 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The objective of this study was to evaluate the effect of a low dose of hCG or FSH on follicle growth and pregnancy per artificial insemination (AI) in crossbreed beef heifers subjected to a split-time AI program. All heifers (n = 386) were subjected to a 7-d Co-synch protocol and an intravaginal controlled internal drug release (CIDR) device (GnRH and CIDR insertion; 7 days later PGF2α, CIDR removal, and application of a tail head estrus detection patch; 36 hours later AI of heifers detected in estrus, 24 hours later timed AI of remaining heifers). At CIDR removal, heifers were randomly assigned to receive either 150 IU of hCG, 20 mg of FSH, or remained as untreated controls. The diameter of the preovulatory follicle and the pregnancy per AI did not differ among treatments. A smaller (P = 0.035) proportion of heifers in the hCG group were detected in estrus compared with control. Additionally, the incidence of premature ovulation was greater (P < 0.01) among heifers treated with hCG compared with control. In conclusion, using a low dosage of hCG or FSH at the time of device removal did not affect pre-ovulatory follicle growth or the proportion of pregnant heifers.
1. Introduction
The primary purposes of using estrus synchronization protocols in cow-calf operations are to concentrate calvings at the beginning of the calving season and increase calf uniformity, leading to greater weaning weights and improving profitability (Baruselli et al. Citation2012, Citation2018; Bó and Baruselli Citation2014). When estrus synchronization is combined with artificial insemination (AI) or timed artificial insemination (TAI), the introduction of new and better genetics to the herd is also a major advantage. However, despite such benefits, the last report of the USDA regarding cow-calf operation management practices in the USA showed that only 11.6% of producers use AI and 7.3% use estrus synchronization (USDA Citation2020).
The Co-synch estrus synchronization protocol and its variations are GnRH-based and used worldwide (Beef reproduction task force Citation2021; Lamb and Mercadante Citation2016). Pregnancy per artificial insemination (P/AI) remains variable when using the Co-Synch protocols, ranging from 42 to 67% and 39% to 68% for cows and heifers, respectively (Martínez et al. Citation2002; Bridges et al. Citation2008; Kasimanickam et al. Citation2008, Citation2020; Marquezini et al. Citation2011; Whittier et al. Citation2013; Madureira et al. Citation2020). The use of exogenous gonadotropins at the time of intravaginal progesterone (P4) device removal to enhance follicular growth by supporting the low release of luteinizing hormone (LH) has become a common adaptation in estradiol-based protocols in South America, for Bos taurus indicus cattle (Baruselli et al. Citation2012) and for Bos taurus cattle (Mínguez and Calvo Citation2020). Equine chorionic gonadotropin (eCG) (Bó and Baruselli Citation2014; Prata et al. Citation2018), human chorionic gonadotropin (hCG) (Santos et al. Citation2001; Prata et al. Citation2018; Souza et al. Citation2019), and follicle-stimulating hormone (FSH) (Santos et al. Citation2007) have shown beneficial results in P/AI when used at different moments in estradiol-based protocols. However, the use of hCG at the time of intravaginal P4 device removal has not been explored in GnRH-based protocols and only Small et al. Citation2008 have reported the use of FSH at the time of intravaginal P4 device removal in GnRH based protocols.
The eCG, which has an FSH – and LH-like actions in cattle, has been shown to increase P/AI from 61.5 to 71.7% (Sales et al. Citation2011). However, eCG is not commercially available for estrus synchronization protocols in the USA (U.S. Food and Drug Administration Citation2019). Therefore, exogenous FSH is an alternative strategy that has been shown to increase P/AI from 29.9 to 58.5% when applied during estradiol-based protocols (Santos et al. Citation2007). Exogenous FSH has been widely used for superovulation protocols in beef and dairy cattle due to its effect on FSH receptors in the granulosa cells, enhancing development of multiple preovulatory follicles allowing more follicles to continue growing past deviation (Bó and Mapletoft Citation2020). In addition, it has also been used in AI protocols to improve P/AI results. Santos et al. Citation2007 evaluated the use of 10 or 20 mg of FSH or 400 IU of eCG at the time of the intravaginal P4 device withdrawal during an estradiol-based protocol in Bos taurus indicus cows. The authors showed an increase in P/AI using either gonadotropin compared to the control group (eCG = 60.2%; 10 mg of FSH = 59.7%; 20 mg of FSH = 58.5%; Control = 29.9%). Both doses of FSH and eCG presented similar P/AI results. In contrast, Sales et al. Citation2011 reported that supplementing cows with 10 mg of FSH at the time of intravaginal P4 device removal did not increase P/AI compared with controls and eCG (10 mg of FSH = 60.0%; eCG = 71.6%; Control: 61.5%).
Alternatively, hCG is another gonadotropin hormone that can enhance follicular growth and P/AI by inducing ovulation, because of its LH-like effect. The main use of hCG has been to treat cystic cows as it has a similar therapeutic effect as GnRH (De Rensis et al. Citation2010). Besides, hCG has been used after AI to promote ovulation and formation of an accessory corpus luteum (CL). Santos et al. Citation2001 showed that after applying 3,300 IU of hCG in dairy cattle five days after AI, plasma progesterone levels increased (Control: 14.8 ng/mL; hCG: 20.7 ng/ml) associated with an increased number of CLs. The hCG has been tested in AI protocols as an ovulation inductor, but results were not satisfactory when used in GnRH-based protocols. Research conducted in beef cattle mainly attempted to replace GnRH with hCG in a Co-Synch protocol. Burns et al. Citation2008 showed that using 1,000 IU of hCG to replace GnRH on day 0 of the estrus synchronization protocol and TAI day decreased the P/AI (GnRH: 53.4% vs. hCG: 38.9%). Geary et al. Citation2001 replaced GnRH with 2,500 IU of hCG on day 0 and at TAI in cows with or without calf removal for 48 h, and no difference was found between the four treatments (GnRH without calf removal: 49%; GnRH with calf removal: 46%; hCG without calf removal: 34%; hCG with calf removal: 35%; P = 0.44). Recently, a new strategy of using a low dose of hCG (150 IU) at the time of the intravaginal P4 device removal was described by Souza et al. Citation2019 using estradiol-based protocols. They found an increase in the P/AI (Control = 43.3% vs. hCG = 53.2%). That study suggested that hCG may provide LH-like stimulus to final stages of follicular growth to ultimately enhance P/AI. However, the effect of hCG to stimulate final follicular growth in GnRH-based protocols has not been tested.
We hypothesize that a small dose of FSH (20 mg) or hCG (150 IU) at the time of the intravaginal P4 device removal enhances follicular growth and P/AI in crossbred beef heifers subjected to a 7-d Co-synch + CIDR protocol.
2 . Material and methods
The experiment was conducted with the approval of the Institutional Animal Care and Use Committee of the University of Florida (Study number 202011004).
2.1. Animals
A total of 386 commercial crossbred yearling (approx. 12-16 month of age) heifers (Bos taurus indicus x Bos taurus taurus) from a commercial farm in Okeechobee, Florida, were enrolled in this experiment during a 90-day breeding season. Heifers were kept on Bahiagrass (Paspalum notatum) and Limpograss (Hemarthria altissima) pastures, supplemented with a mixed ration feed, a mineral salt, and ad libitum access to water. For management purposes, heifers were divided into two groups (G1 n = 197; G2 n = 189).
Heifers were randomly assigned to three treatments within groups (Control n = 134, FSH n = 124, and hCG n = 128) and were allocated to treatments homogeneously, according to reproductive tract score (RTS; details in ultrasonography section; [Anderson et al. Citation1991]), body condition score (BCS; 1 to 9 scale; [Wagner et al. Citation1988]) and pubertal status (cycling: the presence of a corpus luteum, or not cycling: absence of a corpus luteum). Data was organized in an Excel spreadsheet, and heifers were ranked according to the three criteria. Next, a random number was generated for each heifer in Excel, which was used to allocate them to one of the treatments. We wanted to be sure that each treatment received homogenous groups of heifers and that each heifer had the same chance to receive any treatment. Three different sires were used for artificial insemination, and sires were randomly assigned to heifers within the treatments.
2.2. Reproductive management
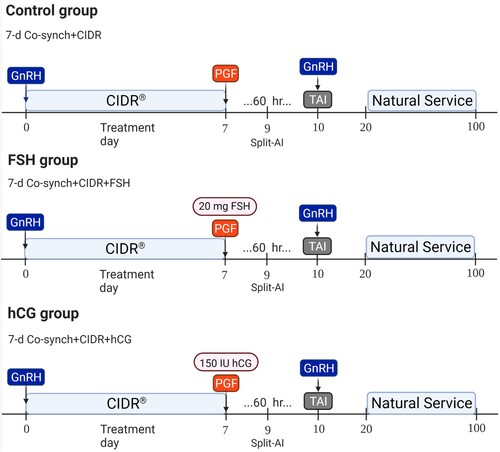
All heifers were subjected to a 90-d breeding season. Estrus in all heifers were synchronized with a 7-d Co-synch + CIDR (controlled internal drug release; Zoetis) protocol (). On day 0, heifers were treated with an intramuscular (IM) injection of 100 µg of GnRH (Factrel, Zoetis) and an intravaginal CIDR device containing 1.38 g of progesterone (Eazi-Breed CIDR, Zoetis) was inserted. On day 7, the intravaginal CIDR device was removed in the afternoon, an injection of 25 mg of dinoprost tromethamine (Prostaglandin (PGF); Lutalyse HighCon Injection, Zoetis) was given IM, and an estrus detection patch (Estrotect™) was placed halfway between the hip and tail head on all heifers. Additionally, heifers in the FSH group received an IM injection of 20 mg of FSH (Folltropin, Vetoquinol). Heifers in the hCG group were treated with an IM injection of 150 IU of hCG (Chorulon, Merck). Estrus detection was carried out 36 hours after the intravaginal P4 device removal. Heifers that presented more than 50% of the estrus detection patch rubbed off were considered in estrus and were artificially inseminated 12 hours later and received an IM injection of 100 µg of GnRH. On day 10, the heifers that presented estrus after 36 hours and heifers that did not present estrus were TAI, 60 hours after intravaginal P4 device removal. All heifers received an IM injection of 100 µg of GnRH at the time of the TAI. Ten days after TAI, the heifers were exposed to bulls for natural service in a ratio of 1 bull per 20 heifers.
Figure 1 . Experimental design and treatments conducted in the present study. A Split-Time AI program was conducted based on the 7 d Co-synch + CIDR protocol. Treatments were applied at the time of intravaginal P4 device removal (FSH group: 20 mg of FSH; hCG group: 150 IU of hCG; Control: no additional treatment). Heifers that presented estrus 36 hours after intravaginal P4 device (CIDR) removal were inseminated 12 hours later. Heifers that presented estrus after 36 hours and those that did not present signs of estrus were TAI 60 hours after CIDR removal.
2.3. Ultrasonography
Measurements of pre ovulatory follicles (POF) were performed on a single, still sonogram of the apparent maximal area for each structure. (Esaote ultrasound, MyLab Delta Vet, with 10-5 MHz transducer). On day 0, presence of CL and RTS on a scale from 1 to 5 (1: no follicles, no CL immature uterine horn; 2: the presence of 8 mm follicles, uterine horn of 20–25 mm diameter, no tone; 3: presence of 8–10 mm follicles, uterine horn of 25–30 mm diameter and slight tone; 4: presence of > 10 mm follicles, 30 mm diameter and good tone; 5: presence of > 10 mm follicles and corpus luteum, > 30 mm diameter and good tone; [Anderson et al. Citation1991]) were determined. On day 7 and at AI (either 12 hours after estrus detection or TAIday 10), the length (L) and width (W) of the dominant follicle were measured to calculate the follicular diameter (D). The follicular diameter was calculated with the formula:
The follicular growth was calculated by subtracting the follicle diameter at AI from the follicle diameter on day 7. Premature ovulation was defined for heifers with a deviated follicle (>5 mm; Cooke et al. Citation2020) on day 7 but without a dominant preovulatory follicle at AI. P/AI and P/AI + natural service (P/AI + NS) were evaluated 30 and 130 days after TAI, respectively. Heifers were considered pregnant if a viable (i.e. presence of heartbeat) embryo or fetus was detected.
2.4. Statistical analyses
Dependent binary variables (P/AI, NS, P/A + NS, estrus detection, and premature ovulation) were analyzed using the GLIMMIX procedure of SAS (SAS Institute, Cary, NC). When a significant effect was found, multiple comparison tests were performed.
The complete model for P/AI accounted for the following fixed independent variables: treatment (Control, FSH, or hCG), follicular growth rate, cyclicity status, BCS, estrus detection and sire. Variables were removed from the model by backward elimination if P > 0.10. Treatment was forced in the final model. Therefore, the final model for P/AI (n = 386), NS (n = 241) and P/AI + NS (n = 386) considered the treatment effect only. The model to evaluate estrus response accounted for treatment effect only and a total of 19 heifers were not included in the analysis because they lost their Estrotect patch. The complete model for premature ovulation accounted for treatment and follicle diameter on day 7.
Dependent continuous variables (follicle diameter on day 7 and at AI, and follicular growth rate) were analyzed by ANOVA using the GLM procedure of SAS. Follicle diameter at day 7 was used as a covariate when follicle diameter at AI and follicular growth rate was analyzed. In addition, continuous data were tested for normality of the residues (Shapiro – Wilk test) and homogeneity of variances (Levene’s and Bartlett’s tests) and transformed when necessary. The final model for follicle diameter on day 7 accounted for the fixed independent variable of treatment only. For the follicle diameter at AI and follicular growth rate the final model accounted for the fixed independent variable of treatment and follicle diameter on day 7. One heifer was not included in the analyses of follicle diameter at day 7 and AI day because she was not scanned. Forty-one heifers were not included in the follicle diameter at AI day as they ovulated before, also six heifers were removed as they did not have a measurable follicle.
We conducted a post hoc power analysis for follicle diameter on day seven and pregnancy per AI (using the PROC POWER procedure of SAS software). Both follicle diameter at day seven and pregnancy per AI had a high power (> 99% and 92%, respectively). P-values are considered significant at <0.05 and tendencies at <0.10.
3. Results
The heifers in the current experiment had on average a BCS of 5, a RTS of 4 and 90% were cycling at the time the P4 intravaginal device was inserted.
3.1 . Estrus detection
A total of 148 (Control n = 57, FSH n = 57, and hCG n = 34) heifers presented estrus 36 hours after removing the intravaginal CIDR. The proportion of heifers detected in estrus decreased (P = 0.03) for hCG compared with control and FSH groups ().
Table 1. Effects of treatment (Control, FSH, or hCG) on estrus response, P/AI, P/AI + NS, proportion of premature ovulations, and follicle diameters at day 7 and AI day.
3.2 . Follicular diameter and growth
Follicle diameter on day 7 (Control: 9.30 ± 0.26 mm; FSH: 9.85 ± 0.27 mm; hCG: 9.34 ± 0.27 mm) and at AI (Control: 11.64 ± 0.17 mm; FSH: 11.69 ± 0.19 mm; hCG: 11.48 ± 0.19 mm) did not differ (P > 0.05) among groups. Additionally, there was no difference (P = 0.78) among groups in the follicular growth from day 7 to AI (Control: 2.77 ± 0.18 mm; FSH: 2.83 ± 0.20 mm; hCG: 3.01 ± 0.21 mm).
The proportion of heifers with premature ovulation was greater (P < 0.01) in the hCG group when compared with the control group, while the FSH group was similar to both the other groups (Control = 3.42%; FSH = 12.18%; hCG = 17.53%; P ≤ 0.01; ).
Independent of the treatments, heifers that were inseminated 12 hours after estrus detection had larger follicle diameters on day 7 and at AI day when compared to heifers that were submitted to TAI at day 10 (). Cyclicity status at the beginning of the protocol did not influence follicle diameters on day 7 and at AI day ().
Table 2. Relationship between AI day (12 hours after estrus detection or TAI on day 10) and estrus response, P/AI, P/AI + NS, and follicle diameters at day 7 and AI day.
Table 3. Relationship between pubertal status (Cyclic, Non-Cyclic) and estrus response, P/AI, P/AI + NS, and follicle diameters at day 7 and AI day.
3.3 . Pregnancy per artificial insemination, natural service, and natural service + P/AI.
There was no difference (P = 0.44) among treatments on the P/AI (Control: 41.80%; FSH: 36.29%; hCG: 34.37%; ). No difference (P = 0.42) was observed among treatments of NS pregnancy results (Control: 92.31%; FSH: 86.08%; hCG: 90.48%; ). Also, NS + P/AI evaluated 130 days after AI was not different (P = 0.78) among treatments (Control: 93.28%; FSH: 91.13%; hCG: 92.97%).
Independently of the treatments, P/AI and NS + P/AI were not influenced by the AI day (12 hours after estrus detection or TAI at day 10; ) or the cyclicity status at the beginning of the protocol ().
4 . Discussion
Estrus synchronization protocols in beef heifers continue to yield highly variable P/AI, ranging from 39 to 65% (Martínez et al. Citation2002; Kasimanickam et al. Citation2020; Madureira et al. Citation2020). Bos taurus indicus influenced cattle have benefited from the administration of exogenous gonadotropins at the time of the intravaginal P4 device removal to enhance follicular growth and P/AI (Baruselli et al. Citation2004; Small et al. Citation2009; Sá Filho et al. Citation2010). The objective of this experiment was to evaluate P/AI and follicular growth in response to an injection of 20 mg of FSH or 150 IU of hCG at the time of intravaginal P4 device removal on a 7-d Co-synch + CIDR protocol in crossbred beef heifers. In this experiment, there was no difference in follicular growth among treatments. No difference was found in the preovulatory follicle diameter at AI day (12 hours after estrus detection or TAI on day 10). Also, no difference was detected among treatments in P/AI or P/AI + NS. However, heifers in the hCG group presented premature ovulation in a greater proportion when compared to the control group and the estrus response was less intense in the hCG group when compared to the control and FSH group.
Administration of FSH or hCG at P4 device removal did not influence follicle growth. A similar result was reported by Sales et al. Citation2011 when they used 10 mg of FSH or 300 IU of eCG at the time of the P4 device removal in an estradiol-based protocol. They found a greater preovulatory follicle diameter in the eCG group (13.9 mm) when compared with the FSH group (12.8 mm) or control (12.9 mm) group. In the same study, eCG promoted an increase in follicular growth from day 8 to day 10, which was greater than the FSH and the control groups (eCG: 1.40 mm/day; FSH: 0.90 mm/day; Control: 0.95 mm/day). However, in the study conducted by Souza et al. Citation2019, a single injection of 150 IU of hCG increased the follicular growth when measured 24 h following the device removal (hCG = 2.04 mm; Control = 1.60 mm). Lack of effect of treatments in follicle growth in the present study was unexpected.
In this experiment, the follicle diameter on day 7 in the FSH and hCG groups was larger than 7 mm. It has been established that the deviation process occurs in follicles > 7 mm, meaning that follicles become LH dependent instead of FSH dependent (Mihm et al. Citation2006; Acosta et al. Citation2018). Therefore, the follicle diameter at day 7 could explain one reason why exogenous FSH did not have an effect. On the other hand, the 150 IU of hCG dosage might have induced ovulation instead of helping follicular growth. However, further research should be conducted to identify if this was the case.
Administration of FSH or hCG at intravaginal P4 device removal did not influence P/AI in beef heifers. There are no reports of using either hormone in a GnRH-based protocol at the time of the intravaginal P4 device removal, as most studies have been conducted with estradiol benzoate-based protocols. Souza et al. Citation2019 evaluated the use of 150 IU of hCG at the time of removal of the intravaginal P4 device when using an estradiol-based protocol. In that study, Bos indicus cows were synchronized by applying 2 mg of benzoate estradiol + intravaginal P4 device containing 750 mg of P4 at Day 0, one injection of PGF, and intravaginal P4 device removal at day 8.5 and TAI 36 to 40 hours after device removal. On day 8.5, animals received an injection of 150 IU of hCG (hCG group) or 300 IU of eCG (eCG group) or nothing (control group). The P/AI was greater in the eCG group (53.8%) and hCG group (53.2%) when compared with the control group (43.3%). Regarding the use of FSH, Santos et al. Citation2007 used an estradiol-based protocol and found an increase in the P/AI when an injection of 400 IU of eCG (60.2% P/AI), 10 mg of FSH (59.7% P/AI), or 20 mg of FSH (58.5% P/AI) was given at the time of the intravaginal P4 device removal when comparing with the control group (29.9% P/AI). In contrast, Sales et al. Citation2011 used an estradiol-based protocol in Nellore cows to evaluate the effect of FSH or eCG at P4 device withdrawal. They applied 10 mg of FSH or 300 IU of eCG and there no difference in P/AI between the FSH (60% P/AI) and the control groups (61.5% P/AI) were detected. However, P/AI was greater in the eCG group (71.6%). Exogenous gonadotropins have been used to stimulate the growth of dominant follicles in Bos Indicus cattle, improving P/AI results. However, the benefits of using this strategy have been mostly in animals presenting anestrous, animals with limited feed intake, low body condition score, or in lactating cows (Sa Filho et al. Citation2009; Sá Filho et al. Citation2010). The heifers in the current study had on average a BCS of 5 on a scale from 1 to 9; also, 90% were cycling at the beginning of the experiment. Thus, the heifers’ physiological stage could help explain why there was no increase in follicle size or follicular growth in this experiment, which resulted in no effect in P/AI.
Premature ovulation was observed in the present study when hCG was injected at intravaginal P4 device removal. Among the animals that ovulated prematurely, 17% were from the hCG group. This could be one reason why P/AI did not increase with the application of 150 IU of hCG, as a proportion of animals were inseminated after ovulation. On the other hand, it can be suggested that there was a reduction in the proestrus phase after premature ovulation. Studies have shown that by reducing the proestrus phase, the P/AI is reduced. (Bridges et al. Citation2010) evaluated the influence of the proestrus length on fertility in lactating and non-lactating beef cows. The proestrus length was managed by changing the PGF-GnRH interval generating two experimental groups: long proestrus (2.25 days of proestrus length) and short proestrus (1.25 days of proestrus length). They found that the P/AI was greater in the long proestrus group (50% of P/AI) than in the short proestrus group (2.6% of P/AI). In the current study, the preovulatory follicle diameter in heifers that presented premature ovulation is unknown; however, there is a possibility that those heifers ovulated smaller or immature follicles (< 10 mm follicles) or they went under atresia. A study reported that animals that ovulated immature follicles had decreased P4 concentrations during the early luteal phase, and fertility was reduced when immature follicles were induced to ovulate (Mussard et al. Citation2007). Studies have shown that animals that ovulated smaller follicles had a reduction in fertility compared with those with larger follicles (Pugliesi et al. Citation2016). Also, the ovulation of small follicles has been related to inappropriate oviductal (Gonella-Diaza et al. Citation2018, Citation2015) and uterine environments (Mesquita et al. Citation2014), resulting in lower embryo receptivity and pregnancy failure.
In the present study, the hCG group expressed a lower estrus response when compared with the other two treatments. This could be explained by the increase in premature ovulations in this group. It has been shown that by decreasing the proestrus phase, there is a reduction in estradiol levels. Bridges et al. Citation2010 showed that estradiol concentration at estrus was greater in the long proestrus group than in the short proestrus group (12.7 pg/ml vs. 9.1 pg/ml, respectively). This could suggest that animals presenting premature ovulation in the present study also had shorter proestrus and possibly lower estradiol concentrations. This could explain the lower estrus response in the hCG group.
In conclusion, the results in this experiment did not support the original hypothesis. Follicular growth and P/AI were not increased when either a low dosage of FSH or hCG were injected at the time of intravaginal P4 device removal when using a 7-d Co-Synch + CIDR protocol. Furthermore, 150 IU of hCG increased the number of heifers that underwent early ovulation and decreased the estrus response. Therefore, further research should be conducted to understand follicular dynamics using these two exogenous gonadotropins.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Acosta T, Beg M, Ginther OJ. 2018. Effects of modified FSH surges on follicle selection and codominance in heifers. Anim. Reprod. 2:28–40.
- Anderson KJ, LeFever DG, Brinks JS, Odde KG. 1991. The use of reproductive tract scoring in beef heifers. Agri-Practice. 12:19–26.
- Baruselli P, Sales JN, Sala R, Vieira L, Sá Filho M. 2012. History, evolution and perspect ives of timed artificial in semination programs in Brazil. Anim. Reprod. 9:139–152.
- Baruselli PS, Ferreira RM, Sá Filho MF, Bó GA. 2018. Review: using artificial insemination v. natural service in beef herds. Animal. 12:s45–s52. doi:10.1017/S175173111800054X.
- Baruselli PS, Reis EL, Marques MO, Nasser LF, Bó GA. 2004. The use of hormonal treatments to improve reproductive performance of anestrous beef cattle in tropical climates. Anim Reprod Sci. 82-83: 479–486. doi:10.1016/j.anireprosci.2004.04.025.
- Beef reproduction task force. 2021. 2021 Estrus synchronization protocols for heifers and cows [WWW Document]. Beef Reprod. task force. https://beefrepro.org/wp-content/uploads/2020/12/Protocols-for-Sire-Directories-2021-Final.pdf (accessed 4.1.21).
- Bó GA, Baruselli PS. 2014. Synchronization of ovulation and fixed-time artificial insemination in beef cattle. Animal. 8:144–150. doi:10.1017/S1751731114000822.
- Bó GA, Mapletoft RJ. 2020. Superstimulation of ovarian follicles in cattle: gonadotropin treatment protocols and FSH profiles. Theriogenology. 150:353–359. doi:10.1016/j.theriogenology.2020.02.001.
- Bridges GA, Helser LA, Grum DE, Mussard ML, Gasser CL, Day ML. 2008. Decreasing the interval between GnRH and PGF2α from 7 to 5 days and lengthening proestrus increases timed-AI pregnancy rates in beef cows. Theriogenology. 69:843–851. doi:10.1016/j.theriogenology.2007.12.011.
- Bridges GA, Mussard ML, Burke CR, Day ML. 2010. Influence of the length of proestrus on fertility and endocrine function in female cattle. Anim Reprod Sci. 117:208–215. doi:10.1016/j.anireprosci.2009.05.002.
- Burns MG, Buttrey BS, Dobbins CA, Martel CA, Olson KC, Lamb GC, Stevenson JS. 2008. Evaluation of human chorionic gonadotropin as a replacement for gonadotropin-releasing hormone in ovulation-synchronization protocols before fixed timed artificial insemination in beef cattle1. J Anim Sci. 86:2539–2548. doi:10.2527/jas.2008-1122.
- Cooke RF, Daigle CL, Moriel P, Smith SB, Tedeschi LO, Vendramini JMB. 2020. Cattle adapted to tropical and subtropical environments: social, nutritional, and carcass quality considerations. J Anim Sci. 98:1–20. doi:10.1093/jas/skaa014.
- De Rensis F, López-Gatius F, García-Ispierto I, Techakumpu M. 2010. Clinical use of human chorionic gonadotropin in dairy cows: An update. Theriogenology. 73:1001–1008. doi:10.1016/j.theriogenology.2009.11.027.
- Geary TW, Salverson RR, Whittier JC. 2001. Synchronization of ovulation using GnRH or hCG with the CO-synch protocol in suckled beef cows. J Anim Sci. 79:2536–2541. doi:10.2527/2001.79102536x.
- Gonella-Diaza AM, Da Silva Andrade SC, Sponchiado M, Pugliesi G, Mesquita FS, Van Hoeck V, De Francisco Strefezzi R, Gasparin GR, Coutinho LL, Binelli M. 2015. Size of the ovulatory follicle dictates spatial differences in the oviductal transcriptome in cattle. PLOS ONE. doi:10.1371/journal.pone.0145321.
- Gonella-Diaza AM, Mesquita FS, Lopes E, da Silva KR, Cogliati B, De Francisco Strefezzi R, Binelli M. 2018. Sex steroids drive the remodeling of oviductal extracellular matrix in cattle†. Biol Reprod. 99:590–599. doi:10.1093/biolre/ioy083.
- Kasimanickam R, Hall JB, Currin JF, Whittier WD. 2008. Sire effect on the pregnancy outcome in beef cows synchronized with progesterone based Ovsynch and CO-synch protocols. Anim Reprod Sci. 104:1–8. doi:10.1016/j.anireprosci.2007.01.003.
- Kasimanickam RK, Kasimanickam VR, Oldham J, Whitmore M. 2020. Cyclicity, estrus expression and pregnancy rates in beef heifers with different reproductive tract scores following progesterone supplementation. Theriogenology. 145:39–47. doi:10.1016/j.theriogenology.2020.01.028.
- Lamb GC, Mercadante VRG. 2016. Synchronization and artificial insemination strategies in beef cattle. Vet Clin North Am - Food Anim Pract. doi:10.1016/j.cvfa.2016.01.006.
- Madureira G, Consentini CEC, Motta JCL, Drum JN, Prata AB, Monteiro PLJ, Melo LF, Gonçalves JRS, Wiltbank MC, Sartori R. 2020. Progesterone-based timed AI protocols for Bos indicus cattle II: reproductive outcomes of either EB or GnRH-type protocol, using or not GnRH at AI. Theriogenology. 145:86–93. doi:10.1016/j.theriogenology.2020.01.033.
- Marquezini GHL, Dahlen CR, Bird SL, Lamb GC. 2011. Administration of human chorionic gonadotropin to suckled beef cows before ovulation synchronization and fixed-time insemination: replacement of gonadotropin-releasing hormone with human chorionic gonadotropin1. J Anim Sci. 89:3030–3039. doi:10.2527/jas.2010-3455.
- Martínez MF, Kastelic JP, Adams GP, Cook B, Olson WO, Mapletoft RJ. 2002. The use of progestins in regimens for fixed-time artificial insemination in beef cattle. Theriogenology. 57:1049–1059. doi:10.1016/S0093-691X(01)00682-3.
- Mesquita FS, Pugliesi G, Scolari SC, França MR, Ramos RS, Oliveira M, Papa PC, Bressan FF, Meirelles FV, Silva LA, et al. 2014. Manipulation of the periovulatory sex steroidal milieu affects endometrial but not luteal gene expression in early diestrus Nelore cows. Theriogenology. 81:861–869. doi:10.1016/j.theriogenology.2013.12.022.
- Mihm M, Baker PJ, Ireland JLH, Smith GW, Coussens PM, Evans ACO, Ireland JJ. 2006. Molecular evidence that growth of dominant follicles involves a reduction in follicle-stimulating hormone dependence and an increase in luteinizing hormone dependence in Cattle1. Biol Reprod. 74:1051–1059. doi:10.1095/biolreprod.105.045799.
- Mínguez C, Calvo A. 2020. Effect of equine chorionic gonadotropin on pregnancy rate in Brown Swiss cows under high altitude conditions. J Appl Anim Res. doi:10.1080/09712119.2020.1741373.
- Mussard ML, Burke CR, Behlke EJ, Gasser CL, Day ML. 2007. Influence of premature induction of a luteinizing hormone surge with gonadotropin-releasing hormone on ovulation, luteal function, and fertility in cattle1. J Anim Sci. 85:937–943. doi:10.2527/jas.2006-592.
- Prata A, Drum J, Melo L, Araujo E, Sartori R. 2018. Effect of different chorionic gonadotropins on final growth of the dominant follicle in Bos indicus cows. Theriogenology. 111:52–55. doi:10.1016/j.theriogenology.2018.01.011.
- Pugliesi G, Santos FB, Lopes E, Nogueira É, Maio JRG, Binelli M. 2016. Improved fertility in suckled beef cows ovulating large follicles or supplemented with long-acting progesterone after timed-AI. Theriogenology. 85:1239–1248. doi:10.1016/j.theriogenology.2015.12.006.
- Sá Filho MF, Torres-Júnior JRS, Penteado L, Gimenes LU, Ferreira RM, Ayres H, Castro E, Paula LA, Sales JNS, Baruselli PS. 2010. Equine chorionic gonadotropin improves the efficacy of a progestin-based fixed-time artificial insemination protocol in nelore (Bos indicus) heifers. Anim Reprod Sci. 118:182–187. doi:10.1016/j.anireprosci.2009.10.004.
- Sa Filho O, Meneghetti M, Peres RFG, Lamb GC, Vasconcelos JLM. 2009. Fixed-time artificial insemination with estradiol and progesterone for Bos indicus cows II: strategies and factors affecting fertility. Theriogenology. 72:210–218. doi:10.1016/j.theriogenology.2009.02.008.
- Sales JNS, Crepaldi GA, Girotto RW, Souza AH, Baruselli PS. 2011. Fixed-time AI protocols replacing eCG with a single dose of FSH were less effective in stimulating follicular growth, ovulation, and fertility in suckled-anestrus Nelore beef cows. Anim Reprod Sci. 124:12–18. doi:10.1016/j.anireprosci.2011.02.007.
- Santos IC, Martins C, Valentim R, Baruselli PS. 2007. Pregnancy rate in FTAI anestrus bos indicus cows treated with a single dose of fshp (folltropin®) (abstract). Acta Sci. Vet. 35:1151.
- Santos JEP, Thatcher WW, Pool L, Overton MW. 2001. Effect of human chorionic gonadotropin on luteal function and reproductive performance of high-producing lactating Holstein dairy cows. J Anim Sci. 79:2881–2894. doi:10.2527/2001.79112881x.
- Small J, Dias F, Pfeifer L, Lightfoot K, Colazo M, Kastelic J, Mapletoft R. 2008. Administration of Follicle-Stimulating Hormone At Cidr Removal Does Not Affect the Pregnancy Rate in a Cidr-Based, Cosynch Protocol in Lactating Beef Cows. Reprod Fertil Dev. 20:90–90. doi:10.1071/RDv20n1Ab20.
- Small JA, Colazo MG, Kastelic JP, Mapletoft RJ. 2009. Effects of progesterone presynchronization and eCG on pregnancy rates to GnRH-based, timed-AI in beef cattle. Theriogenology. 71:698–706. doi:10.1016/j.theriogenology.2008.09.045.
- Souza A, Valenza A, Carneiro T, Zanatta G. 2019. A low dose of hCG (150IU) associated to inducing ovulation with estradiol benzoate can successfully replace eCG in timed AI protocols in Nelore cows 2019.
- USDA. 2020. Beef 2017, Beef Cow-calf management practices in the United States, 2017, Report 1. Fort Collins.
- U.S. Food and Drug Administration. 2019. The Cattle Estrous Cycle and FDA-Approved Animal Drugs to Control and Synchronize Estrus—A Resource for Producers [WWW Document]. https://www.fda.gov/animal-veterinary/product-safety-information/cattle-estrous-cycle-and-fda-approved-animal-drugs-control-and-synchronize-estrus-resource-producers.
- Wagner J, Lusby K, Oltjen J, Rakestraw J, Wettemann R, Walters L. 1988. Carcass composition in mature hereford cows: estimation and effect on daily metabolizable energy requirement during winter. J Anim Sci. 603–612. doi:10.2527/jas1988.663603x
- Whittier WD, Currin JF, Schramm H, Holland S, Kasimanickam RK. 2013. Fertility in angus cross beef cows following 5-day CO-synch + CIDR or 7-day CO-synch + CIDR estrus synchronization and timed artificial insemination. Theriogenology. 80:963–969. doi:10.1016/j.theriogenology.2013.07.019.
