ABSTRACT
Nitrate poisoning due to the consumption of cabbages was diagnosed in a small goat flock, in Qena city, Egypt. The methylene blue 1% antidote (1 mg/kg BW) was given intravenously to the poisoned goats. Nitrate poisoning was confirmed in blood plasma, saliva, urine, and cabbage dry matter samples. The dark brown blood and methemoglobin fraction were 47.25 ± 0.58%. Green cabbage containing 7.1% nitrate on dried materials was fed to the goats. The nitrate concentration in the saliva was (59.50 ± 4.67), compared to the healthy goats reared in the same area and tested positive in the plasma and urine of intoxicated goats. Intoxication induced a significant decline in the Red Blood Cells count, hemoglobin concentration, and HCT % (P < 0.001), while the WBC count (P < 0.001), neutrophils (P < 0.001), and monocyte percentage were elevated (P < 0.002) in intoxicated goats, while the lymphocyte % (P < 0.01) significantly decreased when compared to the control. For the oxidative stress in the red blood cells, nitrate toxicity caused a significant elevation in the concentration of Malondialdehyde, (P < 0.001) while Superoxide Dismutase, Glutathione, and Glutathione S-Transferase levels were significantly reduced (P < 0.001) compared to the control. In this current report, the ingestion of goat to cabbage containing a high level of nitrate exhibited nitrate-nitrite intoxication.
1. Introduction
Nitrate intoxication in the ruminants animals is occurred due to the consumption of high feed materials containing a high concentration of nitrate. The ingested nitrate is subsequently reduced to nitrite by the action of rumen microbes (Roder Citation2002). Regardless of the route of exposure to both nitrate and nitrites, they were fatly reached to the blood circulation. Furthermore, nitrate is gradually oxidized to nitrites which is readily distributed into most body fluids such as urine, saliva, gastric juice, sweat, and intestinal contents (Cortas and Wakid Citation1991). Nitrite intoxication also depends on its concentration in forage, where a nitrate quantity of 0.5% is likely to be threatening and acute toxicity can occur if the nitrate amount is more than 1% (Puschner Citation2000). Ruminants can convert the forage nitrate in the rumen to protein. However, when the nitrate concentrations exceed the conversion rate to protein, they enter the blood circulation in the form of nitrate, which attaches to the circulating red blood cells’ hemoglobin, esulting in methemoglobin formation. The formed methemoglobin is unable to transport blood oxygen, so the deaths of the exposed animals were due to asphyxiations, as the methemoglobin quantity in the bloodstream, may rise to 80% (Arnold et al. Citation2014). Ruminants are more liable to nitrate toxicity as long as the presence of nitrate-reducing bacteria in the rumen converts forage nitrate to toxic nitrites. Both compounds, nitrates (NO-3) and nitrites (NO-2), are produced spontaneously by the ruminal microflora from the oxidation of nitrogen (N) by many sources, including plants, soil, or water. The produced nitrate in the rumen is safer until reduced to nitrite by microbial activity (Beatson Citation1987). The process of nitrate conversion in the rumen compartments ends with the formation of reduced ammonia (NH3), which is used in microbial protein synthesis and has many bacterial biochemical steps and sequences from which the formation of the more toxic nitrite (NO-2). However, produced ammonia is not toxic to ruminants because the amount of ammonia produced is limited (Casteel Citation1997 and Valli Citation1998). On the condition of rapid ingestion of bulk of grass holding a high nitrate concentration, the nitrite ion will be produced and accumulate in the rumen, which is afterward absorbed via the ruminal epithelium and enters the bloodstream, where it combines with the erythrocytes in the exchange of chloride ions. Moreover, within the red blood cells, the nitrite ions oxidize the hemoglobin to form methemoglobin which cannot transport oxygen to body tissues, resulting in hypoxia. Hypoxia is commonly visible when about 30-40% of the hemoglobin content in the red blood cells is converted to methemoglobin (El Bahri et al. Citation1997; Casteel and Evans Citation2004). There are common sources of excess nitrate, including plants containing high toxic nitrate levels that undergo oxidation in the rumen to nitrite quantities plentiful to induce toxicity. From these grasses and green plants are cabbages, potatoes, beets, sugarloaf, leaf lettuce, and carrot contribute to aggregate nitrate ions, mostly when they are cultivated using an elevated amount of nitrogen fertilizers or they have grown up in the highly-fertilized ground with dung or if they grow in environmental dehydration conditions (Dolan et al. Citation2010). The nitrate limits in cabbages deferrers substantially and may exceed 1200 mg/kg of fresh weight and these limits possibly induced toxic consequences in the ruminants. Finally, nitrite ions cause poisoning because of the lethal hypoxia when methemoglobin develops and hemoglobin has low adequacy to carry oxygen to the body (Brkić et al. Citation2017; Kellerman et al. Citation2005). The physiological role of Red Blood Cells (RBCs) is the carrying of oxygen from the lungs to different body organs. Within the blood circulation RBCs are frequently exposed the endogenous and exogenous sources of reactive oxygen species (ROS) which cause disruption and impairment of the RBC functions (Lee et al. Citation2003; Nagababu et al. Citation2013). Exposure to nitrate and nitrite caused oxidative stress which denaturates and aggregates hemoglobin also it leads to decrease the capacity of the cellular antioxidant defense system. Furthermore, it causes injuries to the cellular membrane as well as disturbances in the normal energy metabolism within the erythrocytes which in turn decrease the life span of the circulating erythrocytes in the blood Ansari et al. Citation2015. When NaNO2 was given to the experimental mice it induced increase in the concentration of methemoglobin, the enzymatic the activity of both glutathione reductase and glucose 6-phosphate dehydrogenase with the erythrocytes. On the other hand a reduction in the activity of antioxidant defense system of erythrocytes (superoxide dismutase and catalase) as well as the concentration of lipid peroxidation of the erythrocyte was reported following nitrite poisoning (Shugalei et al. Citation1992). In this study, we would like to suppose a case of nitrate intoxication in groups of eight goats fed cabbages in the local village in Egypt. The case was presented by the owners to the local veterinary staff. First aid was done (antidote, general vitality markers, and sampling), and the case was then presented to the veterinary hospital, Vet-Med Faculty, South Valley University, Qena, Egypt, approximately thirty minutes after first aid.
2. Materials and methods
2.1. Animals of the study
The presented patients were adult Saidi (distributed widely in Upper Egypt) goats, including six females and two males, with ages ranging from 1.5-2 years. They are located in El-Mofragya village, Qous town, which belongs to Qena city, Egypt. The animal's owner said that all the goats on his farm were routinely given 2.5% albendazole per mouth and were prescribed Botox 5% lotion monthly for the removal of external parasites. The goats had no record of any disease in the old days and were in good fitness and unable to eat any plants before the cabbage intake.
2.2. Case history
It was reported that the goats were to graze on their own in the field. At the end of August 2022, a small flock of eight adult Saidi goats in El-Mofragya village, south of Qena city, were fed around twenty-two kilograms (22 kg) of whole cabbage (leaves and roots) brought from a vegetable farm close to the village. Fertilization of the soil and cabbages with nitrogen-based fertilizers (urea and calcium nitrate) were noted to be continuously used for the growth of plants on the farm in the El-Mofragya village area. Approximately two to three hours later, after the cabbage feeding – The animals were presented to the veterinary hospital, Faculty of Veterinary Medicine, South Valley University, Qena, Egypt. All weight goats showed clinical signs of poisoning.
2.3. Clinical signs of poisoning
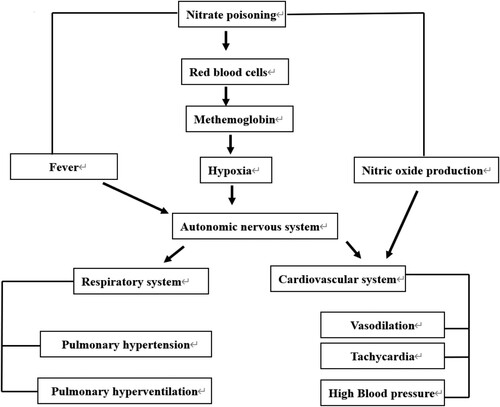
All the general and specific clinical signs were recorded. The most common clinical signs were including ruminal tympani, weakness, congestion of eyes with the blue color sclera, foams, grinding teeth, unbalanced gait with incoordination, abdominal pain, and uncontrolled movements. Furthermore, dyspnea, mild twitching, ataxia, dyspnea, terminal simple convulsions, sunken eye, purple mucous membranes, perioral cyanosis, and frequent urination were recorded and they are mostly related to nitrate poisoning. The schematic cartoon of the experimental design is shown in . Nitrate toxicity induced different responses in the body where the intoxicated goats were presented with fiver as well as nitrate converted to nitrite which enters the circulation and attaches to the RBCs forming the methemoglobin and causing hypoxia. In addition, part of the produced nitrite is converted to nitric oxide. The fever, hypoxia, as well as the produced nitric oxide act, might act directly on the cardiovascular system or via the autonomic nervous system, leading to tachycardia, vasodilatations, and increased blood pressure. Also, the autonomic nervous system acts on the respiratory system via pulmonary hypertension and hyperventilation (increased respiratory rate).
2.4. Estimation of general vital signs and physiological changes
The eight goats were presented to the veterinary hospital, appearing ill, ashen, discolored, and in distress. Body temperature, heart rate, respiratory rate, and systolic blood pressure were recorded. In addition to recording the oxygen saturations using a pulse-oximeter via ear and methemoglobinemia, and the blood pH.
2.5. Laboratory diagnosis
2.5.1. Sample collection
Theoretical identification of nitrate intoxication was also done using samples collected from the poisoned goats. Arterial blood samples were drained from the poisoned animals using heparin anticoagulant. Arterial blood appeared dark brown to chocolate in color. Saliva was collected in clean Falcone 10 mL tubes from the mouth foams and urine were collected in clean plastic cups during frequent urination. Specimens of cabbage leaves and roots were collected and transported to the Department of Veterinary Public Health Laboratory, Faculty of Veterinary Medicine, South Valley University, Qena, Egypt.
2.5.2. The Concentration of Nitrate in Urine and Plasma; general hematological and oxidative stress parameters.
Urine samples and blood plasma were tested for nitrites using a urine test strip (Combi-Screen, Germany). The concentration of nitrite was measured in saliva using a nitric oxide kit (Cat No, 2533). Nitric oxide assay is used as indicator the concentration of endogenous nitrite production in the biological fluids (Montgomery and Dymock Citation1961). Whole blood samples were used for nitrite test in plasma, blood pH, complete blood count (CBC), and erythrocyte oxidative stress markers Malondialdehyde (MDA) (Cat No, MD 2529) Thiobarbituric acid (TBA) reacts with Malondialdehyde (MDA) interact with the thiobarbituric acid with low pH for 30 min producing the thiobarbituric acid reactive product which indicate the membrane lipid damages (Ohkawa et al. Citation1979), glutathione peroxidase (GPx) (Cat No, GP 2524). The cellular glutathione peroxidase (GPx) enzyme protect the cells from the damage resulted from peroxides which undergoes decomposition forming the highly active free radicals mainly lipid peroxidation. (Paglia and Valentine Citation1967). Superoxide dismutase (SOD) (Cat No, SD 2521) Superoxide dismutase (SODs) is considered one important part of cellular antioxidant system. It catalyzes the process of superoxide anion dismutation to molecular oxygen and hydrogen peroxide (Nishikimi et al. Citation1972), and Glutathione S-Transferase (GST) (Cat No, GT 2519) Glutathione S- transferase (GST) conjugate the toxicant in the cells with glutathione which produce water soluble byproducts that further metabolized to mercapturic acid and exerted through the urinary system (Habig et al. Citation1974). Detection. Kits for NO, MDA, GPx, SOD, and GST measurements were purchased from (Biodiagnostic, Dokki, Egypt), and they were measured by a colorimetric method using a spectrophotometer (Mindray Ba-88A, Mindray). The measurement was established according to product manufacturers.
2.5.3. Cabbage dry matter collection
The cabbage samples consumed by the poisoned goats were used for the analysis of nitrate content. Four representative specimens of cabbage leaves and roots were collected, labeled, and transported to the Department of Public Health, Faculty of Veterinary Medicine, South Valley University, Qena, Egypt, for assessment of nitrate concentration.
2.6. Diagnostic therapy
Methylene blue 1%, the recommended antidote for nitrate intoxication, was intravenously injected for several minutes at a dose of 1 mg/kg BW per goat and repeated after 1 h. Symptomatic treatment and fluid therapy were applied. An antihistamine medication was injected per muscle with 70 mL of 10% oxy-tetracycline given in the rumen. In addition to hypotension treatment with an intravenous injection of epinephrine, there was no mortality after treatment was recorded. One day after poisoning, all the goats had completely reclaimed.
2.7. Statistical analyses
The data recorded from the analyzes were assessed statistically by SPSS software with the use of One-way Analysis of Variance (ANOVA) with using tukey's post hoc t test. The significance level was considered when the probability is less than 0.05 (P < 0.05).
3. Results
3.1. Changes in vital signs and physiological parameters due to nitrate poisoning
The changes in the general vital signs and blood changes were recorded in all the poisoned goats. The results showed that the poisoned goats were febrile; the temperature was 41.77 ± 0.26, the heart rate was 107.62 ± 0.70 bpm, the respiratory rate was 27.37 ± 0.46 breaths per minute, and systolic blood pressure was in the range of 110 ± 0.92 (). Oxygen saturations were detected using a pulse-oximeter via ear and oxygen saturation was 82.62 ± 0.53%.
Table 1. Vital signs in healthy and nitrate-poisoned-goats.
The methemoglobinemia ranged from 47.25 ± 0.58 and the blood pH was 6.55 ± 0.10 ().
Table 2. Blood changes related to nitrate toxicity in healthy and nitrate-poisoned-goats.
3.2. Nitrate in urine
The collected samples of urine and blood plasma from the eight poisoned goats tested positive for nitrate when compared with healthy goats ().
Table 3. Nitrate concentration in saliva (μmol/l) and urine in healthy and nitrate- poisoned-goats.
3.3. Nitrate in saliva
All the samples of saliva were examined for detection of the nitrate level in the poisoned goats compared to the controls. Nitrate poisoning is marked by a significant increase in the nitrite concentration of 59.50 ± 4.67 compared to healthy goats at 24.13 ± 1.03 (P < 0.001), shown in .
3.4. Hematological analyses
The presented poisoned cases revealed a significant decrease (P < 0.001) in the RBC counts (8.94 ± 0.39), hemoglobin concentration (Hb) 6.58 ± 0.27, and packed cell volume of red blood cells (20.13%) when compared with healthy goats (). Moreover, intoxication by nitrite induced a significant increase in the white blood cell count (WBCs) of 11.71 ± 0.34, neutrophils 46.35 ± 0.92 and monocytes 12.00 ± 1.13%, while a significant decrease was recorded in the lymphocytes 40.00 ± 1.00% when compared with healthy goats ().
Table 4. Red blood cell count (RBC), hemoglobin (Hb) concentration, and packed cell volume (PCV) of RBC in healthy and nitrate-poisoned-goats.
Table 5. White blood cells (WBC), lymphocytes %, neutrophils %, and monocytes % in healthy and nitrate-poisoned-goats.
3.5. Erythrocytes lipid peroxidation
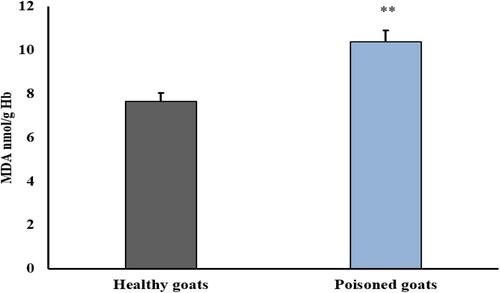
Erythrocytes’ lipid peroxidation was increased by nitrate poisoning, which was marked by a significant increase in the secondary product of oxidation (MDA) (10.37 ± 0.42) – P < 0.001 in comparisons to the healthy animals as shown in ().
Figure 2. Effect of nitrate poisoning on erythrocytic lipid peroxidation (nmol/g Hb) activities in healthy and nitrate-poisoned goats. Nitrate poisoning caused a significant increase in erythrocytes MDA when compared with healthy goats. Bars indicate mean ± SE and asterisks indicate significant differences at P < 0.05.
MDA when compared with healthy goats. Bars indicate mean ± SE and asterisks indicate significantly differences at P < 0.05.
3.6. Anti-Oxidative enzymes in erythrocytes.
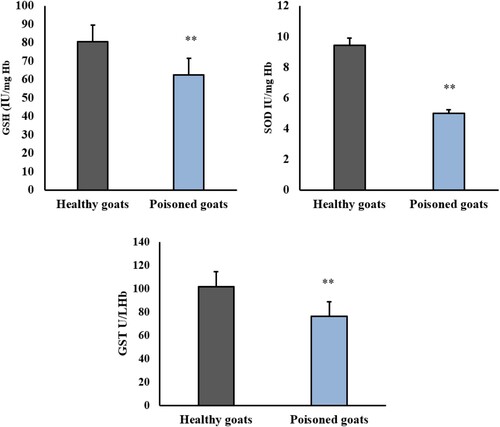
A great antioxidant response was reported in the erythrocytes in response to nitrite toxicity. Poisoning by nitrate caused a significant decrease in GSH (62.37 ± 1.99), SOD (4.98 ± 0.34), and GST (76.15 ± 2.53) when compared to healthy goats, P < 0.001 ().
Figure 3. Effect of nitrate poisoning on erythrocytic superoxide dismutase (SOD) (IU/mg Hb), glutathione peroxidase (GSH-Px) (IU/mg Hb), glutathione S-transferee (GST) (U/L Hb) activities in healthy and nitrate poisoned goats. Nitrate poisoning caused a significant increase in erythrocytes SOD, GSH-Px, and GST activities in comparison to healthy goats. Bars indicate mean ± SE and asterisks indicate significant differences at P < 0.05.
3.7. Nitrate contents in cabbages
Analysis of cabbage samples by radio potentiometry showed an increase in the percentage of nitrate content in the dry matter basis to 7.1%, indicating that nitrate is responsible for the suspected cases of poisoning in goats.
4. Discussion
Nitrate poisoning is rare but is considered an important cause of poisoning in livestock. The incidence of nitrate poisoning mainly occurs in cattle, especially those in the Latin West (Haliburton Citation1999). Regardless of the high levels of nitrate-gathering plants (Aslani Citation2004), the information on nitrate intoxication in Egyptian livestock is still limited. Nitrate is present in most forages, vegetables, and grasses in different quantities, but it can be accumulated to a toxic level in some circumstances, such as dehydration, cloudy weather, and at night (Haliburton Citation1999). In addition, nitrates can also accumulate in the plants after the utilization of large quantities of nitrogen-based fertilizers. In this report, the frequent application of nitrogen fertilizers results in an accumulation of high concentrations of nitrate ions in cabbages that were fed to a small flock of goats. It was reported that nitrate levels of over 15,000 ppm in the dried materials of forages cause intoxication in bovine livestock (El Bahri et al. Citation1997; Radostits et al. Citation2000). A nitrate concentration equal to 0.5% or more is dangerous and can cause acute poisoning, especially when the nitrate level is more than 1% (Puschner Citation2000). In our present study, goats fed cabbage with a high % of accumulated nitrate exhibited signs of poisoning. Our observation agreed with results recorded previously (Valli Citation1998), who reported that nitrate poisoning cases commonly occur following the feeding of goats to plants containing 3-7% nitrate on a dry matter basis, and nitrate accumulation in plants, mainly in stems and leaves (Puschner Citation2000). The most common and vital factor in nitrate toxicity cases is the level of nitrate-containing forages consumed. In our report, the poisoned goats did not eat any other foods before being fed feeding with cabbage, which contains an elevated amount of nitrate ions. Nitrate poisoning symptoms were exhibited when the animals were fed on high nitrate diets. About 20–40% of the blood hemoglobin is switched to methemoglobin, which cannot carry oxygen (Johnson et al. Citation1983; Pfister Citation1988). In our report, the recorded clinical signs are related to nitrate-nitrite poisoning. Most of the clinical signs that appear reflect tissue oxygen deprivation (El Bahri et al. Citation1997; Casteel and Evans Citation2004). This observation comes in agreement with our observation that the methemoglobinemia was 47%. When the percentage of methemoglobin reaches 67–90 percent, death occurs as a result of asphyxiation (Arnold et al. Citation2014). Ruminants are the primary species exposed to nitrate poisoning due to the conversion of nitrate to nitrite ion by the ruminal microflora. The excess nitrite ion enters the circulation and produces methemoglobin, rendering the red blood cells unable to carry oxygen, so hypoxia is noticed (Osweiler et al. Citation1985). Also, the methemoglobin percentage is increase in the blood and appears deeply brown or chocolate in color, the most usual aspect of nitrates or nitrite toxicity. Nitrate poisoning has common clinical symptoms, including abdominal pain and diarrhea, foamy salivation, rapid breath, tremors, incoordination, tachycardia, convulsions, and death may happen in less than 24 h (Osweiler et al. Citation1985). These results agree with our findings, where the poisoned goats exhibited dyspnea, foam, tremors, ataxia, head pressure, and had dark brown arterial blood. Clinical symptoms such as ataxia, tremors, and head pressure were due to central nervous system (CNS) disorders. The CNS disruptions in this study were attributed to methemoglobinemia, a serious CNS deficiency of oxygen, and accompanying hypoxia. When the oxygen concentration becomes less than 17%, tachycardia is directly initiated (Iwasaki et al. Citation2006). In addition, nitrate and nitrite are converted to nitric oxide (Perini et al. Citation1996), which is considered a strong vasodilator product (Vigo et al. Citation2010). The autonomic nervous system (ANS) plays an important roles in acclimatizing the body in response to hypoxia by stimulating and enhancing various autonomic mechanisms in the respiratory and cardiovascular systems. In the cardiovascular system in response to hypoxia, the resting heart rate (HR) is increased with an elevation in cardiac output as well as an increase in blood pressure (Woods et al. Citation2008; Broman et al. Citation2021). The respiratory system responses proceeded through the increase in the rate of pulmonary hypertension and hyperventilation (Ahmad et al. Citation2013). In accordance, the poisoned goats were presented with a febrile condition which increased the heart rate, blood vasodilation (Eddie et al. Citation2010), and respiratory rate (Gadomski et al. Citation1994). In this report, the assessment of nitrate toxicity depends on the recorded clinical signs, the chocolate-dark brown color of arterial blood, and the feeding of the plant gathering nitrate. Moreover, nitrate toxicosis can also be evidenced by the detection of the presence of nitrite content in the urine and serum in the poisoned animals and the cerebrospinal fluid and aqueous humor in dead animals (El Bahri et al. Citation1997; Casteel and Evans Citation2004). In our report, nitrate was measured in the urine, saliva, and blood plasma of nitrite. Testing the urine and blood plasma revealed positive for nitrite using urine test strep. After ingestion of the inorganic nitrates and nitrites, they are widely disseminated via the circulation, mainly in the plasma, because they remain stable for a long time. Also, about 25% of absorbed nitrate reaches the salivary glands (Carlsson et al. Citation2001), and in urine, which rises abruptly after ingestion (Doel et al. Citation2005; Duncan et al. Citation1995; Walker Citation1996). Exposure to nitrate reaches the circulation and is distributed to the different body organs. In our study, a decrease in the RBC count, Hb, and PCV were observed in poisoned animals. This decrease might be attributed to inhibition or defective hematopoiesis, suggesting that nitrate anions induced response effects from cumulative toxicity (Nikolov Citation1983 and Nohl et al. Citation2000). Leukocytosis was represented by monocytosis, neutrophils, and significant lymphopenia such results were also obtained by the previous reports (Iman and Manal Citation2007). Both Catalase (Gaetani et al. Citation1996; Mueller et al. Citation1997), and Glutathione peroxidase (Gaetani et al. Citation1989; Johnson et al. Citation2010) are considered the major members in the antioxidant defense system within the erythrocytes against the ROS where they are playing an important role in decomposing of the hydrogen peroxide H2O2.
In this report nitrate poisoning in the exposed goat induced weakening in the erythrocyte’s antioxidant defense is marked by a decreased concentration of GPx, SOD, and GST. It was accompanied by an increase in lipid peroxidation. This observation agreed with the data reported previously (Ansari et al. Citation2015), and the results were reported by Zavodnik et al. (Citation1999), who mentioned that the toxicity by nitrate induced hyperpolarization to the cell membrane of the erythrocytes resulting from oxidation of the Red Blood Cells and ROS generation, in association to depletion in the concentration of glutathione and increases the lipid membrane peroxidation as well as methemoglobin (metHb) formation. The changes observed in the oxidative system in erythrocytes suggest the enhancement of oxidative stress by nitrate poisoning. Sodium nitrate caused erythrocyte cell wall injuries, which may be attributed to the lower activities of the membrane-bound enzymes, injury to the cell membrane, and also an imbalance in the normal energy metabolism in erythrocytes. This nitrite-induced damage can reduce erythrocyte age in the blood, causing anemia (Ansari et al. Citation2015; Tashla et al. Citation2021).
The poisoned goats were given anticholinergic agents via the intravenous route, such as antihistamines, to reduce the inflammatory aspects associated with nitrate intoxication, while the administration of vasoconstrictor compounds like epinephrine prevented hypotension even before the point of death (El Bahri et al. Citation1997). Moreover, the intraluminal inoculation with oxytetracycline antibiotics stops the productions of nitrite and its entrance into blood circulation. It is also very important to note that food safety policy needs to be based on the principles of an integrated approach, primary responsibility, traceability of food, and its ingredients, transparency, and risk analysis (Vapa Tankosic et al. Citation2022).
5. Conclusions
Depending on the clinical signs recorded on the poisoned goats as well as nitrate concentration assessments in the different body fluids, it was found that nitrate accumulated in cabbages and reduced to nitrite in ruminal microorganisms. The formed nitrite which enters the circulation and causes many changes including fever, hypertension, tachycardia and increase the respiratory rate. Furthermore the produced nitrite induced changes the blood parameters including leukocytosis and anemia, in addition to increases the percentage of methemoglobin. Moreover the toxicity by nitrate induced oxidative stress as well as decreasing the antioxidant capacity in the circulating erythrocytes.
Institutional review board statement
This study was performed according to the Ethical Committee Board and Research Guidelines of the Faculty of Veterinary Medicine, South Valley University, Qena, Egypt, under ethical approval (No.64/19.09.2022).
Acknowledgements
A great acknowledgment to the goat’s owner for his help and kindness in completing this work. Many thanks to the local veterinary staff for first aid procedures and primary sample collections. Moreover, special thanks to the staff of the veterinary hospital of the Faculty of Veterinary Medicine, South Valley University, Qena, Egypt, ‘XXX’, ‘XXX’ for aiding in the primary diagnosis and full treatment of the admitted cases. AMK and HMD share in the hypothesis and the design of the scientific manuscript. IFR, FZ, ŠH, AMK, and HMD were prepared the chemicals and materials which were used in the practical section of this study. ZF writing review and editing of the manuscript. All authors contribute in the experimental steps and analyzes as well as participating in data analyzes in addition to, writing and revision of the manuscript’s draft. The final form of the manuscript was read and approved by all authors and agreed to publish the results generated from this work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Both the quantitative and qualitative information and results were used to complete the writing and formatting of the report and are contained in the manuscript.
Additional information
Funding
References
- Ahmad A, Dempsey SK, Daneva Z, Azam M, Li N, Li PL, Ritter JK. 2013. Role of nitric oxide in the cardiovascular and renal systems. Int J Mol Sci. 19(9):2605. doi:10.3390/ijms19092605. PMID: 30177600; PMCID: PMC6164974.
- Ansari FA, Ali SN, Mahmood R. 2015. Sodium nitrite-induced oxidative stress causes membrane damage, protein oxidation, and lipid peroxidation and alters major metabolic pathways in human erythrocytes. Toxicol In Vitro. 29(7):1878–1886. doi:10.1016/j.tiv.2015.07.022. ISSN 0887-2333.
- Arnold M, Gaskill C, Lehmkuhler J, Smith SR. 2014. Nitrate poisoning. ANRES, 165, https://uknowledge.uky.edu/anr_reports/165.
- Aslani MR. 2004. Poisonous plants of Iran and their effects on animals. Mashhad: Ferdowsi University Press, 72–74.
- Beatson CG. 1987. Methaemoglobinaemia-nitrates in drinking water. Environ Health. 86:31. https://www.scirp.org/(S(351jmbntvnsjt1aadkposzje))/referenced=637118.
- Brkić D, Bošnir J, Bevardi M, Bošković AG, Miloš S, Lasić D, Krivohlavek A, Racz A, Mojsović–Ćuić A, Trstenjak NU. 2017. Nitrate in leafy green vegetables and estimated intake. AJTCAM. 14(3):31–41. doi:10.21010/ajtcam.v14i3.4. PMID: 28480414; PMCID: PMC5412236.
- Broman ME, Vincent J, Ronco C, Hansson F, Bell M. 2021. The relationship between heart rate and body temperature in critically Ill patients. Critical Care Med. 49(3):e327–e331. doi:10.1097/CCM.0000000000004807.
- Carlsson S, Wiklund NP, Engstrand L, Weitzberg E, Lundberg JO. 2001. Effects of pH, nitrite, and ascorbic acid on nonenzymatic nitric oxide generation and bacterial growth in urine. Nitric Oxide. 5(6):580–586. doi:10.1006/niox.2001.0371. PMID: 11730365.
- Casteel S. 1997. Reproductive toxicology. In: Theriogenology R. S. Youngquist, editor. Current, therapy in large animal. 1st ed. Philadelphia, PA: W. B. Saunders Co.; p. 392–398.
- Casteel SW, Evans TJ. 2004. Nitrate. In: Plumlee K, editor. Clinical veterinary toxicology-e-book. St. Louis, USA: Mosby; p. 127–130.
- Cortas NK, Wakid NW. 1991. Pharmacokinetic aspects of inorganic nitrate ingestion in man. Pharmacol Toxicol. 68:192–195. doi:10.1111/j.1600-0773.1991.tb01221.x. https://airep.com.au/wp-content/uploads.
- Doel JJ, Benjamin N, Hector MP, Rogers M, Allaker RP. 2005. Evaluation of bacterial nitrate reduction in the human oral cavity. Eur J Oral Sci. 113(1):14–19. doi:10.1111/j.1600-0722.2004.00184.x.
- Dolan LC, Matulka RA, Burdock GA. 2010. Naturally occurring food toxins. Toxins (Basel). 2(9):2289–2332. doi:10.3390/toxins2092289.
- Duncan C, Dougall H, Johnston P, Green S, Brogan R, Leifert C, Smith L, Golden M, Benjamin N. 1995. Chemical generation of nitric oxide in the mouth from the enter salivary circulation of dietary nitrate. Nat Med. 1(6):546–551. doi:10.1038/nm0695-546. PMID: 7585121.
- Eddie W, Michael H, Jon OL, David SW. 2010. Nitrate-nitrite-nitric oxide pathway: implications for anesthesiology and intensive care. Anesthesiology. 113:1460–1475. doi:10.1097/ALN.0b013e3181fcf3cc.
- El Bahri L, Blouin A, Belguith J. 1997. Toxicology of nitrates and nitrites in livestock,” The Compendium on continuing education for the practicing veterinarian (USA). 19: 5 643–649, ISSN: 0193-1903.
- Gadomski AM, Permutt T, Stanton B. 1994. Correcting respiratory rate for the presence of fever. J Clinic Epidem. 47(9):1043–1049. doi:10.1016/0895-4356(94)90120-1. ISSN 0895-4356.
- Gaetani GF, Ferraris AM, Rolfo M, Mangerini R, Arena S, Kirkman HN. 1996. Predominant role of catalase in the disposal of hydrogen peroxide within human erythrocytes. Blood. 87(4):1595–1599. doi:10.1182/blood.V87.4.1595.bloodjournal8741595. PMID: 8608252.
- Gaetani GF, Galiano S, Canepa L, Ferraris AM, Kirkman HN. 1989. Catalase and glutathione peroxidase are equally active in detoxification of hydrogen peroxide in human erythrocytes. Blood. 73(1):334–339. doi:10.1182/blood.V73.1.334.334. PMID: 2491951.
- Habig WH, Pabst MJ, Jakoby WB. 1974. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 249(22):7130–7139. PMID: 4436300.
- Haliburton JC. 1999. Nitrate poisoning associated with consumption of forages or hay. In: Howard I, editor. Current veterinary therapy, food animal practice. 4th Edition. Philadelphia: Saunders; p. 278–279.
- Iman BS, Manal MM. 2007. Pathomorphological changes and endocrine dysfunctions due to nitrate toxicity in goats. Egypt J Clinic Path. 35:1–18.
- Iwasaki K, Ogawa Y, Aoki K, Saitoh T, Otsubo A, Shibata S. 2006. Cardiovascular regulation response to hypoxia during stepwise decreases from 21% to 15% inhaled oxygen. Aviat Space Environ Med. 77(10):1015–1019. PMID: 17042245.
- Johnson JL, Schneiderman NR, Kelling CL, Doster AR. 1983. Nitrate exposure in perinatal beef calves,” In Proceedings of the American association of veterinary laboratory diagnosticians, 30th annual meeting, Lake City, UT, USA, 167–180, https://agris.fao.org/agris-search.
- Johnson RM, Ho YS, Yu DY, Kuypers FA, Ravindranath Y, Goyette GW. 2010. The effects of disruption of genes for peroxiredoxin-2, glutathione peroxidase-1, and catalase on erythrocyte oxidative metabolism. Free Radic Biol Med. 48(4):51925. doi:10.1016/j.freeradbiomed.2009.11.021. PMID: 19969073; PMCID: PMC2818700.
- Kellerman T, Coetzer TJ, Naud ́e T, Botha C. 2005. Plant poisonings and mycotoxicoses of livestock in Southern Africa, 2nd ed. Cape Town: Oxford University Press. https://hdl.handle.net/10520/EJC99646.
- Lee TH, Kim SU, Yu SL, Kim SH, Park DS, Moon HB, Dho SH, Kwon K-S, Kwon HJ, Han Y-H, et al. 2003. Peroxiredoxin II is essential for sustaining life span of erythrocytes in mice. Blood. 101:5033–5038. doi:10.1182/blood-2002-08-2548.
- Montgomery HAC, Dymock JF. 1961. The determination of nitrate in water. Analyst. 86:414–416.
- Mueller S, Riedel HD, Stremmel W. 1997. Direct evidence for catalase as the predominant H2O2 -removing enzyme in human erythrocytes. Blood. 90:4973–4978. doi:10.1182/blood.V90.12.4973.
- Nagababu E, Mohanty JG, Friedman JS, Rifkind JM. 2013. Role of peroxiredoxin-2 in protecting RBCs from hydrogen peroxide-induced oxidative stress. Free Radic. Res. 47:164–171. DOI: 10.3109/10715762.2012.756138.
- Nikolov I. 1983. Dynamics of paraclinical indices in nitrate poisoning in sheep. Vet Med Nauki. 20(7):34–43. Bulgarian, PMID: 6659353.
- Nishikimi M, Appaji N, Yagi K. 1972. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun. 46(2):849–854. doi:10.1016/S0006-291X(72)80218-3.
- Nohl H, Staniek K, Sobhian B, Bahrami S, Redl H, Kozlov AV. 2000. Mitochondria recycle nitrite back to the bioregulator nitric monoxide. Acta Biochim Pol. 47(4):913–921. doi:10.18388/abp.2000_3946. PMID: 11996114.
- Ohkawa H, Ohishi N, Yagi K. 1979. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 95(2):351–358. doi:10.1016/0003-2697(79)90738-3. PMID: 36810.
- Osweiler G, Carson T, Buck W, Van Gelder G. 1985. Clinical and diagnostic veterinary toxicology-e-book, 3rd ed. Dubuque, IA: Kendall/Hunt Publishing Co. ISBN-13: 978-0840333322.
- Paglia DE, Valentine WN. 1967. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 70(1):158–169. PMID: 6066618.
- Perini R, Milesi S, Biancardi L, Veicsteinas A. 1996. Effects of high-altitude acclimatization on heart rate variability in resting humans. Eur JApplPhysiol. 73:521–528. doi:10.1007/bf00357674. PMID: 8817122.
- Pfister A. 1988. Nitrate intoxication of ruminant livestock. In: L. F. James, M. H. Ralphs, Nielson, editor. The ecology and economic impact of poisonous plants on livestock production. Boulder, CO: Westview Press; p. 233–260. doi:10.1201/9780429310225.
- Puschner B. 2000. Anti-nutritional factors in alfalfa hay, In Proceedings of the national alfalfa symposium, Las vegas, December. NV, USA.
- Radostits OM, Gay CC, Blood DC, Hinchcliff KW. 2000. Bovine mastitis: In veterinary medicine a textbook of the diseases of cattle, sheep, pigs, goats, and horses, 9th ed. London: W.B. Saunders Company Ltd. https://www.scirp.org/(S(351jmbntvnsjt1aadkposzje.
- Roder JD. 2002. Nitrate toxicity, veterinary toxicology. Fall newsletter. Butterworth-Heinemann, 2001, 1–23.
- Shugalei IV, L'vov SN, Tselinskii IV, Baev VI. 1992. Ukr. Biokhim. Zh. (in Russian), 64 111–114, https://link.springer.com/article/10.1134S107036321203022X.
- Tashla TĆM, Kurćubić V, Prodanović R, Puvača N. 2021. Occurrence of oxidative stress in sheep during different pregnancy periods. Acta Agriculturae Serbica. 26(52):111–116. doi:10.5937/aaser2152111t.
- Valli V. 1998. The hematopoietic system, In Pathology of domestic animals, K. V. F. Jubb, P. C. Kennedy, and N. Palmer, Eds., Academic Press, London, 4th ed, 101–264. https://www.elsevier.com/books/pathology-of-domestic-animals/jubb/978-0-12-391606-8.
- Vapa Tankosic J, Puvăca N, Giannenas I, Tufarelli V, Ignjatijevíc S. 2022. Food safety policy in the European Union. J Agron Technol. Eng. Manag. 5:712–717. DOI: 10.55817/EMRK6646.
- Vigo DE, Lloret SP, Videla AJ, Chada DP, Hunichen HM, Mercuri J, Romero R, Siri LCN, Cardinali DP. 2010. Heart rate nonlinear dynamics during sudden hypoxia at 8230 m simulated altitude. Wilderness Environ Med. 20:4–10. doi:10.1016/j.wem.2009.12.022. Epub 2009 Dec 22. PMID: 20591347.
- Walker R. 1996. The metabolism of dietary nitrites and nitrates. Biochem Soc Trans. 24(3):780–785. doi: 10.1042/bst0240780. PMID: 8878847.
- Woods DR, Allen S, Betts TR, Gardiner D, Montgomery H, Morgan JM, Roberts PR. 2008. High altitude arrhythmias. Cardiology. 111:239–246. doi:10.1159/000127445. Epub 2008 Apr 23. PMID: 18434732.
- Zavodnik IB, Lapshina EA, Rekawiecka K, Zavodnik LB, Bartosz G, Bryszewska M. 1999. Membrane effects of nitrite-induced oxidation of human red blood cells. Biochim Biophys Acta. 142:306–316. doi: 10.1016/s0005-2736(99)00136-4.