 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
A 42-day feeding trial was carried out to evaluate the effect of dietary supplementation of zinc and threonine on the integrity of intestinal mucosa and expression of intestinal mucin 5ac (MUC5AC) gene in broiler chickens. A total of 640-day-old Arbor Acres broiler chicks were allocated to four dietary treatments with eight replicates of 20 birds in a completely randomized design. The experimental diets included T0: control (standard diet), T1: standard diet + 40 mg/kg Zinc Sulfate (ZnSO4), T2: standard diet + 2% L-Threonine (L-Thr) and T3: standard diet + (40 mg/kg ZnSO4 + 0.2% L-Threonine (L-Thr)). During the starter phase, the average daily gain and body weight of birds fed the T1 diet was lower (p < 0.05) than T2 and T0. The villus height (VH) of the jejunum in T2 was longer (p < 0.05) than in T0 and T3. In the ileum, VH for T1 was longer (p < 0.05) than for T0. The MUC5AC mRNA expression in the duodenum at the starter phase was lower (p < 0.05) in T3 than in T2 while in the jejunum, T1 was higher (p < 0.05) than the other treatment groups. The supplementation of zinc and threonine in the diet can improve the intestinal integrity of broiler chickens.
Introduction
The digestive system of poultry is a tube containing different tissues including intestinal epithelium, which is the first line of defense against pathogens and toxins (Abreu Citation2010). Its integrity depends on several factors such as the appearance of the intestinal mucosa and mucus whose major component is mucin. Exposure of the body to pathogens gives rise to the sanitary challenge in livestock production, resulting in significant risks in the extensive system breeding currently practised in Africa. Naturally, the organism is equipped with various lines of defense against pathogens. In the gastrointestinal tract, the pathogens face the alkalinity salivary; gastric acidity, intestinal alkalinity and enzyme defense. On the other hand, the intestinal epithelium has three types of capacity to protect the organism against the pathogens such as physics, microbiology and immunology capacity. It is therefore important to improve the immune capacity of the intestinal epithelium to prevent diseases and reduce antibiotic use in poultry farms (Ducatelle et al. Citation2015).
Zinc in all animal species is recognized as an essential trace element for many physiological functions (Park et al. Citation2004; Duffy et al. Citation2023). Zinc deficiency in chickens alters the innate and adaptive immunity (Hojyo and Fukada Citation2016), impairs the intestinal mucosa morphology in broilers (Ahmadi et al. Citation2013), damages cellular function by reducing DNA, RNA synthesis and genes expression (Park et al. Citation2004; Duffy et al. Citation2023). National Research Council (Citation2001), and Ibrahim et al. (Citation2017) showed that at 40 mg/kg incorporation of zinc in broiler diet, growth, health and reproductive traits were not improved. Thus, for better performance of chickens resulting from genetic improvement, feed manufacturing industries opt for a supplement of 100 ppm to 120 ppm of zinc in feed (Mahmoud et al. Citation2020). Furthermore, Azzam and El-Gogary (Citation2015) reported that zinc supplementation improves immunity capacity and growth performance under stress caused by high stocking density. In other hand, Increasing the level of zinc in a broiler’s diet causes a disturbance of the balance of other trace elements such as copper for example (Zhao et al., Citation2014; Byrne and Murphy Citation2022). High levels of zinc supplementation increases the production cost and the excretion of minerals in feces causing environmental pollution (Mahmoud et al. Citation2020). The supplementation of zinc as sulfate of zinc at 40 mg/kg improved the growth performance of broiler chicken and reduce the zinc excretion in environment (Zhang et al. Citation2018).
Threonine is the third most limited amino acid for broilers fed corn-soybean meal basal diets (Kidd et al. Citation1999; Tugay et al. Citation2009 Debnath et al. Citation2019). In supplementation, threonine improves intestinal health in chickens (Dong et al. Citation2017). The recommended level of threonine supplementation in broilers is from 1-3 g/kg of diet (Chen et al. Citation2017). Threonine supplementation at an adequate level improves the immunity capacity, and growth performance in broilers under the stress induces by high stocking density (Azzam and El-Gogary Citation2015). The integrity of the intestinal barrier is most dependent on mucin, which represents the major component of intestinal mucus (Janice et al., Citation2013). The major component of intestinal mucins in broilers is threonine, representing 30% of its total amino acid content (Bortoluzzi et al. Citation2018). Threonine is therefore recognized as an amino acid essential for the production of mucins in the gut of chickens and plays a key role in the maintenance of intestinal barrier integrity (Sandberg et al. Citation2007; Nichols and Bertolo Citation2008).
Recent studies have demonstrated the link between zinc and amino acids including threonine (Te-Jung et al. Citation2005). According to Te-Jung et al. (Citation2005), in the gut, threonine fixes the zinc and there is a reduction in the availability of each nutrient by chelation (Farhadi et al. Citation2021). It is thus, hypothesized that the supplementation of zinc and threonine in the diet of the birds can enhance gut functioning. The objective of this study was to evaluate the effect of dietary threonine and zinc supplementation on the integrity of small intestine mucosa and the expression of intestinal mucin 5ac (MUC5AC) gene in broiler chickens.
Materials and methods
Study area, birds husbandry and experimental design
The experimental procedure was approved by the scientific community of the Animal Eco-Nutrition Lab and conducted at the Poultry Unit of the Shandong Agricultural University of China. Six hundred and forty (640) day-old male Arbor Acres broiler chicks were randomly assigned into four dietary treatments with 8 replicates and 20 birds per cage in a completely randomized design. The chicks were weighed at hatch and individuals with a mean weight of 37.21 ± 0.53 g were selected and randomly placed in windowless environmentally controlled room cages (1 × 0.9 m). The cages had wire flooring and were equipped with one open feeding trough and 2 drinkers. The room temperature was maintained at 33°C during the first 3 days and then decreased gradually by age until reaching 24°C at 21 days. The birds were given access to feed and water ad libitum and light exposure followed 23 h of light during the starter phase (0–21 days) and 16 h of light during the finisher phase (22–42 days). On day 22, the chicks were allotted to their respective cage at 23°C with a surface of 1.54 m2. The experiment lasted for 42 days. Prophylaxes and vaccination of birds were administered as recommended for the Arbor Acres strain (Raach-Moujahed and Haddad Citation2013).
Experimental diets
The experimental diets were formulated with the specification of broiler feed requirements, recommended for Arbor Acres strain (NRC Citation1994). The feed composition and chemical analysis for both the starter phase (0–21 days) and the finisher phase (22–42 days) are presented in . Four experimental diets were formulated namely; T0 (control group): fed a standard diet without Zinc Sulfate (ZnSO4) and L-Threonine (L-Thr) supplementation, T1 (standard diet supplemented with 40 mg ZnSO4/kg diet), T2 (standard diet supplemented with 0.2% L-Thr) and T3 (standard diet supplemented with 40 mg ZnSO4/kg diet and 0.2% L-Thr). These levels employed were in accordance with NRC (Citation1994) recommendations. The ZnSO4 and L-Thr were procured from Shandong Hemeihua Biotechnology Co., Ltd., Shandong, China.
Table 1. Percent diet composition and nutrient levels of standard diets (as-fed basis).
Measurements and data collection
Feed intake (FI) was recorded and birds were weighed individually on a weekly basis for the replicates. Average daily gain (ADG) and feed conversion ratio (FCR) were calculated during both starter (0–21 days) and finisher (22–42 days) phases. On days 21 and 42, one bird from each cage of each treatment was randomly selected (8 birds per treatment), weighed and humanely sacrificed by the cervical dislocation to collect the intestinal sample including the duodenum, jejunum and ileum. The small intestine was isolated (from the stomach-duodenal pylorus at the ileocecal junction) from the digestive tract and divided into three segments, duodenum (from the pylorus to the distal portion of the duodenal loop covering the pancreas), jejunum (from the distal portion of the duodenal loop to Meckel's diverticulum) and ileum (from Meckel's diverticulum to the ileum-caeca junction). Approximately 1 cm from the middle of the duodenum, jejunum and ileum was incised and then fixed in a 4% paraformaldehyde solution for histological measurements. Additionally, 2 cm of each of the duodenum, jejunum and ileum were collected, opened longitudinally, rinsed with a solution of sodium chloride (NaCl) to remove the digesta, and then put in a sealed tube that is placed in liquid nitrogen for the determination of the gene expression of MUC5AC gene. The immune organs such as Bursa and spleen were also carefully isolated and then weighed to determine the relative weight of the immune organs following the procedure of Chen et al. (Citation2017) with slight modification.
Histological measurement
Approximately 0.5 cm of each of the 4% paraformaldehyde-preserved intestine samples was dehydrated and left under a low-pressure tap running water for 24 h to rinse and remove the digesta. After removal of the digesta, the segments were immersed in paraffin in order to immobilize them in a position that will allow a view of the entire structure of the villi and crypts under a microscope. They were then recovered and using a microtome (Fully automatic rotary paraffin HM355S slicer), 5 microns of each segment was cut and slid, and then the paraffin was removed with xylene, rehydrated and stained with hematoxylin and eosin. The villous height (HV), and the crypt depth (CD) of well-oriented villi were measured by section using an inverted phase contrast fluorescence microscope (model TE2000-5, purchased from Nikon Corporation of Japan) following the procedure of Nighot (Citation2008).
mRNA extraction and real-time polymerase chain reaction
About 100 mg of each sample of the intestine preserved in liquid nitrogen, in separate tubes was taken and used for extraction of the mRNA with TRI-zol reagent (TaKaRa, Nojihigashi 7-4-38 Kusatsu, Shiga, Japan 525-0058) by the method described by Zhang et al. (Citation2014). After extraction, the concentration and quality of mRNA of each sample were checked with U-2450 (Shimadzu Corporation, Kyoto, Japan). Subsequently, mRNA samples were diluted with diethyl pyrocarbonate-treated water (DEPC water) to the correct concentration for transcription. The mixture of the reverse transcriptor ‘Roche Transcriptor Kit’ for obtaining the complementary strand of DNA (cDNA) was carried out according to the indications of the kit and then 9 μl of the reverse transcriptor mixed was added to 11 μl of each diluted mRNA sample. The mRNA mixture and reverse transcriptase were used for transcription to obtain cDNA using the Qualitative Gradient PCR instrument. The primer sequence of the target gene is shown in . Real-time Polymerase Chain Reaction (PCR) was performed using the Quantitative Fluorescence (Q5) Real-Time PCR System (Applied Biosystems, CA, U.S.A.). The data were analysed with QuantStudioTM Design & Analysis Software Primer 5.0 software (SPS Inc., CA, U.S.A.) and synthesized by Shanghai Sangon Company (Shanghai, China). The cDNA samples were diluted before their use for Real-time PCR: 4 μl of the cDNA sample with 16 μl of DEPC water. For Real-time PCR, 2 μl of the diluted cDNA was mixed with 0.5 μl forward (F) MUC5AC primer, 0.5 μl reverse (R) MUC5AC primer, 10 μl of SYBR green and 7 μl of DEPC water. The primer sequence of the target gene is shown in . The prepared mixtures for the PCR were made in sufficient quantities to be deposited in 48 wells of the PCR plate. The gene encoding glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a standard in the remaining 48 wells of the plate. Once the reaction media have been gently deposited in the various wells of the plate, it was centrifuged and then positioned in the thermocycler and the Real-time PCR steps (denaturation: 95°C for the 30s per cycle, hybridization: 60°C for 30 s) were launched in 40 cycles. The 2-ΔΔCT method was used to determine the expression level of MUC5AC relative to the sample (Livak and Schmittgen Citation2001). MUC5AC expression level values were grouped by intestinal portion and group to compare duodenum, jejunum and ileum between treatments.
Table 2. Primer sequences for real-time PCR assay.
Statistical analysis
The data were analysed with R software version 3.5.2 (2018-12-20). The mean values of the different parameters were expressed as mean ± standard error. In the first step, a normality test was performed for each parameter. At the baseline, average daily gain, feed conversion ratio, duodenal crypt depth and the level of expression of the MUC5AC gene mRNA did not follow the normal distribution and the non-parametric Kruskal–Wallis test was used to analyse these data. The difference was significant if p < 0.05. The mean values of FI, FCR, ADG, BW and the weight of the Bursa and spleen were compared by the one-way ANOVA and significant averages were submitted to the post-test of Tukey.
Results
Growth performance
The growth performance of chickens supplemented with dietary zinc and threonine is shown in . Similar results on performance (p > 0.05) were found across all the treatment groups during the growth phase. However, during the starter phase, the zinc-supplemented group (T1) recorded the lowest feed intake (p = 0.012) compared to the control group (T0). The average weight gain and body weight of birds in the T0 and L-Thr supplemented group (T2) groups were significantly improved (p = 0.012 and p = 0.01) relative to ZnSO4 supplemented group (T1) group.
Table 3. Growth performance and relative immune organs weight of broilers at 21 and 42 days of age
Relative immune organs weight
Relative immune organs weights at the starter and finisher phases are shown in . At the starter phase, the weight of the Bursa of the birds in the zinc and threonine-supplemented group (T3) was significantly smaller (p = 0.039) than those in the control group (T0). However, on day 42, the birds that received threonine (T2) in the diet had the highest (p = 0.035) weight of Bursa compared to those that received the zinc-supplemented diet (T1).
Intestinal morphology
The supplementation of zinc, threonine or both in the diet did not affect the villi height in the duodenum of the chickens at starter and finisher phases (p > 0.05) () (). In the jejunum, the supplementation of threonine increased the villi height compared with the control group at the starter (p = 0.010) phase but was not significantly different (p > 0.05) at the finisher phase. At the starter phase, the villi height in the ileum of the chickens that were supplemented with zinc increased when compared with the control group (p = 0.044). Additionally, the villi height in the jejunum of chickens fed the diet supplemented with both zinc and threonine decreased significantly (p = 0.010) when compared with the group of chickens that received only threonine in the diet during the starter phase.
Figure 1. Clear micrograph of intestinal segments of broilers from different treatments (T0, T1, T2 and T3). A1, A2 and A3 represent respectively the duodenum, jejunum, and ileum of chickens from treatment T0. B1, B2 and B3 represent respectively the duodenum, jejunum, and ileum of chickens from treatment T1. C1, C2 and C3 represent respectively the duodenum, jejunum, and ileum of chickens from treatment T2. D1, D2 and D3 represent respectively the duodenum, jejunum, and ileum of chickens from treatment T3.
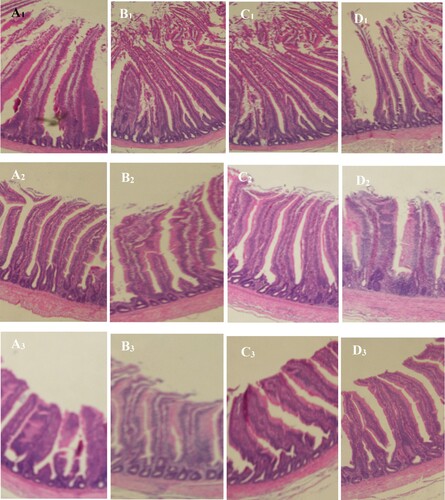
Table 4. Intestinal morphology of broilers at 21 and 42 days of age.
The jejunum of the group of chickens that received the zinc or threonine in supplementation had the lowest (p = 0.004) crypt depth compared with the control group at day 42 of age. Comparably, the group supplemented with both zinc and threonine decreased (p = 0.005) the crypt depth in the duodenum compared with the group of chickens that received the diet supplemented with zinc.
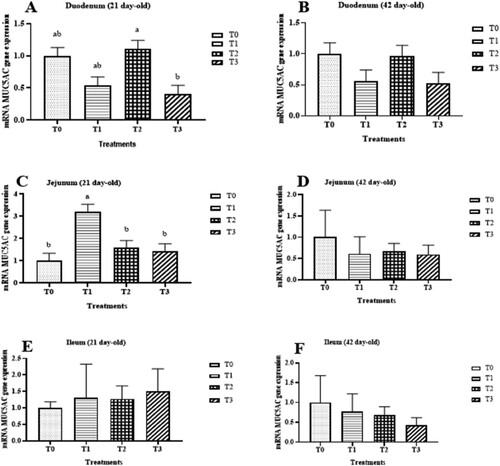
Relative expression of intestinal MUC5AC mRNA
Treatments did not affect the mRNA MUC5AC gene expression in the jejunum and ileum at 0–21 days of age and 22–42 days of age (). However, the level of mRNA MUC5AC gene expression was higher in the duodenum of the group of chickens that received the supplementation of threonine than in the duodenum of chickens that received both zinc and threonine at the starter phase. The group of chickens that received the supplementation of zinc showed a high level of mRNA gene expression in the jejunum of chicken at 21 days of age.
Figure 2. Relative mRNA expression levels of mucin 5ac (MUC5AC) in duodenum at 21 d-old (A), duodenum at 42 d-old (B), jejunum at 21 d-old (C), jejunum at 42 d-old (D), ileum at 21 d-old (E) and ileum 42 d-old (F). Values are represented as means and SEM are represented by vertical bars. Means with different superscripts differ significantly (P < 0.05) (n = 8)
Discussion
In this study, the Zn supplementation as ZnSO4 affected growth performance at a starter phase and this is in line with Zhang et al. (Citation2018) who reported similar result after supplementation of Zn as ZnSO4 at 40 mg/kg in the diet. Zinc is one of the most important trace elements for many physiological functions including growth performance (Park et al. Citation2004; Ogbuewu and Mbajiorgu Citation2023) but when the rate of zinc exceeds the required rate for chickens; feed intake and average daily gain are negatively affected. According to NRC (Citation1994), the recommended diet level of Zn in broilers is 40 g/kg. Reduction in energy intake from low feed intake caused by inadequate dietary zinc is not the primary cause of low growth rate in individuals (MacDonald Citation2000). However, zinc deficiency in chickens causes a disturbance in the concentration of growth hormone (GH) and a reduction in insulin-like growth factor 1 (IGF-I) in the blood (MacDonald Citation2000; Sahin et al. Citation2009). Furthermore, zinc deficiency also affects the membrane signalling system and intracellular second messengers, resulting in disturbance of cell proliferation in response to IGF-I (MacDonald Citation2000). On the other hand, this study showed that zinc supplementation increased the villi height in the jejunum. Wang et al. (Citation2017) obtained the same result after the supplementation of zinc at 80 mg/kg. The authors asserted that zinc supplementation improved the morphology of the intestinal mucous membrane.
L-Thr supplementation has many beneficial effects on broilers, such as increasing growth performance, improving immunity capacity and having a positive effect on the integrity of the intestinal barrier (Dong et al. Citation2017). This study shows that the L-Thr supplementation did not affect the growth performance at the starter and finisher phases. A similar result was obtained by Chen et al. (Citation2017), who reported that the supplementation of Thr at different levels (0, 1 and 3 g/kg) unaffected the growth performance. Our study showed that the L-Thr supplementation improved the villi height in the jejunum at the starter phase, and it is consistent with the finding of Chen et al. (Citation2017). Zinc influences the morphology of the small intestine, improving growth and absorption capacity (Kumar et al. Citation2021). Zinc is also necessary for the division and proliferation of cells, especially for DNA synthesis and mitosis in the mucous membrane (Dosoky et al. Citation2022). Threonine increases intestinal goblet cells and enhances intestinal shape and barrier function (Mao et al. Citation2011). Excessive supplementation of Threonine may lead to a rise of mucin in the colon, which shields the intestinal epithelium from digestion enzymes and acids (Gómez-Guillén and Montero Citation2021). Mucins, which are composed of proline, serine, and Threonine, serve as a physical barrier against pathogens and allow nutrients into the digestive tract (Paone and Cani Citation2020).
The heavier weight observed in the lymphatic tissue (bursa) of the birds supplemented with threonine shows the positive effect of threonine in enhancing immune capacity. Bursa of Fabricius actively contributes to the manufacture of antibodies by promoting the differentiation and amplification of B lymphoid progenitors in its follicular microenvironment, which guarantees the B cell's functional development (Ratcliffe and Härtle Citation2022). It has been reported that in ovo injection of threonine resulted in heavier bursa weight compared to non-injected (Bhanja et al. Citation2012).
Mucin glycoproteins have been involved in mucosal formulation (Ospina-Rojas et al. Citation2013) and intestinal immunity function (Dong et al. Citation2017). Some nutrients level in the diet improves mucin production in the small intestinal by adding Zn at 120 mg/kg (Wen et al. Citation2017) and L-Thr at 0.75% (Azzam and El-Gogary Citation2015). This study showed that the zinc supplementation impaired the FI and ADG, however, the intestinal mucosal morphology was not affected at the starter phase in the duodenum and jejunum. Given the intestinal mucosal morphology is not affected by the Zn supplementation and the FI was affected negatively, that would decrease the ADG. Kim and Patterson (Citation2004) had shown that the Zn supplementation at 100 mg/kg negatively affected the growth performance. On the other hand, our study showed that the Zn supplementation improves the MUC5ac gene expression in the jejunum of the chickens at 21 days. The finding of Wen et al. (Citation2017) supports our results which revealed that Zn level in diet affects MUC5ac gene expression in the jejunum.
The L-Thr supplementation improves MUC5ac gene expression and the intestinal mucosal morphology respectively in the duodenum and jejunum also. The weight of the immunity organs like the Bursa was The weight of the immunity organs like the Bursa was higher with L-Thr supplementation compared to zinc supplementation at the finisher phase. The effects on the intestinal mucosal morphology in this study conform with the results of Debnath et al. (Citation2019) who reported that L-Thr supplementation improved the intestinal morphology through the increasing villus height, crypt depth, villus surface area and mean goblet cell number/villus. The increase in Bursa weight in our study showed that L-Thr supplementation could affect the immunity of chickens.
In this study, the supplementation of zinc and threonine in the same diet adversely affected growth performance, immunity organ weight, intestinal mucosal morphology and MUC5ac mRNA gene expression. That result confirms the chelation link between zinc and threonine illustrated by Te-Jung et al. (Citation2018). It could therefore be inferred that the chelation’s link acted on the availability of both zinc and threonine thereby decreasing the beneficial action of each of them on growth performance, intestinal mucosal morphology and the MUC5ac mRNA gene expression.
Conclusion
Conclusively, zinc or threonine supplementation improved intestinal morphology but overall did not influence growth performance. The combinatory effect of zinc and threonine interacted negatively as one decreased the positive action of the other on the intestinal morphology and mucin MUC5ac gene expression in the duodenum, jejunum and ileum. Further study is warranted to bridge the knowledge gap on the impact of zinc and threonine on other tight-junction (TJ) proteins and on digestive enzymes.
Acknowledgements
The authors are grateful to Jerome Parobali, Wang Minghui, Jiangxue Cai and Zhao Mei for their support and assistance with the animal experiment and guidance with laboratory analysis.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data used is available and can be provided upon reasonable request.
Additional information
Funding
References
- Abreu MT. 2010. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat. Rev. Immunol. 10:131–144. doi:10.1038/nri2707.
- Ahmadi F, Ebrahimnezhad Y, Sis NM, Ghalehkandi JG. 2013. The effects of zinc oxide nanoparticles on performance, digestive organs and serum lipid concentrations in broiler chickens during starter period. International Journal of Bioscience. 3(7):23–29. doi:10.12692/ijb/3.7.23-29.
- Azzam MMM, El-Gogary MR. 2015. Effects of dietary threonine levels and stocking density on the performance, metabolic status and immunity of broiler chickens - scialert responsive version. Asian J. Anim. Vet. Adv. 10:215–225. doi:10.3923/ajava.2015.215.225.
- Bhanja SK, Baran Mandal A, Agarwal SK, Majumdar S. 2012. Modulation of post hatch-growth and immunocompetence through in ovo injection of limiting amino acids in broiler chickens. Indian J Anim Sci. 82(9):993–998.
- Bortoluzzi C, Rochell SJ, Applegate T. 2018. Threonine, Arginine, and Glutamine: influences on intestinal physiology, immunology, and microbiology in broilers. Poult. Sci. 97:937–945. doi:10.3382/ps/pex394.
- Byrne L, Murphy RA. 2022. Relative bioavailability of trace minerals in production animal nutrition: A review. Animals (Basel). 12(15):1981. doi:10.3390/ani12151981.
- Chen YP, Cheng YF, Li XH, Yang WL, Wen C, Zhuang S, Zhou YM. 2017. Effects of threonine supplementation on the growth performance, immunity, oxidative status, intestinal integrity, and barrier function of broilers at the early age. Poult. Sci. 96:405–413. doi:10.3382/ps/pew240.
- Debnath BC, Biswas P, Roy B. 2019. The effects of supplemental threonine on performance, carcass characteristics, immune response and gut health of broilers in subtropics during pre-starter and starter period. J. Anim. Physiol. Anim. Nutr. 103:29–40. doi:10.1111/jpn.12991.
- Dong XY, Azzam MMM, Zou XT. 2017. Effects of dietary threonine supplementation on intestinal barrier function and gut microbiota of laying hens. Poult. Sci. 96:3654–3663. doi:10.3382/ps/pex185.
- Dosoky WM, Al-Banna AA, Zahran SM, Farag SA, Abdelsalam NR, Khafaga AF. 2022. Zinc oxide nanoparticles induce dose-dependent toxicosis in broiler chickens reared in summer season. Environmental Science and Pollution Research. 29(36):54088–54107. doi:10.1007/s11356-022-19156-4.
- Ducatelle R, Eeckhaut V, Haesebrouck F, Immerseel F. 2015. A review on prebiotics and probiotics for the control of dysbiosis: Present status and future perspectives. Anim. Int. J. Anim. Biosci. 9:43–48. doi:10.1017/S1751731114002584.
- Duffy R, Yin M, Redding LE. 2023. A review of the impact of dietary zinc on livestock health. Journal of Trace Elements and Minerals. 5:100085. doi:10.1016/j.jtemin.2023.100085.
- Farhadi JS, Moravej H, Ghaffarzadeh M, Mohammad BE. 2021. Comparison of zinc sulfate and zinc threonine based on zn bioavailability and performance of broiler chicks. Biol Trace Elem Res. 199:2303–2311. doi:10.1007/s12011-020-02354-x.
- Gómez-Guillén MC, Montero MP. 2021. Enhancement of oral bioavailability of natural compounds and probiotics by mucoadhesive tailored biopolymer-based nanoparticles: A review. Food Hydrocolloids. 118:106772. doi:10.1016/j.foodhyd.2021.106772.
- Hojyo S, Fukada T. 2016. Roles of zinc signaling in the immune system. J. Immunol. Res. 2016:1–21. doi:10.1155/2016/6762343.
- Ibrahim D, Ali H, El-Mandrawy S. 2017. Effects of different zinc sources on performance, bio distribution of minerals and expression of genes related to metabolism of broiler chickens. Zagazig Vet. J. 45:292–304. doi:10.5281/zenodo.1000462.
- Janice J, Jąkalski M, Makałowski W. 2013. Surprisingly high number of Twintrons in vertebrates. Biology Direct. 8:1–5. https://doi.org/10.1186/1745-6150-8-4.
- Kidd MT, Lerner SP, Allard JP, Rao SK, Halley JT. 1999. Threonine needs of finishing broilers: growth, carcass, and economic responses. J. Appl. Poult. Res. 8:160–169. doi:10.1093/japr/8.2.160.
- Kim WK, Patterson PH. 2004. Effects of dietary zinc supplementation on broiler performance and nitrogen loss from manure. Poult. Sci. 83:34–38. doi:10.1093/ps/83.1.34.
- Kumar A, Hosseindoust A, Kim M, Kim K, Choi Y, Lee S, Lee S, Lee J, Cho H, Kang WS, Chae B. 2021. Nano-sized zinc in broiler chickens: effects on growth performance, zinc concentration in organs, and intestinal morphology. The journal of poultry science. 58(1):21–29. doi:10.2141/jpsa.0190115.
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 25:402–408. doi:10.1006/meth.2001.1262.
- MacDonald RS. 2000. The role of zinc in growth and cell proliferation. J Nutr. 130(5):1500S–1508S. doi:10.1093/jn/130.5.1500s.
- Mahmoud UT, Abdel-Mohsein HS, Mahmoud MAM, Amen OA, Hassan RIM, Abd-El-Malek AM, Osman MA. 2020. Effect of zinc oxide nanoparticles on broilers’ performance and health status. Trop Anim Health Prod. 52:2043–2054. doi:10.1007/s11250-020-02229-2.
- Mao X, Zeng X, Qiao S, Wu G, Li D. 2011. Specific roles of threonine in intestinal mucosal integrity and barrier function. Frontier Bioscience. E3(4):1192–1200. doi:10.2741/e322.
- National Research Council. 1994. National research council nutrient requirements of poultry. 9th rev. ed. Washington, DC: National Academy Press.
- National Research Council. 2001. Nutrient requirements of poultry. 9th rev. ed. Washinton, DC: National Academic Press10.17226/2114.
- Nichols NL, Bertolo RF. 2008. Luminal threonine concentration acutely affects intestinal mucosal protein and mucin synthesis in piglets. J. Nutr. 138:1298–1303. doi:10.1093/jn/138.7.1298.
- Nighot PK. 2008. Role of ClC-2 and NHE transport proteins in intestinal mucosal barrier. North Carolina: North Carolina State University.
- Ogbuewu IP, Mbajiorgu CA. 2023. Potentials of dietary zinc supplementation in improving growth performance, health status, and meat quality of broiler chickens. Biol Trace Elem Res. 201:1418–1431. doi:10.1007/s12011-022-03223-5.
- Ospina-Rojas IC, Murakami AE, Oliveira CAL, Guerra AFQG. 2013. Supplemental glycine and threonine effects on performance, intestinal mucosa development, and nutrient utilization of growing broiler chickens. Poult Sci. 92:2724–2731. doi:10.3382/ps.2013-03171.
- Paone P, Cani PD. 2020. Mucus barrier, mucins and gut microbiota: the expected slimy partners? Gut. 69(12):2232–2243. doi:10.1136/gutjnl-2020-322260.
- Park SY, Birkhold SG, Kubena LF, Nisbet DJ, Ricke SC. 2004. Review on the role of dietary zinc in poultry nutrition, immunity, and reproduction. Biol. Trace Elem. Res. 101:147–164. doi:10.1385/BTER:101:2:147.
- Raach-Moujahed A, Haddad B. 2013. Performance, livability, carcass yield and meat quality of Tunisian local poultry and fast-growing genotype (Arbor Acres) fed standard diet and raised outdoor access. J Anim Prod Adv. 3(3):75–85. doi:10.5455/japa.20130305122741.
- Ratcliffe MJ, Härtle S. 2022. B cells, the bursa of Fabricius, and the generation of antibody repertoires. In: Toronto C. M., editor. Avian immunology. Canada: Academic Press; p. 71–99.
- Sahin K, Sahin N, Kucuk O, Hayirli A, Prasad AS. 2009. Role of dietary zinc in heat-stressed poultry: a review. Poult Sci. 88(10):2176–2183. doi:10.3382/ps.2008-00560.
- Sandberg FB, Emmans GC, Kyriazakis I. 2007. The effects of pathogen challenges on the performance of naïve and immune animals: the problem of prediction. Anim. Int. J. Anim. Biosci. 1:67–86. doi:10.1017/S175173110765784X.
- Te-Jung L, Chi-Ying FH, Chiun-Jye Y, Yuan-Chii L, Tzeng-Horng L, Wen-Chang C, Te-Ling L, Wen-Yih J, Ming-Derg L. 2005. Zinc ion acts as cofactor for serine/threonine kinase MST3 and has a distinct role in autophosphorylation of MST3. J Inorg Biochem. 99:1306–1313. doi:10.1016/j.jinorgbio.2005.03.003.
- Tugay A, Ferda O, Hatice H. 2009. Threonine requirement of broiler from 22-42 Days [WWW Document].
- Wang C, Zhang L, Su W, Ying Z, He J, Zhang L, Zhong X, Wang T. 2017. Zinc oxide nanoparticles as a substitute for zinc oxide or colistin sulfate: Effects on growth, serum enzymes, zinc deposition, intestinal morphology and epithelial barrier in weaned piglets. PLoS One. 12(7):e0181136. doi:10.1371/journal.pone.0181136.
- Wen M, Zhao H, Liu G, Chen X, Wu B, Tian G, Cai J, Jia G 2017. Effect of zinc supplementation on growth performance, inestinal development, and intestinal barrier-related gene expression in Pekin ducks. Biol Trace Elem Res. 183:351–360. doi:10.1007/s12011-017-1143-7.
- Zhang H, Chen Y, Li Y, Yang L, Wang J, Wang T. 2014. Medium-chain TAG attenuate hepatic oxidative damage in intra-uterine growth-retarded weanling piglets by improving the metabolic efficiency of the glutathione redox cycle. Br. J. Nutr. 112:876–885. doi:10.1017/S000711451400155X.
- Zhang TY, Liu JL, Zhang JL, Zhang N, Yang X, Qu HX, Xi L, Han JC. 2018. Effects of dietary zinc levels on the growth performance, organ zinc content, and zinc retention in broiler chickens. Braz J Poul Sci. 20(01):127–132. doi:10.1590/1806-9061-2017-0604.
- Zhao C-Y, Tan S-X, Xiao X-Y, Qiu X-S, Pan J-Q, Tang Z-X. 2014. Effects of dietary zinc oxide nanoparticles on growth performance and antioxidative status in broilers. Biol. Trace Elem. Res. 160:361–367. doi:10.1007/s12011-014-0052-2.
