization of periparturient Holstein-Friesian cows. J Dairy Sci. 96(10):6423–6439.
- Pushpakumara PGA, Gardner NH, Reynolds CK, Beever DE, Wathes DC. 2003. Relationships between transition period diet, metabolic parameters and fertility in lactating dairy cows. Theriogenology. 60(6):1165–1185.
- Randhawa SNS, Ranjan R, Singh R, Chand N. 2014. Diagnosis and management of negative energy balance and associated production diseases in bovines. Intas Polivet. 15(2):497–503.
- Rhoads RP, Rhoads ML, Boisclair YR. 2005. Implications of a dysfunctional somatotropic axis during the transition period in dairy cattle. Proc. Southwest Nutr. Conf. 4:197–208.
- Rhodes FM, McDougall S, Burke CR, Verkerk GA, Macmillan KL. 2003. Invited review: treatment of cows with an extended postpartum anestrous interval. J. Dairy Sci. 86(6):1876–1894.
- Ribeiro ES, Bisinotto RS, Lima FS, Greco LF, Morrison KA, Thatcher WW, Santos JEP. 2014. Plasma anti-Müllerian hormone in adult dairy cows and associations with fertility. J Dairy Sci. 97(11):6888–6900.
- Rocchetti G, O’Callaghan TF. 2021. Application of metabolomics to assess milk quality and traceability. Current Opinion Food Sci. 40:168–178.
- Roche JR, Burke CR, Crookenden MA, Heiser A, Loor JL, Meier S, … Turner SA. 2018. Fertility and the transition dairy cow. Reprod, Fertility Develop. 30(1):85–100.
- Roche JR, Friggens NC, Kay JK, Fisher MW, Stafford KJ, Berry DP. 2009. Invited review: body condition score and its association with dairy cow productivity, health, and welfare. J Dairy Sci. 92(12):5769–5801.
- Roche JR, Macdonald KA, Schütz KE, Matthews LR, Verkerk GA, Meier S, … Webster JR. 2013. Calving body condition score affects indicators of health in grazing dairy cows. J Dairy Sci. 96(9):5811–5825.
- Roschinsky R, Kluszczynska M, Sölkner J, Puskur R, Wurzinger M. 2015. Smallholder experiences with dairy cattle crossbreeding in the tropics: from introduction to impact. Animal. 9:150–157.
- Rulquin H, Delaby L. 1997. Effects of the energy balance of dairy cows on lactational responses to rumen-protected methionine. J Dairy Sci. 80(10):2513–2522.
- Shapiro BI, Gebru G, Desta S, Negassa A, Negussie K, Aboset G, Mechal H. 2015. Ethiopia livestock master plan: Roadmaps for growth and transformation.
- Shelke SK, Thakur SS, Shete SM. 2012. Protected nutrients technology and the impact of feeding protected nutrients to dairy animals: a review. Int J Dairy Sci. 7:51–62.
- Shin EK, Jeong JK, Choi IS, Kang HG, Hur TY, Jung YH, Kim IH. 2015. Relationships among ketosis, serum metabolites, body condition, and reproductive outcomes in dairy cows. Theriogenology. 84(2):252–260.
- Soares RAN, Vargas G, Muniz MMM, Soares MAM, Cánovas A, Schenkel F, Squires EJ. 2021. Differential gene expression in dairy cows under negative energy balance and ketosis: A systematic review and meta-analysis. J Dairy Sci. 104(1):602–615.
- Sordillo LM, Contreras GA, Aitken SL. 2009. Metabolic factors affecting the inflammatory response of periparturient dairy cows. Anim Health Res Rev. 10(1):53–63.
- Sretenović L, Petrović MP, Aleksić S, Pantelić V, Katić V, Bogdanović V, Beskorovajni R. 2008. Influence of yeast, probiotics and enzymes in rations on dairy cows performances during transition. Biotechnol Anim Husbandry. 24(5-6):33–43.
- Sundrum A. 2015. Metabolic disorders in the transition period indicate that the dairy cows’ ability to adapt is overstressed. Animals. 5(4):978–1020.
- Tadesse G, Bekelle D, Singh B. 2012. Prevalence and clinico-pathology of ketosis in dairy cows in Tigray region of Ethiopia. Momona Ethiopian J Sci. 4(1):115–120.
- Tamminga S. 2006. The effect of the supply of rumen degradable protein and metabolisable protein on negative energy balance and fertility in dairy cows. Anim Reprod Sci. 96(3-4):227–239.
- Tedesco D, Tava A, Galletti S, Tameni M, Varisco G, Costa A, Steidler S. 2004. Effects of silymarin, a natural hepatoprotector, in periparturient dairy cows. J Dairy Sci. 87(7):2239–2247.
- Tesfaye A, Habte Y, Minten B. 2020. COVID-19 is shifting consumption and disrupting dairy value chains in Ethiopia. IFPRI book chapters, 42-45.
- Tesfaye M, Beze A, Degefa K. 2019. Dairy plant processing capacity and challenges in milk processing industry of Ethiopia. European J Biol Sci. 11(3):106–113.
- Turk R, Folnožić I, Đuričić D, Vince S, Flegar-Meštrić Z, Dobranić T, … Samardžija M. 2016. Relationship between paraoxonase-1 activity and lipid mobilisation in transition dairy cows. Veterinarski Arhiv. 86(5):601–612.
- Vanholder T, Leroy JL, Dewulf J, Duchateau L, Coryn M, De Kruif A, Opsomer G. 2005. Hormonal and metabolic profiles of high-yielding dairy cows prior to ovarian cyst formation or first ovulation post partum. Reprod Domest Anim. 40(5):460–467.
- Vries MJ, Veerkamp RF. 2000. Energy balance of dairy cattle in relation to milk production variables and fertility. J. Dairy Sci. 83:62–69.
- Wang Y, Huo P, Sun Y, Zhang Y. 2019. Effects of body condition score changes during peripartum on the postpartum health and production performance of primiparous dairy cows. Animals. 9(12):1159.
- Wathes DC, Brickell JS, Bourne NE, Swali A, Cheng Z. 2008. Factors influencing heifer survival and fertility on commercial dairy farms. Animal. 2(8):1135–1143.
- Wathes DC, Cheng Z, Bourne N, Taylor VJ, Coffey MP, Brotherstone S. 2007. Differences between primiparous and multiparous dairy cows in the inter-relationships between metabolic traits, milk yield and body condition score in the periparturient period. Domest Anim Endocrinol. 33(2):203–225.
- Wathes DC, Cheng Z, Chowdhury W, Fenwick MA, Fitzpatrick R, Morris DG, … Murphy JJ. 2009. Negative energy balance alters global gene expression and immune responses in the uterus of postpartum dairy cows. Phys Genom. 39(1):1–13.
- Wisnieski L, Norby B, Pierce SJ, Becker T, Gandy JC, Sordillo LM. 2019. Predictive models for early lactation diseases in transition dairy cattle at dry-off. Prev Vet Med. 163:68–78.
- Xu W, Van Knegsel A, Saccenti E, Van Hoeij R, Kemp B, Vervoort J. 2020. Metabolomics of milk reflects a negative energy balance in cows. J Proteome Res. 19(8):2942–2949.
- Xu W, Vervoort J, Saccenti E, van Hoeij R, Kemp B, van Knegsel A. 2018. Milk metabolomics data reveal the energy balance of individual dairy cows in early lactation. Sci Rep. 8(1):1–11.
- Zarrin M, De Matteis L, Vernay MC, Wellnitz O, Van Dorland HA, Bruckmaier RM. 2013. Long-term elevation of β-hydroxybutyrate in dairy cows through infusion: effects on feed intake, milk production, and metabolism. J Dairy Sci. 96(5):2960–2972.
- Zebeli Q, Mansmann D, Steingass H, Ametaj BN. 2010. Balancing diets for physically effective fibre and ruminally degradable starch: A key to lower the risk of sub-acute rumen acidosis and improve productivity of dairy cattle. Livestock Sci. 127(1):1–10.
- Zenobi MG, Scheffler TL, Zuniga JE, Poindexter MB, Campagna SR, Gonzalez HC, Farmer AT, Barton BA, Santos JEP, Staples CR. 2018. Feeding increasing amounts of ruminally protected choline decreased fatty liver in nonlactating, pregnant Holstein cows in negative energy status. J Dairy Sci. 101(7):5902–5923.
- Zhang F, Nan X, Wang H, Zhao Y, Guo Y, Xiong B. 2020. Effects of propylene glycol on negative energy balance of postpartum dairy cows. Animals. 10(9):1526.
- Zhou Z, Vailati-Riboni M, Trevisi E, Drackley JK, Luchini DN, Loor JJ. 2016. Better postpartal performance in dairy cows supplemented with rumen-protected methionine compared with choline during the peripartal period. J Dairy Sci. 99(11):8716–8732.
