ABSTRACT
Oxidative stress is caused by an imbalance between the production of harmful oxygen molecules and the body's ability to repair their detrimental implications, leading to reduced growth rates, increased disease susceptibility, and decreased reproductive performance in animals. Vitamin A, comprising retinol, retinal, and retinoic acid, is crucial for normal growth, reproduction, and vision. Vitamin A possesses antioxidant properties by directly scavenging reactive oxygen species, boosting antioxidant enzyme activity, and promoting antioxidant defence mechanisms. Numerous studies have shown that livestock with adequate levels of vitamin A in their diet experience reduced oxidative stress compared to those with vitamin A deficiency. Moreover, vitamin A supplementation can mitigate oxidative stress in animals exposed to stressful conditions like heat stress. Adequate vitamin A status in livestock through dietary interventions and improved animal management practices can significantly benefit animal health and well-being. However, further research is still needed to optimize dosing strategies and fully understand the relationship between vitamin A and oxidative stress in different animal species and production systems. Therefore, continued research efforts are essential to fully harness the potential of vitamin A as an effective tool for mitigating oxidative stress and improving animal welfare.
Introduction
Vitamin A (retinol) encompasses three distinct chemical compounds, each serving specific functions in the body: retinol, an alcohol; retinal, an aldehyde; and retinoic acid (Carazo et al. Citation2021). As a vital micronutrient for animal growth and health, retinol plays a crucial role in livestock production. This fat-soluble vitamin is essential for various metabolic activities, including vision, immune system regulation, and cell differentiation (Herschel Conaway et al. Citation2013). While mammals and birds have the potential to synthesize vitamin A from precursor compounds like carotenoids present in their diets, several factors can impact their actual ability to do so. These factors include diet composition, age, and health status (Green and Fascetti Citation2016). Moreover, it has been reported that only a limited proportion of carotenoids can be enzymatically converted into retinoid precursors of retinol or retinoic acid in animals (Solomons Citation1994). Typically, naturally occurring vitamin A and carotenoid levels in feed ingredients are not considered during diet formulation for practical reasons (Darroch Citation2000). Instead, retinyl acetate is added to the diet to meet the total retinol requirement.
As animal production continues to grow and expand to meet the increasing global demand for protein, the need to maintain animal health and productivity becomes paramount. One of the major challenges faced by the animal agriculture industry is the negative impact of oxidative stress on livestock health and performance (Ponnampalam et al. Citation2022). Oxidative stress, caused by an imbalance between the production of reactive oxygen species (ROS) and antioxidant defence mechanisms, can lead to a wide range of health issues in animals, including reduced growth rates, compromised immune function, and increased susceptibility to infections (Celi and Gabai Citation2015). ROS are highly reactive molecules that can cause oxidative damage to lipids, proteins, and nucleic acids (Checa and Aran Citation2020). Therefore, it is essential to maintain a robust antioxidant system to neutralize ROS and prevent oxidative stress.
Vitamin A, as an essential micronutrient with a broad range of physiological functions, has long been recognized for its potent antioxidant properties. Pioneering research conducted by Monaghan and Schmitt in Citation1932 revealed that retinol could inhibit the oxygen uptake of linoleic acid in vitro for several hours at low concentrations. Furthermore, an in vitro peroxidation system has ranked the antioxidant activities of retinoids in the following order: retinol > retinal > retinyl palmitate > retinoic acid (Das Citation1989). It is noteworthy that Das (Citation1989) also suggested that retinol and its analogs may have stronger antioxidant properties than certain well-known antioxidants, including α-tocopherol. However, the mechanism underlying the antioxidant activity of vitamin A in vivo remains unclear, and the relationship between its structure and function has not been fully elucidated until now.
In recent years, there has been increasing interest in the relationship between vitamin A status and oxidative stress in animals (Jin et al. Citation2014; Gad et al. Citation2018; Hu et al. Citation2020; Hao et al. Citation2021). Better understanding of this relationship can provide insights into the optimal vitamin A requirements of livestock and help mitigate the adverse effects of oxidative stress.
The objective of this paper is to provide a comprehensive review of the current understanding of the relationship between vitamin A status and oxidative stress in animal production. In particular, this review will focus on the following aspects:
The effects of oxidative stress on animal health and performance.
The mechanisms associated with vitamin A antioxidant activity.
Vitamin A’s main antioxidant properties: Direct or indirect?
The influence of vitamin A supplementation on oxidative stress in animals.
Future applications of vitamin A as a therapeutic agent for preventing and treating oxidative stress-related diseases in livestock.
By examining these five topics, this review aims to provide additional insights into the potential benefits of vitamin A as a nutritional strategy for improving animal health and productivity, and to identify areas for future research in this field.
Oxidative stress and its impact on animal well-being
Oxidative stress or redox imbalance refers to the condition where the production of ROS exceeds the body’s capacity to neutralize or detoxify them, leading to cellular damage and disruption of physiological functions (Pizzino et al. Citation2017). While ROS are natural byproducts of cellular metabolism and play important roles in signalling pathways and host defence against pathogens (Dunn et al. Citation2015; Dickson and Zhou Citation2020), their excessive accumulation can occur due to various factors such as environmental stressors, insufficient elimination of free radicals, disease, and substances in feed (Miazek et al. Citation2022).
In animals, oxidative stress can significantly impact their well-being (Ponnampalam et al. Citation2022; Zhong et al. Citation2023). It can cause tissue damage, impair immune function, and contribute to the development of diseases (Colitti et al. Citation2019; De La Riva et al. Citation2022). Furthermore, redox imbalance can affect animal behaviour and reproduction (Pintus and Ros-Santaella Citation2021).
ROS can react with cellular components such as proteins, lipids, and DNA, leading to impairment and dysfunction (Checa and Aran Citation2020). This can affect organ function and lead to chronic illnesses such as liver, mammary gland, and lungs diseases as well as kidney failure in cattle, swine and domestic fowl (Li et al. Citation2015; Tang et al. Citation2018; Guo et al. Citation2021; Li et al. Citation2023). In poultry, oxidative stress causes skeletal muscle damage, resulting in reduced meat quality and productivity (Akbarian et al. Citation2016; Chen et al. Citation2022; ).
Table 1. Impact of hydrogen peroxide (H2O2) exposure on the quality of broiler breast meat (adapted from Chen et al. Citation2022).
Redox imbalance can also have detrimental effects on immune function, rendering animals more vulnerable to infections and diseases. Although white blood cells, including neutrophils and macrophages, generate ROS during phagocytosis, excessive free radicals’ production can cause damage to these immune cells, thereby diminishing their capacity to identify and combat pathogens (Tavassolifar et al. Citation2020; Wang and Xu Citation2022). Consequently, this can result in increased susceptibility to bacterial and viral infections, as well as delayed wound healing. Mastitis, a common udder infection in dairy cattle that can reduce milk production and quality, has been associated with oxidative stress (Laliotis et al. Citation2020).
In addition to tissue damage and impaired immune function, oxidative stress can contribute to the development of chronic diseases in humans and farm animals (Lykkesfeldt and Svendsen Citation2007; Avila-Escalante et al. Citation2020). ROS can promote the formation of cancerous cells and DNA mutations, leading to the development of tumours (Liou and Storz Citation2010). In pigs, oxidative stress has been linked to the development of gastric ulcers, a painful condition that can affect feed intake and growth (Suzuki et al. Citation2011; De Witte et al. Citation2018).
Animal behaviour and reproduction can also be impacted by redox imbalance. ROS induced inflammation can affect neurotransmitter function and lead to changes in behaviour such as increased aggression or reduced activity (Veit et al. Citation2021). In animals, oxidative stress has been shown to reduce reproductive success, as ROS can damage sperm and reduce fertilization rates (Li et al. Citation2022; Pintus and Ros-Santaella Citation2021).
Preventing and mitigating oxidative stress in livestock is important for promoting their well-being. Various approaches can be taken to achieve this, including providing a balanced diet that is rich in antioxidants, reducing exposure to environmental stressors, and ensuring adequate housing and management practices. Fat-soluble antioxidant vitamins, such as vitamins E, D and A, can be used to provide protection against oxidative damage (Ayemele et al. Citation2021).
To summarize, oxidative stress is a multifaceted condition that can have wide-range and long-term impacts on animal health and well-being. It can result in tissue damage, immune system dysfunction, and an increased risk of developing other diseases. Additionally, redox imbalance can affect animal behaviour and reproduction, further highlighting the importance of addressing this condition. Improved overall animal welfare can be promoted by preventing and mitigating oxidative stress.
Antioxidant activity of vitamin A
Recent research has suggested that the primary mediator of vitamin A’s antioxidant effects is its metabolite, all-trans-retinoic acid (ATRA). For example, at present, ATRA is the standard treatment for the management of acute promyelocytic leukemia (APL) in humans (Avvisati and Tallman Citation2003; Deng and Chen Citation2022). Studies have shown that APL cells have higher levels of ROS and decreased antioxidant capacity compared to healthy cells, resulting in increased oxidation rates (Chen et al. Citation2020). Therefore, targeting redox imbalance represents a good therapeutic strategy for the management of APL, and ATRA’s antioxidant properties make it a viable option for the treatment of this disease. Similarly, livestock production is often associated with significant oxidative stress due to various factors, including exposure to temperature-related stress, inferior feed quality, and disease status (Surai et al. Citation2019). Thus, the management of redox imbalance is not only important for the treatment of diseases like APL in humans, but it is also crucial in reducing oxidative stress in livestock production.
Retinol may possess direct antioxidant properties due to the presence of hydrophobic chains composed of polyene units. These polyene units have the ability to quench singlet oxygen, neutralize thiyl radicals, and stabilize peroxyl radicals (Landete Citation2013). However, vitamin A can undergo auto-oxidation when exposed to higher levels of oxygen, and is therefore most effective as antioxidant at physiological oxygen tensions found in tissues (Krinsky and Johnson Citation2005; Dao et al. Citation2017).
According to Palace et al. (Citation1999), vitamin A exhibits direct antioxidant activity through multiple mechanisms. Specifically, it functions as a chain-breaking antioxidant by reacting with peroxyl radicals, thereby preventing the propagation of lipid peroxidation in cells and the generation of hydroperoxides. Furthermore, retinol effectively scavenges peroxyl radicals, as evidenced by its ability to inhibit peroxidation in both homogeneous solutions of methyl linoleate and model phosphatidylcholine liposomes. Liposomes are useful as a model system because they can mimic the lipid bilayer structure of cell membranes. Ciaccio et al. (Citation1993) confirmed these findings in vivo using rat tissues. They observed that increasing the concentration of vitamin A within cell membranes improved the resistance of membrane lipids to endogenously produced peroxidation. Notably, when all-trans-retinol and α-tocopherol are simultaneously incorporated into unilamellar liposomes and peroxidation is induced by the hydrophylic 2,2′-azobis(2-amidinopropane)hydrochloride, a synergistic antioxidant effect is observed (Tesoriere et al. Citation1993). This suggests that all-trans-retinol may regenerate α-tocopherol by interacting with tocopheroxyl radicals. However, the effectiveness of this interaction may depend on molecular features and the relative location of the antioxidants in the bilayer. Despite the evidence supporting the antioxidant properties of retinol, the exact role of retinol in the body’s antioxidative network is not yet fully understood and requires further investigation.
ATRA is a potent transcriptional regulator that affects the expression levels of hundreds of genes through retinoic acid response elements present within these cistrons (Blaner et al. Citation2021). Many of the genes regulated by ATRA are involved in antioxidant-related processes such as glutathione (GSH) metabolism, superoxide dismutase (SOD) activity, and the regulation of oxidative stress response pathways, indicating an indirect mode of action of this vitamin A metabolite compared to retinol (Teixeira et al. Citation1996; Malivindi et al. Citation2018).
GSH metabolism is known to play a critical role in the maintenance of cellular redox balance (Strutynska et al. Citation2023). ATRA has been shown to upregulate the expression of GSH-related genes, including glutathione peroxidase (GSH-PX) and GSH reductase, leading to enhanced cellular antioxidant defence mechanisms (Park et al. Citation2009; El Haddad et al. Citation2012; Brigelius-Flohé and Flohé Citation2020). Furthermore, ATRA has been found to increase SOD activity, which is a critical antioxidant enzyme that neutralizes superoxide radicals (Rao et al. Citation2010). This upregulation of SOD activity may be responsible for the observed antioxidant effects of ATRA in various cellular systems.
Additionally, ATRA has been shown to regulate oxidative stress by modulating the expression of several genes involved in oxidative stress pathways. For instance, a study conducted by Azzam et al. (Citation2022) demonstrated that ATRA downregulates the expression of NADPH oxidase genes, which encode key enzymes involved in the production of ROS, in rat liver cells. This resulted in attenuation of oxidative stress and an improvement in the antioxidant status of the hepatocytes.
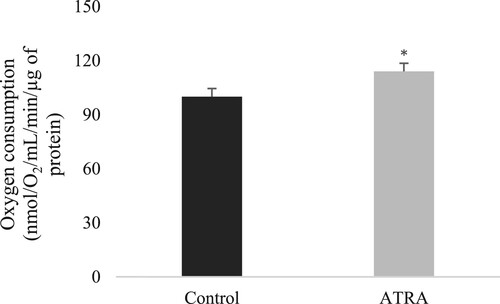
Moreover, several studies have demonstrated that ATRA can enhance mitochondrial antioxidant activity through various mechanisms (Tripathy et al. Citation2016). Tourniaire et al. (Citation2015) demonstrated that ATRA upregulates genes involved in mitochondrial biogenesis and antioxidant defence, resulting in an increase in oxidative phosphorylation capacity and mitochondrial content. These changes in mitochondrial function are likely to have significant implications for the regulation of oxidative stress ().
Figure 1. Oxygen consumption in adipocytes exposed to 2 µM of ATRA was measured using Clarke’s electrode (adapted from Tourniaire et al. Citation2015). Control refers to control cells, which received the vehicle (dimethyl sulfoxide). Data are the mean ± SEM of three independent cultures per treatment condition. The assessment compared ATRA-treated cells to untreated cells and measured their oxygen consumption rates to determine if ATRA-induced gene expression changes altered cellular metabolism. ATRA increased oxygen consumption by 15% (*P < 0.05).
Finally, ATRA has been shown to induce autophagy, a cellular process that enables cells to survive in conditions of nutrient depletion or oxidative stress (Rajawat et al. Citation2010; Choi et al. Citation2013). According to Rajawat et al. (Citation2010), ATRA promotes the maturation of autophagosomes through a pathway that does not involve the classic nuclear retinoid receptors. Retinoic acid causes a redistribution of a receptor called cation-independent mannose-6-phosphate receptor from the trans-Golgi region to the maturing autophagosomal structures, which induces their acidification. Autophagy has been implicated in the regulation of redox imbalance and the maintenance of cellular homeostasis.
Taken together, these findings suggest that ATRA plays a crucial role in the regulation of antioxidant-related genes and pathways, leading to enhanced antioxidant capacity and protection against oxidative stress-induced damage. Despite some evidence suggesting that retinol may function as a direct antioxidant by scavenging ROS, its overall mechanism of action as a free radical quencher remains unclear and further investigation is needed.
Vitamin A’s main antioxidant properties: direct or indirect?
The topic of whether vitamin A acts as a direct or indirect antioxidant has been a subject of debate for a considerable period. Some researchers suggest that vitamin A has direct antioxidant properties, as it can scavenge free radicals (Tesoriere et al. Citation1993; Palace et al. Citation1999; Landete Citation2013). However, others propose that its antioxidant effects are mainly indirect, as it regulates gene expression and encourages the production of other antioxidants (Rajawat et al. Citation2010; El Haddad et al. Citation2012; Brigelius-Flohé and Flohé Citation2020; Azzam et al. Citation2022).
Direct antioxidant properties refer to a substance’s capability to neutralize free radicals by providing electrons or hydrogen atoms to these unstable molecules (Lobo et al. Citation2010). Research indicates that vitamin A can function as a direct antioxidant, mainly in its retinol form (Ciaccio et al. Citation1993; Tesoriere et al. Citation1993). Retinol has been shown to scavenge free radicals both in vitro and in vivo, lowering oxidative stress and safeguarding against cellular damage.
In contrast, indirect antioxidant properties pertain to a substance’s ability to increase the body’s antioxidant enzyme production, such as GSH, SOD, and catalase (CAT) (Treml et al. Citation2021). Vitamin A has been proven to upregulate the expression of these antioxidant enzymes, therefore improving the body’s defence against oxidative stress (Park et al. Citation2009; Malivindi et al. Citation2018).
A group of scientists has recently indicated that vitamin A is not a direct radical quencher (Bohn et al. Citation2022). Instead, it has been suggested that vitamin A mainly functions as an indirect antioxidant by transcriptionally regulating genes that play a role in the body’s canonical antioxidant responses (Blaner et al. Citation2021).
Ultimately, it is clear that vitamin A possesses multifaceted antioxidant properties. While certain studies indicate that vitamin A has the ability to directly scavenge free radicals, others suggest that its antioxidant effects are primarily indirect. In addition, it is important to consider vitamin A’s role in regulating gene expression and promoting the synthesis of other antioxidants, which ultimately aids in protecting cells from oxidative damage. However, further research is necessary to thoroughly comprehend the mechanisms underlying vitamin A’s antioxidant properties and to identify optimal strategies for exploiting these benefits for therapeutic purposes in animal production.
Vitamin A status and oxidative stress
Vitamin A is widely recognized as an essential micronutrient for promoting optimal health, growth, and development in mammals and birds. While its important functions have been extensively studied, recent investigations in swine, cattle, and poultry have uncovered evidence suggesting that vitamin A status may exert a significant influence on oxidative stress within animal production systems (Kucuk et al. Citation2003; Ma et al. Citation2005; Jin et al. Citation2014; Hu et al. Citation2020; Zhou et al. Citation2021). This finding has important implications for various aspects of animal performance, including production efficiency, animal welfare, and the quality of food products.
In a study by Liang et al. (Citation2019), the effect of dietary vitamin A supplementation on dam and offspring was investigated in terms of early growth performance, antioxidant index, and tissue vitamin A content of goslings. The study found that supplementation with 9,000 IU/kg of vitamin A significantly increased the antioxidant enzymes GSH-PX, SOD, total antioxidant capacity (T-AOC), CAT, and tissue retinol content, and decreased malondialdehyde (MDA) levels in offspring (P < 0.05), compared to the no vitamin A supplemented group (). Maternal vitamin A levels also had a significant impact on offspring GSH, GSH-PX, SOD, MDA, T-AOC, and CAT (P < 0.05). Maternal and offspring vitamin A supplementation interacted with weight gain, tissue retinol content, GSH, GSH-PX, SOD, MDA, and CAT of goslings (P < 0.05). Overall, maternal supplementation with 12,000 IU/kg vitamin A and offspring supplementation with 9,000 IU/kg vitamin A were found to be beneficial for gosling growth. These findings highlight the importance of reducing oxidative stress and maintaining optimal antioxidant status through vitamin A supplementation in offspring and maternal geese.
Table 2. Effect of various vitamin A levels in maternal and offspring diets on liver antioxidant index of goslings at one day old and seven days old* (adapted from Liang et al. Citation2019).
Animal tissues are known to be vulnerable to oxidative stress induced by environmental stressors. Kucuk et al. (Citation2003) conducted a study that showed significant improvements in the performance of heat-stressed broiler chickens and a decrease in blood serum levels of MDA with the addition of 15,000 IU of vitamin A per kg of feed compared to the low vitamin A group. MDA is a primary marker of lipid peroxidation and measuring its levels in the blood can provide valuable information about the extent of oxidative stress in the body. Lower MDA levels in tissues indicate lower oxidative damage.
Hu et al. (Citation2020) conducted a study to investigate how gelatin and starch-encapsulated vitamin A affect the growth performance and antioxidant capacity in weaned piglets. The findings indicate that supplementing the diet with retinyl acetate led to an improvement in both antioxidant capacity and growth performance.
Similar findings were reported by Zhou et al. (Citation2021), where their study showed that two different sources of vitamin A significantly increased the antioxidant capacity of piglets between day 1 and 36 after weaning. This was demonstrated by a decrease in the level of MDA and an increase in the level of GSH-PX in the blood serum (). The study findings suggest that vitamin A supplementation has a positive effect on lipid peroxidation.
Table 3. The effects of two different sources of vitamin A on serum biochemical parameters of weaned piglets on day 36 of the study* (adapted from Zhou et al. Citation2021).
Shi et al. (Citation2018) conducted a study to investigate the protective effects of ATRA against lipopolysaccharide (LPS)-induced oxidative stress on bovine mammary epithelial cells (BMECs). BMECs were divided into four groups and treated for 30 h: control, ATRA alone, LPS alone, and ATRA plus LPS. The results showed that LPS treatment led to a significant increase in the concentration of inflammatory cytokines, the gene expression of NF-kBp50 and NF-kBp65, and the levels of intracellular ROS and MDA. However, combining ATRA with LPS improved the antioxidant enzyme activities of selenoproteins, indicating that ATRA has the potential to counteract the immunosuppressive condition of BMECs’ oxidative damage and improve antioxidative function. While the authors suggested that ATRA can be used in feed as a therapeutic agent, further research is needed to determine the safety and efficacy of dietary supplementation with ATRA in vivo.
ROS play a critical role in the functioning of the immune system, but their excessive production can lead to oxidative stress and immune dysregulation (Tavassolifar et al. Citation2020; Wang and Xu Citation2022). To maintain proper immune function and prevent oxidative damage, the body requires adequate levels of antioxidants (Ponnampalam et al. Citation2022). Studies have demonstrated that fat-soluble vitamins, particularly vitamins E and A, can enhance immune responses in individuals exposed to environmental sources of free radicals (Hajian Citation2015). These findings suggest that dietary supplementation with retinol may help to mitigate the negative impact of oxidative stress on immune function.
According to Jin et al. (Citation2014), supplementing dairy cows with vitamin A at twice the amount recommended by the NRC (Citation2001) resulted in significant improvements in immunoglobulins A, M, and G, interleukin-1, GSH-PX, SOD, CAT activities, T-AOC, and the ability to inhibit hydroxyl radicals. The authors also observed a significant reduction in the somatic cell count in milk, soluble CD8, MDA, and ROS in the group supplemented with increased amounts of vitamin A (). These findings suggest that vitamin A supplementation has a beneficial effect on oxidative stress and immune function in dairy cows.
Table 4. Effect of higher vitamin A supplementation on immune and antioxidant function in dairy cows fed experimental diets for 60 d (adapted from Jin et al. Citation2014).
Ma et al. (Citation2005) conducted a study which investigated the effects of vitamin A supplementation on beef cattle. The study found that supplementing beef cattle with 3300 or 4400 IU of vitamin A per kg of dry matter (DM) resulted in a significant increase in GSH-PX and SOD activities in serum, compared to supplementation with 1100 or 2200 IU of vitamin A per kg of DM. Additionally, increasing the supplementation levels to 4400 and 5500 IU/kg DM significantly reduced MDA levels in blood serum. Based on these findings, the authors recommended a vitamin A supplementation level of 3300 IU/kg DM intake for beef cattle. However, to achieve optimal levels of antioxidative protection, an intake of 5500 IU of vitamin A per kg of DM may be necessary.
Thus, vitamin A supplementation has been shown to improve antioxidant capacity and mitigate the negative impact of oxidative stress on immune function in various animals, highlighting the importance of maintaining optimal antioxidant status through vitamin A supplementation. Nonetheless, further research is necessary to determine the adequate dosage and potential risks associated with high levels of supplementation.
Practical implications and future directions
The relationship between vitamin A status and oxidative stress in animal husbandry has important practical implications for livestock health and management. One practical consequence is that improving vitamin A status in animals may help to prevent or mitigate the negative effects of oxidative stress on animal health and production (Jin et al. Citation2014; Liang et al. Citation2019). This can be achieved through dietary interventions, such as adjusting feed formulations to include the appropriate levels of vitamin A and adding other antioxidant vitamins to livestock diets. Additionally, it may also involve improving animal management practices to reduce stress and environmental factors that can contribute to oxidative stress. Cattle, swine and domestic fowl with adequate vitamin A status and optimal environmental conditions are likely to have improved growth rates, reproductive performance, and immune function. Furthermore, reducing oxidative stress help in preventing damage to cells and tissues, leading to improved overall well-being.
Another practical implication is that vitamin A status may serve as a potential useful biomarker for assessing redox imbalance in livestock. Measuring vitamin A levels in animal blood samples by means of HPLC with UV detection could provide a quick and easy way to assess oxidative stress and identify animals that may be at increased risk of health problems (Jansen and Ruskovska Citation2015).
Future investigations could explore additional mechanisms by which vitamin A may influence oxidative stress and animal health. For instance, research could examine the role of other antioxidant systems, beyond GSH and SOD, in mediating the effects of vitamin A on redox imbalance. Moreover, subsequent studies could investigate the potential for vitamin A supplementation to improve animal health and production outcomes, such as growth rates and meat quality.
Some studies have indicated that administering high levels of vitamin A supplementation to rats during aerobic exercise could potentially elevate oxidative stress, which may have adverse effects on their health (Gasparotto et al. Citation2015; Petiz et al. Citation2017). Consequently, there is a need for further research to gain a better understanding of the association between vitamin A and oxidative stress in living organisms. Hence, it is crucial to strike a balance between the advantages of vitamin A and its potential risks by ensuring animals are given appropriate doses of the vitamin in their diet.
Notably, hypervitaminosis A, a condition caused by excessive intake of retinol, is extremely rare in livestock under normal circumstances. This is because the levels of vitamin A in animal feed are usually well regulated. Although hypervitaminosis A can be induced in livestock under certain experimental conditions, it is highly unlikely to occur in practical farming or husbandry scenarios. Nevertheless, it is important to consider the local legal regulations when using vitamin A in practice, and any application should always be conducted in accordance with the legal provisions that are applicable to the specific region or country.
In conclusion, vitamin A status and oxidative stress are closely intertwined in animal production. Vitamin A deficiency can increase oxidative stress and cause health issues. By implementing dietary interventions and improving animal management practices to maintain adequate vitamin A status, animal producers can significantly benefit livestock health and welfare. It is essential to ensure that animals have sufficient vitamin A status to prevent health problems and improve productivity.
Conclusions
In conclusion, the studies highlighted in this review suggest that maintaining optimal vitamin A status through dietary supplementation may play a significant role in reducing oxidative stress and promoting immune function in animal production systems. Vitamin A supplementation has been shown to improve performance, antioxidant capacity, and growth in various animal species, including swine, cattle, and domestic fowl. Additionally, higher levels of vitamin A intake have been associated with a reduction in markers of lipid peroxidation and improvements in immune function. These findings have important implications for animal performance, including the production of high-quality food products and the promotion of animal welfare. Therefore, it is essential to consider the role of vitamin A in animal nutrition and incorporate appropriate supplementation strategies to maintain optimal vitamin A status in livestock production systems.
Acknowledgements
Ute Obermueller-Jevic, PhD is thanked for constructive comments on the draft of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors. However, it is worth mentioning that all three authors are affiliated with BASF, a company that produces vitamins and carotenoids, including vitamin A.
References
- Akbarian A, Michiels J, Degroote J, Majdeddin M, Golian A, De Smet S. 2016. Association between heat stress and oxidative stress in poultry; mitochondrial dysfunction and dietary interventions with phytochemicals. J Anim Sci Biotechnol. 7:37. doi:10.1186/s40104-016-0097-5.
- Avila-Escalante ML, Coop-Gamas F, Cervantes-Rodriguez M, Mendez-Iturbide D, Aranda-Gonzalez II. 2020. The effect of diet on oxidative stress and metabolic diseases-clinically controlled trials. J Food Biochem. 44:e13191. doi:10.1111/jfbc.13191.
- Avvisati G, Tallman MS. 2003. All-trans retinoic acid in acute promyelocytic leukaemia. Best Pract Res Clin Haematol. 16:419–432. doi:10.1016/S1521-6926(03)00057-4.
- Ayemele AG, Tilahun M, Lingling S, Elsaadawy SA, Guo Z, Zhao G, Xu J, Bu D. 2021. Oxidative stress in dairy cows: Insights into the mechanistic mode of actions and mitigating strategies. Antioxidants (Basel). 10(12):1918. doi:10.3390/antiox10121918.
- Azzam MM, Hussein AM, Marghani BH, Barakat NM, Khedr MMM, Heakel NA. 2022. Retinoic acid potentiates the therapeutic efficiency of bone marrow-derived mesenchymal stem cells (BM-MSCs) against cisplatin-induced hepatotoxicity in rats. Sci Pharm. 90:58. doi:10.3390/scipharm90040058.
- Blaner WS, Shmarakov IO, Traber MG. 2021. Vitamin A and vitamin E: Will the real antioxidant please stand up? Annu Rev Nutr. 41:105–131. doi:10.1146/annurev-nutr-082018-124228.
- Bohn T, Böhm V, Dulińska-Litewka J, Landrier J-F, Bánáti D, Kucuk O, Borel P, Canas JA, Rühl R. 2022. Is vitamin A an antioxidant? Int J Vitam Nutr Res. doi:10.1024/0300-9831/a000752.
- Brigelius-Flohé R, Flohé L. 2020. Regulatory phenomena in the glutathione peroxidase superfamily. Antioxid Redox Signal. 33(7):498–516. doi:10.1089/ars.2019.7905.
- Carazo A, Macákova K, Matoušová K, Krčmová K, Protti M, Mladěnka P. 2021. Vitamin A update: forms, sources, kinetics, detection, function, deficiency, therapeutic use and toxicity. Nutrients. 13:1–36. doi:10.3390/nu13051703.
- Celi P, Gabai G. 2015. Oxidant/antioxidant balance in animal nutrition and health: the role of protein oxidation. Front Vet Sci. 2:48. doi:10.3389/fvets.2015.00048.
- Checa J, Aran JM. 2020. Reactive oxygen species: drivers of physiological and pathological processes. J Inflamm Res. 13:1057–1073. doi:10.2147/JIR.S275595.
- Chen Y, Liang Y, Luo X, Hu Q. 2020. Oxidative resistance of leukemic stem cells and oxidative damage to hematopoietic stem cells under pro-oxidative therapy. Cell Death Dis. 11(4):291. doi:10.1038/s41419-020-2488-y.
- Chen Z, Xing T, Li J, Zhang L, Jiang Y, Gao F. 2022. Oxidative stress impairs the meat quality of broiler by damaging mitochondrial function, affecting calcium metabolism and leading to ferroptosis. Anim Biosci. 35(10):1616–1627. doi:10.5713/ab.22.0019.
- Choi AM, Ryter SW, Levine B. 2013. Autophagy in human health and disease. N Engl J Med. 368:1845–1846. doi:10.1056/NEJMc1303158.
- Ciaccio M, Valenza M, Tesoriere L, Bongiorno A, Albiero R, Livrea MA. 1993. Vitamin A inhibits doxorubicin-induced membrane lipid peroxidation in rat tissues in vivo. Arch Biochem Biophys. 302(1):103–108. doi:10.1006/abbi.1993.1186.
- Colitti M, Stefanon B, Gabai G, Gelain ME, Bonsembiante F. 2019. Oxidative stress and nutraceuticals in the modulation of the immune function: current knowledge in animals of veterinary interest. Antioxidants (Basel). 8(1):28. doi:10.3390/antiox8010028.
- Conaway HH, Henning P, Lerner UH. 2013. Vitamin A metabolism, action, and role in skeletal homeostasis. Endocr Rev. 34(6):766–797. doi:10.1210/er.2012-1071.
- Dao DQ, Ngo TC, Thong NM, Nam PC. 2017. Is vitamin A an antioxidant or a pro-oxidant? J Phys Chem B. 121(40):9348–9357. doi:10.1021/acs.jpcb.7b07065.
- Darroch CS. 2000. Vitamin A. In: AJ Lewis, LL Southern, editor. Swine nutrition. New York: CRC Press; p. 263–280.
- Das NP. 1989. Effects of vitamin A and its analogs on nonenzymatic lipid peroxidation in rat brain mitochondria. J Neurochem. 52:585–588. doi:10.1111/j.1471-4159.1989.tb09159.x.
- De La Riva A, Saldaña TLA, González-Hernández JC. 2022. Assessment on oxidative stress in animals: from experimental models to animal production. Importance of oxidative stress and antioxidant system in health and disease. IntechOpen. doi:10.5772/intechopen.109043.
- Deng Q, Chen J. 2022. Potential therapeutic effect of all-trans retinoic acid on atherosclerosis. Biomolecules. 12(7):869. doi:10.3390/biom12070869.
- De Witte C, Ducatelle R, Haesebrouck F. 2018. The role of infectious agents in the development of porcine gastric ulceration. Vet J. 236:56–61. doi:10.1016/j.tvjl.2018.04.015.
- Dickson KB, Zhou J. 2020. Role of reactive oxygen species and iron in host defense against infection. Front Biosci (Landmark Ed). 25(8):1600–1616. doi:10.2741/4869.
- Dunn JD, Alvarez LA, Zhang X, Soldati T. 2015. Reactive oxygen species and mitochondria: a nexus of cellular homeostasis. Redox Biol. 6:472–485. doi:10.1016/j.redox.2015.09.005.
- El Haddad M, Jean E, Turki A, Hugon G, Vernus B, Bonnieu A, Passerieux E, Hamade A, Mercier J, Laoudj-Chenivesse D, Carnac G. 2012. Glutathione peroxidase 3, a new retinoid target gene, is crucial for human skeletal muscle precursor cell survival. J Cell Sci. 125(24):6147–6156. doi:10.1242/jcs.115220.
- Gad A, Abu Hamed S, Khalifa M, Amin A, El-Sayed A, Swiefy SA, El-Assal S. 2018. Retinoic acid improves maturation rate and upregulates the expression of antioxidant-related genes in in vitro matured buffalo (Bubalus bubalis) oocytes. Int J Vet Sci Med. 15:279–285. doi:10.1016/j.ijvsm.2018.09.003.
- Gasparotto J, Petiz LL, Girardi CS, Bortolin RC, de Vargas AR, Henkin BS, Chaves PR, Roncato S, Matté C, Zanotto-Filho A, et al. 2015. Supplementation with vitamin A enhances oxidative stress in the lungs of rats submitted to aerobic exercise. Appl Physiol Nutr Metab. 40(12):1253–1261. doi:10.1139/apnm-2015-0218.
- Green AS, Fascetti AJ. 2016. Meeting the vitamin A requirement: the efficacy and importance of β-carotene in animal species. Sci World J. 2016:7393620. doi:10.1155/2016/7393620.
- Guo Z, Gao S, Ouyang J, Ma L, Bu D. 2021. Impacts of heat stress-induced oxidative stress on the milk protein biosynthesis of dairy cows. Animals (Basel). 11(3):726. doi:10.3390/ani11030726.
- Hajian S. 2015. Positive effect of antioxidants on immune system. Immunopathol Persa. 1:e02.
- Hao Y, Xing M, Gu X. 2021. Research progress on oxidative stress and its nutritional regulation strategies in pigs. Animals (Basel). 11:1384. doi:10.3390/ani11051384.
- Hu Y, Zhang L, Zhang Y, Xiong H, Wang F, Wang Y, Lu Z. 2020. Effects of starch and gelatin encapsulated vitamin A on growth performance, immune status and antioxidant capacity in weaned piglets. Anim Nutr. 6:130–133. doi:10.1016/j.aninu.2020.01.005.
- Jansen E, Ruskovska T. 2015. Serum biomarkers of (anti)oxidant status for epidemiological studies. Int J Mol Sci. 16(11):27378–27390. doi:10.3390/ijms161126032.
- Jin L, Yan S, Shi B, Bao H, Gong J, Guo X, Li J. 2014. Effects of vitamin A on the milk performance, antioxidant functions and immune functions of dairy cows. Anim Feed Sci Technol. 192:15–23. doi:10.1016/j.anifeedsci.2014.03.003.
- Krinsky NI, Johnson EJ. 2005. Carotenoid actions and their relation to health and disease. Mol Asp Med. 26:459–516. doi:10.1016/j.mam.2005.10.001.
- Kucuk O, Sahin N, Sahin K. 2003. Supplemental zinc and vitamin A can alleviate negative effects of heat stress in broiler chickens. Biol Trace Elem Res. 94:225–236. doi:10.1385/BTER:94:3:225.
- Laliotis GP, Koutsouli P, Sotirakoglou K, Savoini G, Politis I. 2020. Association of oxidative stress biomarkers and clinical mastitis incidence in dairy cows during the periparturient period. J Vet Res. 64(3):421–425. doi:10.2478/jvetres-2020-0053.
- Landete JM. 2013. Dietary intake of natural antioxidants: vitamins and polyphenols. Crit Rev Food Sci Nutr. 53(7):706–721. doi:10.1080/10408398.2011.555018.
- Li D, Shen L, Zhang D, Wang X, Wang Q, Qin W, Gao Y, Li X. 2023. Ammonia-induced oxidative stress triggered proinflammatory response and apoptosis in pig lungs. J Environ Sci. 126:683–696. doi:10.1016/j.jes.2022.05.005.
- Li Q, Yang S, Chen F, Guan W, Zhang S. 2022. Nutritional strategies to alleviate oxidative stress in sows. Anim Nutr. 9:60–73. doi:10.1016/j.aninu.2021.10.006.
- Li S, Tan H-Y, Wang N, Zhang Z-J, Lao L, Wong C-W, Feng Y. 2015. The role of oxidative stress and antioxidants in liver diseases. Int J Mol Sci. 16(11):26087–26124. doi:10.3390/ijms161125942.
- Liang JR, Dai H, Yang HM, Yang Z, Wang ZY. 2019. The effect of dietary vitamin A supplementation in maternal and its offspring on the early growth performance, liver vitamin A content, and antioxidant index of goslings. Poult Sci. 98:6849–6856. doi:10.3382/ps/pez432.
- Liou GY, Storz P. 2010. Reactive oxygen species in cancer. Free Radic Res. 44(5):479–496. doi:10.3109/10715761003667554.
- Lobo V, Patil A, Phatak A, Chandra N. 2010. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev. 4(8):118–126. doi:10.4103/0973-7847.70902.
- Lykkesfeldt J, Svendsen O. 2007. Oxidants and antioxidants in disease: oxidative stress in farm animals. Vet. J. 173(3):502–511. doi:10.1016/j.tvjl.2006.06.005.
- Ma XM, Yang ZB, Yang WR, Song ML. 2005. Effect of different vitamin A levels in diets on antioxidant ability of beef cattle. Chin J Anim Nutr. 17:31–35.
- Malivindi R, Rago V, De Rose D, Gervasi MC, Cione E, Russo G, Santoro M, Aquila S. 2018. Influence of all-trans retinoic acid on sperm metabolism and oxidative stress: its involvement in the physiopathology of varicocele-associated male infertility. J Cell Physiol. 233(12):9526–9537. doi:10.1002/jcp.26872.
- Miazek K, Beton K, Śliwińska A, Brożek-Płuska B. 2022. The effect of β-carotene, tocopherols and ascorbic acid as anti-oxidant molecules on human and animal in vitro/in vivo studies: a review of research design and analytical techniques used. Biomolecules. 12(8):1087. doi:10.3390/biom12081087.
- Monaghan BR, Schmitt FO. 1932. The effects of carotene and of vitamin A on the oxidation of linoleic acid. J Biol Chem. 96(2):387–395. doi:10.1016/S0021-9258(18)76278-9.
- NRC (National Research Council). 2001. Nutrient requirements of dairy cattle. 7th ed. Washington (DC): National Academy of Sciences, USA.
- Palace VP, Khaper N, Qin Q, Singal PK. 1999. Antioxidant potentials of vitamin A and carotenoids and their relevance to heart disease. Free Radic Biol Med. 26(5–6):746–761. doi:10.1016/S0891-5849(98)00266-4.
- Park U-H, Han HS, Um E, An X-H, Kim E-J, Um SJ. 2009. Redox regulation of transcriptional activity of retinoic acid receptor by thioredoxin glutathione reductase (TGR). Biochem Biophys Res Commun. 390(2):241–246. doi:10.1016/j.bbrc.2009.09.097.
- Petiz LL, Girardi CS, Bortolin RC, Kunzler A, Gasparotto J, Rabelo TK, Matté C, Moreira JC, Gelain DP. 2017. Vitamin A oral supplementation induces oxidative stress and suppresses IL-10 and HSP70 in skeletal muscle of trained rats. Nutrients. 9(4):353. doi:10.3390/nu9040353.
- Pintus E, Ros-Santaella LJ. 2021. Impact of oxidative stress on male reproduction in domestic and wild animals. Antioxidants. 10(7):1154. doi:10.3390/antiox10071154.
- Pizzino G, Irrera N, Cucinotta M, Pallio G, Mannino F, Arcoraci V, Squadrito F, Altavilla D, Bitto A. 2017. Oxidative stress: harms and benefits for human health. Oxid Med Cell Longev. 2017:8416763. doi:10.1155/2017/8416763.
- Ponnampalam EN, Kiani A, Santhiravel S, Holman BWB, Lauridsen C, Dunshea FR. 2022. The importance of dietary antioxidants on oxidative stress, meat and milk production, and their preservative aspects in farm animals: antioxidant action, animal health, and product quality-invited review. Animals (Basel). 12(23):3279. doi:10.3390/ani12233279.
- Rajawat Y, Hilioti Z, Bossis I. 2010. Autophagy: a target for retinoic acids. Autophagy. 6(8):1224–1226. doi:10.4161/auto.6.8.13793.
- Rao J, Zhang C, Wang P, Lu L, Zhang F. 2010. All-trans retinoic acid alleviates hepatic ischemia/reperfusion injury by enhancing manganese superoxide dismutase in rats. Biol Pharm Bull. 33:869–875. doi:10.1248/bpb.33.869.
- Shi H, Yan S, Guo Y, Shi B, Guo X. 2018. The pre-protective effect of vitamin A on LPS-induced oxidative stress of bovine mammary epithelial cells. Ital J Anim Sci. 17(4):959–966. doi:10.1080/1828051X.2018.1453757.
- Solomons NW. 1994. Micronurients, antioxidants and general mechanisms of disease. In: A Sastre, M Serrano-Rios, editor. Dairy products in human health and nutrition. Netherlands: Taylor & Francis; p. 343–347.
- Strutynska N, Goshovska Y, Mys L, Strutynskyi R, Luchkova A, Fedichkina R, Okhai I, Korkach Y, Sagach V. 2023. Glutathione restores the mitochondrial redox status and improves the function of the cardiovascular system in old rats. Front Physiol. 13:1093388. doi:10.3389/fphys.2022.1093388.
- Surai PF, Kochish II, Fisinin VI, Juniper DT. 2019. Revisiting oxidative stress and the use of organic selenium in dairy cow nutrition. Animals (Basel). 9(7):462. doi:10.3390/ani9070462.
- Suzuki H, Nishizawa T, Tsugawa H, Mogami S, Hibi T. 2011. Roles of oxidative stress in stomach disorders. J Clin Biochem Nutr. 50(1):35–39. doi:10.3164/jcbn.11-115SR.
- Tang S, Zhou S, Yin B, Xu J, Di L, Zhang J, Bao E. 2018. Heat stress-induced renal damage in poultry and the protective effects of HSP60 and HSP47. Cell Stress Chaperones. 23(5):1033–1040. doi:10.1007/s12192-018-0912-3.
- Tavassolifar MJ, Vodjgani M, Salehi Z, Izad M. 2020. The influence of reactive oxygen species in the immune system and pathogenesis of multiple sclerosis. Autoimmune Dis. 2020:5793817. doi:10.1155/2020/5793817.
- Teixeira CC, Shapiro IM, Hatori M, Rajpurohit R, Koch C. 1996. Retinoic acid modulation of glutathione and cysteine metabolism in chondrocytes. Biochem J. 314:21–26. doi:10.1042/bj3140021.
- Tesoriere L, Ciaccio M, Bongiorno A, Riccio A, Pintaudi AM, Livrea MA. 1993. Antioxidant activity of all-trans-retinol in homogeneous solution and in phosphatidylcholine liposomes. Arch Biochem Biophys. 307(1):217–223. doi:10.1006/abbi.1993.1581.
- Tourniaire F, Musinovic H, Gouranton E, Astier J, Marcotorchino J, Arreguin A, Bernot D, Palou A, Bonet ML, Ribot J, Landrier JF. 2015. All-trans retinoic acid induces oxidative phosphorylation and mitochondria biogenesis in adipocytes. J Lipid Res. 56(6):1100–1109. doi:10.1194/jlr.M053652.
- Treml J, Večeřová P, Herczogová P, Šmejkal K. 2021. Direct and indirect antioxidant effects of selected plant phenolics in cell-based assays. Molecules. 26(9):2534. doi:10.3390/molecules26092534.
- Tripathy S, Chapman JD, Han CY, Hogarth CA, Arnold SL, Onken J, Kent T, Goodlett DR, Isoherranen N. 2016. All-trans-retinoic acid enhances mitochondrial function in models of human liver. Mol Pharmacol. 89(5):560–574. doi:10.1124/mol.116.103697.
- Veit C, Janczak AM, Ranheim B, Vas J, Valros A, Sandercock DA, Piepponen P, Dulgheriu D, Nordgreen J. 2021. The effect of LPS and ketoprofen on cytokines, brain monoamines, and social behavior in group-housed pigs. Front Vet Sci. 7:617634. doi:10.3389/fvets.2020.617634.
- Wang T, Xu H. 2022. Multi-faced roles of reactive oxygen species in anti-tumor T cell immune responses and combination immunotherapy. Explor Med. 3:77–98. doi:10.37349/emed.2022.00076.
- Zhong Y, Ma T, Fu Z, Chen A, Yu J, Huang Y, Fu J. 2023. Effects of hydrogen peroxide-induced oxidative stress on intestinal morphology, redox status, and related molecules in squabs. Animals (Basel). 13(4):749. doi:10.3390/ani13040749.
- Zhou HB, Huang XY, Bi Z, Hu YH, Wang FQ, Wang XX, Wang YZ, Lu ZQ. 2021. Vitamin A with L-ascorbic acid sodium salt improves the growth performance, immune function and antioxidant capacity of weaned pigs. Animal. 15:1751–7311.
