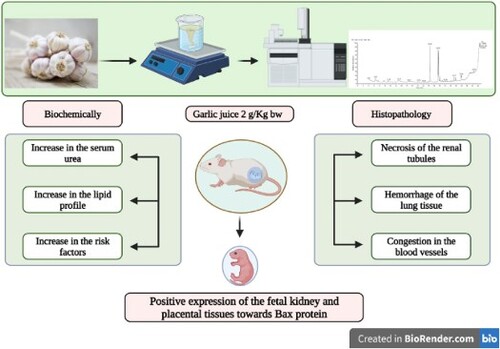
ABSTRACT
Despite the health benefits of herbals, random misuse of such therapy without consult tends to participate in public health issues. This study describes the nephro-and pulmonary toxicity effects of heavy consumption of garlic (Allium sativum) during developmental period. Two groups of ten healthy pregnant female Albino rats each were formed from the 20 in total. Gavages of distilled water were administered to the control group. Since garlic group rats orally received garlic aqueous extract (GAE) 1 ml/rat daily from gestational day (GD) 6 to 19. Kidney functions and lipid biomarkers were assayed along with histopathological investigations. Our results confirmed that the group that received garlic had significantly increased serum levels of urea, cholesterol, triglycerides and LDL. Moreover, the foetal and maternal tissues of the kidney and lung exhibited a varying degree of histopathological manifestations characterized by kidney necrosis and lung haemorrhage, severe congestion, and thickening of the blood vessels. Renal and pulmonary fibrosis were detectable by Masson’s trichrome. A positive expression of the foetal kidney and placental tissues towards Bax protein was noticed. In conclusion, depending on existing findings, the administration of higher concentrations of garlic could explode renal and pulmonary toxicity and decrease the rat’s weight.
1. Introduction
For several years, natural treatments have been used to treat various illnesses (Bhatwalkar et al. Citation2021). According to the World Health Organization (WHO), 80% of people consume herbals in many developing countries (Tugume and Nyakoojo Citation2019). Medicinal plants are growingly expand even among the literate populace (Mahish et al. Citation2016); perhaps because of the increased inefficacy of several modern drugs used the overcome many infections adding to the increase in bacterial resistance to diverse antibiotics and the high cost of drugs (Shinu et al. Citation2022).
Among the herbal-based materials is garlic (Allium sativum L.). Garlic is well-known to possess a variable number of beneficial compounds, allicin, essential oil, flavonoids and tannins. Garlic has a lot of therapeutic effectiveness, comprising antioxidant, antibacterial, antiviral, antifungal, antiparasitic and antimicrobial properties (Fatima et al. Citation2021).
Toxicology is an essential gate in pharmaceutics that deals with the side effects of bioactive agents on living organisms before they are applied in the clinical aspect (Al-Sulaiti et al. Citation2022). Garlic in particular has attracted desirable interest for its prevalent potential medicinal properties (Aly et al. Citation2019). Although its potential medicinal, pharmacological and toxic properties have been reported, little scientific evidence supports them. The demand for a toxicological understanding of the biochemical profile is justified by the increased usage of medicinal plants in alternative medicine. Consequently, the widespread use of garlic as a medicine and the little information on its potential toxicity effect supports the demand to examine garlic’s impact on the body’s vital organs organs (Ozma et al. Citation2023). Therefore, assessment of the toxicity of garlic extract is critical to confirm its safety for clinical administration (Sharwan et al. Citation2015). Garlic consumed in small doses seems to be nontoxic. But the safety of garlic when used at heavy doses must be analysed (Greef et al. Citation2021).
Allium sativum has many beneficial effects, but there is also evidence that it can be harmful to the kidney (Loría Gutiérrez et al. Citation2021). According to scientific investigations, ingestion of raw garlic at higher doses disturbs the digestive functions (El-Saber Batiha et al. Citation2020). Further, remarkable loss of normal heart, liver and kidney architecture was incited after feeding with fresh garlic at 1000 mg/kg/day for 30 days. Consequently, excessive garlic consumption exerts functional renal damage. When administered intraperitoneally, different doses of ethanolic garlic extract raised the serum urea and creatinine levels in comparison to the control (Lawal et al. Citation2016). Following ingestion of garlic at various doses, histological findings in the kidney support the biochemical results (Fowotade et al. Citation2017). However, previously; it was claimed that no toxicity symptoms in rats after dietary garlic extract at 2 g/kg 5 times a week for 5 months (Loría Gutiérrez et al. Citation2021).
The rising heart disease mortality rate was aggravated by the increase in levels of cardiovascular-associated biomarkers and related risk factors (Zou et al. Citation2020). The lipid indices are used as indicators of cardiovascular risks (Vieira et al. Citation2019). Lipid peroxidation is a process that produces free radicals and is involved in the development of atherosclerosis (Salekeen et al. Citation2022). Recent studies have investigated that repeated exposure to herbals is usually capable of predisposing to lipid peroxidation (Chandrasekara and Shahidi Citation2018). On the other hand, earlier reports have highlighted the hypolipidemic of garlic (Allium sativum) (Patiño-Morales et al. Citation2022). In parallel, a significant reduction in serum lipids has been demonstrated following the administration of a large dose of aqueous garlic extract (Olaniyan et al. Citation2013).
The lungs are the main organs for breathing and are the primary part of respiration; they remain a critical organ for life’s survival. Either, rats fed a low dose of garlic or intraperitoneally injected showed little difference in lung function from control animals. Also, a large dose of garlic caused a number of alterations in the rat lung tissues (Dutta et al. Citation2021). It has been recorded that garlic is thought to be possibly safe when taken in low doses. They claimed that while very high doses of garlic extract may have negative effects, though organosulphur S-methylcysteine compound present in garlic does excite some cardiotoxic and hypoxic effects (El-Magd et al. Citation2017). Higher doses of garlic frequently result in significant toxicity (Poppenga Citation2002).
The use of herbs during pregnancy constitutes a major health challenge resulting from inconsequent and random uses (El Hajj and Holst Citation2020). The majority of women resort to using herbal therapy during pregnancy as alternative medicine claiming to they were more effective and own lesser side effects than drug medication (Sumankuuro et al. Citation2022). The uses of medicinal herbs during pregnancy are also striking because various natural products are particularly marketed for symptoms that especially occurred during pregnancy; like vomiting and nausea (Ahmed Citation2021). Cumulative mortality may result from consecutive consumption of garlic even at low concentrations (Erazo-Pagador Citation2021). However, there is a deficiency of randomized controlled trial data on the safety and efficacy of herbs used during pregnancy (Ozioma and Chinwe Citation2019). A study in rats reported that administrated of garlic extract for 21 days during pregnancy (200 mg/kg body weight) was responsible for exactly 60% of fetuses’ mortality, as well as, the live fetuses was seemed of congenital malformations. Also, hepatic injury was observed in the histopathological examination (Kamal et al. Citation2023).
During pregnancy, herbal supplements should be used under extreme prudence (Sarecka-Hujar and Szulc-Musioł Citation2022). At that time, the use of herbal medication is responsible for disadvantageous maternal and foetal outcomes with early preterm birth (Makombe et al. Citation2023). Furthermore, the use of medicinal products during pregnancy poses a great encounter for healthcare suppliers, since pregnant women frequently ingest herbal products without consulting them (Illamola et al. Citation2020). Latterly, the teratogenic potential of garlic preparation was assessed in vitro (Alafiatayo et al. Citation2019).
Herein, this study aimed to determine to what extent might consuming heavily garlic affect the maternal and foetal tissues.
2. Materials and methods
2.1. Preparation of aqueous garlic extract
Local cultivars of garlic (Allium sativum) were obtained from the main market in Qena governorate which belongs to the Egyptian capital, during the dry season, then were identified in Botany Department, Faculty of Science, South Valley University-Qena Herbarium by Dr. Hussein, N. R. A.; as Allium sativum L. Sidds 40, Family: Amaryllidaceae J.St.-Hil. Specimens were deposited at the herbarium. The cloves of the bulb stem were used.
Thereby, an extract of Allium sativum was performed by means of a warm water technique according to (Gatsing et al. Citation2003), as follows. At first, the peeling garlic cloves were followed by washing and mincing 200 g through a juicer/blender device, then adding 150 mL water, and ultimately solution was warmed for 15 min at 45°C using constant stirring. The mixture was filtered herewith fresh natural juice was obtained.
2.2. GC-MS analysis
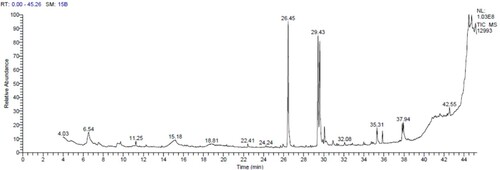
A GC-MS analysis was conducted on garlic samples using a Thermo Scientific TSQ 8000 Triple quadropole mass spectrometer and a TG-5MS fused silica capillary column. The detector and injector temperatures were set at 250°C and 220°C, respectively, with helium as the carrier gas. Samples were dissolved in methanol and introduced into the column, which was initially set at 50°C for 1 min and then gradually raised to 280°C at a rate of 5°C/min. The samples were run for 45 min, and the resulting chromatograms were analysed. Compounds were identified by comparing their relative retention time and mass spectra with reference standards from the NIST library database.
2.3. Experimental animals
In the current study, 20 adult female Albino rats (weighing 200–220 g), were obtained from the Animal House of Sohag University in Egypt. Once animals have come, it immediately checked and then transported in plastic cages to be acclimatized under laboratory conditions for a period of two weeks. The animals were supplied standard diet and water ad-libitum. The animal experimental protocol was approved by the Ethics Committee according to the Faculty of Science, South Valley University, Qena, Egypt (approval no. 019/11/22).
2.4. Experimental design
Prior to experimentation, two female Albino rats were managed in housing cages with a male for induction of pregnancy. Consequently, 20 healthy pregnant female Albino rats within 6-month-old utilized. Accordingly, animals were equally distributed into two groups, with 10 rats/per group. The first group was supplied with distilled water and employed as the control. While the second one was administrated garlic (Allium sativum) juice at 1 ml/rat. Over the exposure period of two weeks, from gestational day 6 to 19, all animals were kept under daily observation.
2.5. Behavioural records
Pregnant females were checked daily for behavioural changes and mortality. Moribund animals were immediately removed and incised. Moreover, the maternal body weights were determined.
2.6. Biochemical analysis
A day after the last dose at GD 20, all pregnant animals were euthanized under anesthetized management using diethyl ether. Blood samples from each animal were withdrawn through the supraorbital technique. The serum was separated by using centrifugation at 3000 rpm for 15 min. Consequent sera were kept deep-frozen pending analysis (Schermer Citation1967). Kidney functions and lipid biomarkers were calorimetrically estimated using kits from Biodiagnostic Company (Dokki, Giza, Egypt). The estimations of urea and creatinine in serum were done according to methods expressed by the previous report (Fawcett and Scott Citation1960; Bartels et al. Citation1972), respectively. Total cholesterol was determined previously (Richmond Citation1973). Serum triglyceride was calculated according to the procedures described previously (Fossati and Prencipe Citation1982). The estimation of high-density lipid-cholesterol (HDL) is determined by the procedures explained previously (Lopez-Virella et al. Citation1977). While the previous report stated that a laboratory method was used to determine serum low-density lipid cholesterol (LDL) based on the previous report (Lee and Niemann Citation1996). Assessment of risk ratios I and II was done as described by the previous report (Carleton et al. Citation1980).
2.7. Tissues samples
Meanwhile, tissue specimens from the mother and their fetuses were collected under caesarean sections at GD 20. The dissected tissues were accurately inspected for any gross lesions. Afterward, biopsies of the kidney, lung and placenta were carefully incised and fixed in 10% neutral buffered formalin for histopathological analyses.
2.8. Histopathological examination
For histological monitoring, the fixed specimens were embedded in paraffinic blocks and successively preceded in ascending series of ethyl alcohol. To be examined under a light microscope, sections measuring 3–4 µm were obtained and stained with Masson’s trichrome stain and Haematoxylin and Eosin (H&E) (Carleton et al. Citation1980).
2.9. Immunohistochemically
Frozen sections for the foetal kidney and placenta of 4 µm thickness using the cryostat microtome technique were thereafter stained with an immunohistochemical reaction for Bax according to (Bressenot et al. Citation2009).
2.10. Statistical analysis
Statistical analysis was carried out using the Statistical Package for Social Sciences (SPSS) of the raw data using a T-Test to compare the two groups. Subsequent results were described as means ± standard deviation (SD). When P < 0.05, statistical differences were considered significant (Borenstein et al. Citation1997).
3. Results
3.1. Characterization of chemical constituents using GC-MS
The GC-MS chromatogram is shown in . The bioactive components were determined by comparing the chromatogram peaks with reference peaks from the NIST Library. The chromatogram showed 20 distinctive peaks and the major bioactive components were Hexandecanoic acid, Trisulphide di-2-propenyl, Linoelaidic acid and cis-Vaccenic acid. Allicin degradation is responsible for the presence of sulphide compounds in oils (Banerjee et al. Citation2003) and is formed when garlic is crushed, and the alliinase enzyme is released. Allicin is then converted into alliin and quickly reacts to form sulphur derivative components due to its instability.
3.2. Mortality and behavioural records
According to this study, excessive oral administration of aqueous garlic extract to pregnant rats was responsible for exactly 10% mortality in the garlic group mothers post-ten days of garlic extract administration. In terms of behaviour, the excessive oral administration of garlic developed signs of clinical abnormalities in both performance and behaviour where animals manifested depression, poor performance, decreased activity, dullness, lethargy, reduced feed intake, anorexia and overcrowding.
3.3. Body weights
The body weight of the pregnant rats at the 1st, 6th, 12th and 15th GD after garlic exposure was significantly reduced (P < 0.05) compared to the control, described in .
Table 1. Effect of GAE on the body weight of the pregnant females (g).
3.4. Biochemical findings
Kidney functions were affected, although the pregnant rats given garlic extract orally experienced a significant (P < 0.05) elevation in the mean urea level in the garlic group when compared with the control ones. Nevertheless, serum creatinine levels showed a non-significant difference in rats dosed with garlic when compared to the control .
Table 2. Effect of GAE on the serum kidney functions (mean ± SD).
Exposure to the garlic at the high dose induced was significant (P > 0.05) rise in serum levels of total cholesterol, triglyceride and LDL following administrations of GAE (A. sativum) when compared to the control rats. Meanwhile, serum HDL didn’t express significant changes in comparison with the control .
Table 3. Effect of GAE on the serum lipid profile (mean ± SD).
Risk factors I and II, Further assessment of garlic on diseases related risk factors I and II as expressed in , implicated in a significant increase (P < 0.05) compared with controls.
Table 4. Effect of GAE on the risk factors (mean ± SD).
3.5. Histopathological results
3.5.1. Hematoxylin and eosin stain
3.5.1.1. Kidney
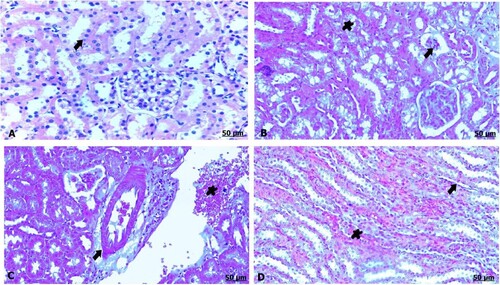
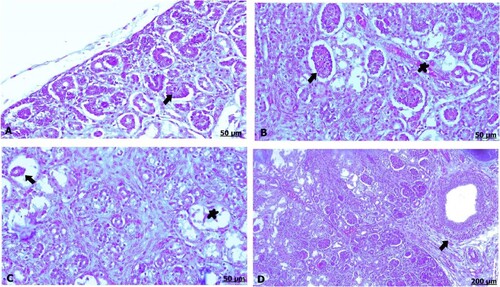
The light microscopic examination of kidney sections from the control mother showed healthy nephrons with intact renal tubules and intact glomeruli (A). Oral administration of GAE caused degeneration and necrosis of the renal tubules, shrinkage and atrophied glomeruli (B). Moreover, thickening and congestion of the blood vessels were noted, and renal fibrosis fundamentally consisted of fibrous tissue formation (C,D).
Figure 2. (A–D) Light photomicrograph of sections from maternal kidney of control (A) and GAE received group (B–D) stained with H & E.: light photomicrograph of (A) shows intact renal tubules and intact glomeruli. Photomicrograph of (B) shows shrinkage and atrophy of glomeruli (arrow), besides necrosis of the renal tubules (star). Photomicrograph of (C) shows thickening and congestion of the blood vessels. Photomicrograph of (D) shows fibrous tissues infiltration (arrow), also congestion of the tubules (arrow).
The light microscopic examination of kidney sections from the control fetus demonstrated renal corpuscles of normal growing tubules and glomeruli (A). Conversely, oral administration of garlic extract exhibited glomerular congestion and hypercellularity in some pregnant females (B), while other mothers suffered from pressure atrophy of the glomeruli resulting from expansion and dilatation in the bowman’s space as well as renal tubular necrosis (C) and perivascular inflammation characterized by mononuclear cell infiltration mainly composed of lymphocytes (D).
Figure 3. (A–D) Light photomicrograph of sections from foetal kidney of control (A) and GAE received group (B–D) stained with H & E.: light photomicrograph of (A) shows normal renal corpuscles. Photomicrograph of (B) shows hypercellularity of glomeruli (arrow), besides congestion of the blood vessels (star). Photomicrograph of (C) shows atrophied glomeruli, as well necrosis of the renal tubules. Photomicrograph of (D) shows perivascular inflammation (arrow).
3.5.1.2. Lung
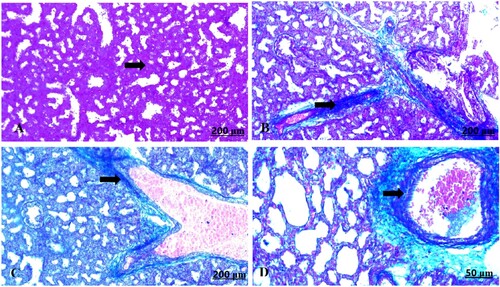
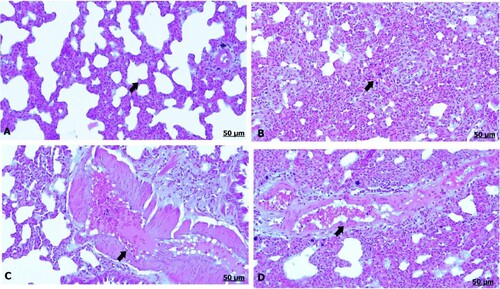
The light section examination of the lung for the control mother announced normal pulmonary parenchyma comprising healthy alveoli and blood vessels (A). However, GAE consumption resulted in severe histological changes characterized by remarkable thickening of the interalveolar septa, which was attributed to interstitial inflammatory cells and erythrocyte infiltration (B), besides thickening and congestion in the blood vessels (C,D).
Figure 4. (A–D) Light photomicrograph of sections from maternal lung of control (A) and GAE received group (B–D) stained with H & E.: light photomicrograph of (A) shows normal parenchymatous tissues of the lung. Photomicrograph of (B) shows prominent thickening of the interalveolar septa. Photomicrograph of (C, D) shows severe thickening and congestion of the blood vessels.
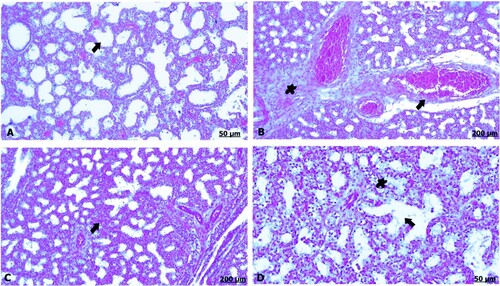
The light section examination of the lung of the control fetus indicated well-developed alveoli and vasculature (A). In a group that received garlic extract, there was prominent congestion and dilatation of the blood vessels (B). Likewise, there was thickening of the interalveolar septa and blood vessels bounded by discrete aggregation of inflammatory cells (C). Furthermore, alveolar emphysema owing to ruptured and anastomosing alveoli was observed (D).
Figure 5. (A–D) Light photomicrograph of sections from foetal lung of control (A) and GAE received group (B–D) stained with H & E.: light photomicrograph of (A) shows intact alveoli and vasculature. Photomicrograph of (B) shows remarkable degree of dilatation and congestion of the blood vessels. Photomicrograph of (C) shows thickening of the interalveolar septa shows severe thickening and congestion of the blood vessels. Photomicrograph of (D) shows erythrocytes infiltration (star), moreover, alveolar emphysema (arrow).
3.5.1.3. Placenta
The light sections examination of the lung for control placenta manifested fully vascular and avascular villi (A). Although garlic extract-induced mothers showed intense hyalinization and dilatation of the villi, the placental villi appeared full of edematous fluid (B). Detectable congestion and engorgement of the blood capillaries with stagnant red blood cells were noted (C).
Figure 6. (A–C) Light photomicrograph of sections from maternal placenta of control (A) and GAE received group (B, C) stained with H & E.: light photomicrograph of (A) shows normal vascular and avascular villi of the placenta. Photomicrograph of (B) shows severe hylanized and dilated villi filled with edematous fluid. Photomicrograph of (C) shows severe congestion of the blood capillaries.
3.5.2. Masson’s trichrome stain
3.5.2.1. Kidney
The light section examination of the control maternal kidney stained with Masson’s trichrome displayed normal features of fibrous tissues among the renal tissues (A). On the other hand, garlic administrated group exhibited significant fibrosis that detected by abundant infiltration of the fibrous tissues around renal blood vessels amount of (B–D).
Figure 7. (A–D) Light photomicrograph of sections from maternal kidney of control (A) and GAE received group (B–D) stained with Masson’s Trichrome: light photomicrograph of (A) shows normal of fibrous tissues. Photomicrograph of (B–D) shows potent infiltration of fibrous tissues with darkish blue coloration.
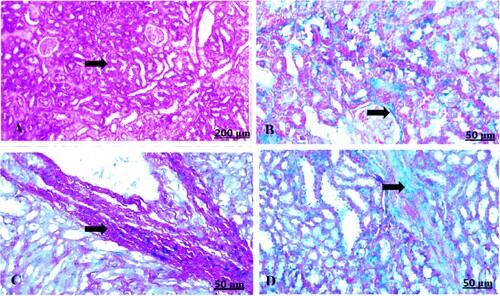
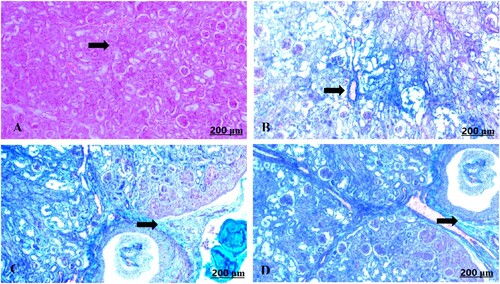
The light section examination of the control foetal kidney stained with Masson’s trichrome revealed minimal fibrous tissue formation (A). Garlic treated group showed heavy distribution of collagen fibres around vasculature (B–D).
Figure 8. (A–D) Light photomicrograph of sections from foetal kidney of control (A) and GAE received group (B–D) stained with Masson’s Trichrome: light photomicrograph of (A) shows minimally deposited collagen fibres. Photomicrograph of (B–D) shows heavily distributed collagen fibres around the blood vessels.
3.5.2.2. Lung
The light section examination of the control maternal lung stained with Masson’s trichrome displayed normally formed fibrous tissues (A). In contrast, the garlic-exposed group manifested perivascular fibrosis with prominent infiltration of the fibrous tissues (B–D).
Figure 9. (A–D) Light photomicrograph of sections from maternal lung of control (A) and GAE received group (B–D) stained with Masson’s Trichrome: light photomicrograph of (A) shows fairly distributed collagen fibres. Photomicrograph of (B–D) shows well defined distribution of the collagen fibres around vasculatures.
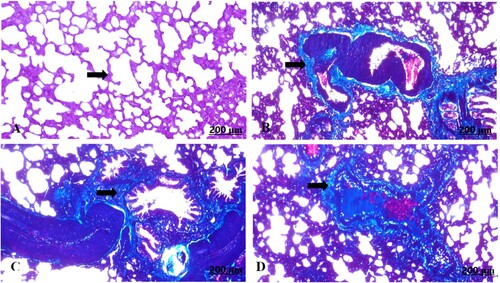
The light section examination of the control foetal lung stained with Masson’s trichrome recorded ill-defined fibrous tissues (A). In contrast, the garlic-exposed group showed enormous accumulation of the fibrous tissues (B–D).
3.6. Bax immunohistochemical reaction
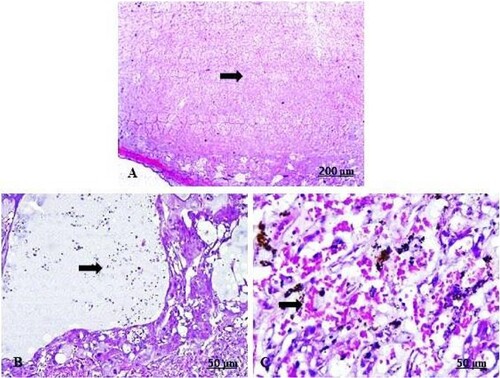
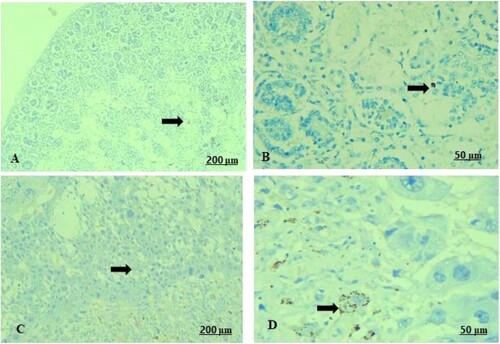
The light sections examination of the control foetal kidney revealed weak reaction of renal tubules towards Bax protein (A). A moderate immunoreactivity of Bax expression in the tubules was recorded in the garlic extract-received group (B). As a parallel to the control foetal kidney, the control placenta expressed a fair Bax immunostaining reaction in the villus (C). Contrariwise, in garlic-consuming rats, there was a strong positive expression of Bax immunostaining (D).
Figure 11. (A–D) Light photomicrograph of sections from foetal kidney (A, B) and placenta (C, D) of the pregnant female by Bax immunostaining. Light section of (A) shows control kidney of minimal reaction of Bax in the tubules. Photomicrograph of (B) shows GAE received kidney with moderate expression of Bax inside the renal tubules. Light section of (C) shows control placenta of fair immunoreactivity of the villi towards Bax. Light section of (D) shows GAE received placenta expressed strong positive expression of Bax protein.
4. Discussion
Lately, there is overwhelming concern of the people in developing countries to medicinal therapies by using traditional product including herbs to have their primary health care demands (Sambo Citation2010). Large scales of indigenous plants are still in use as source of medicinal therapeutics (Tugume and Nyakoojo Citation2019). The toxic and unfavourable effects of these products limit their potential utility (Fowotade et al. Citation2017).
It has recommended the use of these natural products but stresses that when employing medicinal plants as alternatives, safety and toxicity should be taken into account. The particular molecular principles of herbal plants in their natural situations possess variable impact on human physiological and biochemical conditions as triggered interest over their safety (Aware et al. Citation2022).
Garlic (Allium sativum L.) played a significant impact on treating infectious diseases and fighting microorganisms like parasites, bacteria, fungi and viruses (Rouf et al. Citation2020). Garlic afforded several medicinal substances like flavonoid, amino acids, sugars, saponins, mineral, vitamins and microelements (Martins et al. Citation2016). Medicinal characters of garlic involved antioxidant properties (Ramirez et al. Citation2017), antimicrobial (Lawal et al. Citation2016), anticarcinogenic (Petrovic et al. Citation2018), antidiabetic (Emami et al. Citation2017), anti-inflammatory and immunomodulatory (Arreola et al. Citation2015), and cardioprotective (Khatua et al. Citation2013), are due to vital oils inclosing organosulphur compounds, that giving both the unique taste and aroma of garlic.
In the present findings, administration of GAE at higher concentrations initiated a marked rise in serum urea level, which suggested the possibility of renal functioning impairment. Serum urea can be increased consequently to the use of anti-diuretic drugs, dehydration and diet. Moreover, considerable histological changes in kidney following garlic extract administration support the biochemical findings (Fowotade et al. Citation2017). Garlic has the potency to provoke morphological alterations in the liver and kidneys at a high dose, highlighting the requirement to optimize a safe dose of garlic. Moreover, the cumulative consumption of high range of raw garlic resulted in some clinical manifestation (Augusti Citation2020). Alliin and an alliinase enzyme are the most active constituents presented in garlic bulb. When fresh garlic is ground or chewed, these alliin and alliinase compounds interact, forming allicin, which is the essential biologically active ingredient in garlic and responsible for trigging offensive smell and toxicity virtues of the bulb (Tedeschi et al. Citation2022).
Cardiovascular disease is the leading risk factor for global mortality and morbidity. Oxidation of cholesterol fractions, particularly low-density lipoprotein (LDL), plays an important role in atherosclerosis (Borén et al. Citation2020). Cholesterol, esters and triglyceride constituents of the lipoprotein can be oxidized by toxic radicals, wasting their chemical constituents and cellular functions (Feingold Citation2021). Here, garlic elevates serum cholesterol and triglycerides, LDL, and risk factors. An elevation in triglycerides, cholesterol, LDL levels after oral and consecutive administration of herbals was attributed to a rise in the atherogenic index of cholesterol and potential oxidative stress in addition to hepatic injury, according to (Adeyemi and Orekoya Citation2014). It was shown that garlic powder non-significantly lowered blood cholesterol levels (Mulrow et al. Citation2000), might be because of the various amount of allicin potential in the garlic used in the scientific research (Lawson and Wang Citation2001). Otherwise, a significant hypolipidemic effect, notably on triglycerides, was attributed to garlic administration because it inhibited the biosynthesis of fatty acids (Yeh and Liu Citation2001; Olaniyan et al. Citation2013).
The histological results indicated degenerative changes and necrosis of the renal tubules, atrophied glomeruli, pulmonary haemorrhage and emphysema, along with congestion and dilatation in the blood vessels. Moreover, the toxic effect of garlic extract was experimentally confirmed at doses of 300 and 600 mg for 21 days along with histological alterations in male and female rats (Akbar Citation2020). Contrary to the previous report (Njue et al. Citation2015), who recorded non-significant changes for gross necropsy and pathological change in internal organs of garlic received Sprague Dawleys rats. Thence, the significant histopathological alterations in the kidney of the Wistar Albino rats when exposed to 1000 mg/kg/day of fresh garlic homogenate for 30 days announced degeneration of the renal tubules, interstitial nephritis with mononuclear infiltration, glomerular cellularity, tubular dilatation and expansion of the Bowman’s space (Banerjee et al. Citation2001). Intragastric administration of 500 mg/kg body weight in male Albino rats caused profound changes in the lung, characterized by pulmonary congestion and oedema (Rana et al. Citation2011). Accordingly, scientific reviews documented that consecutive consumption of fresh garlic, might exert cytotoxicity impact on cells and tissues, such as lungs, heart, stomach and intestines, resulting in severe cellular damage and dysfunction to such organs, and consequently animals death (Najman et al. Citation2022).
Immunohistochemically, garlic treated rats exhibited an increase in Bax expression indicating increase in the immunoreactivity of apoptosis proteins induced apoptosis attributed to DNA damage and fragmentation in addition to the decrement in apoptosis inhibiting proteins such as Bcl-2 (Shaikh et al. Citation2022).
5. Conclusions
Based on available findings, the present data suggest that relatively high doses of garlic aqueous extract enhanced selective fluctuations in animal performance and biochemical profile. Moreover, profound tissue damage in particular kidneys and lungs was disclosed. Similarly, medicinal herbals, notably garlic, should be administrated at a limited safe range.
Authors’ contributions
ZK and ZAA jointly developed the hypothesis and concept of the study and contributed to the chemical and material preparations, as well as the techniques performed. For this research and scientific paper, AHS, AMA, FZ, ZF, MZ and IFR are involved in the experimental procedures and analyses. MZ, FZ and ZF funding acquisition. All the authors revised and edited the manuscript. All authors have read and approved the final manuscript.
Institutional Review Board statement
This study was performed according to the Ethical Committee Board and Research Guidelines of the Faculty of Science, South Valley University, Qena, Egypt (approval no. 019/11/22).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that supports their conclusions is available from the authors of this manuscript upon request.
Additional information
Funding
References
- Adeyemi OS, and Orekoya BT. 2014. Lipid profile and oxidative stress markers in Wistar rats following oral and repeated exposure to Fijk herbal mixture. J Toxicol. 2014:1–7. doi: 10.1155/2014/876035.
- Ahmed SM. 2021. Medicinal plants and pharmaceutical medicines use in pregnant and lactating women in Ethiopia. http://urn.nb.no/URN:NBN:no-92014.
- Akbar S. 2020. Handbook of 200 medicinal plants: a comprehensive review of their traditional medical uses and scientific justifications. Cham: Springer. doi: 10.1007/978-3-030-16807-0_4.
- Alafiatayo AA, Lai KS, Syahida A, Mahmood M, Shaharuddin NA. 2019. Phytochemical evaluation, embryotoxicity, and teratogenic effects of Curcuma longa extract on Zebrafish (Danio rerio). Evid Based Complement Alternat Med. 2019:1–10. doi: 10.1155/2019/3807207.
- Al-Sulaiti MM, Soubra L, Al-Ghouti MA. 2022. The causes and effects of mercury and methylmercury contamination in the marine environment: a review. Curr Pollut Rep. 8(3): 249–272. doi: 10.1007/s40726-022-00226-7.
- Aly SM, Fetaih HA, Hassanin AA, Abomughaid MM, Ismail AA. 2019. Protective effects of garlic and cinnamon oils on hepatocellular carcinoma in Albino rats. Anal Cell Pathol. 2019:1–15. doi: 10.1155/2019/9895485.
- Arreola R, Quintero-Fabián S, López-Roa RI, Flores-Gutiérrez EO, Reyes-Grajeda JP, Carrera-Quintanar L, Ortuño-Sahagún D. 2015. Immunomodulation and anti-inflammatory effects of garlic compounds. J Immunol Res. 2015:1–13. doi: 10.1155/2015/401630.
- Augusti KT. 2020. Therapeutic and medicinal values of onions and garlic. In: HD Rabinowitch, JL Brewster, editors. Onions and allied crops. Boca Raton, FL: CRC Press; p. 93–108.
- Aware CB, Patil DN, Suryawanshi SS, Mali PR, Rane MR, Gurav RG, Jadhav JP. 2022. Natural bioactive products as promising therapeutics: a review of natural product-based drug development. S Afr J Bot. 151:512–528. doi: 10.1016/j.sajb.2022.05.028.
- Banerjee SK, Maulik M, Manchanda SC, Dinda AK, Das TK, Maulik SK. 2001. Garlic-induced alteration in rat liver and kidney morphology and associated changes in endogenous antioxidant status. Food Chem Toxicol. 39(8):793–797. doi: 10.1016/S0278-6915(01)00018-7.
- Banerjee SK, Mukherjee PK, Maulik SK. 2003. Garlic as an antioxidant: the good, the bad and the ugly. Phytother Res. 17(2):97–106. doi: 10.1002/ptr.1281.
- Bartels H, Böhmer M, Heirli C. 1972. Serum kreatininbestimmung ohne enteiweissen. Clin Chim Acta. 37:193–197. doi: 10.1016/0009-8981(72)90432-9.
- Bhatwalkar SB, Mondal R, Krishna SBN, Adam JK, Govender P, Anupam R. 2021. Antibacterial properties of organosulfur compounds of garlic (Allium sativum). Front Microbiol. 12:1869. doi: 10.3389/fmicb.2021.613077.
- Borén J, Chapman MJ, Krauss RM, Packard CJ, Bentzon JF, Binder CJ, Daemen MJ, Demer LL, Hegele RA, Nicholls SJ, et al. 2020. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 41(24):2313–2330. doi: 10.1093/eurheartj/ehz962.
- Borenstein M, Rothstein H, Cohen J. 1997. Sample power statistics 10. Chicago (IL): SPSS Inc. https://www.utstat.toronto.edu/~brunner/oldclass/378f16/readings/CohenPower.pdf.
- Bressenot A, Marchal S, Bezdetnaya L, Garrier J, Guillemin F, Plénat F. 2009. Assessment of apoptosis by immunohistochemistry to active caspase-3, active caspase-7, or cleaved PARP in monolayer cells and spheroid and subcutaneous xenografts of human carcinoma. J Histochem Cytochem. 57(4):289–300. doi: 10.1369/jhc.2008.952044.
- Carleton HM, Drury RAB, Wallington EA. 1980. Carleton’s histological technique. Oxford University Press. https://www.amazon.com/Carletons-Histological-Technique-Medical-Publications/dp/0192613103.
- Chandrasekara A, Shahidi F. 2018. Herbal beverages: bioactive compounds and their role in disease risk reduction - a review. J Tradit Complement Med. 8(4):451–458. doi: 10.1016/j.jtcme.2017.08.006.
- Dutta A, Dahiya A, Prakash A, Agrawala PK. 2021. Acute toxicity of diallyl sulfide derived from Allium sativum (garlic) in mice and its possible mechanisms. Phytomedicine Plus. 1(3):100084. doi: 10.1016/j.phyplu.2021.100084.
- El Hajj M, Holst L. 2020. Herbal medicine use during pregnancy: a review of the literature with a special focus on sub-Saharan Africa. Front Pharmacol. 11:866. doi: 10.3389/fphar.2020.00866.
- El-Magd MA, Abdo WS, El-Maddaway M, Nasr NM, Gaber RA, El-Shetry ES, Saleh AA, Alzahrani FAA, Abdelhady DH. 2017. High doses of S-methylcysteine cause hypoxia-induced cardiomyocyte apoptosis accompanied by engulfment of mitochondaria by nucleus. Biomed Pharmacother. 94:589–597. doi: 10.1016/j.biopha.2017.07.100.
- El-Saber Batiha G, Magdy Beshbishy A, Wasef L, Elewa YH, Al-Sagan A, Abd El-Hack ME, Prasad Devkota H. 2020. Chemical constituents and pharmacological activities of garlic (Allium sativum L.): a review. Nutrients. 12(3):872. doi: 10.3390/nu12030872.
- Emami S, Rouhani MH, Azadbakht L. 2017. The effect of garlic intake on glycemic control in humans: a systematic review and meta-analysis. Prog Nutr. 19:10–18. doi: 10.23751/pn.v19i1-S.5274.
- Erazo-Pagador G. 2021. Acute toxicity of garlic (Allium sativum) extract to snubnose pompano (Trachinotus blochii) juvenile. In: Proceedings of the international workshop on the promotion of sustainable aquaculture, aquatic animal health, and resource enhancement in Southeast Asia. p. 250–259. http://hdl.handle.net/10862/6278.
- Fatima S, Kumari A, Dwivedi VP. 2021. Advances in adjunct therapy against tuberculosis: deciphering the emerging role of phytochemicals. MedComm. 2(4):494–513. doi: 10.1002/mco2.82.
- Fawcett JK, Scott JE. 1960. A rapid and precise method for the determination of urea. J Clin Pathol. 13(2):156–159. doi: 10.1136/jcp.13.2.156.
- Feingold KR. 2021. Introduction to lipids and lipoproteins. In: Endotext. South Dartmouth (MA): MDText. com, Inc. https://www.ncbi.nlm.nih.gov/books/NBK305896/.
- Fossati P, Prencipe L. 1982. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin Chem. 28(10):2077–2080. doi: 10.1093/clinchem/28.10.2077.
- Fowotade AA, Fowotade A, Enaibe BU, Avwioro GO. 2017. Evaluating toxicity profile of garlic (Allium sativum) on the liver, kidney and heart using Wistar rat model. Int J Trop Dis Health. 26(2):1–12. doi: 10.9734/IJTDH/2017/36282.
- Gatsing D, Aliyu R, Meli WB, Adoga GI, Tchouanguep MF. 2003. Phytochemical profile and antisalmonellal properties of Allium sativum bulb extract. West Afr J Biol Sci. 14:29–36.
- Greef DD, Barton EM, Sandberg EN, Croley CR, Pumarol J, Wong TL, Das N, Bishayee A. 2021. Anticancer potential of garlic and its bioactive constituents: a systematic and comprehensive review. Semin Cancer Biol. 73:219–264. doi: 10.1016/j.semcancer.2020.11.020.
- Illamola SM, Amaeze OU, Krepkova LV, Birnbaum AK, Karanam A, Job KM, Bortnikova VV, Sherwin CMT, Enioutina EY. 2020. Use of herbal medicine by pregnant women: what physicians need to know. Front Pharmacol. 10:1483. doi: 10.3389/fphar.2019.01483.
- Kamal Z, Al-Amgad Z, Mobarak SA. 2023. The relationship between aggravated Allium sativum consumption and cytotoxicity of the pregnant rats with subsequent embryonic growth retardation. Egypt J Zool. doi: 10.21608/ejz.2023.218674.1098.
- Khatua TN, Adela R, Banerjee SK. 2013. Garlic and cardioprotection: insights into the molecular mechanisms. Can J Physiol Pharmacol. 91(6):448–458. doi: 10.1139/cjpp-2012-0315.
- Lawal B, Shittu OK, Oibiokpa FI, Mohammed H, Umar SI, Haruna GM. 2016. Antimicrobial evaluation, acute and sub-acute toxicity studies of Allium sativum. J Acute Dis. 5(4):296–301. doi: 10.1016/j.joad.2016.05.002.
- Lawson LD, Wang ZJ. 2001. Low allicin release from garlic supplements: a major problem due to the sensitivities of alliinase activity. J Agric Food Chem. 49(5):2592–2599. doi: 10.1021/jf001287m.
- Lee R, Niemann D. 1996. Nutritional assessment. Missouri: Mosby. ISBN: 9780815153191، 0815153198.
- Lopez-Virella MF, Stone P, Ellis S, Colwell JA. 1977. Cholesterol determination in high-density lipoproteins separated by three different methods. Clin Chem. 23(5):882–884. doi: 10.1093/clinchem/23.5.882.
- Loría Gutiérrez A, Blanco Barrantes J, Porras Navarro M, Ortega Monge MC, Cerdas Vargas MJ, Madrigal Redondo GL. 2021. General aspects about Allium sativum – a review. Ars Pharm. 62(4):471–481. doi: 10.30827/ars.v62i4.20843.
- Mahish PK, Mahobia R, Yadav J. 2016. Use and awareness of herbal medicines among literate population. Int J Pharma Bio Sci. 7(4):174–178. doi: 10.22376/ijpbs.2016.7.4.p174-178.
- Makombe D, Thombozi E, Chilemba W, Mboma A, Banda KJ, Mwakilama E. 2023. Herbal medicine use during pregnancy and childbirth: perceptions of women living in Lilongwe rural, Malawi – a qualitative study. BMC Women's Health. 23(1):228. doi: 10.1186/s12905-023-02387-z.
- Martins N, Petropoulos S, Ferreira ICFR. 2016. Chemical composition and bioactive compounds of garlic (Allium sativum L.) as affected by pre- and post-harvest conditions: a review. Food Chem. 211:41–50. doi: 10.1016/j.foodchem.2016.05.029.
- Mulrow C, Lawrence V, Ackerman R, Ramirez G, Morbidoni L, Aguilar C, Arterburn J, Block E, Chiquette E, Gardener C. 2000. Garlic: effects on cardiovascular risks and disease, protective effects against cancer, and clinical adverse effects. In: Database of abstracts of reviews of effects (DARE): quality-assessed reviews. http://www.ahrq.gov/clinic/epcsums/garlicsum.htm.
- Najman K, Sadowska A, Buczak K, Leontowicz H, Leontowicz M. 2022. Effect of heat-treated garlic (Allium sativum L.) on growth parameters, plasma lipid profile and histological changes in the ileum of atherogenic rats. Nutrients. 14(2):336. doi: 10.3390/nu14020336.
- Njue LG, Ombui JN, Kanja LW, Gathumbi JK, Nduhiu JG. 2015. Evaluation of oral toxicity level of ethyl acetate extract, from garlic (Allium sativum) in onorrh dawleys rats as per OECD guidelines 423. https://profiles.uonbi.ac.ke/jgitahi/files/evaluation_of_oral_toxicity_level_of_ethyl_acetate_extract_from_garlic_spring_published_article.pdf.http://hdl.handle.net/11295/91629.
- Olaniyan OT, Meraiyebu AB, Arogbonlo A, Dare JB, Shekins O, Shafe MO. 2013. Effects of aqueous extract of garlic (Allium sativum) on blood parameters in adult Wistar rats (Rattus novergicus). Int J Pharm Sci Invent. 2(3):42–45. https://www.edouniversity.edu.ng/oer/journal/effects_of_aqueous_extract_of_garlic_allium_sativum_on_blood_parameters_in_adult_wistar_rats_rattus_novergicus.
- Ozioma E-OJ, Chinwe OAN. 2019. Herbal medicines in African traditional medicine. Herb Med. 10:191–214. doi: 10.5772/intechopen.80348.
- Ozma MA, Abbasi A, Ahangarzadeh Rezaee M, Hosseini H, Hosseinzadeh N, Sabahi S, Noori SMA, Sepordeh S, Khodadadi E, Lahouty M, et al. 2023. A critical review on the nutritional and medicinal profiles of garlic’s (Allium sativum L.) bioactive compounds. Food Rev Int. 39(9):6324–6361. doi: 10.1080/87559129.2022.2100417.
- Patiño-Morales CC, Jaime-Cruz R, Sánchez-Gómez C, Corona JC, Hernández-Cruz EY, Kalinova-Jelezova I, Pedraza-Chaverri J, Maldonado PD, Silva-Islas CA, Salazar-García M. 2022. Antitumor effects of natural Compounds derived from Allium sativum on neuroblastoma: an overview. Antioxidants. 11(1): 48. doi: 10.3390/antiox11010048.
- Petrovic V, Nepal A, Olaisen C, Bachke S, Hira J, Søgaard CK, Røst LM, Misund K, Andreassen T, Melø TM, et al. 2018. Anti-cancer potential of homemade fresh garlic extract is related to increased endoplasmic reticulum stress. Nutrients. 10(4):450. doi: 10.3390/nu10040450.
- Poppenga RH. 2002. Herbal medicine: potential for intoxication and interactions with conventional drugs. Clin Tech Small Anim Pract. 17(1):6–18. doi: 10.1053/svms.2002.27785.
- Ramirez DA, Locatelli DA, González RE, Cavagnaro PF, Camargo AB. 2017. Analytical methods for bioactive sulfur compounds in Allium: an integrated review and future directions. J Food Compos Anal. 61:4–19. doi: 10.1016/j.jfca.2016.09.012.
- Rana SV, Pal R, Vaiphei K, Sharma SK, Ola RP. 2011. Garlic in health and disease. Nutr Res Rev 24(1): 60–71. doi: 10.1017/S0954422410000338.
- Richmond W. 1973. Preparation and properties of a cholesterol oxidase from Nocardia sp. and its application to the enzymatic assay of total cholesterol in serum. Clin Chem. 19:1350–1356. PMID: 4757363. https://pubmed.ncbi.nlm.nih.gov/4757363/.
- Rouf R, Uddin SJ, Sarker DK, Islam, MT, Ali ES, Shilpi JA, Nahar L, Tiralongo E, Sarker SD. 2020. Antiviral potential of garlic (Allium sativum) and its organosulfur compounds: a systematic update of pre-clinical and clinical data. Trends Food Sci Technol. 104:219–234. doi: 10.1016/j.tifs.2020.08.006.
- Salekeen R, Haider AN, Akhter F, Billah MM, Islam ME, Islam KMD. 2022. Lipid oxidation in pathophysiology of atherosclerosis: current understanding and therapeutic strategies. Int J Cardiol Cardiovasc Risk Prev. 14:20014. doi: 10.1016/j.ijcrp.2022.200143.
- Sambo LG. 2010. The decade of African traditional medicine: progress so far. Afr Health Monit. 14:4–6. https://pesquisa.bvsalud.org/portal/resource/pt/biblio-1256269.
- Sarecka-Hujar B, Szulc-Musioł B. 2022. Herbal medicines—Are they effective and safe during pregnancy? Pharmaceutics. 14(1):171. doi: 10.3390/pharmaceutics14010171.
- Schermer S. 1967. The blood morphology of laboratory animal. Longmans, Green and Co. p. 350. https://agris.fao.org/agris-search/search.do?recordID=US201300588372.
- Shaikh IA, Alshabi AM, Alkahtani SA, Orabi MAA, Abdel-Wahab BA, Walbi IA, Habeeb MS, Khateeb MM, Shettar AK, Hoskeri JH. 2022. Apoptotic cell death via activation of DNA degradation, caspase-3 activity, and suppression of Bcl-2 activity: an evidence-based Citrullus colocynthis cytotoxicity mechanism toward MCF-7 and A549 cancer cell lines. Separations. 9(12):411. doi: 10.3390/separations9120411.
- Sharwan G, Jain P, Pandey R, Shukla SS. 2015. Toxicity profile of traditional herbal medicine. J Ayurvedic Herb Med. 1(3):81–90. doi: 10.31254/jahm.2015.1306.
- Shinu P, Mouslem AKA, Nair AB, Venugopala KN, Attimarad M, Singh VA, Nagaraja S, Alotaibi G, Deb PK. 2022. Progress report: antimicrobial drug discovery in the resistance era. Pharmaceuticals. 15(4): 413. doi: 10.3390/ph15040413.
- Sumankuuro J, Baatiema L, Crockett J, Young J. 2022. Women’s use of non-conventional herbal uterotonic in pregnancy and labour: evidence from birth attendants. BMC Pregnancy Childbirth. 22(1):1–12. doi: 10.1186/s12884-022-04934-2.
- Tedeschi P, Nigro M, Travagli A, Catani M, Cavazzini A, Merighi S, Gessi S. 2022. Therapeutic potential of allicin and aged garlic extract in Alzheimer’s disease. Int J Mol Sci. 23(13):6950. doi: 10.3390/ijms23136950.
- Tugume P, Nyakoojo C. 2019. Ethno-pharmacological survey of herbal remedies used in the treatment of paediatric diseases in Buhunga parish, Rukungiri District, Uganda. BMC Complement Altern Med. 19(1):1–10. doi: 10.1186/s12906-019-2763-6.
- Vieira JN, Braz MAD, Gomes FO, Silva PRD, Santos OTDM, Rocha IMGD, Sousa IMD, Fayh APT. 2019. Cardiovascular risk assessment using the lipid accumulation product index among primary healthcare users: a cross-sectional study. Sao Paulo Med J. 137:126–131. doi: 10.1590/1516-3180.2018.0293240119.
- Yeh YY, Liu L. 2001. Cholesterol-lowering effect of garlic extracts and organosulfur compounds: human and animal studies. J Nutr. 131(3):989S–993S. doi: 10.1093/jn/131.3.989S.
- Zou Z, Cini K, Dong B, Ma Y, Ma J, Burgner DP, Patton GC. 2020. Time trends in cardiovascular disease mortality across the BRICS. Circulation. 141(10):790–799. doi: 10.1161/CIRCULATIONAHA.119.042864.