ABSTRACT
Effects of polyherbal mixture on hepatorenal injury and dyslipidaemia in alloxan-induced diabetic rats were evaluated. Alloxan monohydrate (120 mg/kg, i.p.) was injected into Wistar rats to induce diabetes. Animals were allotted to six groups (n = 6) and treatments were administered for consecutive six weeks as normal and diabetic controls, glibenclamide and polyherbal mixture (200, 400 and 600 mg/kg), respectively. Ameliorative effects of the polyherbal mixture were investigated by assessing FBG levels, changes in body and organ (liver and kidney) weights, biochemical parameters, oxidative stress along with inflammatory parameters in addition to histopathological examination of the liver and kidney. The results showed that alloxan-injected rats had significant (P < 0.05) hyperglycaemia in addition to elevated serum levels of hepatorenal injury indices (ALT, AST, ALP, bilirubin, total protein, albumin, creatinine, BUN and uric acid) and hyperlipidaemia evidenced by increased TC, TG, LDL-C, VLDL-C and decreased HDL-C levels. Furthermore, diabetes induction caused an increase in lipid peroxidation (MDA) and a reduction in antioxidant markers (SOD and CAT activities) as well as inflammation (TNF-α) in hepatic and renal tissues. Polyherbal mixture remarkably improved the aforementioned parameters in a dose-dependent manner. Histopathological findings supported the biochemical results. Conclusively, this study has provided novel insights into the efficacy of polyherbal mixtures in managing hyperglycaemia and its secondary complications in diabetes mellitus.
Introduction
Diabetes mellitus (DM) is one of the leading diseases that are responsible for increased morbidity and mortality worldwide (Alam et al. Citation2021). In 2019, nearly 463 million individuals (aged 20–79 years) were diagnosed with DM (Sinclair et al. Citation2020). Persistent hyperglycaemia, due to defective insulin secretion or/and insulin action, affects the metabolism of carbohydrates, lipids and proteins. Also, it causes the excessive generation of reactive oxygen species (ROS). The exceptional sensitivity of pancreatic β-cells to physiological changes subsequently leads to a decrease in β-cell numbers and aggravates DM symptoms (Singh et al. Citation2022).
Chronic hyperglycaemia-associated oxidative stress may result in the development of secondary complications such as dyslipidaemia and hepatorenal damage (Nellaiappan et al. Citation2022). Hepatopathies, including liver fibrosis, glycogen and fat deposition and hepatic enzyme induction, have been reported in DM. Excessive ROS generation and suppression of the antioxidant defence system play a key role in hepato-pathological conditions in diabetic patients (Coman et al. Citation2021). Additionally, arterial hypertension in diabetic patients results in renal damage. The prominent signs of diabetic nephropathy are glomerular damage and proteinuria which are attributed to the reduction in glomerular filtration capacity and renal inflammation (Sugahara et al. Citation2021).
Current pharmacotherapies are effective in the management of hyperglycaemia but their usage is often accompanied by weight gain (insulin therapy) and gastrointestinal complications (metformin). Also, these medicines do not thoroughly address the increased ROS level and fail to manage the progression of secondary complications in diabetic patients. Furthermore, factors include high costs, territorial inaccessibility and socioeconomic status limiting the use of standard medicines (Dowarah and Singh Citation2020; Padhi et al. Citation2020). The increasing burden of the healthcare system necessitates the development of easily accessible and cost-effective novel agents (Waqas et al. Citation2022). Its solution might lie in functional foods and/or medicinal plant-derived supplements. Ethno-pharmacological therapies have been used for their antioxidant, hypoglycaemic, lipid-lowering and hepatorenal protective properties to manage diabetes and related secondary complications (Naveen et al. Citation2021). Even today, the consumption of herbal medicines is booming regardless of major advances in pharmacotherapy (Waqas et al. Citation2022).
Traditional medicine practitioners prefer polyherbal formulations over the use of a single medicinal plant to treat complex diseases like DM (Anwar et al. Citation2022). Polyherbal preparations exert synergistic effects that result in better therapeutic outcomes with fewer side effects. Based on the literature review on the effectiveness of polyherbal preparations (Sulaiman et al. Citation2021; Paul and Majumdar Citation2022), four plants include Cassia absus (Chaksu), Gymnema sylvestre (Ghurmar boti), Nigella sativa (Kalonji) and Piper nigrum (Kali mirch) with considerable nutritional values, hypoglycaemic and antioxidant properties (Mahmoodi and Mohammadizadeh Citation2020; Sidra et al. Citation2018; Khan et al. Citation2019; Takooree et al. Citation2019; Aslam and Hussain Citation2021) were selected for preparing a polyherbal mixture and aimed to evaluate its palliative impact on dyslipidaemia, hepatorenal dysfunction and oxidative stress in alloxan-induced diabetic rats.
Materials and methods
Chemicals, drugs and kits
Alloxan monohydrate (Sigma-Aldrich®, USA) and Glibenclamide (Sanofi-Aventis®, Pakistan) were purchased. Biochemical assays were performed using commercially available kits (QCA®, Spain). Tissue levels of TNF-α were determined using an ELIZA kit (Bioassay Technology®, China).
Plant materials
The whole plant of Gymnema sylvestre and seeds of Cassia absus, Nigella sativa and Piper nigrum were collected from the Botanical Garden, University of Agriculture, Faisalabad (UAF), Pakistan and authenticated (herbarium no. Cassia absus: 212-1-18, Gymnema sylvestre: 213-1-18, Nigella sativa: 214-1-18 and Piper nigrum: 215-1-18).
Preparation of polyherbal mixture
Coarsely powdered dry plant materials (100 g of each plant) were separately macerated in 2 L of water: methanol (3:7, v/v) solvent mixture for three days. Extracts were filtered (Whatman filter paper No. 1) and the solvent evaporated using a rotary evaporator (Heidolph Rotacool®, Germany). An equal ratio (1:1:1:1) of each extract was combined to prepare the polyherbal mixture (Prabhakaran et al. Citation2017).
Phytochemical analysis
Qualitative tests were performed according to standard protocols to confirm the presence of various bioactive phytochemicals (Aslam and Hussain Citation2021). The Folin-Ciocalteau method for total phenolic (TP) content and the AlCl3 calorimetric method to estimate the total flavonoid (TF) content of a polyherbal mixture were used (Sultana et al. Citation2009). TP and TF contents were quantified as mg GAE/g of mixture and mg CE/g of mixture, respectively, using the gallic acid calibration curve (R2 = 0.9955; y = 0.0116x + 0.0927) and catechin calibration curve (R2 = 0.9938; y = 0.0027x + 0.1609).
Experimental animals
Thirty-six female Wistar rats (5–6 weeks aged; body weight range 150–180 g) were procured and kept at the animal house facility of the Institute of Microbiology, UAF, Pakistan. Animals were cared for according to the guidelines of Care and Use of Laboratory Animals by maintaining standard husbandry conditions (25 ± 2°C temperature, 40-60% humidity and 12 h light/dark cycles). Animals were supplied with standard rat food pellets and water was provided ad libitum. The study protocols were approved by the Institutional Biosafety Committee, UAF, Pakistan (D. No. 3507/ORIC).
Experimental design
Rats were allocated to two groups based on body weight: normal control and a second group comprising thirty rats, intended for diabetes induction (n = 6), were given freshly prepared alloxan monohydrate (120 mg/kg, i.p.) after overnight fasting. Diabetic rats were given 5% glucose solution ad libitum for the next 72 h to avoid hypoglycaemia. Rats with a blood glucose level ≥ 250 mg/dL (Ramzan et al. Citation2020) were selected and orally treated for the next six weeks as follows: healthy and diabetic controls with normal saline (3 mL/kg/day), glibenclamide (0.6 mg/kg/day) and polyherbal mixture at 200, 400 and 600 mg/kg/day, respectively.
Fasting blood glucose (FBG) levels were measured weekly using a commercially available glucometer (Saturn® blood glucometer, Taiwan). Initial and final body weights were used to observe the change in body weight.
Sampling of blood and organs
Blood samples were collected through a cardiac puncture under the influence of mild anaesthesia, stored in gel clot activator tubes, incubated (for 30 min at 37°C) and centrifuged (at 3000 rpm for 5 min). Separated serum was stored at −20°C for biochemical analyses. Liver and kidney tissues were collected and weighed to calculate relative organ weight (Porwal et al. Citation2017) using the following formula: Relative organ weight (%) = [(Organ weight/Final body weight) x 100].
Biochemical parameters
Serum levels of ALT, AST, ALP, bilirubin, total protein, albumin, creatinine, blood urea nitrogen (BUN) and uric acid were determined following the instructions of the kit’s manufacturer. Lipid profile was examined by determining TC, TG, LDL-C, VLDL-C (TG/5) and HDL-C. Atherogenic index (AI) was calculated using the formula AI = (TC – HDL-C)/HDL-C, while cardiovascular risk index (CRI) was calculated as CRI = TC/HDL-C (Madić et al. Citation2021).
Determination of oxidative stress markers
Hepatic and renal tissues were minced and mixed in ice-cold phosphate buffer (pH 7.4) to prepare tissue homogenates (10% w/v). Samples were centrifuged for 10 min at 3000 rpm and supernatant was collected to determine the MDA level and activities of SOD and CAT (Hussain et al., Citation2021a,Citationb). Lipid peroxidation (MDA level) was analyzed through the thiobarbituric acid reacting substances (TBRAS) method and estimated as nmol/mg protein. Superoxide dismutase (SOD) activity was determined by measuring pyrogallol autoxidation inhibition capacity in an alkaline medium and expressed in U/mg protein. Catalase (CAT) activity was assessed in tissue homogenates using the H2O2 reduction method and quantified as U/mg protein.
Estimation of inflammatory marker
Tumour necrosis factor-α (TNF-α) levels in liver and kidney tissues were determined using an ELIZA kit (Bioassay Technology®, China), according to the manufacturer’s protocol.
Histopathological examination
Hepatic and renal tissues fixed in 10% buffered formalin were paraffin-embedded and cut into 5–6-μm thickness sections. Tissues were stained with haematoxylin and eosin (H&E) dyes. Histopathological changes were observed under a light microscope (IRMECO®, Germany). Photomicrographs were captured using the attached camera (TOUPCAM®, China).
Statistical analysis
Data were statistically analyzed by applying one-way/two-way ANOVA followed by Duncan’s multiple comparisons (DMR) test using SPSS® software (v23). The results were presented as mean ± SEM. A statistical difference of P < 0.05 was considered significant.
Results
Phytochemical screening of the polyherbal mixture
Phytochemicals identified in the polyherbal mixture are given in .
Table 1. Phytocompounds identified in the mixture through HPLP.
TP and TF contents of the polyherbal mixture
Spectrophotometric analysis revealed that the polyherbal mixture contained 105.52 ± 1.41 mg GAE/g mixture of TP content while TF content contained 101.14 ± 1.28 mg CE/g mixture.
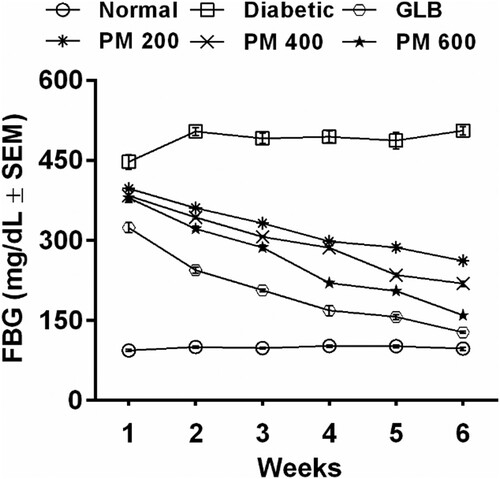
Effect of the polyherbal mixture on fasting blood glucose level
illustrates the antihyperglycaemic efficacy of the polyherbal mixture in diabetic rats by measuring the serum levels of fasting blood glucose (FBG) for six weeks. Alloxan injection significantly (P < 0.05) raised FBG levels in diabetic rats. The polyherbal mixture at 200, 400 and 600 mg/kg gradually lowered FBG levels to normoglycemic levels in a dose-dependent manner. The polyherbal mixture (600 mg/kg) reflected distinguished hypoglycaemic potential in comparison to the glibenclamide-treated group.
Effect of the polyherbal mixture on body and organ weights
As shown in , diabetes induction caused a significant (P < 0.05) reduction in body weight. Diabetic rats treated with graded doses of polyherbal mixture exhibited a significant (P < 0.05) gain in body weight with a considerably higher effect at 600 mg/kg. In addition, organ weights of the liver and kidney of diabetic control were found significantly (P < 0.05) increased in comparison to normal control. While treatment with glibenclamide and polyherbal mixture normalized the liver and kidney weight.
Table 2. Effect of the polyherbal mixture on body, liver and kidney weights of alloxan-induced diabetic rats.
Effect of the polyherbal mixture on hepatorenal function indices
indicates the ameliorative effects of glibenclamide and graded doses of the polyherbal mixture on liver function biomarkers in alloxan-injected diabetic rats. A significant (P < 0.05) increase in serum levels of ALT, AST and ALP with a corresponding increase in bilirubin concentration was seen in diabetic control. However, polyherbal mixture-treated diabetic groups significantly (P < 0.05) alleviated liver function parameters compared to diabetic control. Also, diabetes induction led to a significant (P < 0.05) reduction in total protein and albumin concentrations compared to normal control. Treatment of diabetic rats with different doses of polyherbal mixture significantly (P < 0.05) raised the total protein and albumin concentrations. The results demonstrated a significant (P < 0.05) elevation in serum levels of creatinine, BUN and uric acid in diabetic control when compared to normal control. Polyherbal mixture administration significantly (P < 0.05) declined these parameters in treated diabetic rats. In addition, the polyherbal mixture (600 mg/kg) displayed higher attenuated effects on hepatorenal function indices.
Table 3. Effect of the polyherbal mixture on hepatorenal function parameters in alloxan-induced diabetic rats.
Effect of the polyherbal mixture on hyperlipidaemia
The lipid profile of normal and diabetic groups was determined by assessing the serum levels of TC, TG, LDL-C, VLDL-C and HDL-C (). A mark (P < 0.05) increase was noted in TC, TG, LDL-C and VLDL-C and a reduction in HDL level in alloxan-induced diabetic rats in comparison to the normal control, after six weeks of the experiment. Treatment with the polyherbal mixture demonstrated an anti-lipidaemic effect and significantly (P < 0.05) ameliorated serum lipid profile. Furthermore, higher AI and CRI of diabetic control were significantly (P < 0.05) lower in polyherbal mixture-treated groups.
Table 4. Effect of the polyherbal mixture on lipid profile of alloxan-induced diabetic rats.
Effect of the polyherbal mixture on oxidative stress in hepatic and renal tissues
The results indicated the attenuating effects of glibenclamide and polyherbal mixture on lipid peroxidation (MDA level) and antioxidants (SOD and CAT activities) in the liver and kidney tissues of diabetic rats (). A significant (P < 0.05) elevation in MDA level and reduction in SOD and CAT activities in diabetic control was observed after six weeks of diabetes induction. Glibenclamide and polyherbal mixture administration significantly (P < 0.05) decreased MDA level and improved SOD and CAT activities in hepatic and renal tissues of diabetic rats. Moreover, the polyherbal mixture at 600 mg/kg showed profound ameliorating effects on lowering lipid peroxidation (MDA levels) and raising antioxidant status (SOD and CAT activities), compared to glibenclamide.
Table 5. Effect of the polyherbal mixture on tissue lipid peroxidation/antioxidants of alloxan-induced diabetic rats.
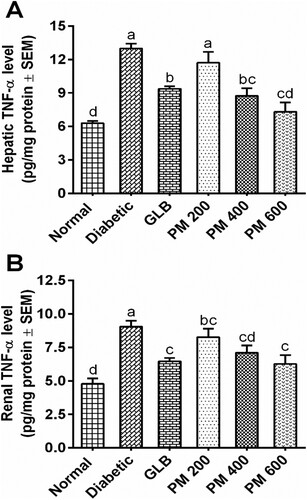
Effect of the polyherbal mixture on hepatic and renal inflammatory markers
indicates the inflammatory response of the liver and kidney tissue to alloxan injection and the attenuating effect of the polyherbal mixture in diabetic rats. Significantly (P < 0.05) increased TNF-α levels in hepatorenal tissues were noticed in diabetic control compared to normal control. Diabetic rats given a polyherbal mixture exhibited significantly (P < 0.05) lowered TNF-α levels, indicating a reduction in hepatorenal inflammation.
Figure 2. Effect of the polyherbal mixture on inflammatory cytokine (TNF-α) in hepatic and renal tissues of alloxan-induced diabetic rats. Different superscripts (a-d) indicate significant (P < 0.05) differences between groups. Data presented as mean ± SEM (n = 6). GLB: glibenclamide, PM: polyherbal mixture.
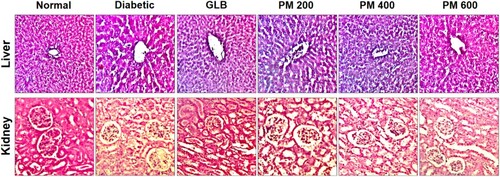
Histopathological findings
Liver and kidney histopathological examination revealed normal histoarchitecture in normal control (). Diabetic control exhibited pathological aberrations such as inflammatory cell infiltration, necrotic hepatocytes and sinusoid dilatation with hepatocyte vacuolation in the liver. Prominent features of nephropathy include glomerulus congestion, vacuolar degeneration and inflammatory cell infiltration in interstitial tissue were observed in the diabetic group. Treatment with a polyherbal mixture markedly ameliorated the aforementioned hepatorenal degenerative changes. Furthermore, polyherbal mixture at 400 and 600 mg/kg effectively improved the hepatic and renal histology that is comparable to glibenclamide.
Discussion
In DM, hyperglycaemia is a consequence of the altered metabolism of glucose, proteins and lipids along with an increase in ROS production that is involved in the development of DM complications (Ohiagu et al. Citation2021). Assessment of FBG is considered an essential biomarker for the diagnosis of diabetic conditions (Egan and Dinneen Citation2019). In this study, alloxan injection disrupted pancreatic β cells, leading to decreased cellular uptake of glucose, thereby creating a hyperglycaemic state, as observed in diabetic rats (Macdonald Ighodaro et al. Citation2017). Following six weeks of treatments, the polyherbal mixture markedly reduced the FBG levels which could be referred to as a decrease in ROS generation induced by alloxan (Volpe et al. Citation2018). Medicinal plants, which exert hypoglycaemic activity, usually contain phytochemicals such as polyphenols, alkaloids, sterols, tannins and terpenoids (Lankatillake et al. Citation2019; Jan et al. Citation2021). In this study, significant quantities of phenolics and flavonoids were found in the polyherbal mixture. Phenolic compounds exert antioxidant activities by scavenging ROS and inhibiting lipid peroxidation (Fiore et al. Citation2020; Mushtaq et al. Citation2021). Flavonoids cause cAMP phosphodiesterase inhibition that modulates insulin secretion, which explains the significant decline in FBG levels in diabetic rats (Wałkuski et al. Citation2018).
Continuous body weight loss is the most peculiar feature of DM caused by a reduction in peripheral glucose uptake and glycogen synthesis, negative nitrogen balance, muscle wasting and glycosuria. Also, alloxan-induced diabetes is linked with progressive body weight loss which may be due to excessive protein catabolism in tissue or increased muscle wasting (Ghauri et al. Citation2021). The results showed that a polyherbal mixture increased the body weight of diabetic rats in a dose-dependent manner, suggesting that the polyherbal mixture had substantially improved metabolic activities and general health status. Also, treatment of diabetic rats with the polyherbal mixture demonstrated a reversal of hepatic and renal hypertrophy evidenced by a decrease in liver and kidney weights.
The liver is the major organ for insulin clearance which helps to maintain normal fasting and post-prandial glucose levels (Ahrén and Foley Citation2016). Altered hepatic functions indicate hepatopathy in DM. In the present study, a significant rise in ALT, AST, ALP and bilirubin levels was observed in diabetic control, indicating the release of cytosolic enzymes into the blood circulation (Aissaoui et al. Citation2017). Also, elevated bilirubin levels may be a consequence of increased bilirubin production from haemolysis, conjugation and/or a decline in liver uptake (Ikewuchi et al. Citation2017). Polyherbal mixture administration caused a marked reduction in ALT, AST, ALP and bilirubin levels, indicating a return to normal liver function and this effect could be attributed to the antioxidant and hepatoprotective activities of the polyherbal mixture since antioxidants are known to reduce the development of chemically-induced hepatic injury (Abenavoli et al. Citation2018).
The results also showed a significant decline in total protein and albumin concentrations after diabetes induction. This decrease in protein levels could be linked to increased conversion of amino acids to glucose (Barti and Ram Citation2021). Treatment with the polyherbal mixture triggered a marked increase in total protein and albumin concentrations in diabetic rats. The polyherbal mixture (600 mg/kg) demonstrated the most promising effects on total protein and albumin levels compared to glibenclamide, which could be attributed to increased amino acid uptake in the liver, increased protein synthesis and/or decreased proteolysis (Barti and Ram Citation2021; Madić et al. Citation2021).
The estimation of serum creatinine, BUN and uric acid levels, is a useful biomarker for assessing renal function in diabetic conditions (Nowak et al. Citation2018). In this study, the results indicated significantly elevated serum levels of creatinine, BUN and uric acid following diabetes induction, thus indicating impaired renal function. Administration of a polyherbal mixture alleviated these parameters in a dose-dependent manner. These findings could be correlated to the presence of bioactive phytocompounds such as polyphenols and tannins that are known to protect against the damaging effects of alloxan action (Kang et al. Citation2020).
Hyperlipidaemia, due to altered metabolic activities, is frequently found in diabetic patients with characteristic elevated serum levels of TC, TG, LDL-C and VLDL-C and reduced HDL-C. It further increases the risk of cardiovascular complications and thus necessitates control over dyslipidaemia (Beverly and Budoff Citation2020). The present study showed a significant increase in TC, TG, LDL-C and VLDL-C levels and a decrease in HDL-C levels in diabetic rats. However, repeated treatment with a polyherbal mixture markedly ameliorated the lipid profile and decreased the AI and CRI in diabetic rats. This indicates that the polyherbal mixture might have interfered with the cholesterol and fatty acid synthesis pathway, resulting in hypolipidaemic effects (Renganathan and Pillai Citation2020). In agreement with previous studies, the polyherbal mixture exhibited significant hypolipidaemic activity which may be linked to its polyphenol contents responsible for enhanced insulin secretion from β-pancreatic cells, glucose oxidation and lipid synthesis pathway (Madić et al. Citation2021; Hassan et al. Citation2022).
Oxidative stress plays a crucial role in the pathogenesis of DM due to impaired glutathione metabolism, lipid peroxidation and altered antioxidant enzymatic systems. Host immune response to alloxan action and breakdown of RBCs are among the main factors that accelerate ROS generation in diabetes, subsequently causing depletion of antioxidant enzymes and increased lipid peroxidation which results in liver damage (Ighodaro Citation2018). In addition, oxidative stress along with arterial hypertension in DM results in renal injury (Ikewuchi et al. Citation2017). Lipid peroxidation also contributes to the excessive generation of ROS and leads to severe cellular damage in diabetes (Renganathan et al. Citation2020). In our study, a marked increase in lipid peroxidation (MDA level) and suppression of antioxidant markers in liver and kidney tissues was observed in diabetic rats induced by alloxan. The polyherbal mixture, particularly at 400 and 600 mg/kg, led to a decline in MDA levels and an increase in SOD and CAT activities in both organs as reported in a previous study (Renganathan and Pillai Citation2020). Antioxidants in the polyherbal mixture could be responsible for the improvement in the antioxidant status of treated diabetic rats (Murtaza et al. Citation2021).
Studies reported that the overproduction of ROS with subsequent inflammation is involved in liver and kidney injury (Ighodaro Citation2018). Hyperglycaemia-induced oxidative stress influences the expression of pro-inflammatory cytokines including tumour necrosis factor-α (TNF-α), which is a major contributing factor in diabetic tissue injury (Sierra-Mondragon et al. Citation2018). In the current study, significantly high levels of TNF-α were observed in hepatic and renal tissues. Administration of a polyherbal mixture in diabetic rats markedly reversed the pro-inflammatory cytokine levels in both organs induced by alloxan injection and our findings are concomitant with earlier studies (Safhi et al. Citation2019). Flavonoids have been reported to suppress the NF-κB and MAPK signalling pathways and ultimately down-regulate TNF-α expression (Yin et al. Citation2019).
Hepatic and renal histopathological examination revealed the damaging effects of alloxan on hepatocytes and renal structures in diabetic rats. An upsurge in serum levels of liver function markers indicates extensive hepatocyte injury. Liver histology of diabetic rats showed structural alterations such as hepatocyte necrosis, vacuolization and infiltration of inflammatory cells as a result of diabetes induction. Polyherbal mixture partially reversed these damage effects which could be attributed to its capability to normalize hepatic function parameters and antioxidant status of treated diabetic rats, thereby leading to improved liver histology as seen in a previous study (Yazdi et al. Citation2019). The kidney is among the major organs susceptible to diabetes-associated oxidative stress. Renal histology showed glomerular and tubular damage in diabetic control, indicating the primary and secondary effects of diabetes on renal histology which could be linked to a persistent hyperglycaemic environment and responsible for proximal and distal tubule dilatation in the renal cortex (Madić et al. Citation2021). The restoration of hepatic and renal histology to nearly normal levels following polyherbal mixture treatment may be attributed to the presence of regenerative agents such as polyphenols, alkaloids and terpenoids (Hussain et al., Citation2021a,Citationb).
Conclusion
This study’s findings revealed that diabetes induction caused hyperglycaemia, altered hepatorenal function indices and hyperlipidaemia. Furthermore, a marked increase in oxidative stress and inflammatory markers in hepatorenal tissues was noticed. Supplementation with a polyherbal mixture remarkably reversed the alloxan-induced changes evidenced by hypoglycaemic, lipid-lowering, antioxidant and anti-inflammatory activities in diabetic rats. Additionally, the polyherbal mixture showed higher efficiency than glibenclamide in attenuating hepatorenal injury. Therefore, this polyherbal mixture could be a potential candidate for the management of diabetes and its associated secondary complications.
Consent to Participate
All authors are agreed to submit the article to this journal.
Consent to Publish
All authors are agreed to publish in this journal.
Authors Contributions
Bilal Aslam: research methodology, sampling; Asif Hussain: conceptualization, Zia-uddin Sindhu: sampling and analysis, Ibad Ullah Jan and Sahar Nigar: sampling and analysis, Rifat Ullah Khan: editing and reviewing and Abdulwahed Fahad Alrefaei5, Mohammed Fahad Albeshr and Vincenzo Tufarelli: Editing and revising.
Competing interests
The authors declare that they have no relevant competing interest in the contents of this article.
Acknowledgement
We extend our appreciation to the Researchers Supporting Project (No. RSP2023R218), King Saud University, Riyadh, Saudi Arabia.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
On request.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
References
- Abenavoli L, Izzo AA, Milić N, Cicala C, Santini A, Capasso R. 2018. Milk thistle (Silybum marianum): a concise overview on its chemistry, pharmacological, and nutraceutical uses in liver diseases. Phytother Res. 32(11):2202–2213. doi:10.1002/ptr.6171.
- Ahrén B, Foley JE. 2016. Improved glucose regulation in type 2 diabetic patients with DPP-4 inhibitors: focus on alpha and beta cell function and lipid metabolism. Diabetologia. 59(5):907–917. doi:10.1007/s00125-016-3899-2.
- Aissaoui O, Amiali M, Bouzid N, Belkacemi K, Bitam A. 2017. Effect of Spirulina platensis ingestion on the abnormal biochemical and oxidative stress parameters in the pancreas and liver of alloxan-induced diabetic rats. Pharm Biol. 55(1):1304–1312. doi:10.1080/13880209.2017.1300820.
- Alam S, Hasan MK, Neaz S, Hussain N, Hossain MF, Rahman T. 2021. Diabetes mellitus: insights from epidemiology, biochemistry, risk factors, diagnosis, complications and comprehensive management. Diabetology. 2(2):36–50. doi:10.3390/diabetology2020004.
- Anwar S, Kausar MA, Parveen K, Zahra A, Ali A, Badraoui R, Snoussi M, Siddiqui WA, Saeed M. 2022. Polyherbal formulation: The studies towards identification of composition and their biological activities. J King Saud Univ-Sci. 34(7):102256. doi:10.1016/j.jksus.2022.102256.
- Aslam B, Hussain A. 2021. Phytochemical characterization and solvent fraction depending in vitro antioxidant activities of Cassia absus, Gymnema sylvestre, Nigella sativa and Piper nigrum. Rev Chim. 72:38–49. doi:10.37358/RC.21.2.8418.
- Barti H, Ram V. 2021. Development and evaluation of polyherbal formulation for antidiabetic potential in streptozotocin-induced diabetic rats. Prog Nucl Energy 6 Biol Sci. 1(1):46–52. doi:10.55006/biolsciences.2021.1105.
- Beverly JK, Budoff MJ. 2020. Atherosclerosis: pathophysiology of insulin resistance, hyperglycemia, hyperlipidemia, and inflammation. J Diabetes. 12(2):102–104. doi:10.1111/1753-0407.12970.
- Coman LI, Coman OA, Bădărău IA, Păunescu H, Ciocîrlan M. 2021. Association between liver cirrhosis and diabetes mellitus: a review on hepatic outcomes. J Clin Med. 10(2):262. doi:10.3390/jcm10020262.
- Dowarah J, Singh VP. 2020. Anti-diabetic drugs recent approaches and advancements. Bioorg Med Chem. 28(5):115263. doi:10.1016/j.bmc.2019.115263.
- Egan AM, Dinneen SF. 2019. What is diabetes? Medicine. 47(1):1–4. doi:10.1016/j.mpmed.2018.10.002.
- Fiore M, Messina MP, Petrella C, D'Angelo A, Greco A, Ralli M, Ferraguti G, Tarani L, Vitali M, Ceccanti M. 2020. Antioxidant properties of plant polyphenols in the counteraction of alcohol-abuse induced damage: impact on the Mediterranean diet. J Funct Foods. 71:104012. doi:10.1016/j.jff.2020.104012.
- Ghauri S, Raza SQ, Imran M, Saeed S, Rashid M, Naseer R. 2021. Assessment of α-amylase and α-glucosidase inhibitory potential of citrus reticulata peel extracts in hyperglycemic/hypoglycemic rats. 3 Biotech. 11:1–9. doi:10.1007/s13205-021-02717-8.
- Hassan F, Aslam B, Muhammad F, Faisal MN. 2022. Hypoglycemic properties of Sphaeranthus indicus and Nigella sativa in alloxan induced diabetes mellitus in rats; A new therapeutic horizon. Pak Vet J. 42(2):141–146. doi:10.29261/pakvetj/2022.011.
- Hussain A, Aslam B, Muhammad F, Faisal MN, Kousar S, Mushtaq A, Bari MU. 2021a. Anti-arthritic activity of Ricinus communis L. and Withania somnifera L. extracts in adjuvant-induced arthritic rats via modulating inflammatory mediators and subsiding oxidative stress. Iran J Basic Med Sci. 24:951–961. doi:10.22038/ijbms.2021.55145.12355.
- Hussain Z, Khan JA, Arshad MI, Muhammad F, Abbas RZ. 2021b. Protective effects of cinnamon, cinnamaldehyde and kaempferol against Acetaminophen-induced acute liver injury and apoptosis in mouse model. Pakistan Veterinary Journal. 41:25–32. doi:10.29261/pakvetj/2020.090.
- Ighodaro OM. 2018. Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed Pharmacother. 108:656–662. doi:10.1016/j.biopha.2018.09.058.
- Ikewuchi CC, Ikewuchi JC, Ifeanacho MO. 2017. Restoration of plasma markers of liver and kidney functions/integrity in alloxan-induced diabetic rabbits by aqueous extract of Pleurotus tuberregium sclerotia. Biomed Pharmacother. 95:1809–1814. doi:10.1016/j.biopha.2017.09.075.
- Jan I, Munir I, Khan I, Suhail SM, Iqbal A. 2021. Anti-inflammatory, antipyretic, analgesic and acute toxicity studies of dosiflavone using animal models of inflammation and pain. Sarhad Journal of Agriculture. 37:1201–1210. doi:10.17582/journal.sja/2021/37.4.1201.1210.
- Kang GG, Francis N, Hill R, Waters D, Blanchard C, Santhakumar AB. 2020. Dietary polyphenols and gene expression in molecular pathways associated with type 2 diabetes mellitus: a review. Int J Mol Sci. 21(1):140. doi:10.3390/ijms21010140.
- Khan F, Sarker MMR, Ming LC, Mohamed IN, Zhao C, Sheikh BY, Tsong HF, Rashid MA. 2019. Comprehensive review on phytochemicals, pharmacological and clinical potentials of Gymnema sylvestre. Front Pharmacol. 10:1223. doi:10.3389/fphar.2019.01223.
- Lankatillake C, Huynh T, Dias DA. 2019. Understanding glycaemic control and current approaches for screening antidiabetic natural products from evidence-based medicinal plants. Plant Methods. 15(1):1–35. doi:10.1186/s13007-019-0487-8.
- Macdonald Ighodaro O, Mohammed Adeosun A, Adeboye Akinloye O. 2017. Alloxan-induced diabetes, a common model for evaluating the glycemic-control potential of therapeutic compounds and plants extracts in experimental studies. Medicina. 53(6):365–374. doi:10.1016/j.medici.2018.02.001.
- Madić V, Petrović A, Jušković M, Jugović D, Djordjević L, Stojanović G, Vasiljević P. 2021. Polyherbal mixture ameliorates hyperglycemia, hyperlipidemia and histopathological changes of pancreas, kidney and liver in a rat model of type 1 diabetes. J Ethnopharmacol. 265:113210. doi:10.1016/j.jep.2020.113210.
- Mahmoodi MR, Mohammadizadeh M. 2020. Therapeutic potentials of Nigella sativa preparations and its constituents in the management of diabetes and its complications in experimental animals and patients with diabetes mellitus: a systematic review. Complement Ther Med. 50:102391. doi:10.1016/j.ctim.2020.102391.
- Murtaza S, Khan JA, Aslam B, Faisal MN. 2021. Pomegranate peel extract and quercetin possess antioxidant and hepatoprotective activity against Concanavalin A-induced liver injury in mice. Pak Vet J. 41:197–202. doi:10.29261/pakvetj/2020.097.
- Mushtaq A, Aslam B, Muhammad F, Khan JA. 2021. Hepatoprotective activity of Nigella sativa and Piper nigrum against Concanavalin A-induced acute liver injury in mouse model. Pak Vet J. 41:78–84. doi:10.29261/pakvetj/2020.076.
- Naveen YP, Urooj A, Byrappa K. 2021. A review on medicinal plants evaluated for anti-diabetic potential in clinical trials: present status and future perspective. J Herb Med. 28:100436. doi:10.1016/j.hermed.2021.100436.
- Nellaiappan K, Preeti K, Khatri DK, Singh SB. 2022. Diabetic complications: an update on pathobiology and therapeutic strategies. Curr Diabetes Rev. 18(1):31–44. doi:10.2174/1573399817666210309104203.
- Nowak N, Skupien J, Smiles AM, Yamanouchi M, Niewczas MA, Galecki AT, Duffin KL, Breyer MD, Pullen N, Bonventre JV, Krolewski AS. 2018. Markers of early progressive renal decline in type 2 diabetes suggest different implications for etiological studies and prognostic tests development. Kidney Int. 93(5):1198–1206. doi:10.1016/j.kint.2017.11.024.
- Ohiagu FO, Chikezie PC, Chikezie CM. 2021. Pathophysiology of diabetes mellitus complications: metabolic events and control. Biomed Res Ther. 8(3):4243–4257. doi:10.15419/bmrat.v8i3.663.
- Padhi S, Nayak AK, Behera A. 2020. Type II diabetes mellitus: a review on recent drug based therapeutics. Biomed Pharmacother. 131:110708. doi:10.1016/j.biopha.2020.110708.
- Paul S, Majumdar M. 2022. Comparative study of six antidiabetic polyherbal formulation for its multimodal approaches in diabetes management. 3 Biotech. 12(5):114. doi:10.1007/s13205-022-03166-7.
- Porwal M, Khan NA, Maheshwari KK. 2017. Evaluation of acute and subacute oral toxicity induced by ethanolic extract of Marsdenia tenacissima leaves in experimental rats. Sci Pharm. 85(3):29. doi:10.3390/scipharm85030029.
- Prabhakaran G, Bhore SJ, Ravichandran M. 2017. Development and evaluation of poly herbal molluscicidal extracts for control of apple snail (Pomacea maculata). Agriculture. 7(3):22. doi:10.3390/agriculture7030022.
- Ramzan T, Aslam B, Muhammad F, Faisal MN, Hussain A. 2020. Influence of Ocimum sanctum (L.) extract on the activity of gliclazide in alloxan-induced diabetes in rats. Rev Chim. 71(10):101–110. doi:10.37358/RC.20.11.8379.
- Renganathan S, Pillai RG. 2020. Antioxidant activities of Dhanwantaram Kashayam–an Ayurvedic poly herbal formulation alleviates diabetic complications in rats. J Diab Metabol Disord. 19:1345–1355. doi:10.1007/s40200-020-00655-5.
- Renganathan S, Srivastava A, Pillai RG. 2020. Dhanwantaram Kashayam, an ayurvedic polyherbal formulation, reduces oxidative radicals and reverts lipids profile towards normal in diabetic rats. Biochem Biophys Rep. 22:100755. doi:10.1016/j.bbrep.2020.100755.
- Safhi MM, Qumayri HM, Masmali AU, Siddiqui R, Alam MF, Khan G, Anwer T. 2019. Thymoquinone and fluoxetine alleviate depression via attenuating oxidative damage and inflammatory markers in type-2 diabetic rats. Arch Physiol Biochem. 125(2):150–155. doi:10.1080/13813455.2018.1443141.
- Sidra K, Shahzad H, Hussain A, Tipu MK, Humaira F, Madiha A. 2018. Brassica oleracea L. var. Italica Plenck and Cassia absus L. extracts reduce oxidative stress, alloxan induced hyperglycemia and indices of diabetic complications. Pak J Bot. 50(2):775–784.
- Sierra-Mondragon E, Molina-Jijon E, Namorado-Tonix C, Rodríguez-Muñoz R, Pedraza-Chaverri J, Reyes JL. 2018. All-trans retinoic acid ameliorates inflammatory response mediated by TLR4/NF-κB during initiation of diabetic nephropathy. J Nutr Biochem. 60:47–60. doi:10.1016/j.jnutbio.2018.06.002.
- Sinclair A, Saeedi P, Kaundal A, Karuranga S, Malanda B, Williams R. 2020. Diabetes and global ageing among 65–99-year-old adults: findings from the international diabetes federation diabetes atlas. Diabetes Res Clin Pract. 162:108078. doi:10.1016/j.diabres.2020.108078.
- Singh A, Kukreti R, Saso L, Kukreti S. 2022. Mechanistic insight into oxidative stress-triggered signaling pathways and type 2 diabetes. Molecules. 27(3):950. doi:10.3390/molecules27030950.
- Sugahara M, Pak WLW, Tanaka T, Tang SC, Nangaku M. 2021. Update on diagnosis, pathophysiology, and management of diabetic kidney disease. Nephrology. 26(6):491–500. doi:10.1111/nep.13860.
- Sulaiman CT, Anju K, Anandan EM, Balachandran I. 2021. Synergistic interactions of phytochemicals in polyherbal formulation enhance the chemical transformations of active constituents. J Appl Spectrosc. 88:181–186. doi:10.1007/s10812-021-01156-w.
- Sultana B, Anwar F, Ashraf M. 2009. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules. 14:2167–2180. doi:10.3390/molecules14062167.
- Takooree H, Aumeeruddy MZ, Rengasamy KR, Venugopala KN, Jeewon R, Zengin G, Mahomoodally MF. 2019. A systematic review on black pepper (Piper nigrum L.): from folk uses to pharmacological applications. Crit Rev Food Sci Nutr. 59(1):210–243. doi:10.1080/10408398.2019.1565489.
- Volpe CMO, Villar-Delfino PH, Dos Anjos PMF, Nogueira-Machado JA. 2018. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 9(2):119. doi:10.1038/s41419-017-0135-z.
- Wałkuski M, Szwed O, Lendzioszek M, Terlikowska KM. 2018. Polyphenols and flavonoids in the prevention and treatment of diabetes type 2. Prog Health Sci. 8:174–180. doi:10.5604/01.3001.0012.8343.
- Waqas S, Akram M, Panda AK, Elbossaty WF, Hegazil AG, Ghasemian AB, Aharwal RP, Wiwanitkit V. 2022. Current trends and future prospect of medicinal plants derived nutraceuticals: A. Curr Trends Pharm Pharmaceut Chem. 4(1):1–5. doi:10.18231/j.ctppc.2022.001.
- Yazdi HB, Hojati V, Shiravi A, Hosseinian S, Vaezi G. 2019. Liver dysfunction and oxidative stress in streptozotocin-induced diabetic rats: protective role of Artemisia turanica. J Pharmacopuncture. 22(2):109. doi:10.3831/KPI.2019.22.014.
- Yin M, Jiang N, Guo L, Ni Z, Al-Brakati AY, Othman MS, Moneim AEA, Kassab RB. 2019. Oleuropein suppresses oxidative, inflammatory, and apoptotic responses following glycerol-induced acute kidney injury in rats. Life Sci. 232:116634. doi:10.1016/j.lfs.2019.116634.