ABSTRACT
From the perspective of animal welfare, sows housed in group housing systems are recommended, which is conducive to health problems and the release of natural behaviour, but can lead to other problems such as social pressure due to competition for limited resources. Sow natural behaviour can be used to improve physiological and mental health to ameliorate reproductive performance. However, the natural behaviour of sow is ignored in modern intensive housing groups. The challenges faced by social behaviour, maternal behaviour and dietary behaviour of sows in a group-housing system are discussed in this paper. Furthermore, many management strategies will affect sow behaviour, such as diet management and housing systems, providing adequate nutrition, and a reasonable environment for sows. These can be considered the basis for the successful farrowing of high-production sows. Therefore, sow performance can be improved by influencing sow behaviour through management strategies such as reasonable stocking density, a good feeding environment and suitable feed for the growth stage.
1. Background
In the natural environment, the behaviour and group life of sows are not disturbed by humans. However, they do not receive the same treatment in modern intensive housing groups (Peltoniemi et al. Citation2016). Increasingly intensive animal agricultural practices have led to changes in the lives of animals in diet and living habits. Many normal sow behaviours are also restricted and the long-term physical discomfort of the sow can cause chronic stress, and further affects the physiological function and reproductive performance of sows (Peltoniemi et al. Citation2021). In adapting to these changes, animals are often subjected to unpredictable physical and psychological pressures, causing sows to have emergency behaviour and even a decrease in litter size (Holyoake et al. Citation1995; Vasdal et al. Citation2011; Pandolfi et al. Citation2017). In 2008, the World Organization for Animal Health (OIE) highlights that the need for animals to be kept for good animal welfare includes keeping animals healthy, comfortable, safe, well-fed, able to express natural behaviours, and free from pain and fear (Commission). In addition, due to sows needing different nutrients during perinatal, early gestation, late gestation, and farrowing, managers often feed the same diets, resulting in sows often experiencing low reproductive performance or reproductive failure. It is therefore important to optimize sow management practices to avoid unnecessary stress (Peltoniemi et al. Citation2016). Several management issues must be considered, including normal behaviours, environmental and dietary issues of sow. Addressing these problems is crucial for successful farrowing and lactation (Peltoniemi et al. Citation2021). This review explores recent advances in the understanding of sow behaviour. The influence of sow behaviour is examined, to review the effects of sow behaviour on reproductive performance. This review also highlights the challenges and outstanding research questions to be addressed in future studies.
2. Social behaviour
In recent years, due to animal welfare concerns, the housing system for pregnant sows has attracted considerable attention. It is considered that if sows are confined to individual feeding systems during gestation, there will be adverse effects such as constrained behaviour, decreased physiological performance, and reproductive performance of sows (Averós et al. Citation2010; Zhang et al. Citation2013). This is also detrimental to sow welfare. The welfare regulations for pig husbandry systems have resulted in changes in European Union regulations (Directive 2008/1120/EC, ): during gestation, in the European Union sows must be housed in a group housing system from 1 January 2013. Currently, the directive has been widely adopted in countries such as the United States, Canada, Australia and New Zealand (Spoolder and Vermeer Citation2015). The group breeding system can provide sufficient space for activities and free behaviour expression, conforming to the habits of sows living in groups. The gestation group housing system was beneficial to farrowing rate, where reduced farrowing duration and increased total number of piglets born and those born alive of the sows were observed (Ferket and Hacker Citation1985; Bates et al. Citation2003; Morgan et al. Citation2018). However, some challenges have arisen with the use of group housing systems, such as social behaviour (Tsuma et al. Citation1996).
Table 1. The main regulations in Directive 2008/120/EC on the welfare of pregnant sows, adapted from Maes et al. (Citation2016).
Excessive social interaction can result in potential confrontation among sows, predominantly caused by competition for resources and territory that establishes the social hierarchy (Maes et al. Citation2016). The group housing pen can also lead to adverse social interactions, leading to poorer health, welfare and reproductive outcomes of gestating sows. Frequent aggression of short duration is often observed when competing for food, however, more intense aggression is associated with establishing a social hierarchy. We can find that the intensity of aggression usually increases in the first few days of introducing new animals (Arey Citation1999). Although aggression is beneficial for maintaining the social stability of the animals and the healthy development of the population, most aggressive encounters between sows result in skin injuries and lameness, leading to physiological and psychological stress, which can also cause immunosuppression (Tuchscherer and Manteuffel Citation2000), and can lead to adverse effects on health and litter size (Maes et al. Citation2016; Peltoniemi et al. Citation2016). Chronic stress can keep cortisol elevated, causing reproductive disruption in sows (Turner et al. Citation2005). The suppression of LH secretion by stress factors generated under excessive social behaviours leads to suppression of corpus luteum function for more than 2 days, which may lead to the end of pregnancy. Social stress can lead to a decreased standard of welfare, and reduced longevity, and can compromise productivity in animals (Borell et al. Citation2007; Spoolder et al. Citation2009). Therefore, it is necessary to carry out reasonable group feeding of sows to reduce social stress and an increasing need for more friendly models of grouping sows (Kemp and Soede Citation2012; van Nieuwamerongen et al. Citation2014). Further studies are needed to examine how to mitigate the effects of aggressive interactions between sows in the group housing system (Munsterhjelm et al. Citation2008; Bunter et al. Citation2015).
The two most important issues related to the reproductive function of group-housed sows are the stress associated with group-housed during mating and gestation and the occurrence of lactation estrus when sows are housed in groups during lactation. Einarsson et al. (Citation2014) proposed grouping the sows during the location in one phase of production (Einarsson et al. Citation2014). The stress associated with grouping sows at any point after weaning and in the next gestation can be avoided by grouping sows during lactation. There are recommendations based on a previous study (Peltoniemi et al. Citation2016): (1) Separating sows from piglets, constant sow-to-piglets contact may be stressful to sows in traditional single-feeding environments. The burden of lactation can be somewhat lessened when kept away from piglets in group lactation systems (Hultén et al. Citation2006); (2) Sows were grouped immediately after weaning and artificial insemination (AI), not during gestation. Grouping immediately after weaning avoids estrus caused by sow mixing after insemination and female social interaction. On the other hand, group-housed sows with AI can freely display estrus, which may improve the likelihood of observing estrus behaviour. Grouping sows when they are in estrus and AI not only allows the sows to interact and come into estrus freely, improving animal welfare but also reduces the risk of insemination of estrus-free sow. Reduce social pressure to group during gestation and establish social hierarchies in advance. In large-scale comparisons, grouping all sows after weaning appears to do more good than bad for reproductive performance (Peltoniemi et al. Citation2016). In conclusion, sows may exhibit better estrus behaviour when grouped after weaning. Furthermore, avoiding the potential problems of maintaining early pregnancy and maintaining a high level of reproductive performance is more about management and expertise than group housing, such as electric-feeding systems that provide undisturbed feeding, should be considered to avoid unnecessary stress (Kemp and Soede Citation2012; Maes et al. Citation2016).
3. Maternal behaviour
3.1. Nest building behaviour
Nest-building by prepartum sows is an important part of maternal behaviour in domestic pigs manifested in the form of rooting, clawing and foraging, which is regarded as an intrinsic behaviour pattern that is stimulated both internally and externally during the parturition period (Yun and Valros Citation2015; Peltoniemi et al. Citation2021). In a more natural state, sows would dig out hay with their mouths to block the leaks around them to build up a nest. The onset of nesting occurs approximately 24 h before farrowing, peaking between 6 and 12 h and the duration of nest-building behaviour is affected by the environment in which the animal is housed (Wischner et al. Citation2009; Yun and Valros Citation2015). Even without nesting material, sows exhibit nest-building behaviours such as rooting, nosing and pawing, due to an instinct to build an environment that protects the piglets (Lawrence et al. Citation1994; Illmann et al. Citation2016). The restriction of nest-building behaviour adversely affects sow welfare (Plush et al. Citation2021). Sow behaviour can be negatively affected if no nesting material is available, including more oral-nasal stereotypes and stress reactions, decreased nursing duration, and a longer duration of parturition and postural changes (Damm et al. Citation2003; Chaloupková et al. Citation2011a; Herskin et al. Citation2016). In addition, the intensity of sow nesting behaviour was positively correlated with the number of litters and permits sows to express their nest-building behaviour shortened the duration of farrowing (Oliviero et al. Citation2008; Malmkvist et al. Citation2012). Therefore, we can know that nesting behaviour is related to the reproductive performance of sows.
The initial phase of nest-building is mainly based on the decrease in progesterone levels in the sow and the increase in prostaglandin F2α levels, which causes an increase in oxytocin (Jensen Citation1986; Widowski and Curtis Citation1989; Algers and Uvnäs-Moberg Citation2007). The nesting behaviour gradually stops approximately 4 h before farrowing, possibly related to a sharp rise in oxytocin. The initiation of sow nesting behaviour is triggered by an increase in the concentration of prolactin (PRL), and a decrease in progesterone (Castrén et al. Citation1993). The second phase is driven by the sow's nesting behaviour and depends predominantly on the nesting material that can be obtained. The more suitable the nesting material, the shorter the duration of nesting behaviour (Damm et al. Citation2000). In intensive pig farming systems, due to lack of space and nesting materials or both, it may be difficult for sows to perform nesting-related behaviours. This may reduce blood flow to the piglets during gestation, increasing the connection with parturition, lactation performance and stress levels (Chaloupková et al. Citation2011b; Langendijk and Plush Citation2019). Due to a lack of nesting material, inhibiting nest-building behaviour in sows leads to increased endogenous opioids, resulting in the inhibition of oxytocin secretion and delaying the farrowing process and early lactation (Zanella et al. Citation1996; Liu et al. Citation2021). This can increase the stress level of the sow and the likelihood of piglet crushing (Oliviero et al. Citation2010; Yun et al. Citation2014).
Managers should provide prenatal sows with a supply of extra nesting materials in the loosely housed pen (such as sawdust and straw) to minimize the level of stress of the sow around the time of farrowing. The provision of straw and other bedding materials might reduce the risk of crushing by allowing the sow to build a nest and increase the duration and frequency of prenatal nest-building behaviour, and stimulate the sow's motivation to care for her newborn piglets, particularly in the case of deep bedding (Baxter et al. Citation2009). Interestingly, colostrum intake was significantly higher in piglets born to sows using nesting material, possibly stimulating an increase in sow prolactin and thus colostrum production (Plush et al. Citation2021). Providing nesting material can provide extra warmth to newborn piglets on the floor, reducing the damage they may suffer from hypothermia, which would have strong implications for survivability (Vasdal et al. Citation2011; Baxter et al. Citation2015). Therefore, allowing the sows to perform nest-building behaviours will improve sow health and welfare and increase piglet survival and growth.
3.2. Nursing and suckling behaviour
Nursing and suckling behaviour is a survival-critical behaviour derived from the interaction between the sow and the litter. It is the only way for the piglet to obtain nutrition after birth and is the most important behaviour for the survival of the piglet (Mellor and Lentle Citation2015). Nursing piglets would approach the body of the sow and suckle from the nipples to obtain colostrum after birth, as the same time, the sow calls to the piglets with a typical nursing grunt so that the piglets can obtain colostrum (van Nieuwamerongen et al. Citation2014). The lactation response is caused by the hum of the sow in penal lactation. Although a large number of behaviourally well-developed piglets are capable of suckling on their own, the milk needs to be distributed evenly among all the piglets for the fitness of the large litters (Algers Citation1993).
At the beginning of lactation, the sow would lie down, and the frequency of grunting increases to release a signal to the piglets massaging the udder segment (Fraser Citation1980). The piglets stimulate the udder receptors by massaging the udder of the sow and the sow then receives a signal through HPA, causing the release of mammogenic hormones such as prolactin and oxytocin. These hormones reach the breast through the blood and stimulate the contraction of the myoepithelial cells. This allows the acinar milk to flow into the duct system, and the udder sphincter muscles then relax, resulting in the release of milk from the sow (Barb et al. Citation2008). Piglets can directly absorb the immunoglobulin (IgG) from milk via blood circulation to enter the immune system, because the colostrum of sows contains high levels of IgG to protect piglets from exogenous disease microorganisms and reduce the risk of mastitis in sows (Danielsen et al. Citation2006; Kielland et al. Citation2015). Although the sow can continuously release milk due to the action of oxytocin during lactation, there is an interval between the release of oxytocin; the sow only breastfed the piglet at intervals (Algers and Uvnäs-Moberg Citation2007). The lactation interval of the sow and the length and intensity of massage allowed by the sow after the piglets were sucked would affect the intake of milk by the piglets. It is only through effective cooperation between the sow and piglets, that the piglets can obtain milk.
In the group housing system, sows do not produce enough milk, which can potentially affect the growth of piglets before weaning (Farmer Citation2019). Previous studies have shown that once the number of piglets born alive exceeds the number of teats, the piglets tend to fight fiercely to obtain teats, which can prevent adequate milk intake (Rutherford et al. Citation2013). Sometimes the sow unsuccessfully releases milk, leading to piglets having low weaning weight, poor growth and development, low immunity, and even death during the peak weaning period (Castrén et al. Citation1989; Illmann and Madlfousek Citation1995). Therefore, mammary gland development in the sow is crucial for optimal milk production. Good management strategies stimulate the release of maternal oxytocin, such as the sow oxytocin injections to stimulate milk ejection, thereby increasing the total number of functional glands, which may be important in improving mammary gland development in postpartum sows (Martineau et al. Citation1992; Peltoniemi et al. Citation2021). It is also possible to use fostering to meet the necessary nutritional needs of all postpartum piglets, intending to balance piglet numbers or normalize litter size (Baxter et al. Citation2013). Fostering is usually required immediately after birth and individual piglets may be fostered with sows that farrow around the same time and has smaller litter sizes. Alternatively, they can be grouped into a new litter to be placed onto a foster sow. This is done to maximize access to the teat for weak piglets and sows who wean their piglets early can provide extra milk from other piglets. Several studies suggested the more complex practice of cross-fostering, intending to improve the competitiveness of smaller piglets (English and Smith Citation1995; Kecman and Waehner Citation2016). Whether fostering or cross-fostering is practiced, routine fostering of piglets should be carried out as early as possible to ensure that the fostered piglets receive colostrum, and the negative impact on feeding piglets is often greatly reduced. In commercial practice, it has also been previously recommended that the provision of milk substitutes or glucose could be used as a supplement to improve weak piglets or the litters of sows with hypogalactia survival without jeopardizing the welfare of the sow and her piglets, but this would be said to depend on the skill of the stockperson and increase the financial cost to managers (Kobek-Kjeldager et al. Citation2020). These management strategies are used to optimize the survival and growth of large litters of piglets, especially during the lactation period (Peltoniemi et al. Citation2021).
3.3. Maternal behaviour related to piglet crushing
3.3.1. Postural change behaviour
Postural change behaviour of the sow is an important part of maternal behaviour, which not only provides a comfortable environment for the piglets but also a signal that encourages the piglets to approach the sow's udder during lactation (Pedersen et al. Citation2003). The nutrition of piglets after birth is completely dependent on the milk from sows. Therefore, the expression of side-lying behaviour in sows is of great significance for improving the survival rate of piglets during lactation. However, the postural change behaviour of the sow increases the squeezing of the piglets, which is one of the main reasons for piglet loss (a) (Marchant et al. Citation2000b; Kilbride et al. Citation2012). The majority of piglets die within the first 2–4 days of life due to crushing by the sow (b) (Marchant et al. Citation2000a), because they lack sufficient mobility to avoid dangerous situations, particularly for weaker piglets that are less alert or mobile (English and Smith Citation1995). Each change in the posture and behaviour of the sow increases the likelihood of more than two dead piglets during lactation (Tanaka and Koketsu Citation2007).
Figure 1. Causes of death in piglets from birth to weaning. Adapted from Oliviero and Peltoniemi (Citation2020). Distribution of causes of death from birth to weaning in piglets (a); Distribution of piglet mortality from birth to day seven (b).
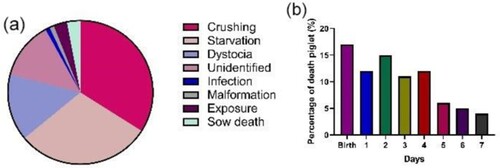
In the group-housing system, sows have more postural changes postpartum, and greater attentiveness to piglets compared to sows in the group-individual system (Thodberg et al. Citation2002). Pens with farrowing crates can effectively reduce the loss of piglets from being crushed by sows, but they prevent the sows from performing natural behaviours and also cause animal welfare problems (Nicolaisen et al. Citation2019). Free farrowing pens would increase sow activity and negatively affect piglet crushing rate and mortality in piglets (Kirkden et al. Citation2013; Koketsu and Iida Citation2017). Therefore, sows need some space to check their offspring before lying down during farrowing and early lactation.
Managers should improve the problem of poor pen design and insufficient space allocation for sows. Because postnatal piglet mortality caused by crushing could be associated with a different behavioural pattern and maternity in the sow in different feeding spaces (Kirkden et al. Citation2013). Less than 5 m2 of space in a free-range system may disrupt piglet aggregation behaviour and increase piglet mortality (Lambertz et al. Citation2015). Furthermore, the provision of bedding material to permit nest-building behaviour before farrowing may act to increase maternal behaviour and reduce stress. Sows housed in farrowing crates with straw had significantly lower postural change behaviours than sows without straw, possibly due to the farrowing pen with straw can enhance the maternal behaviour and the lactation performance of the sows (Yi et al. Citation2019). Additionally, managers can supervise farrowing during the postpartum phase when the risk of crushing is greatest, and if the stockperson is present, they can manually intervene to rescue trapped piglets (Spicer et al. Citation1986). It is also possible to group the piglets with the sow when the sow is before lying down so that the sow has increased attention to the piglets or move them away from the lying area of the sow during feeding. Other recommendations focus on improving sow health and piglet vitality in the first 3 days after farrowing, such as timely culling of older sows and ensuring that all piglets have access to colostrum (Kirkden et al. Citation2013).
3.3.2. Responsiveness to piglet screams
Sows were more sensitive to screaming for help from crushed piglets compared with screaming for help from piglets while fighting, and screaming for help was life-threatening in the case of being trapped, but while fighting was only a kind of competition between piglets in the same litter (Illmann et al. Citation2008). The piglet is easily trapped under the sow and became a threat to life, which is exacerbated by the sow being confined, making it possible to increase the chances of piglets being crushed if sows in the narrow space. The sow responds to the trapped piglet's screaming behaviour within a minute, and it generally survives (Weary et al. Citation1996a; Weary et al. Citation1996b). Therefore, the sow needs to respond auditorily to piglet screams. However, there are individual differences in the sow's response to the piglet screaming for help, which may be due to oxytocin that it affects maternal behaviour by regulating the response of the brain to significant auditory signals (Algers and Uvnäs-Moberg Citation2007). Some sows do not respond after being pressed against the piglet, while some sows respond immediately, allowing the piglet to escape (Wülbers-Mindermann et al. Citation2015). Approximately 50% of sows respond to the piglet's cry for help 9–10 h after farrowing, and only 25% respond after 24 h. The reactivity of sows within 1 week after farrowing is stronger than at 19 days after farrowing. Thus it can be seen that the response of the sow to the piglet screaming for help decreases as the postpartum time increases (Grandinson et al. Citation2003; Chaloupková et al. Citation2008).
Another question is whether a 100% sow response to piglets’ screams would be an adaptive behaviour. A sow may make a trade-off between lying down and standing, mainly depending on the sow's responsiveness and concentration (Hutson et al. Citation1993). Increased weight and leg damage would make it difficult for them to stand up, resulting in decreased reactivity and increased piglet mortality (Pluym et al. Citation2013). Therefore, managers should observe the physical health status of sows in time and treat them accordingly, ensuring that sows are not overweight during lactation. Recommended strategies to increase sow response to piglet squeal include improving sow behaviour by genetic selection (Vangen et al. Citation2005). Another finding is the screaming of the sow to the piglets would also depend on the level of maternity (Melišová et al. Citation2014). As far as we know the addition of nesting material to the group rearing system significantly reduced the occurrence of abnormal behaviour and increase maternal behaviour in sows (Illmann et al. Citation2008). Therefore, nesting material appears to be essential in the group housing system. Furthermore, compared with sows kept indoors, outdoor farrowing huts facilitate seem a less restricted maternal behaviour in sows, it is suggested that sows in outdoor huts are more responsive towards playbacks of piglet screams than sows in pens, which could better prevent fatal piglet crushing (Wülbers-Mindermann et al. Citation2015). We also recommend that managers can keep sows in outdoor farrowing huts.
3.3.3. Sow communication with piglets
Although the sow does not establish a connection with the piglet by licking it (Johnsen et al. Citation2015), it establishes an attachment relationship through behaviours such as olfactory (sniffing), vocal (grunting) and tactile (nudging) cues. This keeps the piglets nearby, with contact being maintained for at least 1 s to protect them from danger (Andersson et al. Citation2011; Ocepek et al. Citation2017; Ocepek and Andersen Citation2017). Nose contact is a common social behaviour between sows and piglets, which improves the mother-baby relationship and physical development of piglets (Portele et al. Citation2019). There are examples of sows exhibiting maternal behaviour during lactation and communication with piglets. This can significantly increase the weaning weight and survival rate of piglets (Koketsu and Dial Citation1997; Brand et al. Citation2004). The piglets being close to the sow outside of the lactation period would increase the risk of being crushed, and sow and piglet communication can bring benefits to the piglets, such as calories, milk and protection, resulting in an offsetting risk of being crushed (Kratinová Citation2008; Burri et al. Citation2009; Melišová et al. Citation2011). In the first 2 days after farrowing, piglets had highly active communication with sows, and piglets had also a lower mortality rate (Ocepek and Andersen Citation2018). The sows may have been more aware of their presence and protected them to a greater extent.
When the sow communicates with the piglets, the sow can locate the piglets, which helps the sow keep the piglets nearby and protect them from danger such as low temperature and starvation (without stepping on or lying on them). It can be achieved by selecting these specific maternal traits and stimulating sows to become more attentive through environmental factors (i.e. nesting material, good handling to prevent fear, etc.) (Ocepek and Andersen Citation2018). Allowing sows to move around and communicate freely with their offspring and motivating sows to care for their young is critical to ensuring the future welfare and sustainability of pig production. Therefore, managers can provide nesting materials or soft-textured floor types to improve piglet hypothermia. Another strategy is to provide heat close to the sow, which should help prevent weak piglets from getting cold at the birth site and ensure that all piglets can meet their two most pressing needs heating and colostrum in the same place.
3.3.4. Lack of maternal behaviour
The lack of maternal behaviour by sows was manifested by sows biting or killing newborn piglets during the farrowing period or within 24 h after farrowing. A survey of sows found that approximately 5.3% exhibited behaviours that hurt piglets, with the frequency of primiparous sows being as high as 10–13% (Steen et al. Citation1988). The lack of maternal behaviour is affected by factors such as environment, hormone levels, farrowing experience and genetic factors (Steen et al. Citation1988). This has brought huge economic losses to the pig industry and severely affects the welfare of piglets. At present, the mechanism and influencing factors of the sow's injury to the piglets are not very clear.
4. Dietary behaviour
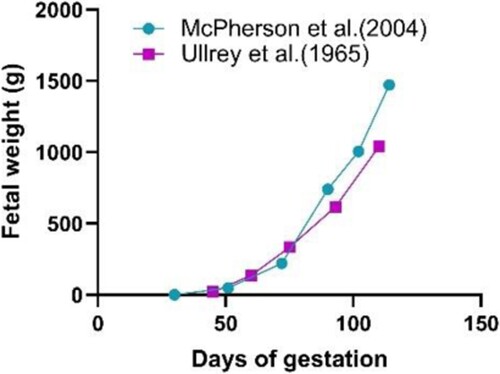
Over the last few decades, sow litter size has increased by three pigs. The fetal weight of pigs has increased by 40% (; Kim et al. Citation2013). Improvements in a reproductive performance increase the metabolic demands on sows during gestation and lactation. A sow needs to produce an ample amount of milk to meet the needs of large and fast-growing litters. However, the number of functional teats on the sow is often insufficient to feed the whole litter (Peltoniemi et al. Citation2021). Dissatisfaction with energy supply would hurt lactation ability and litter size growth. Sow milk production has also increased fourfold from 1935 to 2010, indicating that the sow's mammary glands have also gradually adapted to the increased demand for milk production (Kim et al. Citation2013). But it's still not enough for the whole litters. Milk synthesis occurs in mammary epithelial cells. Strategies to increase milk production should therefore consider achieving optimal mammary gland development, and increasing the number of mammary epithelial cells during pregnancy and lactation (Sánchez et al. Citation2021). At farrowing, the sow appears to prioritize glucose to the mammary gland above the uterus, whereby insufficient dietary energy may compromise the farrowing process (Theil et al. Citation2022). Undernutrition increases the risks of weight loss in sows during lactation, as well as extending the interval between weaning and estrus (Macias and Hinck Citation2012; Mudd et al. Citation2016). Therefore, providing adequate levels of fibre and protein in the late-pregnancy diet is essential to ensure optimal mammary development in pregnancy and lactation of sows.
Figure 2. Growth patterns of a porcine fetus. Adapted from Ullrey et al. (Citation1965) and McPherson et al. (Citation2004).
There were early indications that diet deprivation followed by over allowance during the gestation phases could be beneficial for milk yield in sows (Crenshaw et al. Citation1989). However, more recent studies have suggested that this may not be the case in sows. Excessive feeding restrictions by sows result in a decrease in their voluntary feed intake and make it difficult to produce satiety, showing lying, vacuum chewing and frequent rising during pregnancy, which leads to a negative impact on reproductive performance (Sun et al. Citation2015). The management strategy factor is the precise nutritional diet of the sow. It is important to ensure the normal growth of the sow, increase the nutrient digestibility of the diet, and reduce feed waste and reproductive performance during sow production (Quesnel and Farmer Citation2019). Typically, sows are on a high-fibre diet during gestation and then receive a certain amount of lactation feed 2–3 days before farrowing. Feeding pregnant sows a high-fibre diet can prevent constipation, increase satiety, and maintain normal reproductive performance (Jarrett and Ashworth Citation2018). Almost all studies have found that adding dietary fibre that a variety of sources of fibre-rich components and types to sow diets during pregnancy significantly increased the total litter birth, weaning litter weights, and milk fat content in colostrum (Veum et al. Citation2009; Loisel et al. Citation2013; Li et al. Citation2021). On the other hand, the lactating diet should have a higher amino acid, higher energy diet than the gestation diet because lactating sows require a high-nutrient diet to support mammary gland development and increase the number of functional teats (Tokach et al. Citation2019). Lactating sows need to utilize up to 70% of dietary protein to synthesize milk protein and providing amino acids in the diet close to the requirements of lactating sows can reduce body protein mobilization and potentially increase litter size (Trine et al. Citation2016). Increasing the energy concentration of the lactation diet from 12.8 MJ ME/kg to 13.4 MJ ME/kg improved energy intake, resulting in reduced weight loss and increased litter size during lactation (Xue et al. Citation2012). Moreover, dietary lipids are widely used as a source of energy and essential fatty acids in sow diets. Supplementation of specific lipids during lactation can improve subsequent reproductive performance in sows, because lipid supplementation increases average daily energy intake and sows exhibited higher milk fat production, and improved litter growth rates. In conclusion, different diets should therefore be used for sows at different periods according to their physical condition.
5. Other behaviours
Sexual behaviours such as oral and nose contact can promote the secretion of oxytocin in sows, thereby improving reproductive performance (Kemp et al. Citation2005). However, more than 90% of intensive animal husbandry now uses artificial insemination technology, resulting in a lack of sexual behaviour. Lack of sexual behaviour during artificial insemination is a key factor that affects the effect of artificial insemination (Rault et al. Citation2014). In conventional artificial insemination, the sows lack sexual stimulation, therefore, the sows cannot secrete enough effective oxytocin. This affects the release of pituitary LH and the time of ovulation not being concentrated results in decreased uterine contraction ability and impacts the conception rate in sows. This not only leads to a decrease in the quality of animal welfare but also reduces reproductive efficiency. To improve the effect of artificial insemination in pigs, some sexual behaviours should be retained, including the use of bionic insemination, keeping boars to indirectly stimulate sows, as well as supplementing exogenous hormones.
Another type, known as stereotyped behaviour, is a behaviour that occurs because the sow cannot satisfy diet and behaviour needs in the restricted pen. This mostly occurs in the sow during pregnancy (Cronin and Wiepkema Citation1984). The symptoms include empty mouth chewing, biting the pen, licking the ground, and depression-like behaviour. Sows with stereotyped behaviours would reduce reproductive performance in individual sows because of excessive fat deposition during farrowing and weaning, leading to in lower birth weight and survival rate of the piglets (van der Staay et al. Citation2010). The welfare and feed of sows in production is, therefore, a matter of concern. A diet with high fibre and trace elements can prevent the occurrence of the pica phenomenon due to stereotyped behaviour, reduce stereotyped behaviour of sows during pregnancy, increase progesterone content, and therefore lower the stillbirth rate in sows (DeDecker et al. Citation2014; Huang et al. Citation2020).
6. Conclusion
It was concluded that sow behaviour before and after delivery can show significant differences in the improvement of sow health. This can lead to improvements in sow production efficiency, reproductive performance, and piglet growth rate. The open breeding environment is more similar to the natural environment for pigs. Group breeding helps reduce fighting behaviours and stereotyped behaviour of sows stimulates positive social behaviours and also improves animal welfare. It is helpful to encourage sows’ maternal behaviour and associated stable hormonal levels. This can help to reduce the mortality of piglets. However, group breeding would cause injuries to the limbs. Therefore, innovative pig house designs and management strategies are required, such as the sows being grouped earlier during pregnancy. It is also concluded that the diet should have different composition ratios at different stages for the sows to meet their physical needs. The establishment of a feeding strategy can improve sow performance, and increase the survival rate of embryos during early pregnancy and pregnancy.
Disclosure statement
No potential conflict of interest was reported by the author(s ).
Additional information
Funding
References
- Algers B. 1993. Nursing in pigs: communicating needs and distributing resources. J Anim Sci. 71(10):2826–31.
- Algers B, Uvnäs-Moberg K. 2007. Maternal behavior in pigs. Horm Behav. 52:78–85.
- Andersson A, Nismaa R, Huusko J, Jensen P. 2011. Behaviour of European wild boar (Sus scrofa) in connection with farrowing in an enclosure. Mamm Biol. 76:332–338.
- Arey DS. 1999. Time course for the formation and disruption of social organisation in group-housed sows. Appl Anim Behav Sci. 62:199–207.
- Averós X, Brossard L, Dourmad JY, de Greef KH, Edge HL, Edwards SA, Meunier-Salaün MC. 2010. Quantitative assessment of the effects of space allowance, group size and floor characteristics on the lying behaviour of growing-finishing pigs. Animal. 4:777–783.
- Barb CR, Hausman GJ, Lents CA. 2008. Energy metabolism and leptin: effects on neuroendocrine regulation of reproduction in the gilt and sow. Reprod Domest Anim. 43(Suppl 2):324–330.
- Bates RO, Edwards DB, Korthals RL. 2003. Sow performance when housed either in groups with electronic sow feeders or stalls. Livest Prod Sci. 79:29–35.
- Baxter EM, Adeleye OO, Jack MC, Farish M, Edwards SA. 2015. Achieving optimum performance in a loose-housed farrowing system for sows: The effects of space and temperature. Appl Anim Behav Sci. 169:9–16.
- Baxter EM, Jarvis S, Sherwood L, Robson SK, Ormandy E, Farish M, Smurthwaite KM, Roehe R, Lawrence AB, Edwards SA. 2009. Indicators of piglet survival in an outdoor farrowing system. Livest Sci. 124:266–276.
- Baxter EM, Rutherford KM, D’Eath RB, Turner SP, Jarvis S.. 2013. The welfare implications of large litter size in the domestic pig II: management factors. Animal Welfare. 2:22–26.
- Borell EV, Dobson H, Prunier A. 2007. Stress, behaviour and reproductive performance in female cattle and pigs. Hormones & Behavior. 52:130–138.
- Brand H, Schouten W, Kemp B. 2004. Maternal feed intake, but not feed composition affects postural behaviour and nursing frequency of lactating primiparous sows. Appl Anim Behav Sci. 86:41–49.
- Bunter KL, Lewis CR, Newman S. 2015. Social genetic effects influence reproductive performance of group-housed sows. J Anim Sci. 93:3783–3793.
- Burri M, Wechsler B, Gygax L, Weber R. 2009. Influence of straw length, sow behaviour and room temperature on the incidence of dangerous situations for piglets in a loose farrowing system. Appl Anim Behav Sci. 117:181–189.
- Castrén H, Algers B, Jensen P. 1989. Occurrence of unsuccessful sucklings in newborn piglets in a semi-natural environment. Appl Anim Behav Sci. 23:61–73.
- Castrén H, Algers B, Passillé A-M, Rushen J, Uvnäs-Moberg K. 1993. Preparturient variation in progesterone, prolactin, oxytocin and somatostatin in relation to nest building in sows. Appl Anim Behav Sci. 38:91–102.
- Chaloupková H, Illmann G, Neuhauserová K, Simecková M, Kratinová P. 2011a. The effect of nesting material on the nest-building and maternal behavior of domestic sows and piglet production. J Anim Sci. 89:531–537.
- Chaloupková H, Illmann G, Neuhauserová K, Simecková M, Kratinová P. 2011b. The effect of nesting material on the nest-building and maternal behavior of domestic sows and piglet production. J Anim Sci. 89:531–537.
- Chaloupková H, Illmann G, Pedersen LJ, Malmkvist J, Šimečková M. 2008. Sow responsiveness to human contacts and piglet vocalization during 24 h after onset of parturition. Appl Anim Behav Sci. 112:260–269.
- Crenshaw JD, Park CS, Swantek PM, Keller WL, Zimprich RC. 1989. Growth, reproduction and lactation response of gilts to a phased feeding regimen designed to induce compensatory growth. J Anim Sci. 67:107–108.
- Cronin GM, Wiepkema PR. 1984. An analysis of stereotyped behaviour in tethered sows. Ann Rech Vet. 15:263–270.
- Damm BI, Lisborg L, Vestergaard KS, Vanicek J. 2003. Nest-building, behavioural disturbances and heart rate in farrowing sows kept in crates and Schmid pens. Livest Prod Sci. 80:175–187.
- Damm BI, Vestergaard KS, Schrøder-Petersen DL, Ladewig J. 2000. The effects of branches on prepartum nest building in gilts with access to straw. Appl Anim Behav Sci. 69:113–124.
- Danielsen M, Thymann T, Jensen BB, Jensen ON, Sangild PT, Bendixen E. 2006. Proteome profiles of mucosal immunoglobulin uptake in inflamed porcine gut. Proteomics. 6:6588–6596.
- DeDecker AE, Hanson AR, Walker PM, Salak-Johnson JL. 2014. Space allowance and high fiber diet impact performance and behavior of group-kept gestating sows. J Anim Sci. 92:1666–1674.
- Einarsson S, Sjunnesson Y, Hultén F, Eliasson-Selling L, Dalin AM, Lundeheim N, Magnusson U. 2014. A 25 years experience of group-housed sows-reproduction in animal welfare-friendly systems. Acta Vet Scand. 56:37–45.
- English PR, Smith WJ. 1995. Some causes of death in neonatal piglets. J Anim Sci. 15–22.
- Farmer C. 2019. Review: mammary development in lactating sows: the importance of suckling. Animal. 13:s20–s25.
- Ferket SL, Hacker RR. 1985. Effect of forced exercise during gestation on reproductive performance of sows [microform]. Can J Anim Sci. 65:851–859.
- Fraser D. 1980. A review of the behavioural mechanism of milk ejection of the domestic pig. Appl Anim Ethol. 6:247–255.
- Grandinson K, Rydhmer L, Strandberg E, Thodberg K. 2003. Genetic analysis of on-farm tests of maternal behaviour in sows. Livest Prod Sci. 83:141–151.
- Herskin MS, Jensen KH, Thodberg K. 2016. Influence of environmental stimuli on nursing and suckling behaviour in domestic sows and piglets. Anim Sci. 68:27–34.
- Holyoake PK, Dial GD, Trigg T, King VL. 1995. Reducing pig mortality through supervision during the perinatal period. J Anim Sci. 73:3543–3551.
- Huang S, Wei J, Yu H, Hao X, Zuo J, Tan C, Deng J. 2020. Effects of dietary fiber sources during gestation on stress status, abnormal behaviors and reproductive performance of sows. Animals (Basel). 10(1):141–147.
- Hultén F, Wallenbeck A, Rydhmer L. 2006. Ovarian activity and oestrous signs among group-housed, lactating sows: influence of behaviour, environment and production. Reprod Domest Anim. 41:448–454.
- Hutson GD, Price EO, Dickenson LG. 1993. The effect of playback volume and duration on the response of sows to piglet distress calls. Appl Anim Behav Sci. 37:31–37.
- Illmann G, Chaloupková H, Melišová M. 2016. Impact of sow prepartum behavior on maternal behavior, piglet body weight gain, and mortality in farrowing pens and crates. J Anim Sci. 94:3978–3986.
- Illmann G, Madlfousek J. 1995. Occurrence and characteristics of unsuccessful nursings in minipigs during the first week of life. Appl Anim Behav Sci. 44:9–18.
- Illmann G, Neuhauserová K, Pokorná Z, Chaloupková H, Šimečková M. 2008. Maternal responsiveness of sows towards piglet’s screams during the first 24 h postpartum. Appl Anim Behav Sci. 112:248–259.
- Jarrett S, Ashworth CJ. 2018. The role of dietary fibre in pig production, with a particular emphasis on reproduction. J Anim Sci Biotechnol. 6(9):59–67.
- Jensen P. 1986. Observations on the maternal behaviour of free-ranging domestic pigs. Applanimbehav. 16:131–142.
- Johnsen JF, de Passille AM, Mejdell CM, Bøe KE, Grøndahl AM, Beaver A, Rushen J, Weary DM. 2015. The effect of nursing on the cow–calf bond. Appl Anim Behav Sci. 163:50–57.
- Kecman J, Waehner M. 2016. Management of large litters in piglet production. Tierarztliche Praxis. Ausgabe G GroBtiere. 44:318–325.
- Kemp B, Soede NM. 2012. Reproductive issues in welfare-friendly housing systems in pig husbandry: a review. Reprod Domest Anim. 47(Suppl 5):51–57.
- Kemp B, Soede NM, Langendijk P. 2005. Effects of boar contact and housing conditions on estrus expression in sows. Theriogenology. 63:643–656.
- Kielland C, Rootwelt V, Reksen O, Framstad T. 2015. The association between immunoglobulin G in sow colostrum and piglet plasma. J Anim Sci. 93:4453–4462.
- Kilbride AL, Mendl M, Statham P, Held S, Harris M, Cooper S, Green LE. 2012. A cohort study of preweaning piglet mortality and farrowing accommodation on 112 commercial pig farms in England. Prev Vet Med. 104:281–291.
- Kim SW, Weaver AC, Shen YB, Zhao Y. 2013. Improving efficiency of sow productivity: nutrition and health. J Anim Sci Biotechnol. 4:8–26.
- Kirkden RD, Broom DM, Andersen IL. 2013. Invited review: piglet mortality: management solutions. J Anim Sci. 91:3361–3389.
- Kobek-Kjeldager C, Moustsen VA, Theil PK, Pedersen LJ. 2020. Effect of litter size, milk replacer and housing on production results of hyper-prolific sows. Animal. 14:824–833.
- Koketsu Y, Dial GD. 1997. Factors associated with prolonged weaning-to-mating interval among sows on farms that wean pigs early. J Am Vet Med Assoc. 211:894–898.
- Koketsu Y, Iida R. 2017. Sow housing associated with reproductive performance in breeding herds. Mol Reprod Dev. 84:979–986.
- Kratinová I. 2008. Carefulness and flexibility of lying down behaviour in sows during 24 h post-partum in relation to piglet position. Appl Anim Behav Sci. 114:346–358.
- Lambertz C, Petig M, Elkmann A, Gauly M. 2015. Confinement of sows for different periods during lactation: effects on behaviour and lesions of sows and performance of piglets. Animal. 9:1373–1378.
- Langendijk P, Plush K. 2019. Parturition and its relationship with stillbirths and asphyxiated piglets. Animals (Basel). 9:885–891.
- Lawrence B, Petherick C, Lean M, Deans A, Chirnside J, Gaughan A, Clutton E, Terlouw EMC. 1994. The effect of environment on behaviour, plasma cortisol and prolactin in parturient sows. Appl Anim Behav Sci. 39:313–330.
- Li H, Yin J, Tan B, Chen J, Zhang H, Li Z, Ma X. 2021. Physiological function and application of dietary fiber in pig nutrition: A review. Anim Nutr. 7:259–267.
- Liu X, Song P, Yan H, Zhang L, Wang L, Zhao F, Gao H, Hou X, Shi L, Li B, et al. 2021. A comparison of the behavior, physiology, and offspring resilience of gestating sows when raised in a group housing system and individual stalls. Animals (Basel). 11:2076–2081.
- Loisel F, Farmer C, Ramaekers P, Quesnel H. 2013. Effects of high fiber intake during late pregnancy on sow physiology, colostrum production, and piglet performance. J Anim Sci. 91:5269–5279.
- Macias H, Hinck L. 2012. Mammary gland development. Wiley Interdiscip Rev Dev Biol. 1:533–557.
- Maes D, Pluym L, Peltoniemi O. 2016. Impact of group housing of pregnant sows on health. Porcine Health Manag. 2:17–22.
- Malmkvist J, Pedersen L, Kammersgaard T EJ. 2012. Influence of thermal environment on sows around farrowing and during the lactation period. J Anim Sci. 90:3186–3199.
- Marchant JN, Rudd AR, Mendl MT, Broom DM, Meredith MJ, Corning S, Simmins PH. 2000a. Timing and causes of piglet mortality in alternative and conventional farrowing systems. Vet Rec. 147:209–214.
- Marchant JN, Rudd AR, Mendl MT, Broom DM, Meredith MJ, Corning S, Simmins PH. 2000b. Timing and causes of piglet mortality in alternative and conventional farrowing systems. Vet Rec. 147:209–214.
- Martineau GP, Smith BB, Doizé B. 1992. Pathogenesis, prevention, and treatment of lactational insufficiency in sows. Vet Clin North Am Food Anim Pract. 8:661–684.
- McPherson RL, Ji F, Wu G, Blanton JR Jr., Kim SW. 2004. Growth and compositional changes of fetal tissues in pigs. J Anim Sci. 82:2534–2540.
- Melišová M, Illmann G, Andersen IL, Vasdal G, Haman J. 2011. Can sow pre-lying communication or good piglet condition prevent piglets from getting crushed? Appl Anim Behav Sci. 134:121–129.
- Melišová M, Illmann G, Chaloupková H, Bozděchová B. 2014. Sow postural changes, responsiveness to piglet screams, and their impact on piglet mortality in pens and crates. J Anim Sci. 92:3064–3072.
- Mellor DJ, Lentle RG. 2015. Survival implications of the development of behavioural responsiveness and awareness in different groups of mammalian young. N Z Vet J. 63:131–140.
- Morgan L, Klement E, Novak S, Eliahoo E, Younis A, Sutton GA, Abu-Ahmad W, Raz T. 2018. Effects of group housing on reproductive performance, lameness, injuries and saliva cortisol in gestating sows. Prev Vet Med. 160:10–17.
- Mudd AT, Alexander LS, Johnson SK, Getty CM, Malysheva OV, Caudill MA, Dilger RN. 2016. Perinatal dietary choline deficiency in sows influences concentrations of choline metabolites, fatty acids, and amino acids in milk throughout lactation. J Nutr. 146:2216–2223.
- Munsterhjelm C, Valros A, Heinonen M, Hlli O, Peltoniemi O. 2008. Housing during early pregnancy affects fertility and behaviour of sows. Reprod Domest Anim. 43:584–591.
- Nicolaisen T, Lühken E, Volkmann N, Rohn K, Kemper N, Fels M. 2019. The effect of sows’ and piglets’ behaviour on piglet crushing patterns in Two different farrowing Pen systems. Animals (Basel). 9(8):538–542.
- Ocepek M, Andersen IL. 2017. What makes a good mother? Maternal behavioural traits important for piglet survival. Appl Anim Behav Sci. 193:29–36.
- Ocepek M, Andersen IL. 2018. Sow communication with piglets while being active is a good predictor of maternal skills, piglet survival and litter quality in three different breeds of domestic pigs (Sus scrofa domesticus). PLoS One. 13:e0206128.
- Ocepek M, Rosvold EM, Andersen-Ranberg I, Andersen IL. 2017. Can we improve maternal care in sows? maternal behavioral traits important for piglet survival in loose-housed sow herds. J Anim Sci. 95:4708–4717.
- Oliviero C, Heinonen M, Valros A, Hälli O, Peltoniemi OA. 2008. Effect of the environment on the physiology of the sow during late pregnancy, farrowing and early lactation. Anim Reprod Sci. 105:365–377.
- Oliviero C, Heinonen M, Valros A, Peltoniemi O. 2010. Environmental and sow-related factors affecting the duration of farrowing. Anim Reprod Sci. 119:85–91.
- Oliviero C, Peltoniemi O. 2020. Troubled Process of Parturition of the Domestic Pig. 12:35–46.
- Pandolfi F, Edwards SA, Robert F, Kyriazakis I. 2017. Risk factors associated with the different categories of piglet perinatal mortality in French farms. Prev Vet Med. 137:1–12.
- Pedersen LJ, Damm BI, Marchant-For De JN, Jensen KH. 2003. Effects of feed-back from the nest on maternal responsiveness and postural changes in primiparous sows during the first 24 h after farrowing onset. Appl Anim Behav Sci. 83:109–124.
- Peltoniemi O, Björkman S, Maes D. 2016. Reproduction of group-housed sows. Porcine Health Manag. 2:15–26.
- Peltoniemi O, Han T, Yun J. 2021. Coping with large litters: management effects on welfare and nursing capacity of the sow. J Anim Sci Technol. 63:199–210.
- Plush KJ, McKenny LA, Nowland TL, van Wettere W. 2021. The effect of hessian and straw as nesting materials on sow behaviour and piglet survival and growth to weaning. Animal. 15:100273–100277.
- Pluym LM, Van Nuffel A, Van Weyenberg S, Maes D. 2013. Prevalence of lameness and claw lesions during different stages in the reproductive cycle of sows and the impact on reproduction results. Animal. 7:1174–1181.
- Portele K, Scheck K, Siegmann S, Feitsch R, Maschat K, Rault JL, Camerlink I. 2019. Sow-piglet nose contacts in free-farrowing pens. Animals Basel. 9(8):513–518.
- Quesnel H, Farmer C. 2019. Review: nutritional and endocrine control of colostrogenesis in swine. Animal. 13:s26–s34.
- Rault JL, Morrison RS, Hansen CF, Hansen LU, Hemsworth PH. 2014. Effects of group housing after weaning on sow welfare and sexual behavior. J Anim Sci. 92:5683–5692.
- Rutherford KM, Berg L, Roehe AB, Arnott E, Sandoe AS. 2013. The welfare implications of large litter size in the domestic pig I: biological factors. Universities Federation for Animal Welfare. 2:15–19.
- Sánchez C, Franco L, Regal P, Lamas A, Cepeda A, Fente C. 2021. Breast milk: a source of functional compounds with potential application in nutrition and therapy. Nutrients. 13:1026–1034.
- Spicer EM, Driesen SJ, Fahy VA, Horton BJ, Sims LD, Jones RT, Cutler RS, Prime RW. 1986. Causes of preweaning mortality on a large intensive piggery. Aust Vet J. 63:71–75.
- Spoolder H, Geudeke MJ, Peet-Schwering C, Soede NM. 2009. Group housing of sows in early pregnancy: a review of success and risk factors. Livest Sci. 125:1–14.
- Spoolder HAM, Vermeer HM. 2015. Gestation group housing of sows: the gestating and lactating sow. 3:78–92.
- Steen AH, Schaeffer LR, Jong H, Groot PN. 1988. Aggressive behaviour of sows at parturition. Journal of Animal Ence. 66:271–279.
- Sun HQ, Tan CQ, Wei HK, Zou Y, Long G, Ao JT, Xue HX, Jiang SW, Peng J. 2015. Effects of different amounts of konjac flour inclusion in gestation diets on physio-chemical properties of diets, postprandial satiety in pregnant sows, lactation feed intake of sows and piglet performance. Anim Reprod Sci. 152:55–64.
- Tanaka Y, Koketsu Y. 2007. A field study of the associations between behaviors and reproductive performance in lactating sows. J Vet Med Sci. 69:1229–1233.
- Theil PK, Farmer C, Feyera T. 2022. Review: physiology and nutrition of late gestating and transition sows. J Anim Sci. 100(6):176–182.
- Thodberg K, Jensen KH, Herskin MS. 2002. Nursing behaviour, postpartum activity and reactivity in sows: effects of farrowing environment, previous experience and temperament. Appl Anim Behav Sci. 77:53–76.
- Tokach MD, Menegat MB, Gourley KM, Goodband RD. 2019. Review: nutrient requirements of the modern high-producing lactating sow, with an emphasis on amino acid requirements. Animal. 13:2967–2977.
- Trine F, Pedersen T, Sønderby B, Takele F, Uffe K. 2016. A two-diet feeding regime for lactating sows reduced nutrient deficiency in early lactation and improved milk yield. Livest Sci. 191:165–173.
- Tsuma VT, Einarsson S, Madej A, Kindahl H, Lundeheim N. 1996. Effect of food deprivation during early pregnancy on endocrine changes in primiparous sows. Anim Reprod Sci. 41:267–278.
- Tuchscherer M, Manteuffel G. 2000. The effect of psycho stress on the immune system. Another Reason for Pursuing Animal Welfare (Review). Archiv fur Tierzucht. 43:547–560.
- Turner AI, Hemsworth PH, Tilbrook AJ. 2005. Susceptibility of reproduction in female pigs to impairment by stress or elevation of cortisol. Domest Anim Endocrinol. 29:398–410.
- Ullrey DE, Sprague JI, Becker DE, Miller ER. 1965. Growth of the swine fetus. J Anim Sci. 24:711–717.
- van der Staay FJ, Schuurman T, Hulst M, Smits M, Prickaerts J, Kenis G, Korte SM. 2010. Effects of chronic stress: a comparison between tethered and loose sows. Physiol Behav. 100:154–164.
- Vangen O, Holm B, Valros A, Lund MS, Rydhmer L. 2005. Genetic variation in sows’ maternal behaviour, recorded under field conditions. Livest Prod Sci. 93:63–71.
- van Nieuwamerongen SE, Bolhuis JE, van der Peet-Schwering CM, Soede NM. 2014. A review of sow and piglet behaviour and performance in group housing systems for lactating sows. Animal. 8:448–460.
- Vasdal G, Østensen I, Melišová M, Bozděchová B, Illmann G, Andersen IL. 2011. Management routines at the time of farrowing—effects on teat success and postnatal piglet mortality from loose housed sows. Livest Sci. 136:225–231.
- Veum TL, Crenshaw JD, Crenshaw TD, Cromwell GL, Easter RA, Ewan RC, Nelssen JL, Miller ER, Pettigrew JE, Ellersieck MR. 2009. The addition of ground wheat straw as a fiber source in the gestation diet of sows and the effect on sow and litter performance for three successive parities. J Anim Sci. 87:1003–1012.
- Weary DM, Pajor EA, Fraser D, Honkanen AM. 1996a. Sow body movements that crush piglets: a comparison between two types of farrowing accommodation. Appl Anim Behav Sci. 49:149–158.
- Weary DM, Pajor EA, Thompson BK, Fraser D. 1996b. Risky behaviour by piglets: a trade off between feeding and risk of mortality by maternal crushing? Anim Behav. 51:619–624.
- Widowski TM, Curtis SE. 1989. Behavioral responses of periparturient sows and juvenile pigs to prostaglandin F2 alpha. J Anim Sci. 67:3266–3276.
- Wischner D, Kemper N, Krieter J. 2009. Nest-building behaviour in sows and consequences for pig husbandry. Livestock Ence. 124:1–8.
- Wülbers-Mindermann M, Berg C, Illmann G, Baulain U, Algers B. 2015. The effect of farrowing environment and previous experience on the maternal behaviour of sows in indoor pens and outdoor huts. Animal. 9:669–676.
- Xue L, Piao X, Li D, Li P, Zhang R, Kim SW, Dong B. 2012. The effect of the ratio of standardized ileal digestible lysine to metabolizable energy on growth performance, blood metabolites and hormones of lactating sows. J Anim Sci Biotechnol. 3:11–17.
- Yi R, Wang C, Zhang X, Zhao P, Zhang M, Li X, Cui S, Liu H, Bao J. 2019. Maternal behavior, posture change, and production performance of lactating sows housed in an enriched environment. J Appl Anim Welf Sci. 22:298–308.
- Yun J, Swan KM, Farmer C, Oliviero C, Peltoniemi O, Valros A. 2014. Prepartum nest-building has an impact on postpartum nursing performance and maternal behaviour in early lactating sows. Appl Anim Behav Sci. 160:31–37.
- Yun J, Valros A. 2015. Benefits of prepartum nest-building behaviour on parturition and lactation in sows - a review. Asian-Australas J Anim Sci. 28:1519–1524.
- Zanella AJ, Broom DM, Hunter JC, Mendl MT. 1996. Brain opioid receptors in relation to stereotypies, inactivity, and housing in sows. Physiol Behav. 59:769–775.
- Zhang ZF, Li J, Park JC, Kim IH. 2013. Effect of vitamin levels and different stocking densities on performance, nutrient digestibility, and blood characteristics of growing pigs. Asian-Australas J Anim Sci. 26:241–246.
