ABSTRACT
A 90-day feeding trial was conducted to investigate the effects of phytase supplemented Distiller’s Dried Grains with Soluble (DDGS) based diet on the production performance, tissue composition, nutrient digestibility and activity of anti-oxidant enzymes in the gills of mrigal (Cirrhinus mrigala) juveniles. Six experimental diets were formulated to contain increasing levels of supplemental phytase, 0 (D1), 250 (D2), 500 (D3), 750 (D4), 1000 (D5), and 1250 (D6) phytase (FTU/kg). Each experimental diet was randomly assigned and fed three times daily to triplicate groups of 180 fish. Significant increase in body weight gain and specific growth rate, feed conversion and protein efficiency ratios of groups fed up to 750 FTU/kg were observed. Broken line analysis of Specific Growth Rate showed that the optimal dietary phytase level of mrigal juveniles is 750 FTU /kg or higher than this dose. Results among protein, lipid, moisture, ash and P contents of whole body, muscle, liver and viscera were not significantly different as phytase level increased from 250 FTU/kg to 1250 FTU/kg (D2 to D6). It was concluded that phytase at the rate of 750 FTU /kg is the optimum dose for the enhanced growth in juvenile mrigal.
Introduction
Distiller’s Dried Grains with Soluble (DDGS) is a by-product of ethanol distillation and an enriched feedstuff containing valuable nutrients for the growth and support to gastrointestinal and immune functions of fish and has a lower cost on a protein-unit basis compared to other protein sources used in aquaculture (Jacob et al. Citation2008; Slominski Citation2012; Swiatkiewicz et al. Citation2016; Akhtar et al. Citation2020). Several studies have reported improved fish growth when employing diets for Nile Tilapia that include DDGS (Oreochromis niloticus) and channel catfish (Ictalurus punctatus) (Schaeffer et al. Citation2009; Li et al. Citation2010; Chevanan et al. Citation2010).
Phosphorus (P) is an important component for fish in various ways as a main constituent of nucleic acids, cell membranes, and fish bones. A diet with considerable amounts of low-bioavailable P may hinder fish growth and increase pollution of receiving waters (Ganga et al. Citation2015; Maas et al. Citation2021). In plants, 50–80% of P is generally stored in the form of IP6, also known as phytate (C6H18O24P6) (Francis et al. Citation2001; Frank et al. Citation2007). Plant-based proteins such as soybean meal are concentrated in protein and amino acids and have been widely utilized in fish feeds in recent decades (Ng and Romano Citation2013). However, phytate comprises the main storage form of P in plants and is virtually indigestible to monogastric animals, including fish. Moreover, data shows that the adverse effects of dietary IP6 in monogastrics extend beyond limited P availability. For instance, IP6 is known to act as a chelating agent of some divalent minerals, thereby decreasing their bio-availability due to the non-absorbable nature of IP6-mineral complexes from the gastrointestinal tract (Greiner and Konietzny Citation2006; Cao et al. Citation2007). Because fish lack endogenous phytase (myo-inositol hexakisphosphate phosphohydrolase), the enzyme required for phytate breakdown, phytase has been added to fish diets containing phytate-bearing ingredients to mediate hydrolysis and increase availability of phytate-P (Sajjadi and Carter Citation2004). Worldwide, various studies were reported on dietary phytase supplementation and their effects on different fish species such as Labeo rohita, Oncorhynchus mykiss, Oreochromis niloticus, and Psetta maxima (Cao et al. Citation2007; Adeoye et al. Citation2016; Von Danwitz et al. Citation2016; Dersjant-Li et al. Citation2017; Maas et al. Citation2021).
Antioxidant enzymes have been utilized as indicators of the antioxidant status of organisms and as biomarkers for assessing oxidative stress (Ayhan and Zeliha Citation2009; İbrahim et al. Citation2011; Kakoolaki et al. Citation2013). The enzymes namely Catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPx) have been identified in the tissues of many teleosts, such as salmonids, lutjanids, pomadaysids, sciaenids, mullets, seabreams, and tunas (Fuat et al. Citation2014). Moreover, Zeliha (Citation2018) states that these enzymes play a crucial role in providing antioxidant protection against cellular damages. Therefore, the presence of these antioxidant enzymes plays a significant role in preserving a relatively low concentration of the hydroxyl radical, which is known to be reactive and detrimental to biological systems (Kakoolaki et al. Citation2013; Zeliha Citation2018). The organs of interest for assessing the impact of tissue damage include gills, liver, and muscle (Korkmaz et al. Citation2009). The monitoring of tissue damage resulting from dietary phytase supplementation can be conveniently conducted due to the constant exposure of fish gills to the surrounding environment (Fuat et al. Citation2014; Zeliha Citation2018).
Cirrhinus mrigala belonging to the family Cyprinidae is the major cultured species of Pakistan. They are bottom feeder and mainly feed on decaying vegetable materials. It has a great commercial value, grow in semi-intensive polyculture setup and generally feed on formulated diet prepared with plant byproducts (Hussain et al. Citation2011, Citation2018). It is very necessary to prepared economically promoted cost effective diet to improve carp farming (Chen et al. Citation2022). Therefore, the study aims to determine the optimum dose of microbial phytase in DDGS-based diets for mrigal fish based on growth, nutrient digestibility, tissue composition, and activity of antioxidant enzymes in gills.
Materials and methods
A total of 108 Mrigal juveniles weighing (12.14 ± 1.52 g) were collected from the Government Fish Seed Hatchery, Bahawalpur, and brought to the laboratory located in the Department of Zoology, Government Sadiq College Women University, Bahawalpur, Pakistan. They were acclimatized in the laboratory for 30 days and initially fed with 33% protein diet. Experimental tanks (40× 30 × 30 cm, L × W × H, 120-L) were designed specifically for the purpose of fecal matter collection of the fish from water media. In each tank (replicate), 18 juveniles were kept and fed with the basal diet thrice a day as described in previous digestibility studies (Allan and Rowland Citation1992). The temperature (24.9–28.7°C), pH (7.4–8.6), and dissolved oxygen (D.O; 5.8–7.3 mg L−1) were monitored and sustained within acceptable range throughout the study by using Jenway pH and temperature digital meter (model 3510), and dissolved oxygen (DO) digital meter (model 970). Proper aeration through aeration capillaries was maintained in the tanks.
Components of feed and preparation of experimental diets
The ingredients for the experimental diets were obtained from a nearby feed store and phytase (Ronozyme, NOVOZYME®, DSM Nutritional Products, Switzerland) was purchased as powder form and mixed in distilled water to make a solution. Six experimental diets (designated as D1, D2, D3, D4, D5, and D6) were formulated using DDGS at 540 g /kg comprises 33% crude protein (CP) and 10.0% crude lipid (CL). The supplemental phytase level included in the diet as D1 (0 FTU/kg), D2 (250 FTU/kg), D3 (500 FTU/kg), D4 (750 FTU/kg), D5 (1000 FTU/kg) and D6 (1250 FTU/kg) as shown in . Ingredients were mixed with the water (400 mL /kg) then cold press extruded to produce pellets (2 mm diameter). Chromic oxide (0.55) was mixed as an indicator for digestibility determination (Abbas and Siddiqui Citation2013). The diets were dried at 45°C in an air convection oven. The tentative feed was kept in sealed bags before use, while, moisture content was checked using AOAC (Citation2000) methods.
Table 1. Formulation and chemical investigation of the experimental diets.
Feeding protocol and sample collection
Mrigal juveniles were hand fed thrice daily (7:00, 12:00, and 17:00 h) for 90 days on 2% regular ration, which was adjusted fortnightly on the basis of total body weight. Regular feed provided was noted and un-eaten feed was collected for the investigation of total feed intake and feed efficiency ratio. The quantity of food to be supplied was modified based on bi-monthly sampling for weight and length measurements per treatment (Debnath et al. Citation2005; Abbas and Siddiqui Citation2013). Light regime of photoperiod of 12L: 12D phase was maintained throughout the experimental period.
Nutrient digestibility determination
Fish fecal matter was collected from each tank once a day every morning prior to feeding till the end of experiment. Fecal waste was carefully removed from the fecal collection tube at the bottom of the tank by simple filtration. The samples were than dried, grounded and stored at −18°C for further analysis. Fecal samples were collected during the whole trial except for the first week.
Growth indices
The growth efficiency of the juveniles were determined by using the following parameters: Weight gain (WG) = 100 × [(final body weight – initial body weight) / initial body weight]; Specific growth rate (SGR) = 100 × [ln final body weight – ln initial body weight / time in days]; Feed intake (FI) = total feed fed as % body weight – total uneaten feed; Feed conversion ratio (FCR) = total feed fed (g) / total wet weight gain (g); Protein efficiency ratio (PER) = weight gain / protein intake; Apparent digestibility coefficient (ADC) of nutrient or energy = 100 × [1 − (dietary Cr2O3 / fecal Cr2O3) × (fecal nutrient or energy/ dietary nutrient or energy)]; Condition factor (CF) = 100 × (weight / length3); Viscerosomatic index (VSI) = 100 × [wet weight of visceral organs and associated fat tissue (g) / wet body weight (g)]; Hepatosomatic index (HSI) = wet liver weight (g) / empty fish weight (g) × 100; Mesenteric fat index (MFI) = 100 × [mesenteric fat weight (g) / wet body weight (g)]; Nutrient gain = nutrient in whole body of final fish – nutrient in whole body of initial fish; Non-faecal excreted nutrient = digestible nutrient – nutrient gain; Nutrient retention efficiency (%) = nutrient gain / digestible nutrient intake × 100.
Chemical analysis
Five juvenile fish from each tank were randomly selected and dissected to obtain their liver and visceral mass for the investigation of somatic indices (Hepatosomatic index, Viscerosomatic index, and Mesenteric fat index) and samples of liver and viscera were weighed and stored (−20°C) for subsequent proximate analysis. Furthermore, 5 fish juveniles were pooled and euthanized from each tank and stored (−20°C) for whole body proximate investigation according to AOAC (Citation2000) method. For the calculation of moisture content, samples were dried into an oven (Labostar-LG 122, Japan) for about twelve hours at 105°C for the determination of crude protein (N × 6.25) using micro Kjeldahl method after an acid digestion (Buchi 430/323, Switzerland). Crude fat content was determined using the petroleum ether (PE) extraction procedure (Soxhlet HT2 1045 method) outlined by Bligh and Dyer (Citation1959). Ash determination by burning in a muffle furnace (Isuzu, Japan) at 550°C for total 18 h. Phosphorus was determined by spectrophotometric method using molybdovandate reagent. Acid detergent fiber was used to examine crude fiber and gross energy was estimated by bomb-calorimeter (Model-1265, USA). Whereas, chromic oxide (Cr2O3) with fecal samples of the fish was evaluated by wet-acid digestion method reported by Furukawa and Tsukahara (Citation1966) and apparent digestibility (AD) approximations was calculated using technique of Bureau et al. (Citation1999).
Gills antioxidant enzymes
The dissected and separately stored gills of Cirrhinus mrigala were washed with the help of phosphate buffer (PBS) having pH 6.5 (0.2M) in order to eliminate RBCs. It was then thoroughly normalized in PBS (1:4 w/v) using a mixer. Later on, the mixture was mounted onto centrifuge and set for 10,000 rpm at 4°C. After 15 min of centrifugation the supernatants were separated and preserved at -80°C for enzyme activity of Catalase, superoxide dismutase (SOD) and glutathione peroxidase (GPx).
Statistical investigation
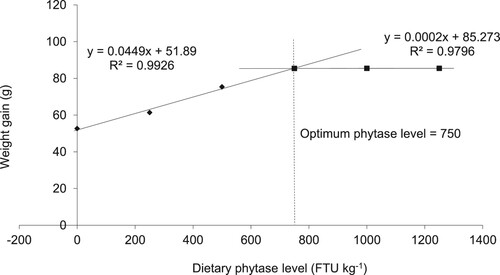
All data were subjected to one-way analysis of variance (ANOVA) using Costate Computer Software (Version 6.303). The student Newman Kaul’s significant difference test was used to compare the deviations between mean values at significance level of P < 0.05. The optimal dietary phytase level for SGR was estimated by the broken line regression model (Robbins et al. Citation1979).
Results
Growth profile
As shown in , final weight, total WG and SGR of juvenile mrigal improved significantly (P < 0.05) with dietary phytase levels (D4, D5 and D6) supplementation compared to the control (). The FI and FCR, and PER were significantly (P < 0.05) reduced in supplemented groups compared to the fish in the control group. On the other hand, PER significantly (P < 0.05) increased in D4 compared to the control.
Table 2. The growth rate, feed utilization efficiency and apparent digestibility of protein, lipid and energy % of juvenile mrigal fed different dietary phytase levels for 90 days.
The ADC of dry matter, protein, lipid, phosphorus and energy were significantly (P < 0.05) increased by graded level of phytase inclusion in the experimental diet. No significant (P < 0.05) change was noted in fish from the level of 750 FTU /kg to 1250 FTU /kg (D4 to D6) in most cases except ADC of energy.
shows non-significant (P > 0.05) results among protein, lipid, moisture, ash and P contents of whole body, muscle, liver and viscera as increased phytase level from 250 FTU/kg to 1250 FTU/kg (D2 to D6). The CF value did not show significant difference among different experimental groups. However, VSI, HIS and MFI were significantly (P < 0.05) decreased in fish fed upgraded dietary phytase level at 500 FTU/kg and above.
Table 3. Total proximate composition (% on wet weight basis) of whole body, muscle, liver and viscera of juvenile mrigal fed various dietary phytase levels for 90 days.
describes the effects of graded levels of phytase supplementation on SOD, CAT, and GPx activity in the gills of mrigal juveniles fed with DDGS based diet. No significant difference was found in concentration of these enzymes in fish.
Table 4. Enzymatic activity of juvenile mrigal fed different dietary phytase levels for 90 days.
The body WG showed the optimal dietary phytase level of juveniles Cirrhinus mrigala is 750 FTU/kg (). No mortality was experimented, as all fish performed healthy during the trial period.
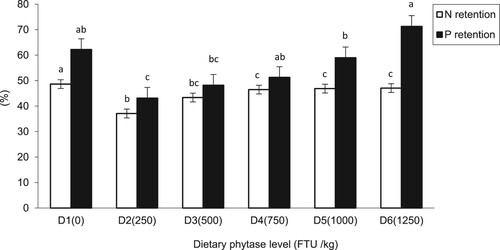
shows the retention of N and P in response to phytase supplementation in fish. The results showed that N retention was significantly lower in the supplemented groups compared to the control. Similarly, P retention was significantly (P < 0.05) higher in the control fish and those supplemented with 750 and 1250 FTU /kg. Nitrogen retention in fish juveniles was significantly decreased by phytase supplementation into investigational diets from 48.63% in D1 diet to 37.08% in D2 diet. However, upgraded phytase levels increased nitrogen retention efficacy from D3 (43.36%) to D6 (47.05%). Whereas, P retention was also decreased in fish fed D2 (43.13%) from D1 (62.21%) and gradually improved by increasing dietary phytase level from (48.19%) in fish fed D3 to D6 (71.29%).
Discussion
This study investigated the effects of several dietary phytase levels on the growth of mrigal juveniles on growth, digestibility and antioxidant status. Among the six phytase levels tested, it was found that the ideal level was 750 FTU/kg. The supplemented range of phytase (750–1000 U /kg) in the fish diet has been described by Cao et al. (Citation2007) and Hussain et al. (Citation2014) as having positive effects on various aspects of fish performance, including higher BWG, improved feed conversion efficiency, enhanced nutrient digestibility, increased mineral absorption, and enhanced protein deposition. Hussain et al. (Citation2011), Von Danwitz et al. (Citation2016), Rachmawati et al. (Citation2017), Mahmoud et al. (Citation2019), Akhtar et al. (Citation2020), and Miller et al. (Citation2021) have demonstrated that an appropriate dietary phytase content, which is comparable or higher, is beneficial for several fish species. In addition, Sajjadi and Carter (Citation2004) observed increased growth rates in Atlantic salmon that were fed a diet supplemented with phytase, regardless of the presence or absence of phytic acid. The optimal phytase level can vary depending on the specific species of fish, different sources and products, feed formulation (particularly substrate), and other response parameters. Previous studies have reported different recommended levels of phytase for various fish species, such as 250–500 U /kg for Zctalurus punctatus and Clarias gariepinus (Weerd Citation1999), 1000 U /kg for Morone saxatilis (Papatryphon et al. Citation1999), 500–1500 U /kg for Oreochromis niloticus (Liebert and Portz Citation2005), 800–1000 U /kg for Cyprinus carpio (Bai et al. Citation2003), 1000 U /kg for Sebastes schlegeli (Yoo et al. Citation2005), and 500–1000 U /kg for Pangasius pangasius (Debnath et al. Citation2005).
In our investigation, we observed a significant correlation between a higher feed intake (FI) and a lower dietary phytase level (0, 250, and 500 FTU /kg) in mrigal fish juveniles. This finding suggests that these juveniles consumed a greater amount of dry matter diet in order to adjust the phytase intake according to their specific needs. According to Adeoye et al. (Citation2016), the addition of phytase aids in the hydrolysis of phytate, hence enhancing the digestion of nutrients in fish. Nevertheless, it has been observed that an elevated dosage can impede the growth of fish and perhaps result in the competitive inhibition of essential minerals such as magnesium (Mg), zinc (Zn), iron (Fe), and cations during the process of assimilation (Roy and Lall Citation2003; Cong-mei et al. Citation2021). Several studies have provided evidence to suggest that the inclusion of dietary phytase significantly increased the feeding intake of fish compared to a diet without phytase supplementation (Vielma et al. Citation2004; Von Danwitz et al. Citation2016). However, this claim has been contradicted in the case of rainbow trout, Oncorhynchus mykiss (Barnes et al. Citation2012), Nile tilapia (Oreochromis niloticus), and Atlantic salmon (Salmo salar) by Maas et al. (Citation2021) and Sajjadi and Carter (Citation2004), respectively.
In the current investigation, the parameters of FCR, SGR, and PER exhibited a progressive increase as the dietary phytase level was incrementally raised to 750 FTU /kg, coinciding with an elevation in body growth. Previous studies have documented similar findings in relation to other fish juveniles, such as tilapia (Liebert and Portz Citation2005; Adeoye et al. Citation2016; Maas et al. Citation2018), channel catfish (Li et al. Citation2010; Li et al. Citation2011), African catfish (Yildirim and Turan Citation2010), largemouth bass (Miller et al. Citation2021), Japanese seabass (Ai et al. Citation2007), turbot (Von Danwitz et al. Citation2016), carp (Nwanna and Schwarz Citation2007), and rohu (Hussain et al. Citation2011), with the exception of Nile tilapia (Maas et al. Citation2021). Therefore, the increased availability of dietary P resulted in higher FCR, SGR, and PER compared to a P-deficient diet, as reported by Vielma et al. (Citation2004) and Cong-mei et al. (Citation2021). The survival rate of C. mrigala juveniles in our experiment was found to be 100%, which is consistent with the findings of previous studies conducted by Adeoye et al. (Citation2016) and Maas et al. (Citation2021). These studies explored the effects of feeding Nile tilapia (O. niloticus) with either a single or a combination of phytase supplemented diets for a duration of 36 and 42 days, respectively.
Phytase assessment has been thoroughly established in the literature during the previous two decades. Early and contemporary investigations on phytase addition in fish diets have consistently found that it improves growth performance and nutrient digestibility. According to the literature, the effects of phytase at high doses are connected to the enzyme's ability to release bounded phosphorus in low non-phytic phosphorus diets (Miller et al. Citation2021), hence boosting phosphorus bioavailability (Cozannet et al. Citation2023). Furthermore, our study revealed enhanced digestibility as shown by increased ADC of dry matter, protein, fat, P, and energy when phytase supplementation levels were elevated. Vandenberg et al. (Citation2012), Von Danwitz et al. (Citation2016), Maas et al. (Citation2021) and Miller et al. (Citation2021) had comparable results. They claimed that there had been a considerable rise in the ADC of protein, fat, and phosphorus, which had benefited growth and may have improved the utilization of plant protein sources in fish diet. Similarly, multiple studies have demonstrated that adding phytase to various types of fish meals successfully boosted the nutritional content of all nutrients (Cowieson et al. Citation2006).
In this study, a marginal decrease in the lipid content of fish was observed when the dietary phytase level was increased. However, no statistically significant differences were found in the lipid content and P levels among the entire body, muscle, liver, and viscera. Von Danwitz et al. (Citation2016) observed similar findings in additional fish species. Numerous studies have employed several indices such as CF, HSI, VSI, and MFI to assess the nutritional value of fish. The indicators observed in the current investigation exhibited a statistically significant decrease as the level of phytase supplementation exceeded the established requirement. This phenomenon could potentially be attributed to a decrease in the consumption of feed. In contrast, Adeoye et al. (Citation2016) reported that the addition of phytase in young tilapia (O. niloticus) did not yield any statistically significant effects on growth indicators.
The present research demonstrated that the addition of phytase had no impact on the antioxidant levels in the gills of juvenile C. mrigala. The outcomes of the study conducted by Miller et al. (Citation2021) likewise confirmed the absence of statistically significant effects on SOD and CAT in largemouth bass. In contrast, Ye et al. (Citation2016) reported a beneficial impact on the activity of CAT, but found no significant impacts on the activity of SOD in the puffer fish species Takifugu obscurus. Another study, which investigated the impact of yeast phytase supplementation over a span of 14 days, arrived at comparable findings. It demonstrated that incorporating phytase into the diet did not yield a noteworthy effect on catalase activity in Mycteroperca rosacea (Reyes-Becerril et al. Citation2008). Conversely, a few other studies indicated that using phytase as a dietary supplement did have a significant impact on the oxidative activities of fish gills (Zhu et al. Citation2014; Adeshina et al. Citation2023). The variation in antioxidant activity among different fish species may stem from factors such as the dosage, duration, and source of phytase, in addition to the specific species of fish used in the experiment. Supporting evidence may be found in several additional studies that have examined the activities of antioxidant enzymes in fish and their role in reducing oxidative stress (Ayhan and Zeliha Citation2009; Korkmaz et al., 2009; İbrahim et al. Citation2011; Fuat et al. Citation2014). According to Gulhan and Selamoglu (Citation2016) as well as Zeliha (Citation2018), it has been noted that overcrowding and other stress-related factors have the potential to create an unfavorable physiological state, hence increasing the vulnerability to infectious diseases. In addition, the impact of nutrition on the health and immune responses of fish is significant. Consequently, there has been a rise in studies focusing on the development of dietary immunostimulant supplements, including organic, inorganic, and synthetic substances. These supplements are incorporated into fish feeds, with various natural antioxidants being among the agents utilized in their formulation.
Conclusion
It was concluded that phytase at the rate of 750 FTU /kg is the optimum dose for the enhanced growth in juvenile mrigal.
Acknowledgements
We extend our appreciation to the Researchers Supporting Project (No. RSP2023R191), King Saud University, Riyadh, Saudi Arabia. Authors are thankful to the Chairman of Zoology Department, The Government Sadiq College Woman University Bahawalpur for providing the laboratory facilities.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Abbas G, Siddiqui PJA. 2013. The effects of varying dietary protein level on growth, feed conversion, body composition and apparent digestibility coefficient of juvenile mangrove red snapper, Lutjanus argentimaculatus (Forsskal 1775). Aquac Res. 44:807–818. doi:10.1111/j.1365-2109.2012.03096.x.
- Adeoye AA, Jaramillo-Torres A, Fox SW, Merrifield DL, Davies SJ. 2016. Supplementation of formulated diets for tilapia (Oreochromis niloticus) with selected exogenous enzymes: overall performance and effects on intestinal histology and microbiota. Anim Feed Sci Technol. 215:133–143. doi:10.1016/j.anifeedsci.2016.03.002.
- Adeshina I, Akpoilih BU, Tiamiyu LO, Badmos AA, Emikpe BO, Abdel-Tawwab M. 2023. Effects of dietary supplementation with microbial phytase on the growth, bone minerals, antioxidant status, innate immunity and disease resistance of African catfish fed on high soybean meal-based diets. J Anim Physiol Anim Nutr. 107:733–745. doi:10.1111/jpn.13765.
- Ai Q, Mai K, Zhang W, Xu W, Tan B, Zhang C, Li H. 2007. Effects of exogenous enzymes (phytase, non-starch polysaccharide enzyme) in diets on growth, feed utilization, nitrogen and phosphorus excretion of Japanese seabass, Lateolabrax japonicus. Comp Biochem Physiol A Mol Integr Physiol. 147(2):502–508. doi:10.1016/j.cbpa.2007.01.026.
- Akhtar S, Zahoor I, Yahya S, Waseem N, Khan S, Khalid A, Malik S. 2020. Effect of phytase supplementation on labeo rohita fingerlings that are fed by phytase supplemented DDGS based diet. International Journal of Aquaculture and Fishery Sciences. 6(2):061–067. doi:10.17352/2455-8400.000058.
- Allan GL, Rowland SJ. 1992. Development of an experimental diet for silver perch (Bidyanus bidyanus). Austasia Aquacult. 6:39–40.
- AOAC. 2000. Official methods of analysis of association of official analytical chemists (Vol. I.) 17th ed. Arlington, USA: Association of Official Analytical Chemists; p. 684.
- Ayhan D, Zeliha S. 2009. Biochemical changes and sensory assessment on tissues of carp Cyprinus carpio Linnaeus 1758 during sale conditions. Fish Physiol Biochem. 35(6):709–714.
- Bai DQ, Qiao XT, Wei D, Guo L, Qi HL. 2003. Effects of phytase on utilization ratio of nutrient composition (calcium, phosphorus, etc.) of Carp (Cyprinus carpio L.). J Tianjin Agric Col. 10:6–11.
- Barnes EM, Brown ML, Rosentrater KA. 2012. Juvenile rainbow trout responses to diets containing distillers dried grain with solubles, phytase, and amino acid supplements. Open J Anim Sci. 02:69–77. doi:10.4236/ojas.2012.22011.
- Bligh EG, Dyer WJ. 1959. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 37:911–917. doi:10.1139/y59-099.
- Bureau DP, Harris AM, Cho CY. 1999. Apparent digestibility of rendered animal protein ingredients for rainbow trout (Oncorhynchus mykiss). Aquaculture. 180:345–358. doi:10.1016/S0044-8486(99)00210-0.
- Cao L, Wang W, Yang C, Yang Y, Diana J, Yakupitiyage A, Li D. 2007. Application of microbial phytase in fish feed. Enzyme Microb Technol. 40(4):497–507. doi:10.1016/j.enzmictec.2007.01.007.
- Chen L, Xu J, Sun X, Xu P. 2022. Research advances and future perspectives of genomics and genetic improvement in allotetraploid common carp. Reviews in Aquaculture. 14:957–978. doi:10.1111/raq.12636.
- Chevanan N, Rosentrater KA, Muthukumarappan K. 2010. Effects of processing conditions on single screw extrusion of feed ingredients containing DDGS. Food Bioprocess Technol. 3:111–120. doi:10.1007/s11947-008-0065-y.
- Cong-mei X, Hai-rui Y, Zhang Q, Chen BB, Li LY, Qiu XY, Qi T, Liu JQ, Shan LL. 2021. Dietary phosphorus requirement of coho salmon (Oncorhynchus kisutch) alevins cultured in freshwater. Aquac Nutr. 27(6):2427–2435. doi:10.1111/anu.13374.
- Cowieson AJ, Acamovic 1T, Bedford MR. 2006. Phytic acid and phytase: implications for protein utilization by poultry. Poul Sci. 85:878–885.
- Cozannet P, Jlali M, Moore D, Archibeque M, Preynat A. 2023. Evaluation of phytase dose effect on performance, bone mineralization, and prececal phosphorus digestibility in broilers fed diets with varying metabolizable energy, digestible amino acids, and available phosphorus concentration. Poult Sci. 102(7):102755. doi:10.1016/j.psj.2023.102755.
- Debnath D, Pal AK, Sahu NP, Jain KK, Yengkokpam S, Mukherjee SC. 2005. Effect of dietary microbial phytase supplementation on growth and nutrient digestibility of Pangasius pangasius (Hamilton) fingerlings. Aquac Res. 36(2):180–187. doi:10.1111/j.1365-2109.2004.01203.x.
- Dersjant-Li Y, Wealleans AL, Barnard LP, Lane S. 2017. Effect of increasing Buttiauxella phytase dose on nutrient digestibility and performance in weaned piglets fed corn or wheat based diets. Anim Feed Sci Technol. 234:101–109. doi:10.1016/j.anifeedsci.2017.09.008.
- Francis G, Makkar HPS, Becker K. 2001. Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish. Aquaculture. 199(3-4):197–227. doi:10.1016/S0044-8486(01)00526-9.
- Frank DN, Amand ALS, Feldman RA, Boedeker EC, Harpaz N, Pace NR. 2007. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. 104:13780–13785. doi:10.1073/pnas.0706625104.
- Fuat GM, Zeliha S, Kenan E, İbrahim Ö. 2014. The effect of propolis on gill liver muscle tissues of rainbow trout oncorhynchus mykiss exposed to various concentrations of cypermethrin. Iranian Journal of Fisheries Sciences. 13(3):684–701.
- Furukawa A, Tsukahara H. 1966. On the acid digestion method for the determination of chromic oxide as an index substance in the study of digestibility of fish feed. Nippon Suisan Gakkaishi. 32:502–506. doi:10.2331/suisan.32.502.
- Ganga R, Tibbetts SM, Wall CL, Plouffe DA, Bryenton MD, Peters AR, Lall SP. 2015. Influence of feeding a high plant protein diet on growth and nutrient utilization to combined ‘all-fish’ growth-hormone transgenic diploid and triploid Atlantic salmon (Salmo salar L.). Aquaculture. 446:272–282. doi:10.1016/j.aquaculture.2015.05.010.
- Greiner R, Konietzny U. 2006. Phytase for food application. Food Technol. Biotechnol. 44(2):125–140.
- Gulhan MF, Selamoglu Z. 2016. Comparison of the effects of propolis and pollen extracts in the same concentrations on some biochemical and hematological parameters in rainbow trout (oncorhynchus mykiss). Journal of Survey in Fisheries Sciences. 3(1):18.
- Hussain S, Afzal M, Rana SA, Javid A, Iqbal M. 2011. Effect of phytase supplementation on growth performance and nutrient digestibility of labeo rohita fingerlings fed on corn gluten meal-based diets. Int J Agric Biol. 13(6):916–922.
- Hussain SM, Ahmad N, Javid A, Shahzad MM, Hussain M, Arsalan MZUH. 2018. Effects of phytase and citric acid supplemented corn gluten (30%) meal-based diets on the mineral digestibility of Cirrhinus mrigala fingerlings. Turkish Journal of Fisheries and Aquatic Sciences. 18(4):501–507. doi:10.4194/1303-2712-v18_4_01.
- Hussain SM, Hameed T, Afzal M, Mubarik MS, Asrar M, Shah SZH, Khichi TAA. 2014. Effects of phytase supplementation on mineral digestibility in Cirrhinus mrigala fingerlings fed on sunflower meal-based diets. International Journal of Biosciences (IJB). 5(12):173–181. doi:10.12692/ijb/5.12.173-181.
- İbrahim Ö, Zeliha S, Aysel AU. 2011. Modulating effect of selenium on gills of fish exposed to heavy metals. Fresenius Environ Bull. 20(1):104–108.
- Jacob ME, Fox JT, Drouillard JS, Renter DG, Nagaraja TG. 2008. Effects of dried distillers’ grain on fecal prevalence and growth of Escherichia coli O157 in batch culture fermentations from cattle. Appl Environ Microbiol. 74(1):38–43. doi:10.1128/AEM.01842-07.
- Kakoolaki S, Zeliha S, Oğuz C, Osman C, İlknur Ö. 2013. Role of propolis on oxidative stress in fish brain. Basic Clin Neurosci. 4(2):45–50.
- Korkmaz N, Cengiz EI, Unlu E, Uysal E, Yanar M. 2009. Cypermethrin -induced histopathological and biochemical changes in Nile tilapia (Oreochromis niloticus), and the protective and recuperative effect of ascorbic acid. Environ Toxicol Pharmacol. 28:198–205.
- Li MH, Oberle DF, Lucas PM. 2011. Evaluation of corn distillers dried grains with solubles and brewers yeast in diets for channel catfish Ictalurus punctatus (Rafinesque). Aquac Res. 42:1424–1430. doi:10.1111/j.1365-2109.2010.02734.x.
- Li MH, Robinson EH, Oberle DF, Lucas PM. 2010. Effects of various corn distillers by-products on growth, feed efficiency, and body composition of channel catfish, ictalurus punctatus. Aquac Nutr. 16(2):188–193. doi:10.1111/j.1365-2095.2009.00650.x.
- Liebert F, Portz L. 2005. Nutrient utilization of Nile tilapia Oreochromis niloticus fed plant based low phosphorus diets supplemented with graded levels of different sources of microbial phytase. Aquaculture. 248(1-4):111–119. doi:10.1016/j.aquaculture.2005.04.009.
- Maas RM, Verdegem MC, Dersjant-Li Y, Schrama JW. 2018. The effect of phytase, xylanase and their combination on growth performance and nutrient utilization in Nile tilapia. Aquaculture. 487:7–14. doi:10.1016/j.aquaculture.2017.12.040.
- Maas RM, Verdegem MC, Lee CN, Schrama JW. 2021. Effects and interactions between phytase, xylanase and β-glucanase on growth performance and nutrient digestibility in Nile tilapia. Anim Feed Sci Technol. 271:114767. doi:10.1016/j.anifeedsci.2020.114767.
- Mahmoud N, Eid A, Wahdan AA, Enany ME, El-Nab A, Asmaa S. 2019. Effect of phytase and citric acid on growth performance, feed utilization and its antibacterial activity against fish pathogens of Nile tilapia fingerlings. Egyptian Journal for Aquaculture. 0(4):0–0. doi:10.21608/eja.2019.47194.
- Miller KK, Rossi Jr W, Habte-Tsion HM. 2021. Assessment of total dietary phosphorus requirement of juvenile largemouth bass, Micropterus salmoides, using soybean meal-based diets: effects on production performance, tissue mineralization, physiological parameters in plasma and intestine and expression of head-kidney genes. Aquac Nutr. 27(1):116–128. doi:10.1111/anu.13169.
- Ng WK, Romano N. 2013. A review of the nutrition and feeding management of farmed tilapia throughout the culture cycle. Reviews in Aquaculture. 5(4):220–254. doi:10.1111/raq.12014.
- Nwanna LC, Schwarz FJ. 2007. Effect of supplemental phytase on growth, phosphorus digestibility and bone mineralization of common carp (Cyprinus carpio L). Aquac Res. 38(10):1037–1044. doi:10.1111/j.1365-2109.2007.01752.x.
- Papatryphon E, Howell RA, Soares Jr JH. 1999. Growth and mineral absorption by striped bass Morone saxatilis fed a plant feedstuff based diet supplemented with phytase. J World Aquacult Soc. 30(2):161–173. doi:10.1111/j.1749-7345.1999.tb00863.x.
- Rachmawati D, Istiyanto S, Maizirwan M. 2017. Effect of phytase on growth performance, diet utilization efficiency and nutrient digestibility in fingerlings of Chanos chanos (Forsskal 1775). Philipp J Sci. 146(3):237–245.
- Reyes-Becerril M, Tovar-Ramírez D, Ascencio-Valle F, Civera-Cerecedo R, Gracia-López V, Barbosa-Solomieu V. 2008. Effects of dietary live yeast Debaryomyces hansenii on the immune and antioxidant system in juvenile leopard grouper Mycteroperca rosacea exposed to stress. Aquaculture. 280:39–44. doi:10.1016/j.aquaculture.2008.03.056.
- Robbins KR, Norton HR, Baker DH. 1979. Estimation of nutrient requirements from growth data. J Nutr. 109:1710–1714. doi:10.1093/jn/109.10.1710.
- Roy PK, Lall SP. 2003. Dietary phosphorus requirement of juvenile haddock (Melanogrammus aeglefinus L.). Aquaculture. 221:451–468. doi:10.1016/S0044-8486(03)00065-6.
- Sajjadi M, Carter CG. 2004. Effect of phytic acid and phytase on feed intake, growth, digestibility and trypsin activity in Atlantic salmon (Salmo salar, L.). Aquac Nutr. 10(2):135–142. doi:10.1111/j.1365-2095.2003.00290.x.
- Schaeffer TW, Brown ML, Rosentrater KA. 2009. Performance characteristics of Nile tilapia (oreochromis niloticus) fed diets containing graded levels of fuel-based distillers dried grains with solubles. Journal of Aquaculture Feed Science and Nutrition. 1(4):78–83. doi:10.3923/joafsnu.2009.78.83.
- Slominski BA. 2012. New generation enzymes. In: Proceedings of the 33rd Western Nutrition Conference. p. 156–170.
- Swiatkiewicz S, Swiatkiewicz M, Arczewska-Wlosek A, Jozefiak D. 2016. Efficacy of feed enzymes in pig and poultry diets containing distillers dried grains with solubles: a review. J Anim Physiol Anim Nutr. 100(1):15–26. doi:10.1111/jpn.12351.
- Vandenberg GW, Scott SL, De La Noüe J. 2012. Factors affecting nutrient digestibility in rainbow trout (Oncorhynchus mykiss) fed a plant protein–based diet supplemented with microbial phytase. Aquac Nutr. 18(4):369–379. doi:10.1111/j.1365-2095.2011.00901.x.
- Vielma J, Ruohonen K, Gabaudan J, Vogel K. 2004. Top-spraying soybean meal-based diets with phytase improves protein and mineral digestibilities but not lysine utilization in rainbow trout, Oncorhynchus mykiss (Walbaum). Aquac Res. 35(10):955–964. doi:10.1111/j.1365-2109.2004.01106.x.
- Von Danwitz A, van Bussel CG, Klatt SF, Schulz C. 2016. Dietary phytase supplementation in rapeseed protein based diets influences growth performance, digestibility and nutrient utilisation in turbot (Psetta maxima L.). Aquaculture. 450:405–411. doi:10.1016/j.aquaculture.2015.07.026.
- Weerd JV. 1999. Balance trials with African catfish Clarias gariepinus fed phytase-treated soybean meal-based diets. Aquac Nutr. 5(2):135–142. doi:10.1046/j.1365-2095.1999.00100.x.
- Ye CX, Wan F, Sun ZZ, Cheng CH, Ling RZ, Fan LF, Wang AL. 2016. Effect of phosphorus supplementation on cell viability, anti-oxidative capacity and comparative proteomic profiles of puffer fish (Takifugu obscurus ) under low temperature stress. Aquaculture. 452:200–208. doi:10.1016/j.aquaculture.2015.10.039.
- Yildirim YB, Turan F. 2010. Effects of exogenous enzyme supplementation in diets on growth and feed utilization in African catfish, Clarias gariepinus. Journal of Animal and Veterinary Advances. 9(2):327–331. doi:10.3923/javaa.2010.327.331.
- Yoo GY, Wang X, Choi S, Han K, Kang JC, Bai SC. 2005. Dietary microbial phytase increased the phosphorus digestibility in juvenile Korean rockfish Sebastes schlegeli fed diets containing soybean meal. Aquaculture. 243(1-4):315–322. doi:10.1016/j.aquaculture.2004.10.025.
- Zeliha S. 2018. Selenium compounds for fish health: an update. Journal of Survey in Fisheries Sciences. 4(2):1–4.
- Zhu Y, Qiu X, Ding Q, Duan M, Wang C. 2014. Combined effects of dietary phytase and organic acid on growth and phosphorus utilization of juvenile yellow catfish Pelteobagrus fulvidraco. Aquaculture. 430:1–8. doi:10.1016/j.aquaculture.2014.03.023.