?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The objective of the study was to establish baseline thermographic information on body and udder skin surface temperature (USST) of lactating cows in different stages of lactation, milk yield, parity, breed and season. Holstein Friesian crossbred (n = 19 cows) and Deoni lactating cows (n = 14 cows) were monitored for body (i.e. eye) and USST prior to milking using a forward looking infra-red (FLIR) camera. It was observed that the mean body and USST of both crossbred and Deoni cows did not differ significantly. The body and USST of both the breeds were significantly higher by 0.9–1.0°C during evening than morning milking. There was no difference in body and USST between days and between udder quarters. Similarly, stage of lactation, milk yield and parity did not show any influence over body and USST. The body and USST were higher in summer (1.1°C) than in spring and winter seasons. Deoni cows had 1.0°C lesser body and USST than crossbred cows. It is concluded that baseline thermographic information on body and USST would be useful in developing breed-specific thermographic signature for individual animal.
Introduction
In recent years, the application of infra-red thermography in veterinary medicine is gaining more interest. In veterinary medicine, IRT was primarily used for diagnostic purposes, assessment of animal welfare and feed utilization efficiency.
In most of the studies, IRT was applied to detect changes in udder skin surface temperature (USST) caused by milking, environmental temperature, exercise (Berry et al. Citation2003; Kennedy et al. Citation2003), and for early detection of naturally occurring and lipopolysaccharide-induced subclinical and clinical mastitis in cows (Sathiyabarathi et al. Citation2016, Citation2018; Willard et al. Citation2007; Metzner et al. Citation2014; Polat et al. Citation2010; Colak et al. Citation2008; Hovinen et al. Citation2008).
There are no studies available that used the IRT technique over a period of time to establish baseline thermographic information on body and USST of lactating cows at different stages of lactation, milk yield, parity, breed and season. Therefore, the study was undertaken to establish baseline thermographic information on body and USST of HF crossbred and Deoni cows in different stages of lactation, milk yield, parity, breed and season.
Materials and methods
The experiment was conducted on lactating crossbred (Holstein Friesian × Bos indicus) and Deoni (Bos indicus) cows maintained at Livestock Research Centre (LRC), Southern Regional Station (SRS) of ICAR-National Dairy Research Institute (NDRI), Bengaluru (Karnataka).
Experimental animals and their management practices
Seventy-six udder quarters of apparently healthy lactating HF crossbred (Holstein Friesian × Bos indicus) cows (n = 19) of first to sixth parity with an average body weight of 451 ± 19 kg and milk yield of 14.4 ± 0.2 kg, 56 udder quarters of lactating Deoni cows (n = 14) with an average body weight of 365 ± 21 kg and milk yield of 3.5 ± 0.1 kg cows were maintained at SRS of ICAR-NDRI under loose housing system were monitored twice a day at 05:30 and 17:30 h for body and USST continuously for 28 days. Both the breeds were milked twice daily, HF crossbred and Deoni cows were milked by machine and hand milking, respectively. The cows were provided with sufficient concentrate feed and roughages and had free access to water.
Weather parameters and temperature humidity index (THI)
Temperature and relative humidity (RH) were recorded during milking time throughout the experimental period using a dry and wet bulb thermometer which was installed at milking shed. The temperature and RH data were used in the analysis of thermogram. The THI was calculated using the following equation (National Research Council Citation1971) ()
where Tdb is the dry bulb temperature of air (°C) and Twb is the wet bulb temperature of air.
Table 1. Mean ambient temperature, RH and THI during the study period (www.imdbangalore.gov.in).
Thermal imaging and analysis
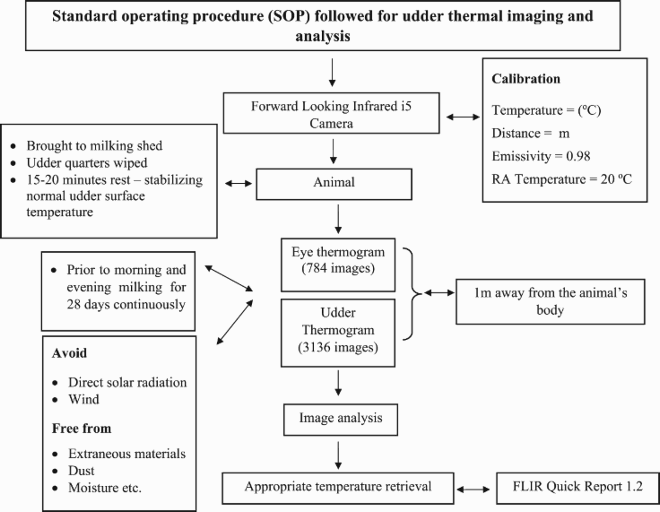
Thermal images of eye and udder surface were taken using a forward looking infra-red (FLIR) i5 camera (FLIR Systems, Inc., Wilsonville, OR, USA) to monitor the changes in the USST of the individual udder quarter throughout the experimental period. Prior to capturing the IRT image, the camera was calibrated to ambient temperature, and the temperature measurement was adjusted to degree Celsius and distance to metres. The values of emissivity and reflected apparent temperature were kept constant for all the images taken at 0.98°C and 20.0°C, respectively. A lateral thermographic image at a distance of 1.0 m from the animal’s head around the eye region including the ocular globe, inner canthus, the skin surrounding the ocular cavity and the lacrimal gland was recorded to observe body temperature of the cows.
Thermographic images of udder were taken prior to morning and evening milking at a distance of 1.0 m from the udder. Thermographic images were captured from the lateral side for fore quarters and posterior or lateral side for hind quarters of the udder (). A total of 784 eye images and 3136 udder images were taken throughout 28 days of the experimental period. The thermographic images were analysed by FLIR Quick Report 1.2 software. The temperature of the inner canthus of eye and the average temperature of the udder surface in a particular image were recorded and used in the analysis.
Statistical analysis
Paired t-test was employed to test the significance of the differences between the mean body (i.e. eye temperature) and USST of the lactating cows and mean body and USST difference between crossbred and indigenous cows. Repeated measure ANOVA was employed to test the significance of the differences in USST between udder quarters of an individual animal, between days and within a day and the effect of stage of lactation, parity and milk yield of crossbred cows on USST. All the above statistical analysis was made using SPSS 16.0 (IBM Corporation, Armonk, NY, USA) and Sigma plot 11 (SYSTAT, Salano, CA, USA).
Results
The mean body and USST of non-mastitis crossbred and Deoni cows during morning and evening milking did not show any significant difference throughout the experimental period. The mean (±SD) body and USST of HF crossbred cows were 37.23 ± 0.06°C and 37.20 ± 0.03°C, respectively, and Deoni cows were 36.15 ± 0.05°C and 36.18 ± 0.07°C (), respectively. The body and USST of crossbred and Deoni cows during evening milking increased significantly by 0.96–1.0°C than the morning milking ().
Figure 2. Body and udder skin surface temperature (mean ± SD) pattern of non-mastitis Holstein Friesian crossbred and Deoni cows during morning and evening milking. BT: body temperature; USST: udder skin surface temperature.
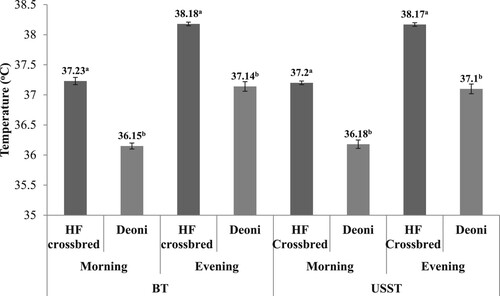
Table 2. Udder skin surface temperature difference between days, udder quarters and within a day of Holstein Friesian crossbred cows (n = 19 cows) and Deoni cows (n = 14 cows).
There was no significant difference in USST of all the cows between days during the study period (). In the present study, preliminary observation revealed that interquartile USST of both HF crossbred and Deoni cows did not differ significantly throughout the duration of observation, indicating body and USST were maintained relatively constant under the normal thermostatic mechanism ().
In the present study, the stage of lactation for crossbred and Deoni cows was divided as early, mid and late lactation with days in milk of <100, 100–200, >200 and <90, 90–180 and 180 days, respectively. The mean USST of crossbred and Deoni cows in early, mid and late stages of lactation was 37.18 ± 0.01°C, 37.17 ± 0.01°C and 37.17 ± 0.01°C and 36.19 ± 0.01°C, 36.19 ± 0.01°C and 36.20 ± 0.01°C, respectively (). Similarly, the parity and milk yield did not influence both body and USST. The mean USST of crossbred primiparous and multiparous cows was 37.15 ± 0.01°C and 37.14 ± 0.01°C, respectively.
Table 3. Effect of stage of lactation on body and udder skin surface temperature of non-mastitis Holstein Friesian crossbred and Deoni cows.
The USST of high and low producing crossbred was 37.14 ± 0.01°C and 37.15 ± 0.01°C and Deoni was 36.19 ± 0.01 and 36.16 ± 0.01°C cows, respectively (). In this study, body temperatures of Deoni and crossbred cows were 36.15 ± 0.05°C and 37.23 ± 0.06°C, respectively and Deoni cows showed lesser body temperature than crossbred cows by 1.08°C. Similarly, the USST was 36.18 ± 0.07°C and 37.20 ± 0.03°C in Deoni and crossbred cows, respectively and Deoni cows showed 1.02°C lesser USST than crossbred cows (; ).
Table 4. Effect of milk yield on body and udder skin surface temperature of non-mastitis HF crossbred and Deoni cows.
Table 5. Body and udder skin surface temperature (mean ± SD) difference between Holstein Friesian crossbred (n = 19 cows; 5440 quarter images) and Deoni cows (n = 14 cows; 3640 quarter images).
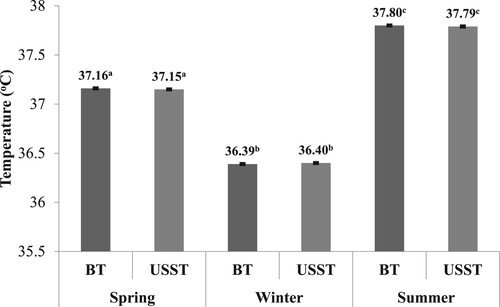
The mean body and USST of HF crossbred cow during spring; winter and summer season was 37.16 ± 0.01°C, 36.39 ± 0.01°C and 37.80 ± 0.01°C and 37.15 ± 0.01°C, 36.40 ± 0.01°C and 37.79 ± 0.01°C, respectively. The body and USST differentials during summer (1.1°C) were significantly higher than in the spring and winter seasons ().
Discussion
IRT is a non-invasive diagnostic tool for assessing changes in skin surface temperature which are influenced by internal conditions of tissues and organs accompanied by fluctuations in the amount and rate of capillary blood flow (Purohit and McCoy Citation1980; Metzner et al. Citation2014).
Body and USST pattern of lactating cows
Schaefer et al. (Citation2003) collected thermographic scans from several anatomical sites on the calves including the lateral side, dorsal side, and hooves, as well as facial areas, such as the ears, nose and eye, which included the inner canthus and lacrimal gland for early diagnosis of bovine diarrhoea infection in calves. The eye temperature was more consistent than the temperature of any other anatomical region and may correlate with core body temperature (Poikalainen et al. Citation2012; Ng and Kaw Citation2006). IRT eye temperatures of ponies were found to be associated with body temperature, as measured rectally or by microchip (Johnson et al. Citation2011). The differences of the temperatures measured using IRT among the body regions, i.e. eye (mean: 37.0°C), back of the ear (35.6°C), shoulder (34.9°C) and vulva (37.2°C), were significant, except between eye and vulva (Hoffmann et al. Citation2012). In this study, it was found that the mean body and USST of non-mastitis crossbred and Deoni cows during morning and evening milking did not show any significant difference throughout the experimental period.
Circadian changes in USST of lactating cows
Bitman et al. (Citation1984) reported that continuous temperature monitoring showed that the deep body temperature of lactating dairy cattle is characterized by a diphasic circadian rhythm with a 12-h period and an ultradian rhythm with a higher frequency with a 90-min period. Similarly, Berry et al. (Citation2003) found that USST appeared to have a distinct pattern within a day, indicating a possible associated monophasic circadian rhythm. In this study, the body and USST of crossbred and Deoni cows during evening milking increased significantly by 0.96–1.0°C than the morning milking. The increased environmental temperature during day time and increased animal’s activity in the loose housing system might have contributed to circadian changes in body and USST.
Day-to-day variation in USST of lactating cows
In the present study, there was no significant difference in USST of all the cows between days during the study period. On the contrary, Berry et al. (Citation2003) found that USST was predictably varying between days. In our study, the body and USST did not differ significantly since no significant difference was observed in ambient temperature and RH.
USST difference between udder quarters of lactating cows
Chun-he et al. (Citation2015) found the normal temperature distribution between rear left and rear right quarters. The variation of the rear left and rear right quarter skin temperature in non-mastitis cows was 35.57 ± 1.3°C and 35.51 ± 1.34°C, respectively. As the left and right quarter temperature difference increases the mean somatic cell count (SCC) in milk had a rising trend. This study showed that subclinical mastitis could be detected initially when the skin temperature difference between left and right quarters was over 1.5℃ and SCC was above 3 × 105 cells per ml for the detection of subclinical mastitis. Similarly, Berry et al. (Citation2003) concentrated on recording posterior udder quarter surface temperature which was more exposed to the environment than the front quarters and the fact that the majority of the mastitis cases occurred in the hind quarters. Metzner et al. (Citation2014) reported a positive correlation between the body and USST of hind quarters and opined that front quarters might display possibly different patterns of surface temperature, especially since surface temperature may be more or less affected by thermal radiation emanating from the medial aspects of the adjacent hind legs. However, in the present study, preliminary observation revealed that interquartile USST of both HF crossbred and Deoni cows did not differ significantly throughout the duration of observation, indicating body and USST were maintained relatively constant under the normal thermostatic mechanism.
Influence of stage of lactation, parity, milk yield, breed and season on USST of lactating cows
Berry et al. (Citation2003) and Polat et al. (Citation2010) suggested that management and seasonal influence may exist on USST. Furthermore, information on the influence of different stages of lactation, parity, milk yield, breed and season is essential to develop a definite predictive mastitis detection model. There are no reports available that compared all these parameters. In the present study, the stage of lactation for crossbred/Deoni cows was taken as early (<100/90 days), mid (100–200/90–180 days) and late (>200/180 days). The mean USST of crossbred and Deoni cows in early, mid and late stages of lactation was 37.18 ± 0.01°C, 37.17 ± 0.01°C and 37.17 ± 0.01°C and 36.19 ± 0.01°C, 36.19 ± 0.01°C and 36.20 ± 0.01°C, respectively. Similarly, the parity and milk yield did not influence both body and USST. The mean USST of crossbred primiparous and multiparous cows was 37.15 ± 0.01°C and 37.14 ± 0.01°C, respectively. The USST of high and low producing crossbred was 37.14 ± 0.01 and 37.15 ± 0.01°C and Deoni was 36.19 ± 0.01 and 36.16 ± 0.01°C cows, respectively. However, the present finding differs from Araki et al. (Citation1987), who reported that USST differs in different stages of lactation.
In this study, body temperatures of Deoni and crossbred cows were 36.15 ± 0.05°C and 37.23 ± 0.06°C, respectively and Deoni cows showed lesser body temperature than crossbred cows by 1.08°C. Similarly, the USST was 36.18 ± 0.07 and 37.20 ± 0.03°C in Deoni and crossbred cows, respectively and Deoni cows showed 1.02°C lesser USST than crossbred cows. This finding agrees with work done by De Netra et al. (Citation2015) (livestocktrail.illinois.edu.) who found low udder temperature of Jersey cows than Holstein cows, it might be related to the effect of body size on basal metabolic rate. It is interesting to observe in the present study that the mean differentials between body and USST of Bos indicus (Deoni) cows were lesser than Bos taurus (crossbred HF cows) indicating the existence of breed difference in thermographic profile and thermoregulatory mechanism. The lesser body temperature in Deoni cattle (Bos indicus) could be attributed to white coat colour (), playing a significant role in greater thermoregulatory capability than Bos taurus. Hansen (Citation2004) reported that Bos indicus cattle produce less heat and have an increased capacity to lose heat towards the environment or a contribution of both mechanisms. The lower metabolic rate, increased capacity for heat loss, body size, reduced growth rate and low to medium milk yield might contribute for better thermoregulatory mechanism in Bos indicus cows than Bos taurus (Finch Citation1985 and Hansen Citation2004). Furthermore, it was suggested that properties of hair coat in Zebu cattle enhance conductive and convective heat loss and reduce the absorption of solar radiation (Hansen Citation2004). Deoni (Bos indicus) cows used in the present study have got white coat colour and evolutionary adaptation mechanism to thrive and perform under tropical ecosystem. This phenotypic character would have significantly contributed in better thermoregulatory mechanism and lower body and udder surface temperature in these breeds which is further supported by the fact that cattle with lighter coat colour, shorter hair and greater hair diameter are more acclimatized to heat stress than animals have darker colours and longer hair coats (Bernabucci et al. Citation2010). Black-coloured skin and hair coat increase the solar heat load on the skin surface due to enhanced solar absorption (Collier and Gebremedhin Citation2015) (). The surface temperature of Holstein cows (with mixed coat colour of black and white) increased by 4.8°C than that of white coat coloured cows where there was an increase of 0.7°C only when they were exposed to direct sunlight (Hillman et al. Citation2001). The higher solar absorption by black coat colour than white could be attributed to the temperature difference in these cattle. The rectal temperature also increased at a rate of 0.7 and 0.3°C/h in black and white cows, respectively. Similarly, a study concluded that cattle with light-hair coats exhibited much higher reflectivity than dark-hair coats (Da Silva et al. Citation2003).
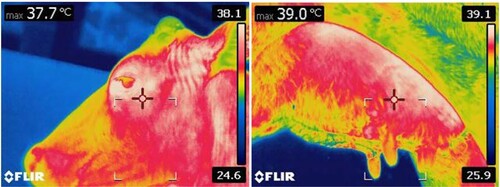
Figure 4. Visual image and infra-red thermogram showing body surface temperature pattern at different regions of white coat coloured Deoni (Bos indicus) cow.

Figure 5. Visual image and infra-red thermogram showing body surface temperature pattern at different regions of black and white coat coloured Holstein Friesian crossbred (Bos taurus × Bos indicus) cow.

B. taurus tropical cattle (Senepol and Carona) are characterized by this phenotype, and this dominant gene is accompanied by an elevated sweating rate in homozygous cattle during heat stress periods (Mariasegaram et al. Citation2007). Another recent classical study about Zebra stripes on thermoregulation in Kenya by Cobb and Cobb (Citation2019) revealed that the black and white stripes showed a significant temperature difference that increased as the day heats up and as the sunshine starts, a relatively steady temperature differential (of 12–15°C) between the black and white stripes were established by mid-morning and was maintained for the next 7 h or so. This indicates that the primary function of the stripes may be thermoregulation.
In the present study, the mean body and USST of HF crossbred cow during spring; winter and summer season was 37.16 ± 0.01°C, 36.39 ± 0.01°C and 37.80 ± 0.01°C and 37.15 ± 0.01°C, 36.40 ± 0.01°C and 37.79 ± 0.01°C, respectively. The body and USST differentials during summer (1.1°C) were significantly higher than in the spring and winter seasons.
Conclusion
Baseline thermographic information on body and USST differentials was established for crossbred and indigenous cows. The existence of breed-specific skin surface temperature differences clearly indicates their adaptability towards thermoregulatory mechanisms and this clearly substantiates the need for establishing a breed-specific thermographic signature and predictive model for early detection of mastitis.
Acknowledgements
The authors thank the Head, Southern Regional Station, Director, Indian Council of Agriculture Research–National Dairy Research Institute for providing needful facilities and post-graduate research fellowship support from National Dairy Research Institute. The authors are grateful to the staff of milking parlour Livestock Research Centre of SRS-NDRI for their assistance to carry out the study.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Araki CT, Nakamura RM, Kam LWG. 1987. Diurnal temperature sensitivity of dairy cattle in a naturally cycling environment. J Therm Biol. 12:23–26.
- Bernabucci U, Lacetera N, Baumgard LH, Rhoads RP, Ronchi B, Nardone A. 2010. Metabolic and hormonal acclimation to heat stress in domesticated ruminants. Animal. 4:1167–1183.
- Berry RJ, Kennedy AD, Scott SL, Kyle BL, Schaefer AL. 2003. Daily variation in the udder surface temperature of dairy cows measured by infrared thermography: potential for mastitis detection. Can J Anim Sci. 8:687–693.
- Bitman J, Lefcourt AM, Wood DL, Stroud B. 1984. Circadian and ultradian rhythms of lactating dairy cows. J Dairy Sci. 67:1014–1023.
- Chun-he Y, Xian-hong G, Zhenghui CAO, Xiaojun Z, Yue H, Yunxiang L, Lei S, Yutao Z. 2015. Study on possibility of left and right quarter skin temperature difference as a detecting indicator for subclinical mastitis in dairy cows. ActaVeterinaria Et ZootechnicaSinica. 46(9):1663–1670.
- Cobb A, Cobb S. 2019. Do zebra stripes influence thermoregulation? J Nat Hist. 53:13–14.
- Colak A, Polat B, Okumus Z, Kaya M, Yanmaz LE, Hayirli A. 2008. Short communication: early detection of mastitis using infrared thermography in dairy cows. J Dairy Sci. 91(11):4244–4248.
- Collier RJ, Gebremedhin KG. 2015. Thermal biology of domestic animals. Annu Rev Anim Biosci. 3:513–532.
- Da Silva RG, La Scala N, Jr Tonhati H. 2003. Radiative properties of the skin and haircoat of cattle and other animals. Trans ASAE. 46(3):913–918.
- De Netra, Griffin C, Michael R, Murphy James H, Baltz Cowles KE. 2015. Variation in the udder surface temperature of dairy cows measured by thermal imaging. Available online: http://www.livestocktrail.illinois.edu. Accessed 20 August 2015.
- Finch VA. 1985. Comparison of non-evaporative heat transfer in different cattle breeds. Aus J Agr Res. 38:497.
- Hansen PJ. 2004. Physiological and cellular adaptations of zebu cattle to thermal stress. Anim Reprod Sci. (Jul. 82-83):349–360. doi:10.1016/j.anireprosci.2004.04.011.
- Hillman PE, Lee CN, Carpenter JR, Baek KS, Parkhurst A. 2001. Impact of hair color on thermoregulation of dairy cows to direct sunlight. ASAE Paper, 014031, St. Joseph.
- Hoffmann G, Schmidt M, Ammon C, Rose-Meierhofer S, Burfeind O, Heuwieser W, Berg W. 2012. Monitoring the body temperature of cows and calves using video recordings from an infrared thermography camera. Vet Res Commun. 372:91–99.
- Hovinen M, Siivonen J, Taponen S, Hanninen L, Pastell M, Aisla AM, Pyorala S. 2008. Detection of clinical mastitis with the help of a thermal camera. J Dairy Sci. 91:4592–4598.
- Johnson SR, Rao S, Hussey SB, Morley PS, Traub-Dargatz JL. 2011. Thermographic eye temperature as an index to body temperature in ponies. J Equine Vet Sci. 31:63–66.
- Kennedy AD, Berry RJ, Scott SL, Kyle BL, Schaefer AL. 2003. Daily variation in the udder surface temperature of dairy cows measured by infrared thermography: potential for mastitis detection. Can J Anim Sci. 83:687–693.
- Mariasegaram M, Chase Jr CC, Chaparro JX, Olson TA, Brenneman RA, Niedz RP. 2007. The slick hair coat locus maps to chromosome 20 in senepol-derived cattle. Anim Genet. 38:54–59.
- Metzner M, Sauter-Louis C, Seemueller Petzl W, Klee W. 2014. Infrared thermography of the udder surface of dairy cattle: characteristics, methods, and correlation with rectal temperature. The Vet J. 199:57–62.
- National Research Council (NRC). 1971. A guide to environmental research on animals. Washington, DC, USA: National Academic Science.
- Ng EYK, Kaw GJL. 2006. Infrared images and fever monitoring devices: physics, physiology and clinical accuracy. In: Bronizino JD, editor. Medical devices and systems: biomedical engineering handbook. Third ed. Boca Raton: CRC Press; p. 1–20.
- Poikalainen V, Praks J, Veermae I, Kokin E. 2012. Infrared temperature patterns of cow’s body as an indicator for health control at precision cattle farming. Agr Res Biosys Eng. 1:187–194.
- Polat B, Colak A, Cengiz M, Yanmaz LE, Oral H, Bastan A, Kaya S, Hayirli A. 2010. Sensitivity and specificity of infrared thermography in detection of subclinical mastitis in dairy cows. J Dairy Sci. 93:3525–3532.
- Purohit RC, McCoy MD. 1980. Thermography in the diagnosis of inflammatory processes in the horse. Animal. 4(5):692–701.
- Sathiyabarathi M, Jeyakumar S, Manimaran A, Pushpadass HA, Sivaram M, Ramesha KP, Das DN, Kataktalware MA. 2018. Infrared thermal imaging of udder skin surface temperature variations to monitor udder health status in Bos indicus (Deoni) cows. Infrared Phys Tech. 88:239–244.
- Sathiyabarathi M, Jeyakumar S, Manimaran A, Pushpadass HA, Sivaram M, Ramesha KP, Das DN, Kataktalware MA, Jayaprakash G, Tapas Kumar P. 2016. Infrared thermography: investigation of body and udder skin surface temperature differentials as an early indicator of mastitis in Holstein Friesian crossbred cows using digital infrared thermography technique. Vet World. 9:1386–1391.
- Schaefer L, Cook N, Tessaro SV, Deregt D, Desroches G, Dubeski PL, Tong AKV, Ggodson DL. 2003. Early detection and prediction of infection using infrared thermography. Can J Anim Sci. 84:73–80.
- Willard S, Dray S, Farrar R, McGee M, Bowers S, Chromiak A, Jones M. 2007. Use of infrared thermal imaging to quantify dynamic changes in body temperature following lipopolysaccharide (LPS) administration in dairy cattle. J Anim Sci. 85(2):26.