?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The effects of cadmium (Cd) and nickel (Ni) toxicity on embryonic and larval development of Hypophthalmichthys molitrix and Ctenopharyngodon idella was determined. Different concentrations (0.1, 0.3, and 0.5 mg/l) of Cd and Ni were administered in separate trials to the fish after spawning to 168 h post-hatching (hph) period. Cd was more toxic to the embryos of both fish, as highest embryonic mortality (%) of H. molitrix and C. idella was observed. However, Ni was found to be more toxic to the larvae of H. molitrix and C. Idella with highest larval mortality at 168 hph. However, Ni was found to be more toxic to the larvae of H. molitrix and C. idella with highest larval mortality at 168 hph. The study found a significant effect of heavy metal and metal concentration for causing deformities in H. molitrix. However, in C. idella only the effect of metal concentration on fish deformities was significant. Furthermore, Ni was found to cause more deformities as compared to Cd in H. molitrix. While Cd was found to cause more deformities as compared to Ni in C. idella. In conclusion, the study suggests that Cd and Ni may cause serious deformities in fish.
Introduction
The contamination of aquatic ecosystems with various trace elements is a worldwide issue, posing health risks to both marine life and humans (Naz et al. Citation2023) . Run-off from industrial and agricultural areas flows into rivers, introducing trace elements into the water, sediments, and plankton (Naz et al. Citation2022). Toxicants like heavy metals in water are considered as a major threat to aquatic creatures as well as public health. These toxins enter the food chain through water, microbes, aquatic plants and fish, and eventually into our bodies via consumption of water and foods (Abbas et al. Citation2019). Recent research has shown the deposition of different metals on the liver and kidneys of animals, particularly in restricted waters like the Mediterranean, and have highlighted the necessity for continuous scene monitoring. Exposure to heavy metals has been linked to substantial threats to human and environmental health as a result of human activities such as industrial and agricultural drainages (Green et al. Citation2010). Heavy metals often collect in both the soft and hard parts of fish due to bioaccumulation. The presence of these metals in fish can indicate their levels in water bodies. Moreover, these metals can be transferred from fish to the organisms that consume them in the food chain (Naz et al. Citation2023). Different heavy metals such as Cu, Zn, and Cd, among other hazardous heavy metals, are widely recognized to exist in high amounts in many aquatic ecosystems, causing harm to living animals (Meybeck et al. Citation2007; Naz et al. Citation2021).
Fish provide essential proteins and nutrients to various organisms including human. Yet, the rise in environmental pollution, primarily from industrial and agricultural growth, is leading to the increased presence of trace elements in these fish. Consequently, freshwater species accumulate these toxins in various organs, including the kidneys, liver, and muscles (Chatha et al. Citation2023). Fishes are regarded to be the most important biomonitors in aquatic systems for estimating metal pollution levels because they are very sensitive to the accumulation of trace elements and are big enough to analyse the concentration of various trace elements in their various organs (Lamas et al. Citation2007). Furthermore, because fish are at the bottom of the aquatic food chain, they may store metals and pass them on to humans through food, resulting in chronic or acute toxicity (Al-Yousuf et al. Citation2000). Although, other environmental factors like water temperature, oxygen, pH, hardness, salinity, and alkalinity may also affect and play significant roles in the accumulation and toxicity into fish, studies showed that accumulation of heavy metals in tissue is primarily dependent on metal concentrations in the water and exposure time (Jitar et al. Citation2015). Pollutants are preferentially accumulated in fatty tissues like the liver of fish, and the consequences are observed when concentrations in these tissues exceed a certain level (Khalil et al. Citation2016). This buildup, however, is contingent on their intake, storage, and excretion from the body (Abdallah and Elmagd Morsy Citation2013). This indicates that metals with a high absorption rate but poor elimination rate in fish tissues are likely to accumulate to greater levels (Idriss and Ahmad Citation2015).
Cadmium (Cd) is a common aquatic environmental contaminant linked to a wide range of human activities and products, including plastics, ceramics, glass, and automobile tires. In fish, heavy metals can function as endocrine disruptors; for example, cadmium has been shown to lower thyroid hormone levels (Buha et al. Citation2018). It reduces thyroid hormone production by altering iodine metabolism, inhibiting estrogen receptors and disrupting growth hormone expression (Nugegoda and Kibria Citation2017). Metal ions’ prooxidative characteristics may cause oxidative stress in fish and oxidative damage to cell membranes. Fish are also genotoxic to cadmium, copper, mercury, and lead (Cavas Citation2008). High concentrations of Cd in the environment pose a significant risk to the health and sustainable growth of the aquaculture sector, limiting the advancement of the food processing industry (Liu et al. Citation2022). Nickel (Ni) is primarily utilized in creating alloys, such as stainless steel, and its use in electric vehicle battery production is on the rise. The introduction of Ni into ecosystems is worrisome due to its potential toxicity to aquatic life (He et al. Citation2023). A low concentration of nickel in freshwater bodies tends to be less harmful. However, it might still induce morphological alterations or chromosomal irregularities within cells. When fish species are exposed to high concentrations of nickel in water bodies, it can be toxic to them (Naz et al. Citation2021).
Heavy metals have a profound impact on the embryonic and larval stages of fish development. They can lead to various complications, including elevated heart rate, diminished cardiac function, higher mortality rates, malformed shapes, and deformities of the vertebral column, among other issues at different stages of embryonic development (Taslima et al. Citation2022). During the whole fish life cycle, the embryonic and larval stages are often regarded to be the most vulnerable in terms of toxicity (Osman et al. Citation2007), and these abnormalities impair fish survival, development rate, well-being, and external morphology. For instance, the vertebral column (Sfakianakis et al. Citation2006), the swim bladder (Witeska et al. Citation2014), the cephalic region (Georgakopoulou et al. Citation2007), the fins, and the lateral line are some of the organs with the most prevalent malformations (Sfakianakis et al. Citation2015). The ones in the vertebral column, particularly lordosis (V-shaped dorsal–ventral curvature), kyphosis (-shaped dorsal–ventral curvature), and scoliosis, are the most common. Fish abnormalities (particularly skeletal ones) are significant because they impair the organism's capacity to interact with its surroundings. A notable example is the loss of swimming capacity (Sfakianakis et al. Citation2011), which is the most significant quality in carrying out life-sustaining activities. Heavy metals can interfere with a variety of metabolic processes in growing fish (particularly embryos), causing developmental delays, morphological and functional abnormalities, and even death. Furthermore, heavy metals stimulate energy-intensive detoxifying processes, allowing inebriated fish to utilize less energy for development. Most heavy metals investigations on developing fish (embryos or larvae) show significant rates of death, hatching delays, changed body form, and body abnormalities (Jezierska et al. Citation2009).
Materials and methods
Study site
The current study was conducted to explore the survival potential and Assessment of deformities in embryo and larvae of Chinese carps (Hypophthalmichthys molitrix and Ctenopharyngodon idella) under acute exposure of Cd and Ni. The study was conducted at the department of Zoology, Government Sadiq College Women University, Bahawalpur from February 2023 to May 2023. The fish species for spawning purposes were obtained from Fish Seed Hatchery, Bahawalpur, Punjab Pakistan.
Induced breeding
Chinese carps, H. molitrix with an average length: 61.61 ± 4.24 cm and average weight: 4.23 ± 1.05 kg and C. idella with an average length:78.61 ± 6.24 cm and average weight: 4.83 ± 1.35 kg were utilized as spawners in this experiment. Females and males were kept apart for 5 days before spawning. Female and male in a 2:1 ratio were used for spawning the day before eggs needed for experimentation. After breeding, the adult fish were removed and the eggs were retrieved with a nylon net with the mesh size of 0.5 mm. After 2 h of oviposition, eggs were shifted to a petri dish and were examined under microscope (Olympus B12). Non-fertilize eggs and inactive embryos were observed and removed. Only fertilized eggs with active embryo were selected for experiments.
Preparation of stock solution
Stock solutions of Cd and Ni were prepared separately by dissolving sufficient amounts of their respective analytical grade chlorides, namely CdCl2 and NiCl2 (99.99%, Sigma Aldrich, Germany) in double distilled deionized water.
Toxicity test on fish embryo
300 fertilized eggs (2 h post oviposition) of H. molitrix and C. idella were separated in 500 ml beaker with 250 ml dechlorinated water for cleaning. After cleaning, the embryos were exposed to different concentrations (0.1, 0.3, and 0.5 mg/l) of Cd and Ni, dissolved in 15 l distilled water separately in bath tubs (45 cm diameter and 30 cm height) for a period of 12 h under 25 ± 2°C temperature, 7.5 ± 0.58 pH, 600 ± 24 µS/cm electric conductivity, 178 ± 16 mg/dm3 as CaCO3 total hardness, 5.56 ± 0.27 mg/l dissolved oxygen. The bath tubs were preferred over fish aquarium to provide constant stirring of the water in a circular pattern to maintain the viability of fish eggs. Water was continuously stirred and aerated. In the control, the embryos were fully incubated in clean water. Three replicates and one control (without heavy metals) were included in this experiment. After 12 h, the embryos were observed under microscope and dead embryo were recoded for each concentration of Cd and Ni. Furthermore, the alive embryos were further studied for deformities caused by Cd and Ni toxicity.
Toxicity test on fish larvae
A total of 300 / concentration newly hatched larvae of H. molitrix and C. idella were exposed to different concentrations (0.1, 0.3, and 0.5 mg/l) of Cd and Ni dissolved in 15 l distilled water in bath tubs. The experimental conditions were same as described above. The experiment was conducted for a period of 168 h. During experiment, the mortality of fish larvae was observed and dead larvae were counted and removed at various stages (6, 30, 48, 72, 96, 144, 168 h) of larval development. After 168 h of exposure, the dead larvae were collected and preserved in ethanol solution for further study on embryonic abnormalities caused by heavy metal toxicity for 168 h exposure. There was one control treatment and three replications for each concentration of Cd as well as Ni.
Morphological observations
After 168 h of exposure, the percentage of abnormalities and type of abnormalities in old fish larvae were assessed. Larvae were photographed using a camera fitted to a light microscope (Olympus B12, 2.5×4 magnification). Body length (BL), body perimeter area excluding yolk sac, yolk-sac perimeter area, head perimeter area, tail curvature, and spinal cord defect were all measured. The ratio of body perimeter area to length was computed for newly hatched and 168-hour-old larvae to assess the impact of Cd and Ni on larval form. The deformed percentage of fish was estimated with the following equation.
where Dp = Deformed percentage (%), Dl = Deformed length (mm), Tl = Total length (mm).
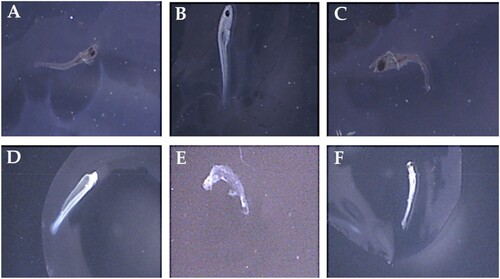
The total and deformed length of exposed larvae were measured using scale fitted in eye piece of microscope. Various deformities like lateral spine curvature-scoliosis, axial spine curvature-lordosis, cardiac enema, yolk-sac malformation, C-shaped body, and spine curvature-lordosis were observed in this study.
Statistical analysis
Various statistical analyses were carried out to explore the adverse effects of Cd and Ni on studies fish species. The mortality (%) of fish species during embryonic and larval period was analysed with one-way analysis of variance (ANOVA) and further explained with Tukey's range test using IBM SPSS (version 25). Survival probability of both fish species against Cd and Ni toxicity was carried out with Kaplan–Meier survival curve in R (version 4.3.1). Two-way ANOVA for the statistical significance of Cd and Ni exposure on deformities (%) in both fish species was carried out using IBM SPSS (version 25).
Results
As shown in , at the end of the experiment (168 h) post-hatching stage, the obtained results showed that the lowest mortality (% mean ± SE) during embryonic stage was observed in the control group; 6.67 ± 0.33 and 7.50 ± 0.29 for H. molitrix and C. idella, respectively. In H. molitrix, the maximum mortality (15.17 ± 0.44) as result of Cd toxicity was observed at 0.5 mg/l concentration followed by 0.3 mg/l and the lowest morality (10.33 ± 0.44) was found at 0.1 mg/l concentration of Cd exposure. Similarly, the maximum mortality (12.83 ± 0.33) as result of Ni toxicity was observed at 0.5 mg/l concentration followed by 0.3 mg/l and the lowest morality (9.17 ± 0.44) was found at 0.1 mg/l concentration of Ni exposure. Similar results were also found in C. idella, with the maximum mortality (16.33 ± 0.17) observed at 0.5 mg/l concentration of Cd, followed by 0.3 mg/l and the lowest morality (13.33 ± 0.44) was found at 0.1 mg/l concentration of Cd exposure. likewise, the maximum mortality (16.00 ± 0.29) as a result of Ni toxicity was observed at 0.5 mg/l concentration followed by 0.3 mg/l and the lowest morality (10.67 ± 0.33) was found at 0.1 mg/l concentration of Ni exposure. Overall highest mortality in fish embryo was caused by 0.5 mg/l concentration of Cd in C. idella while the lowest mortality was caused by 0.1 mg/l concentration of Ni in H. molitrix. The obtained results showed that C. idella embryo were more susceptible to heavy metal toxicity than H. molitrix.
Table 1. Percentage (%) mortality (Mean ± SE) among different developmental stages of H. molitrix and C. idella exposed to different concentrations (0.1, 0.3, and 0.5 mg/l) of Cd and Ni under laboratory conditions.
The results of larval exposure to different concentrations of Cd and Ni toxicity showed that the lowest mortality (% mean ± SE) during larval stage was observed in the control group (1.11 ± 0.31) and (1.30 ± 0.50) at 6 h post-hatching (hph) stage for H. molitrix and C. idella respectively. In H. molitrix, the maximum mortality (89.26 ± 3.70) as result of Cd toxicity was observed at 0.5 mg/l concentration at 168 hph stage and the lowest morality (1.92 ± 0.19) was found at 0.1 mg/l concentration of Cd exposure at 6 hph stage. Similarly, the maximum mortality (90.41 ± 3.90) as a result of Ni toxicity was observed at 0.5 mg/l concentration at 168 hph stage and the lowest morality (3.28 ± 0.21) was found at 0.1 mg/l concentration of Ni exposure at 6 hph stage. Similar results were also found in C. idella, with the maximum mortality (79.19 ± 4.41) observed at 0.5 mg/l concentration of Cd at 168 hph stage and the lowest morality (2.44 ± 1.22) was found at 0.1 mg/l concentration of Cd exposure at 6 hph stage. likewise, the maximum mortality (83.53 ± 5.89) as result of Ni toxicity was observed at 0.5 mg/l concentration at 168 hph stage and the lowest morality (0.59 ± 0.07) was found at 0.1 mg/l concentration of Ni exposure at 6 hph stage. Overall highest mortality in fish larvae was caused by 0.5 mg/l concentration of Ni at 168 hph stage in H. molitrix while the lowest mortality was caused by 0.1 mg/l concentration of Ni at 6 hph stage in C. idella. The obtained results showed that H. molitrix larvae were more susceptible to heavy metal toxicity than C. idella ().
The survival analysis of 168 hph stage larvae of H. molitrix and C. idella at various concentrations (0.1, 0.3, and 0.5 mg/l) of Cd and Ni was conducted. The obtained results showed that in H. molitrix, there was a significant difference (p < .00) in survival probability against different concentrations of Cd toxicity over 168 hph period. Furthermore, the highest survival probability (∼75%) of H. molitrix against Cd toxicity was found at 0.1 mg/l concentration of Cd and the lowest survival probability (∼10%) of H. molitrix against Cd toxicity was found at 0.5 mg/l concentration of Cd (Figure S1). Similarly, like Cd toxicity in H. molitrix, there was a significant difference (p < .00) in survival probability against different concentrations of Ni over 168 hph period of exposure. The highest survival probability (∼60%) of H. molitrix against Ni toxicity was found at 0.1 mg/l concentration of Ni and the lowest survival probability (∼10%) of H. molitrix against Ni toxicity was found at 0.5 mg/l concentration of Ni (Figure S2). Overall, in H. molitrix Ni had comparatively more adverse effect on its survival as compared to Cd. Like H. molitrix, there was a significant difference (p < .00) in survival probability in C. idella against different concentrations of Cd toxicity over 168 hph exposure duration. Moreover, the highest survival probability (∼65%) of C. idella against Cd toxicity was found at 0.1 mg/l concentration of Cd and the lowest survival probability (∼15%) of C. idella against Cd toxicity was found at 0.5 mg/l concentration of Cd (Figure S3). Likewise, there was a significant difference (p < .00) in survival probability in C. idella against different concentrations of Ni over 168 hph exposure period. The highest survival probability (∼80%) of C. idella against Ni toxicity was found at 0.1 mg/l concentration of Ni and the lowest survival probability (∼15%) of H. molitrix against Ni toxicity was found at 0.5 mg/l concentration of Ni. Overall, in C. idella Cd had more adverse effect for its survival as compared to Ni (Figure S4).
Two-way ANOVA was conducted to estimate deformities (%) caused by various concentrations of Cd and Ni at the end of 168 hph exposure duration. The results showed that there was a significant effect of type of heavy metal (df = 1, F = 15.149, p < .00) and heavy metal concentration (df = 2, F = 4.315, p = .018) on deformities caused in H. molitrix. However, the interaction of heavy metal and metal concentration had a non-significant effect (df = 2, F = 0.259, p = .773) on deformities caused in H. molitrix. On the other hand, there was a significant effect of heavy metal concentration (df = 2, F = 10.200, p < .00) on deformities caused in C. idella. However, the type of heavy metal (Cd or Ni) and the interaction of heavy metal and metal concentration had a non-significant effect on deformities caused in C. idella with p = .169 and .841, respectively (). The deformities (%) analysis showed that in H. molitrix Ni caused more deformities as compared to Cd. Furthermore, the most prominent deformities (%mean ± SE) were caused by 0.5 mg/l of Ni (62.12 ± 3.97) while the least deformities in H. molitrix were caused by 0.1 mg/l of Cd (28.57 ± 4.78) (). However, in C. idella Cd caused more deformities than Ni at all three concentrations. Moreover, the most prominent deformities (%±SE) were caused by 0.5 mg/l of Cd (47.12 ± 4.47) while the least deformities were caused by 0.1 mg/l of Cd (22.44 ± 2.26) in C. idella ().
Figure 1. Deformity (%) observed in H. molitrix (mean ± SE) when exposed to different doses of Cd and Ni toxicity for 168 hph.
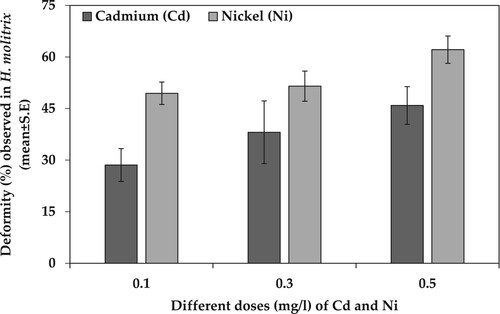
Figure 2. Deformity (%) observed in C. idella (mean ± SE) when exposed to different doses of Cd and Ni toxicity for 168 hph.
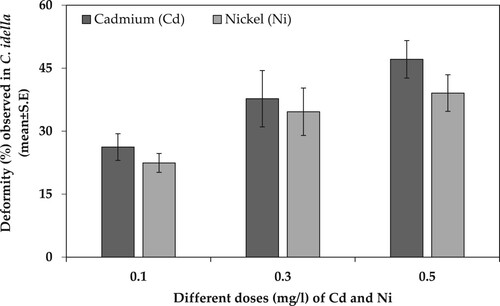
Table 2. Two-way analysis of variance (ANOVA) for percentage deformities caused by different concentrations (mg/l) of Cd and Ni in H. molitrix and C. idella under laboratory conditions.
Heavy metals (Cd and Ni) induced malformations that were observed in 168 hph larvae of in H. molitrix and C. idella. The exposure of Cd and Ni for 168 hph caused major abnormalities in fish morphology. Among these malformations, yolk-sac malformation was observed at all exposure concentration (0.1, 0.3, and 0.5 mg/l) of Cd and Ni. Similarly, spinal cord malformation like lateral spine curvature-scoliosis, axial spine curvature-lordosis, and spine curvature-lordosis spine were also very prominent during heavy metal toxicity of Cd and Ni. Furthermore, other deformities such as cardiac enema and C-shaped body were also found in various cases as a result of Cd and Ni toxicity for a period of 168 hph (). The obtained results clearly demonstrated that Cd and Ni are serious threat to fish embryo and larvae and it may induce mortality and deformities even at very low concentrations like 0.1 mg/l and at very short durations such as 168 h of exposure.
Discussion
The findings reveal that heavy metals (Cd and Ni) exposure to Chinese carp (Hypophthalmichthys molitrix and Ctenopharyngodon idella) embryos and larvae decreased the survival rate and had significant adverse effect on their development. This effect observed considerably more harmful by cadmium than nickel at embryonic stages and vice versa at larval stages. Numerous studies reported the adverse effects on the oncogenic development embryos and larvae by the single heavy metal because open water is contaminated with heavy metals from anthropogenic and geogenic sources. Therefore, it is necessary to evaluate the combined effects of heavy metals on embryonic and larval development of fish which are found in the natural environment. The early developmental phases of fish, especially embryos and larvae, are more vulnerable to contaminants like heavy metals compared to juveniles and adults. These stages are often utilized as biological markers to assess the harmful effects of these chemicals on aquatic life (Taslima et al. Citation2022). Based on the studies examining the combined effects of Cu–Zn and Cd–Zn by Kazlauskiene and Vosyliene (Citation2008) and El-Greisy and El-Gamal (Citation2015) in rainbow trout (Oncorhynchus mykiss) and common carp (Cyprinus carpio) embryos, respectively, and physical deformities (e.g. vertebral column deformities) and high embryonic mortality were revealed. On the other hand, Samson and Shenker (Citation2000) and Osman et al. (Citation2007) reported that mercury (Hg) and lead (Pb) toxicity resulted in defects of important organs of fish such as abnormal and irregular fins, head, tails and several spinal complications, respectively. In the present study, it was reported that control group had the lowest larval mortality at the end of the trial (on 168 h) post-hatching stage, whereas the Cd group had the greatest mortality at the embryonic stages before hatching, while after hatching, Ni had more significant adverse effects. Studies revealed that larvae are less tolerant to heavy metals than the embryonic stage of fish because the embryo carries protective hard chorion layers and perivitelline fluid that can hinder the entry of heavy metals in their body (Mhadhbi et al. Citation2010; Kong et al. Citation2013). Hypophthalmichthys molitrix mortality was also detected in Ni but at a lower rate than cadmium metal exposure during embryonic stages. Studies reported that heavy metals cause toxicity in early stages of fish due to their persisted and non-biodegradable properties in the natural environment. But somehow different developmental stages of fish respond differently to the intoxication, vary from species to species, type of heavy metals and their mode of actions, concentration and also their exposure time (Taslima et al. Citation2022). Similar findings for cyprinid fishes were reported by Jezierska et al. (Citation2009) who found that heavy metals such as Cd and copper (Cu) were toxic to Cyprinus carpio eggs and larvae, as Barbus barbus early life stages (Meybeck et al. Citation2007). Sikorska and Wolnicki (Citation2010) found a similar impact in Tinca tinca larvae. Cadmium increased the prevalence of body deformity and increased the mortality of freshly born larvae in a greater extent than Cu. Williams and Holdway (Citation2000) found body abnormalities in Melanotaenia fluviatilis larvae pulse-exposed to Cd. African catfish (Clarias gariepinus) hatching and embryo survival showed no adverse effects when exposed to Cd levels between 0.05 and 5 mg/l. Conversely, a different study revealed that the hatching, embryo, and larval survival of Ide (Leuciscus idus) were considerably impacted by Cd exposure at 100 μg/l (Witeska et al. Citation2014). Similarly, Zn pollution adversely impacts the hatching rate and survival of various fish species, and it also interferes with the normal development and colouration of several organs (Gárriz and Miranda Citation2020).
The suppression of acetylcholinesterase activity caused vertebral abnormalities in Fundulus heteroclitus larvae exposed to copper pyrithione (Mochida et al. Citation2009). Various authors have observed that the genotoxic effect of Cd and other heavy metals can cause developmental defects (Özkan et al. Citation2011). At every developmental stage (such as blastula, gastrula, segmentation, hatching, etc.), fish embryos/larvae exhibit varied reactions to toxic exposure. These reactions can differ based on the fish species, the specific metal involved, its mechanism of action, the concentration of the heavy metals, and the duration of exposure (Ashaf-Ud-Doulah et al. Citation2021). In the present study, Cd exposure resulted in a rise in the number of malformed larvae among freshly hatched larvae. Different forms of morphological abnormalities (lateral spine curvature-scoliosis, axial spine curvature-lordosis, cardiac enema, yolk-sac malformation, C-shaped body, and spine curvature-lordosis) were identified after heavy metal exposure during post-hatching developmental stages in the present investigation. The findings of this study corroborate those of other researchers. Xia et al. (Citation2020) found a significant decrease in survival and prominent deformities in locomotive organs of Danio rerio when exposed to 5 μg/L of Cd for a period of 7 days. In the current study, increased yolk-sac area was observed after exposure to the maximum cadmium concentration and combined exposure to greater nickel and cadmium concentrations, which was likely owing to the embryo’s reduced yolk metabolism. Numerous literatures reported about the lethal and sub-lethal effects of heavy metals such as lower embryonic and larval survival, stunted growth, delayed hatching, vascular system abnormalities, skeletal deformities, reduce pigmentation, eye anomalies, and others (Samson and Shenker Citation2000; Nguyen and Janssen Citation2002; Osman et al. Citation2007; Cao et al. Citation2009).
Conclusion
In conclusion, this study highlights the differential toxic effects of Cd and Ni on the embryonic and larval development of Hypophthalmichthys molitrix and Ctenopharyngodon idella. Cadmium exhibited greater toxicity towards embryos, leading to higher mortality rates, whereas Ni proved more detrimental to larvae, resulting in elevated larval mortality. Furthermore, heavy metal exposure was significantly associated with deformities in both species, with metal concentration playing a crucial role. Notably, Ni induced more deformities in H. molitrix, while Cd had a greater deformity-inducing effect in C. idella. These findings underscore the importance of understanding species-specific responses to heavy metal toxicity for effective aquatic ecosystem management.
Supplemental Material
Download MS Word (2.3 MB)Acknowledgements
We extend our appreciation to the Researchers Supporting Project (no. RSP2023R191), King Saud University, Riyadh, Saudi Arabia. Authors are thankful to the Chairman of Zoology Department, The Government Sadiq College Woman University Bahawalpur for providing the laboratory facilities.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Abbas M, Chand N, Khan RU, Ahmad N, Pervez U, Naz S. 2019. Public health risk of heavy metal residues in meat and edible organs of broiler in an intensive production system of a region in Pakistan. Environ Sci & Pollut. 26(22):23002–23009. doi:10.1007/s11356-019-05639-4.
- Abdallah MAM, Elmagd Morsy FA. 2013. Persistent organochlorine pollutants and metals residues in sediment and freshwater fish species cultured in a shallow lagoon, Egypt. Environ Technol. 34:2389–2399. doi:10.1080/09593330.2013.770561.
- Al-Yousuf M, El-Shahawi M, Al-Ghais S. 2000. Trace metals in liver, skin and muscle of Lethrinus lentjan fish species in relation to body length and sex. Sci Total Environ. 256:87–94. doi:10.1016/S0048-9697(99)00363-0.
- Ashaf-Ud-Doulah M, Islam SMM, Zahangir MM, Islam MS, Brown C, Shahjahan M. 2021. Increased water temperature interrupts embryonic and larval development of Indian major carp rohu Labeo rohita. Aquac Int. 29:711–722. doi:10.1007/s10499-021-00649-x.
- Buha A, Matovic V, Antonijevic B, Bulat Z, Curcic M, Renieri EA, Tsatsakis AM, Schweitzer A, Wallace D. 2018. Overview of Cadmium thyroid disrupting effects and mechanisms. Int J Mol Sci. 19. doi:10.3390/ijms19051501.
- Cao L, Huang W, Shan X, Xiao Z, Wang Q, Dou S. 2009. Cadmium toxicity to embryonic-larval development and survival in red sea bream Pagrus major. Ecotoxicol Environ Saf. 72:1966–1974. doi:10.1016/j.ecoenv.2009.06.002.
- Cavas T. 2008. In vivo genotoxicity of mercury chloride and lead acetate: micronucleus test on acridine orange stained fish cells. Food Chem Toxicol: Int J Pub Br Ind Biol Res Assoc. 46:352–358. doi:10.1016/j.fct.2007.08.015.
- Chatha AMM, Naz S, Mansouri B, Nawaz A. 2023. Accumulation and human health risk assessment of trace elements in two fish species, Cirrhinus mrigala and Oreochromis niloticus, at Tarukri Drain, District Rahimyar Khan, Punjab, Pakistan. Environ Sci Pollut Res Int. 30:56522–56533. doi:10.1007/s11356-023-26337-2.
- El-Greisy ZA, El-Gamal AHA. 2015. Experimental studies on the effect of cadmium chloride, zinc acetate, their mixture and the mitigation with vitamin C supplementation on hatchability, size and quality of newly hatched larvae of common carp, Cyprinus carpio. Egypt J Aquat Res. 41:219–226. doi:10.1016/j.ejar.2015.03.007.
- Gárriz Á, Miranda LA. 2020. Effects of metals on sperm quality, fertilization and hatching rates, and embryo and larval survival of pejerrey fish (Odontesthes bonariensis). Ecotoxicology. 29:1072–1082. doi:10.1007/s10646-020-02245-w.
- Georgakopoulou E, Angelopoulou A, Kaspiris P, Divanach P, Koumoundouros G. 2007. Temperature effects on cranial deformities in European sea bass, Dicentrarchus labrax (L.). J Appl Ichthyol. 23:99–103. doi:10.1111/j.1439-0426.2006.00810.x.
- Green ID, Diaz A, Tibbett M. 2010. Factors affecting the concentration in seven-spotted ladybirds (Coccinella septempunctata L.) of Cd and Zn transferred through the food chain. Environ Pollut. 158:135–141. doi:10.1016/j.envpol.2009.07.032.
- He J, Wang C, Schlekat CE, Wu F, Middleton E, Garman E, Peters A. 2023. Validation of nickel bioavailability models for algae, invertebrates, and fish in Chinese surface waters. Environ Toxicol Chem. 42:1257–1265. doi:10.1002/etc.5595.
- Idriss AA, Ahmad AK. 2015. Heavy metal concentrations in fishes from Juru river, estimation of the health risk. Bull Environ Contam Toxicol. 94:204–208. doi:10.1007/s00128-014-1452-x.
- Jezierska B, Ługowska K, Witeska M. 2009. The effects of heavy metals on embryonic development of fish (a review). Fish Physiol Biochem. 35:625–640. doi:10.1007/s10695-008-9284-4.
- Jitar O, Teodosiu C, Oros A, Plavan G, Nicoara M. 2015. Bioaccumulation of heavy metals in marine organisms from the Romanian sector of the Black Sea. New Biotechnol. 32:369–378. doi:10.1016/j.nbt.2014.11.004.
- Kazlauskiene N, Vosyliene M. 2008. Characteristic features of the effect of Cu and Zn mixtures on rainbow trout Oncorhynchus mykiss in ontogenesis. Pol J Environ Stud. 17:291.
- Khalil I, Colombara DV, Forouzanfar MH, Troeger C, Daoud F, Moradi-Lakeh M, Bcheraoui CE, Rao PC, Afshin A, Charara R, et al. 2016. Burden of diarrhea in the Eastern Mediterranean Region, 1990-2013: findings from the global burden of disease study 2013. Am J Trop Med Hyg. 95:1319–1329. doi:10.4269/ajtmh.16-0339.
- Kong X, Jiang H, Wang S, Wu X, Fei W, Li L, Nie G, Li X. 2013. Effects of copper exposure on the hatching status and antioxidant defense at different developmental stages of embryos and larvae of goldfish Carassius auratus. Chemosphere. 92:1458–1464. doi:10.1016/j.chemosphere.2013.04.004.
- Lamas S, Fernández J, Aboal J, Carballeira A. 2007. Testing the use of juvenile Salmo trutta L. as biomonitors of heavy metal pollution in freshwater. J Chemosphere. 67:221–228. doi:10.1016/j.chemosphere.2006.10.040.
- Liu Y, Chen Q, Li Y, Bi L, Jin L, Peng R. 2022. Toxic effects of cadmium on fish. Toxics. 10:622. doi:10.3390/toxics10100622.
- Meybeck M, Lestel L, Bonté P, Moilleron R, Colin JL, Rousselot O, Hervé D, de Pontevès C, Grosbois C, Thévenot DR. 2007. Historical perspective of heavy metals contamination (Cd, Cr, Cu, Hg, Pb, Zn) in the Seine River basin (France) following a DPSIR approach (1950–2005). Sci Total Environ. 375:204–231. doi:10.1016/j.scitotenv.2006.12.017.
- Mhadhbi L, Boumaiza M, Beiras R. 2010. A standard ecotoxicological bioassay using early life stages of the marine fish Psetta maxima. Aquat Living Resour. 23:209–216. doi:10.1051/alr/2010014.
- Mochida K, Ito K, Harino H, Tanaka H, Onduka T, Kakuno A, Fujii K. 2009. Inhibition of acetylcholinesterase by metabolites of copper pyrithione (CuPT) and its possible involvement in vertebral deformity of a CuPT-exposed marine teleostean fish. Comp Biochem Physiol C Toxicol Pharmacol. 149:624–630. doi:10.1016/j.cbpc.2009.01.003.
- Naz S, Chatha AM, Danabas D, Khan MF, Xu Y, Zhu P, Shafique L. 2023. Bioaccumulation pattern and health risk assessment of heavy metals in Cirrhinus mrigala at Panjnad Headworks, Bahawalpur, Pakistan. Toxics. 11. doi:10.3390/toxics11070596.
- Naz S, Chatha AMM, Khan RU, Iqbal S, Amjad N, Kiran A, Javed A, Lateef M, Nawaz A. 2023. Current status of fish diversity and abundance at Panjnad headworks Bahawalpur, Punjab, Pakistan. Pak J Zool. 55(6). doi:10.17582/journal.pjz/20220411080448.
- Naz S, Chatha AMM, Saeed S. 2021. Acute nickel toxicity responses of Labeo rohita and Cirrhinus mrigala. Punjab Univ J Zool. 36:63–69. doi:10.17582/journal.pujz/2021.36.1.63.69.
- Naz S, Hussain R, Ullah Q, Chatha AMM, Shaheen A, Khan RU. 2021. Toxic effect of some heavy metals on hematology and histopathology of major carp (Catla catla). Environ Sci. & Pollut. 28(6):6533–6539. doi:10.1007/s11356-020-10980-0.
- Naz S, Mansouri B, Chatha AMM, Ullah Q, Abadeen ZU, Khan MZ, Khan A, Saeed S, Bhat RA. 2022. Water quality and health risk assessment of trace elements in surface water at Punjnad Headworks, Punjab, Pakistan. Environ Sci Pollut Res Int. 29:61457–61469. doi:10.1007/s11356-022-20210-4.
- Nguyen LT, Janssen CR. 2002. Embryo-larval toxicity tests with the African catfish (Clarias gariepinus): comparative sensitivity of endpoints. Arch Environ Contam Toxicol. 42:256–262. doi:10.1007/s00244-001-0007-4.
- Nugegoda D, Kibria G. 2017. Effects of environmental chemicals on fish thyroid function: implications for fisheries and aquaculture in Australia. Gen Comp Endocrinol. 244:40–53. doi:10.1016/j.ygcen.2016.02.021.
- Osman AGM, Mekkawy IA, Verreth J, Kirschbaum F. 2007. Effects of lead nitrate on the activity of metabolic enzymes during early developmental stages of the African catfish, Clarias gariepinus (Burchell, 1822). Fish Physiol Biochem. 33:1–13. doi:10.1007/s10695-006-9111-8.
- Özkan F, Gündüz SG, Berköz M, Hunt AÖ. 2011. Induction of micronuclei and other nuclear abnormalities in peripheral erythrocytes of Nile tilapia, Oreochromis niloticus, following exposure to sublethal cadmium doses. Turk J Zool. 35:585–592. doi:10.3906/zoo-0907-77.
- Samson JC, Shenker J. 2000. The teratogenic effects of methylmercury on early development of the zebrafish, Danio rerio. Aquat Toxicol. 48:343–354. doi:10.1016/S0166-445X(99)00044-2.
- Sfakianakis DG, Georgakopoulou E, Papadakis IE, Divanach P, Kentouri M, Koumoundouros G. 2006. Environmental determinants of haemal lordosis in European sea bass, Dicentrarchus labrax (Linnaeus, 1758). Aquaculture. 254:54–64. doi:10.1016/j.aquaculture.2005.10.028.
- Sfakianakis DG, Leris I, Laggis A, Kentouri M. 2011. The effect of rearing temperature on body shape and meristic characters in zebrafish (Danio rerio) juveniles. Environ Biol Fishes. 92:197–205. doi:10.1007/s10641-011-9833-z.
- Sfakianakis DG, Renieri E, Kentouri M, Tsatsakis AM. 2015. Effect of heavy metals on fish larvae deformities: a review. Environ Res. 137:246–255. doi:10.1016/j.envres.2014.12.014.
- Sikorska J, Wolnicki J. 2010. Cadmium and copper toxicity to tench Tinca tinca (L.) larvae after a short-term exposure. Rev Fish Biol Fish. 20:417–423. doi:10.1007/s11160-009-9145-y.
- Taslima K, Al-Emran M, Rahman MS, Hasan J, Ferdous Z, Rohani MF, Shahjahan M. 2022. Impacts of heavy metals on early development, growth and reproduction of fish – a review. Toxicol Rep. 9:858–868. doi:10.1016/j.toxrep.2022.04.013.
- Williams ND, Holdway DA. 2000. The effects of pulse-exposed cadmium and zinc on embryo hatchability, larval development, and survival of Australian crimson spotted rainbow fish (Melanotaenia fluviatilis). Environ Toxicol. 15:165–173. doi:10.1002/1522-7278(2000)15:3<165::AID-TOX3>3.0.CO;2-Q.
- Witeska M, Sarnowski P, Ługowska K, Kowal E. 2014. The effects of cadmium and copper on embryonic and larval development of ide Leuciscus idus. Fish Physiol Biochem. 40:151–163. doi:10.1007/s10695-013-9832-4.
- Xia Y, Zhu J, Xu Y, Zhang H, Zou F, Meng X. 2020. Effects of ecologically relevant concentrations of cadmium on locomotor activity and microbiota in zebrafish. Chemosphere. 257:127220. doi:10.1016/j.chemosphere.2020.127220.