ABSTRACT
Coenurosis caused by Coenurus cerebralis poses a significant economic threat to sheep. To date, no effective treatment has been identified for this parasitic infection once it has settled in the brain. This study aimed to investigate the surgical removal of C. cerebralis cysts from the brains of infected sheep. Twenty sheep were utilized in the experiment, and the animals underwent a 24-hour fasting period before the surgical procedure. Various biochemical parameters, including TP, ALP, BUN, AST, CK, LDH, RBC, WBC, Hb, and Hct, were measured in the animals both before and one month after the operation. Anesthesia was induced by administering 2.2 mg/kg IV ketamine hydrochloride, five minutes after the administration of 0.1 mg/kg IV xylazine hydrochloride. Following anesthesia, the trepanation site was determined through percussion. Subsequently, an incision was made in the skin, exposing the skull bone. The skull was opened using a scalpel through trepanation, and the cysts were carefully removed. The results demonstrated that the presented surgical technique effectively treated C. cerebralis cysts in the sheep's brains, restoring normal motor and metabolic functions. Given these outcomes, it is concluded that the proposed surgical technique holds promise for practical application in the field.
Introduction
Coenurus cerebralis predominantly affects sheep, with occasional occurrences reported in goats, cattle, camels, other wild ruminants, horses, pigs, rabbits, and even humans. This parasitic infection can manifest in various organs, including the lung, spleen, liver, and heart, resembling tuberculosis. Coenurosis, attributed to the larval stage of the cestode Taenia multiceps, referred to as C. cerebralis, typically resides in the small intestine of dogs and wild carnivores. The significance of this disease lies in its zoonotic nature, causing substantial tissue damage, decreased production, reproductive losses, and noteworthy economic implications. In severe cases, especially when it impacts the tissues of ruminants, coenurosis can lead to fatal outcomes. The prepatent period for this parasite is typically 1.5–2 months (Avcioglu et al. Citation2011; Mohammed Citation2020; Varcasia et al. Citation2022).
The adult stage of Taenia multiceps is a parasite that takes residence in the small intestine of carnivores, serving as the definitive host. Infection is transmitted to intermediate hosts, including ruminants and humans, through the ingestion of food or water contaminated with dog feces containing T. multiceps eggs (Toparlak and Tuzer Citation2012). Coenurus larvae exhibit a slow growth rate and have an incubation period of 6–8 months, reaching their maximum size at the conclusion of this period (Toparlak and Tuzer Citation2012).
The pressure exerted on the brain by C. cerebralis can give rise to a spectrum of neurological symptoms, including circling, bruxism, incoordination, and torticollis. Moreover, the condition may lead to brain atrophy, softening of the skull bone, and thinning of the skull, ultimately culminating in fatality (Achenef et al. Citation1999; Ozmen et al. Citation2005; Guçlu et al. Citation2006; Sharma and Chauhan Citation2006; Schlafer et al. Citation2007; Godara et al. Citation2011; Yılmaz et al. Citation2014; Mohammed Citation2020; Júnior et al. Citation2021; Mohammadi et al. Citation2021; Varcasia et al. Citation2022). While clinical manifestations may raise suspicions of the disease, a definitive diagnosis is established through the identification of parasitic cysts in the brain during necropsy. Initially causing purulent meningoencephalitis, C. cerebralis can progress to induce symptoms related to the central nervous system, ultimately leading to fatal outcomes upon cyst growth (Sharma and Chauhan Citation2006; Yılmaz et al. Citation2018). Reports indicate a predominant concentration of cysts in the parieto-occipital region (Schlafer et al. Citation2007; Johri et al. Citation2023).
In the current literature, there is a notable absence of surgical procedures utilizing a similar technique for the treatment of C. cerebralis once it has established itself in the brain. Previous recommendations in literature propose surgical techniques that are both long-term and costly, necessitating the opening of the skull bones (Scott Citation2012). This study aims to introduce an economical and uncomplicated technique developed specifically for the treatment of C. cerebralis cysts observed in sheep. The presented method is designed to be practical under field conditions, providing a viable alternative to the more elaborate and expensive procedures suggested in the past.
Materials and methods
Study animals
The study involved twenty sheep of various ages and weights suspected of being affected by coenurosis. These sheep were sourced from Kyrgyzstan Turkey Manas University Veterinary Faculty Clinics and private clinics in the Issyk Kul Region. To ensure uniform conditions, animals with comparable feeding regimens and housed in the same area were selected for the study. Specifically, male animals of varying ages present similar symptoms were chosen. Initially, all animals underwent treatment for Listeriosis and Echinococcus. Those that did not respond to the treatment were identified as potentially infected with C. cerebralis and were subsequently included in the study. The selection process involved assessing sheep displaying most of the clinical signs for the palpebral reflex, with the side opposite to the non-reflex side determined as the surgical site.
Samples collection
Blood samples were collected both prior to and one month following the operation. Hematological parameters including white blood cell (WBC) count, red blood cell (RBC) count, hemoglobin (Hb), and hematocrit (Hct), were assessed, alongside biochemical parameters such as creatine kinase (CK), glucose, lactate dehydrogenase (LDH), total protein, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and urea. These comprehensive blood analyses were conducted to monitor the physiological changes associated with the surgical procedure.
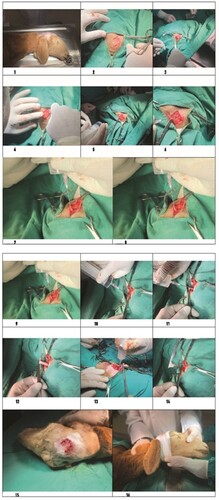
The surgical site was identified by discerning a distinctive sound upon percussion. The criterion for selection was the presence of a sound indicative of a fluid-filled cystic structure, distinguishable from the normal bone sound. The designated area was meticulously prepared for surgery, as depicted in and . Iodine preparation was employed to ensure aseptic and antiseptic conditions.
Figure 2. a: Calvarium, b: Duramater, c: Coenurus Cerabralis cyst membrane, d: Coenurus cerabralis cyst fluid, e: Brain, f: Injector, g: Extracted cyst fluid, h: Hemostatic clamp used to fix the protruding cyst part, i: stretching movements for the release of the cyst, j: the cyst is completely protruding, k: the sutured closure of the opened skin flap, l: the bandage for protection, →: the direction of movement of the cyst fluid
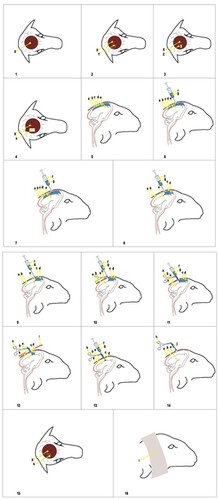
For anesthesia, the animals received intravenous administration of 2.2 mg/kg ketamine hydrochloride (Ketasol %10 Richter Pharma, Interhas, Turkiye) five minutes after 0.1 mg/kg of Xylazine (Xylazinbio %2 Bioveta Plc Czech Republic). A precise skin incision was made ( and ), with the size and direction determined based on the cystic structure identified through percussion. The skin was then separated as a flap to expose the underlying bone ( and ). Carefully using a scalpel, the thinned and deformed bone was removed ( and ), revealing the dura mater ( and ). A sterile syringe was used to pass through the dura mater into the calvarium, and cyst aspiration confirmed the correct location ( and ). The cyst was then drained using a needle and syringe ( and ), with no liquid pressure encountered during the second application ( and ).
Upon reentry, resistance was observed, indicating adherence of the cyst membrane to the syringe tip ( and ). The membrane was secured with hemostats ( and ) to prevent escape, and gentle traction in alternating directions freed the cystic wall ( and ). Following the removal of the remaining cyst fluid with a syringe ( and ), the cyst was fully extracted using gentle movements ( and ). The incised skin flap was sutured under sterile conditions using a vertical mattress suture technique with vicryl 2–0 ( and ), and the area was covered with a protective bandage ( and ).
Post-operatively, animals received long-acting antibiotic treatment (20 mg/kg intramuscular oxytetracycline, Tenaline LA, Ceva Sante Animale, France) and an antiparasitic drug (0.2 mg/kg subcutaneous ivermectin, Iveroxin ICB Turkiye).
Data were collected from the animal owners, and the animals were closely monitored after the operation.
Histopathological tests
Ten non-operated animals present signs of C. cerebralis underwent autopsy. The skulls of these ten sheep were euthanized by sacrification and subsequently opened using established procedures. Tissue samples were meticulously collected from specific regions of the central nervous system where C. cerebralis was observed. These samples were then fixed in 10% buffered formalin for subsequent histopathological examinations.
Thin sections, measuring 5 µm in thickness, were obtained from the paraffin blocks prepared using standard laboratory techniques with a microtome. All sections underwent staining with Hematoxylin and Eosin (H&E) and were meticulously examined under a binocular-capped light microscope (Ok et al. Citation2022).
Hematological and biochemical analyses
Biochemical parameters were measured using an Olympus AU400 analyzer in conjunction with Beckman Coulter. The biochemistry panel consisted of the following parameters: total protein, ALP, BUN, AST, CK, and LDH. All analyses were performed using a spectrophotometric method. Hematological parameters, including RBC, WBC, Hb, and Hct, were determined using the automated hematological analyzer Advia 120 SIEMENS.
Statistical analyses
For the statistical analysis of the obtained blood parameters, the Wilcoxon analysis method was employed to assess the significance of differences between dependent groups. This approach was chosen due to the relatively small number of groups and the incomplete fulfilment of assumptions required for parametric tests. The SPSS 22.0 programme was utilized to conduct these analyses.
Results
and display the biochemical parameters (CK, glucose, LDH, total protein, AST, ALT, ALP, and urea) along with the hematological values (WBC, RBC, hemoglobin, and hematocrit).
Table 1. Biochemical parameters of C. cerebralis infected sheep before and 1 month after the operation (mean ± SE).
Table 2. Hematological parameters of C. cerebralis infected sheep before and 1 month after the operation (mean ± SE).
In the statistical analysis performed using the Wilcoxon method, changes in glucose, LDH, ALT, ALP, and BUN values among the biochemical parameters were deemed nonsignificant. However, statistically significant differences (p < 0.001) were observed in the values of total protein, AST, and CK.
In terms of hematological parameters, no significant changes were detected in WBC values. However, significant differences were observed in RBC and hemoglobin values (p < 0.005), as well as hematocrit values (p < 0.001).
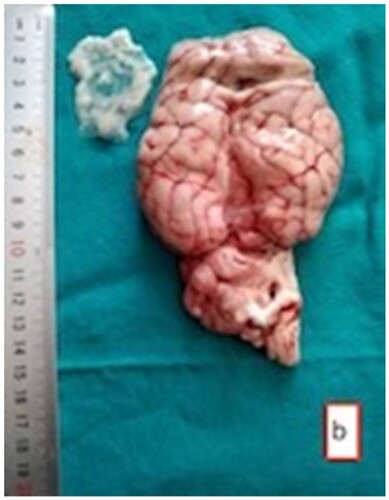
In sheep subjected to the established skull-opening method (), cysts of varying sizes were observed containing transparent fluid. The sizes of these cysts ranged from the smallest, measuring 2 × 2.5 cm, to the largest, measuring 5 × 4.5 cm. Within some of these cysts, dense white scolexes were visible (). While certain cysts were noticeable on the surface of the brain tissue, upon sectioning, it was observed that some cysts were located within the brain tissue itself. Cysts were found in the cerebellum, and in one case, a cyst was situated between the brain and the cerebellum. Instances where cysts were located in the hemisphere ventricles showed enlarged ventricles, and in most cases, the pressure exerted by the cysts in their respective regions led to atrophy of the brain tissue ( and ).
Figure 3. Fluctuant Coenurus cerebralis cyst (arrow) filled with transparent fluid in the frontal region of the brain.
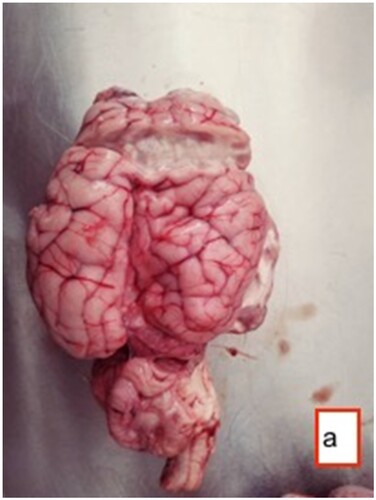
Figure 4. The cyst (arrow) in the frontal region of the brain with evacuated fluid and white scolexes.
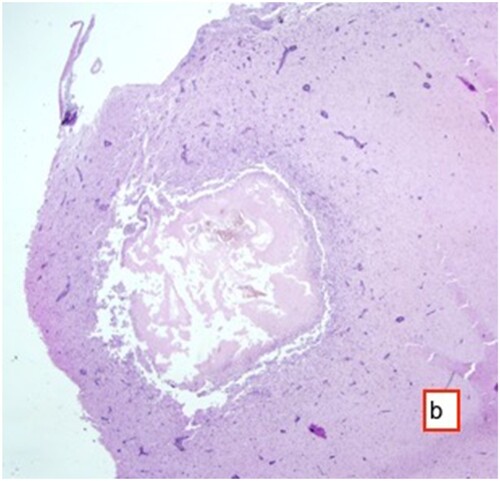
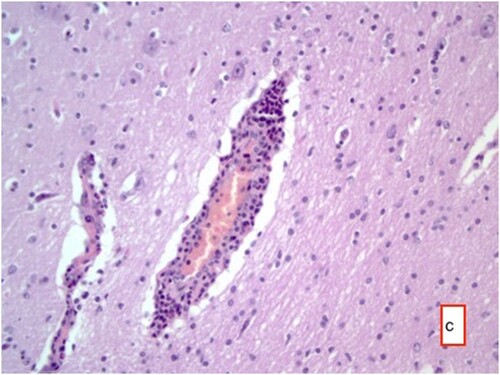
Histopathological examination of the tissue samples obtained from the regions where the cysts were identified revealed several findings. These included the presence of cyst membranes and necrotic areas, along with mononuclear cell infiltrations within these regions. Additionally, eosinophil granulocytes ( and ) and a few foreign body giant cells were observed. Hyperemia was noted in the blood vessels, accompanied by perivascular mononuclear cell infiltrations. In the brain and cerebellum tissues adjacent to the areas with cyst membranes, findings such as neuronal necrosis, neuronophagia, and gliosis were observed (). In certain sections, thickening of the meninges due to mononuclear cell infiltration was also observed.
Figure 5. Cyst membrane (arrow) and mononuclear cell infiltrate in these areas with occasional eosinophil granulocytes, and perivascular mononuclear cell infiltrates HE ×200.
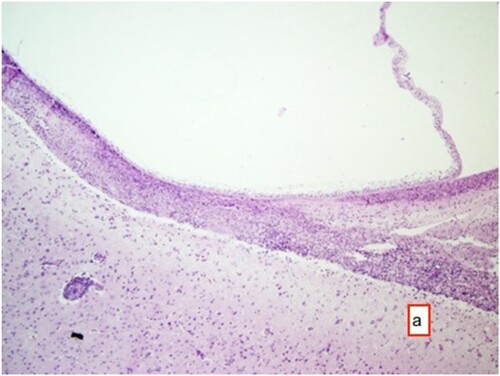
Figure 7. Hyperemia in vessels, perivascular mononuclear cell infiltrates, necrosis in some neurons and gliosis with neuronophagia, HE ×400.
Nervous symptoms, including incoordination, torticollis, spinning around, teeth grinding, constant lying on the ground, weakness, and loss of appetite, were observed in the animals before the operation. However, following the surgical intervention, all of these symptoms completely resolved. The animals regained normal function and returned to their regular activities. Information obtained from the owners of the affected animals indicates that no recurrence of symptoms occurred.
Discussion
The literature underscores that C. cerebralis is considered an incurable disease once it establishes itself in the brain, posing a significant threat that may lead to the death of the affected animal. In this study, our objective was to explore a potential solution for this challenging condition. Remarkably, existing literature lacks any operations employing a comparable technique for the treatment of C. cerebralis once it has entrenched itself in the brain. Surgical techniques recommended in some prior literature are described as prolonged and costly procedures, often necessitating the intricate process of opening the skull bones (Scott Citation2012).
The extent of atrophy in the brain, as well as the thinning and softening of the skull bones, is intricately linked to the size and location of the cysts. Cysts can manifest in various sizes and may be situated in superficial or deeper tissues (Ozmen et al. Citation2005; Schlafer et al. Citation2007; Mohammadi et al. Citation2021). In our study, trepanation was conducted in regions where the bones housing C. cerebralis cysts were identified to be softened. This observation regarding the softening of skull bones is consistent with findings reported in prior research.
Calvarial surgery can be approached through endoscopic procedures via the nose or conventional trepanation (Sadeghian Chaleshtori et al. Citation2020). Other approaches include trans-cochlear and infra-temporal methods (Papadopoulos Citation2021). However, the techniques employed by the researchers mentioned above are economically costly and require a significant amount of equipment. In contrast, the method utilized in our study involves a swift operation, is cost-effective, and has minimal complications.
According to several researchers, elevated creatine kinase (CK) values can result from stress in the body induced by various factors such as infection, trauma, malnutrition, heat, extreme cold, parasitic infestation, and transportation, among others (Avcioglu et al. Citation2011; Avcı et al. Citation2013; Comba et al. Citation2017). In our study, prior to the operation, the CK value was measured at 91.89 ± 14.27 (U/L). However, 1-month post-operation, it significantly decreased to 65.70 ± 9.02 (U/L). This notable reduction is believed to be attributable to alleviating stress caused by the cyst's pressure on the brain. Consequently, it is hypothesized that the decrease in CK values following the operation results from removing the stress factor. These findings align with prior investigations.
In their study on Karagul and Norduz sheep, Karagul and Norduz (Comba et al. Citation2017) reported the following values: WBC (12.77 ± 5.82, 12.23 ± 2.38 × 10^9/L), RBC (10.11 ± 0.77, 10.52 ± 1.22 × 10^12/L), hemoglobin (10.30 ± 0.43, 9.80 ± 1.31 g/dL), and hematocrit (28.30 ± 1.06, 26.40 ± 2.78%). In another study, Smith et al. (Avcı et al. Citation2013) documented the following values indicative of infection: AST (155 ± 26.0 U/L), ALT (19.5 ± 1.40 U/L), BUN (42.5 ± 6.90 mg/dL), and LDH (1303 ± 115 U/L). Additionally, in a study on parasitic diseases in sheep, Johnson et al. (Ayaz et al. Citation2006) found CK and Total Protein values to be 255.43 ± 57.4 U/L and 8.21 ± 0.42, respectively.
In the present study, changes in glucose, LDH, ALT, ALP, and BUN values, examined as part of the biochemical parameters, were not found to be significant. However, statistically significant changes were observed in Total Protein and AST values. These alterations in the parameters are believed to arise from the metabolic imbalances induced by the stress exerted by the cyst on the animal, in accordance with existing literature data.
C. cerebralis settles in the central nervous system of intermediate hosts, inducing neural symptoms such as incoordination, torticollis, self-rotation, and teeth grinding. While C. cerebralis initially triggers purulent meningoencephalitis, it may lead to central nervous system-related symptoms resulting in death after cyst growth (Scott Citation2012). In a study (Guçlu et al. Citation2006), it was reported that bilateral bone perforations attributed to C. cerebralis were identified in a sheep. However, in the current study, no perforations were detected in the sheep's skull bones. The size, location, and macroscopic appearance of the observed cysts in this study were consistent with findings from previous research conducted on sheep (Ozmen et al. Citation2005; Yılmaz et al. Citation2014; Yılmaz et al. Citation2018). Histological sections of the tissue revealed similar characteristics to those reported in other studies regarding nonpurulent meningoencephalitis (Ozmen et al. Citation2005; Schlafer et al. Citation2007; Yılmaz et al. Citation2014; Yılmaz et al. Citation2018; Mohammadi et al. Citation2021). While necrotic changes were observed in certain cases within the cystic areas, it was noted that cellular reactions were more prominent in other instances.
As a result, it was observed that the symptoms caused by C. cerebralis cysts in the brain disappeared with the surgical technique presented in this study.
Accordingly, we recommend the following measures for addressing C. cerebralis disease:
- Medication for Dogs: Emphasize the importance of administering medication to dogs, as they serve as definitive hosts. Implementing an effective deworming strategy can help prevent the transmission of the parasite.
- Fecal Management: Advocate for the proper disposal of feces from medicated dogs to prevent environmental contamination and further spread of the parasite.
- Veterinary Examination: Encourage the regular examination of animals by veterinarians, especially those showing suspicious symptoms. Early detection can aid in timely intervention and treatment.
- Prompt Surgical Intervention: Stress the importance of timely operations for animals diagnosed with C. cerebralis. Swift surgical procedures can contribute to better outcomes and the resolution of symptoms.
- Comprehensive Evaluation: During operations, recommend a thorough evaluation, including an assessment of brain and cerebrospinal fluid (CSF) characteristics, in addition to the parameters examined in the current study. This comprehensive approach can provide valuable insights into the condition.
- Applicability to Cattle: Highlight that the presented operation technique is not limited to sheep and can be applied to cattle as well. This broader application can enhance the effectiveness of the intervention.
- Prioritize Protective Measures: Emphasize the importance of prioritizing protective measures, including preventive strategies and early intervention, to mitigate the impact of C. cerebralis on animal health and prevent economic losses.
Authors’ contributions
AT: conception of the work and drafted the manuscript enrolled animals; IS: responsible for follow-up visits; FH: performed histopathological examination; HHA: conception of the work, interpreted the data; AR: reviewed the manuscript; RS: responsible for blood sampling; NO: performed statistical tests; MS: performed operations; ST: performed operations; NAU: performed laboratory analyses; AAK: laboratory analyses. All authors approved the final manuscript.
Ethics approval
Ethical approval for the study was obtained from the Kyrgyzstan Turkey Manas University Experimental Animals Local Ethics Committee (decision dated 18.02.2021 and numbered 2021/01).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Achenef M, Markos T, Feseha G, Hibret A, Tembely S. 1999. Coenurus cerebralis infection in Ethiopian highland sheep: incidence and observations on pathogenesis and clinical signs. Trop Anim Health Prod. 31:15–24. doi:10.1023/A:1005125316275.
- Avcı O, Yavru S, Bulut O. 2013. Plasma biochemical values in sheep with foot and mouth disease. Eurasian J Vet Sci. 29:216–219.
- Avcioglu H, Yildirim A, Duzlu O, Inci A, Kapakin Terim KA, Balkaya I. 2011. Prevalence and molecular characterization of bovine coenurosis from Eastern Anatolian region of Turkey. Vet Parasitol. 176:59–64. doi:10.1016/j.vetpar.2010.10.033.
- Ayaz E, Ertekin A, Özdal N, Taş Z. 2006. Some biochemical parameters in sheep infected with endoparasites (Fasciola spp., Dicrocoelium dendriticum, hydatid cysts, Trichostrongylidae and Protostrongylidae). Turkiye Parazitol Derg. 30:57–61.
- Comba B, Mert H, Comba A, Mis L, Mert N. 2017. The some hematological and biochemical parameters in Karakul and Norduz Sheep. Van Vet J. 28:137–140.
- Godara R, Katoch R, Yadav A, Khajuria JK, Borkataki S. 2011. Coenurosis in small ruminants: an overview. Vet Pract. 12:102–105.
- Guçlu F, Uslu U, Ozdemir O. 2006. Bilateral bone perforation caused by Coenurus cerebralis in a sheep: Case report. Turkiye Parazitol Derg. 30:282–284.
- Johri A, Singh D, Kumar A, Tiwari K, Arora N. 2023. Coenurosis in sheep and goat. Indian Farmer. 10:415–416i.
- Júnior SFV, Dorneles RF, Stigger AL, Fontoura EG, Feranti JPS. 2021. Cenurosis in a sheep with neurological signs-diagnosis with computed tomography. Acta Sci Vet. 49.
- Mohammadi P, Zakian A, Farjanikish G, Yeganeh FF, Raisi A, Samadipoor M. 2021. Clinical report of Coenurosis cerebralis outbreak in Lori sheep. Comp Clin Path. 30:729–733.
- Mohammed NH. 2020. Prevalence, morphological and biochemical study of larval stage Coenurus cerebralis of Taenia multiceps in sheep. Iraqi J Vet Sci. 34:159–163.
- Ok M, Naseri A, Ates MB, Ider M, Uney K, Sevinc M, Iyigun SS. 2022. The usefulness of serum brain damage biomarkers in detection and evaluation of hypoxic ischemic encephalopathy in calves with perinatal asphyxia. Animals. 12:3223. doi:10.3390/ani12223223.
- Ozmen O, Sahinduran S, Haligur M, Sezer K. 2005. Clinicopathologic observations on Coenurus cerebralis in naturally infected sheep. Scweiz Arch Tierheilk. 147:129–134. doi:10.1024/0036-7281.147.03.129.
- Papadopoulos E. 2021. Atlas of parasites in sheep. Grupo Asís Biomedia SL.
- Sadeghian Chaleshtori S, Abdollahi M, Shokrpoor S, Ashrafi-Tamai I, Hashemian M. 2020. Case report of concurrent occurrence of coenurosis and listerial encephalitis in a sheep. Vet Clin Pathol. 14:301–314.
- Schlafer DH, Miller RB, Maxie MG. 2007. Jubb, Kennedy, and Palmer’s pathology of domestic animals. St. Louis: Elsevier.
- Scott PR. 2012. Diagnosis and treatment of coenurosis in sheep. Vet Parasitol. 189:75–78. doi:10.1016/j.vetpar.2012.03.034.
- Sharma DK, Chauhan PPS. 2006. Coenurosis status in Afro-Asian region: a review. Small Rumin Res. 64:197–202. doi:10.1016/j.smallrumres.2005.05.021.
- Toparlak M, Tuzer E. 2012. Veteriner Helmintology. İstanbul Üniv. Vet. Fak. Masaüstü Yayımcılık Ünitesi, İstanbul.
- Varcasia A, Tamponi C, Ahmed F, Cappai MG, Porcu F, Mehmood N, Dessi G, Scala A. 2022. A proteomics informed by transcriptomics insight into the proteome of Ornithodoros erraticus adult tick saliva. Parasit Vectors. 15:1–18. doi:10.1186/s13071-021-05118-1.
- Yılmaz R, Özyıldız Z, Yumuşak N. 2014. Pathomorphological findings of Coenurus cerebralis in sheep. Harran Üniv Vet Fak Derg. 3:73–77.
- Yılmaz R, Yumusak N, Yilmaz B, Ayan A, Aysul N. 2018. Histopathological, immunohistochemical, and parasitological studies on pathogenesis of Coenurus cerebralis in sheep. J Vet Res. 62:35–41. doi:10.1515/jvetres-2018-0005.