ABSTRACT
The effects of C-type natriuretic peptide (CNP) pre-treatment on the in vitro maturation (IVM) and developmental competence of yak (Bos grunniens) oocytes were evaluated. The results showed that 100 nM CNP could effectively maintain meiotic arrest of yak oocytes in vitro within 6 h. Yak oocytes matured in two-step IVM (pre-IVM with 100 nM CNP for 6 h followed by conventional IVM for 28 h) yielded a significantly (P < .05) increased cleavage rate, blastocyst rate after in vitro fertilization with cattle spermatozoa compared to the conventional one-step IVM method. The density of transzonal projections (TZPs) decreased after 6 h standard IVM (P < .05), whereas, COCs pre-IVM with 100 nM CNP for 6 h maintained the same density of TZPs compared to the fresh COCs. This pre-IVM resulted in lesser reactive oxygen species level (P < .05). Two-step IVM resulted in the increased glutathione levels, mRNA expression levels of BCL2, BMP15, and GDF9 (P < .05) in oocytes. In conclusion, these results indicate that CNP treatment (100 nM, 6 h) before IVM could enhance the maturation of yak oocytes and developmental competence of their crossbred embryos. This may have important applications in in vitro production of yak crossbred embryos.
1. Introduction
Yaks (Bos grunniens) are the principal livelihood provider for people in the Qinghai-Tibetan Plateau where few other domestic animal species live at such high altitude, predominantly due to the exposure to hypoxic conditions (Wiener et al. Citation2003). However, their fertility (Zi Citation2003), meat performance (Cao et al. Citation2019) and milk performance (Xia et al. Citation2018) are inferior to those of improved cattle breeds. Hybridizing of yak females with improved breeds of beef and dairy breeds is widely practised to increase meat and milk output of their progeny (F1). F1 hybrids have greater meat production and milk performance than yaks (Wiener et al. Citation2003; Cao et al. Citation2019). However, the hybrids are only mated back to either yak or cattle males because the F1 males are sterile, but later generations of backcrosses have poor performance (Wiener et al. Citation2003). The first successful live offspring production following transfer of hybrid F1 embryos derived from in vitro fertilization (IVF) between yak oocytes and Jersey spermatozoa to F1 females has been obtained by Xiong et al. (Citation2019a), which provides a novel approach to produce valuable offspring by F1 females, but the IVF system is still not well developed compared with cattle (Xiong et al. Citation2018; Zi et al. Citation2018; Yang et al. Citation2019; Xiong et al. Citation2019a).
The fertilization and normal development of the embryo depend on the cytoplasmic and nuclear maturity of the oocyte (Strączyńska et al. Citation2022). During in vitro maturation (IVM), oocytes resume meiosis before reaching optimal cytoplasmic maturity (Edwards Citation1965). This asynchronous maturation between nuclear and cytoplasm decreases the subsequent developmental competence of oocytes (Blondin et al. Citation1997). The cumulus cells (CCs) of the follicle maintain contact with oocytes using transzonal projections (TZPs), which penetrate through the zona pellucida reaching the oocyte surface (Matzuk et al. Citation2002). The bidirectional exchange of ions and small molecules between the oocyte and CCs through TZPs is essential for the oocyte to achieve cytoplasmic maturation (Russell et al. Citation2016; Yin et al. Citation2020). Reactive oxygen species (ROS) accumulation during IVM is associated with apoptosis and reduced developmental competence in oocytes and embryos (Rodríguez-Nuevo et al. Citation2022; Zhang et al. Citation2022), while glutathione (GSH) is involved in oocyte protection against ROS-induced oxidative damage during IVM (Xiong et al. Citation2018; Silva and Silva Citation2022). There is a high positive correlation between the GSH concentration in IVM oocytes and the blastocyst development rate of bovine oocytes (Hidaka et al. Citation2018). In addition, numerous genes play critical roles in oocyte maturation, notably, growth differentiation factor 9 (GDF9), bone morphogenetic protein 15 (BMP15) (Xiong et al. Citation2019b; Morikawa et al. Citation2021), DNA methyltransferase 1 (DNMT1) (Pan et al. Citation2019; Xiong et al. Citation2022), anti-apoptotic (BCL2) and pro-apoptotic (BAX) genes (Veshkini et al. Citation2018).
A temporary delay in meiosis resumption during IVM could have beneficial effects. C-type natriuretic peptide (CNP), which is expressed at high levels in granulosa cells (GCs), can temporarily maintain the meiotic arrest of oocytes (Zhang et al. Citation2010; Xi et al. Citation2018, Citation2021). High levels of cAMP within the oocyte maintain meiotic arrest. CNP acts on its receptor (NPR2) in cumulus cells to generate cGMP, cyclic GMP then enters the oocyte and regulates the levels of cAMP, thus maintaining oocytes meiotic arrest (Zhang et al. Citation2010; Xi et al. Citation2018). A period treatment in pre-maturation culture media containing CNP has been shown to improve maturation and developmental competence of oocytes in vitro from mouse (Wei et al. Citation2017), pigs (Zhang et al. Citation2017a), goats (Zhang et al. Citation2015; Soto-Heras et al. Citation2019a), cattle (Zhang et al. Citation2017b; Soto-Heras et al. Citation2019b; Jia and Wang Citation2020), sheep (Zhang et al. Citation2018) and human (Sanchez et al. Citation2019). However, the effect of CNP on the maturation and developmental competence of yak oocytes remains unclear. Therefore, the aim of this study was to design a pre-IVM using CNP that sustains meiotic arrest and CC-oocyte communication to improve yak oocyte IVM and embryo developmental competence.
2. Materials and methods
2.1. Animals and chemicals
All animal procedures were conducted according to the guiding principles of the Animals Care and Ethics Committee of Southwest Minzu University (Approval code: 117 SMU-CAVS-220601001).
Unless otherwise stated, all chemicals and reagents used were purchased from Sigma-Aldrich (St. Louis, MO, USA).
2.2. Oocyte recovery and in vitro maturation (IVM)
Ovaries from female yaks were obtained at a local slaughterhouse (Chengdu, China) from October to December, maintained at 35–37°C in Dulbecco’s phosphate-buffered saline (DPBS) supplemented with 1% pen-strep and transported to the laboratory within 3 h after slaughter. Cumulus-oocyte complexes (COCs) were aspirated from healthy follicles (2–8 mm diameter) using an 18-gauge needle attached to a 10-mL syringe containing 1 mL aspiration medium – DPBS supplemented with 6 mg/mL bovine serum albumin (BSA) and 5% fetal calf serum (FCS, Gibco, Life Technologies Inc., Grand Island, NY, USA) and collected under a stereomicroscope (20×, Leica MZ75, Germany). The COCs were rinsed three times in aspiration medium and twice in IVM medium – TCM 199 supplemented with 10% FCS, 1 μg/mL oestradiol-17β, 0.09 mg/mL l-glutamine, 23 µg/mL sodium pyruvate, 1% pen-strep (Zi et al. Citation2018), 0.05 IU/mL FSH (Vetoquinol SA, Lure cedex, France), and 0.05 IU/mL LH (Cedarlane Labs, Canada) (Blaschka et al. Citation2019). Approximately 30 COCs were placed in each 600-μL of IVM drop covered with 300 μL mineral oil and were matured at 38.5°C in a humidified incubator with 5% CO2 (Zi et al. Citation2018). The culture period of oocytes in depended on the experiment group (Section 2.8).
2.3. In vitro embryo production (IVEP)
IVEP was performed according to the previously described procedure (Zi et al. Citation2018). Briefly, after IVM, the COCs were further washed three times in IVFTM (Vitrolife, Sweden), and then approximately 30 COCs were transferred to a 70-μL microdrop of IVFTM covered with mineral oil per drop. Frozen semen from the same Jersey bull was thawed and motile spermatozoa were selected by the swim-up technique in IVFTM. Sperm were then added to produce a final concentration of 2 × 106 sperms/mL in IVF microdrop. After a period of 24 h coincubation, presumptive zygotes were cultured in 50-μL drops of G1™ for 72 h and then cultured in G2TM (Vitrolife, Sweden) covered with mineral oil in a humidified incubator with 90% N2 and 5% CO2 in air. Each culture droplet contained approximately 20 embryos. Cleavage was recorded at 48 h post-insemination (hpi) and blastocyst rate at 168 hpi.
2.4. Assessment of meiotic progression
After three washes with DPBS, CCs were removed from COCs by using hyaluronidase (0.1%) in PBS and repeated pipetting for 1 min. Denuded oocytes were fixed in paraformaldehyde (4%) for 30 min, permeabilized using 0.2% Triton-100 in PBS for 30 min (Jia and Wang Citation2020), sealed with Vaseline at 37°C for 1 h, and stained for 10 min with Hoechst 33342 (10 μg/mL) (Zhang et al. Citation2015). Chromatin configurations were assessed using a confocal laser scanning microscope (ZISS, LSM880, Germany). Chromatin configurations were classified as follows (Zhang et al. Citation2018): immature or germinal vesicle (GV) – the vesicle was clearly visible; resumption of meiosis or GV break down (GVBD) – the chromatin was dispersed and initiating condensation; first metaphase (MI) stage, when chromosomes were condensed and present in equatorial view; and mature or second metaphase (MII) stage – manifested by the presence of chromosomes in the second metaphase plate, with the first polar body (PB) extruded.
2.5. Assessment of transzonal projections (TZPs)
TZPs were assessed by fluorescein isothiocyanate (FITC) conjugated phalloidin, which stains actin filaments using an adapted protocol from Soto-Heras et al. (Citation2019a). Briefly, 10 COCs for each group were denuded, then fixed in cold paraformaldehyde (4%, v/v) for 20 min. At room temperature, COCs were permeabilized in 0.25% Triton X-100 in PBS with 4 mg/mL BSA for 30 min at 38.5°C in an atmosphere of 5% CO2 in humidified air and stained with 5 μg/mL phalloidin-FITC solution in PBS with 4 mg/mL BSA for 60 min. Three washes with 0.4% PBS-BSA were performed between each step. COCs were mounted in a poly-l-lysine-treated coverslip with a drop of Vectashield mounting medium (Vector Laboratories, CA, USA) within a reinforcement ring, and kept at –20°C until analysis. TZPs fluorescence signals were examined with a confocal laser scanning microscope (ZISS, LSM880, Germany) with λex 520 nm and λem 488 nm emission. Images were processed with Image J software. TZPs were observed as continuous filaments between the oocyte and CCs. The TZP density was determined by measuring the mean pixel intensity within the zona pellucida, delimited by the polygon selection tool.
2.6. Assessment of reactive oxygen species (ROS) and glutathione (GSH) levels
Intra-oocyte ROS level was measured by staining with 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA; Invitrogen, CA, USA) and intra-oocyte GSH content was measured with monochlorobimane (MCB) according to previously described procedures (Soto-Heras et al. Citation2019a). Briefly, oocytes were denuded, washed three times in 0.4% BSA-PBS and incubated for either 20–30 min with H2DCF-DA (10 μM) at 37°C or 15 min with MCB (12.5 μM) in 0.4% BSA-PBS at 38.5°C. Oocytes were washed in 0.1% BSA-PBS for two to three times, and immediately transferred with a 10-μL drop to a slide and observed under a confocal laser scanning microscope (ZISS, LSM880, Germany) with 100× magnification (H2DCF-DA: λex 460 nm, λem 525 nm; MCB: λex 370 nm, λem 460 nm). Average fluorescence intensity per oocyte was measured with Image J software and normalized to the background average intensity.
2.7. RNA extraction and quantitative real-time RT–PCR
Three batches of yak oocytes which were completely denuded of CCs via gentle pipetting with a fine bore glass pipette in hyaluronidase (0.1%) were collected and washed three times in DPBS, then immediately frozen and stored at −80°C until use. Total RNA was isolated from oocytes with Cells-to-CT kit (Ambion) following manufacturer’s instructions. Extracted RNA was treated with DNase I to exclude any potential genomic DNA contamination. The isolated RNA was entirely and immediately used for reverse transcription polymerase chain reaction (RT–PCR) using RNA reverse transcription kit (Thermo Fisher Scientific, Invitrogen, USA). RT–qPCR was performed by a real-time Detection System (Bio-Rad, CFX96, USA) along with the SYBR Green Master Mix (Thermo Fisher Scientific, Invitrogen, USA) according to the manufacturer’s protocol using specific primers for each gene (). Using GAPDH as a reference gene, RT–qPCR data were analysed by the 2−ΔΔct method (Livak and Schmittgen Citation2001).
Table 1. Information of primers used in RT–qPCR.
2.8. Experimental design
2.8.1. Exp. 1: Effect of CNP concentration and culture time on germinal vesicle (GV) rate
To determine the effect of CNP pre-treatment yak oocytes on nuclear meiosis, dose–response and time-dependent effect of CNP pre-treatment on meiotic resumption of yak oocytes was conducted. CNP concentrations and culture times were based on previous reports in other animal species (Zhang et al. Citation2015; Wei et al. Citation2017; Zhang et al. Citation2018). The pre-IVM medium is composed of IVM medium and different concentrations of CNP. Yak COCs were cultured in pre-IVM medium with 0 (control), 50, 100 or 200 nM human CNP for 0, 4, 6, 8, 12, 16 or 24 h in each concentration of CNP medium. At the end of each culture period, oocytes with germinal vesicle (GV) were evaluated. A total of 344–364 oocytes were assessed per treatment and time point (three replicates).
2.8.2. Exp. 2: Effect of CNP pre-IVM on oocyte maturation and embryo development
To determine the effect of CNP pre-treatment yak oocytes on oocyte maturation, dose–response of CNP pre-treatment and time-dependent effect of the subsequent IVM on maturation and embryo development of yak oocytes were evaluated. CNP concentrations and culture times were based on previous reports in other animal species (Zhang et al. Citation2015; Wei et al. Citation2017; Zhang et al. Citation2017b, Citation2018). Yak COCs were cultured in pre-IVM medium with 0 (control), 50, 100 or 200 nM CNP for 6 h, followed by 24, 26 or 28 h IVM for each concentration of CNP medium. At the end of each culture period, a proportion of oocytes were randomly examined for evidence of maturation (the presence of chromosomes in the second metaphase plate, with the first PB extruded) and the remaining were used for IVF and IVC to investigate the effect of pre-treatment with CNP on the developmental competence of yak oocytes.
2.8.3. Exp. 3: Effect of CNP pre-IVM on cumulus-oocyte communication
A two-step IVM system was compared to control IVM. Based on the results of Exp. 2, for two-step IVM, yak COCs were cultured for 6 h in pre-IVM medium with 100 nM CNP, followed by 28 h standard IVM medium. For control IVM, oocytes were cultured for 24 h in a standard IVM medium (Zi et al. Citation2018). COCs were fixed and stained with phalloidin-FITC and their TZPs structure and density were assessed at different time points: after oocyte recovery (0 h IVM), 6 h IVM in standard IVM medium (6 h IVM), 6 h pre-IVM in IVM medium with 100 nM CNP (6 h pre-IVM), 24 h IVM in standard IVM medium (24 h IVM), and 6 h pre-IVM plus 28 h IVM in standard IVM medium (two-step IVM).
2.8.4. Exp. 4: Effect of two-step IVM on intra-oocyte levels of ROS and GSH
To evaluate the effect of treatment with CNP before IVM on the ROS and GSH content of yak oocytes, the intra-oocyte levels of ROS and GSH were compared at different time points of IVM: 6 h IVM, 6 h pre-IVM, 24 h IVM, and 6 h pre-IVM plus 28 h IVM.
2.8.5. Exp. 5: Effect of CNP pre-IVM on gene expression of oocytes
The mRNA expression levels BAX, BCL2, DNMT1, BMP15 and GDF9 in yak oocytes were assessed by RT-qPCR after follicle 0 h IVM, 24 h IVM, and 6 h pre-IVM plus 28 h IVM in order to understand the molecular mechanism by which CNP pre-IVM regulate maturation of yak oocytes.
2.9. Statistical analyses
All experiments were performed at least three times, and values are shown as mean ± standard error (SE). Nuclear stage and maturation rate were analysed with two-way ANOVA followed by Tukey’s multiple-comparison test (SPSS, Chicago, IL, USA). Embryo production, ROS content, GSH levels, TZPs density, mRNA expression levels were analysed with one-way ANOVA followed by Tukey’s multiple-comparison test. Data from the nuclear stage and embryo development did not present a normal distribution and were square root arcsine transformed prior to ANOVA. Results were considered statistically significant when P < .05.
3. Results
3.1. CNP treatment induces meiotic arrest of yak oocytes
GVBD is a significant indicator of the resumption of meiosis in oocytes. To test the inhibitive effect of CNP on the meiotic resumption of yak oocytes, COCs were cultured in a medium containing different concentrations (0, 50, 100 and 200 nM) of CNP, and the proportion of oocytes with GV was evaluated after 0, 4, 6, 8, 12, 16, 24 h of culture, respectively. For COCs freshly isolated from ovaries (0 h), there were approximately 79–83% oocytes with GV. After 4 h of culture, the proportions of oocytes with GV in the control group significantly decreased (P < .05), but the GV oocyte rates in CNP groups did not decrease (P > .05) compared with freshly isolated oocytes. After 6 h of culture, in the 100 nM CNP groups, the proportion of oocytes with GV did not decrease compared with freshly isolated oocytes (P > .05) and was significantly higher than that in the other groups (P < .05). After 8 h of culture, the GV oocyte rates in all of the groups were lower than those in freshly isolated oocytes (P < .05). After IVM for 24 h, only approximately 25–32% oocytes were arrested in the GV stage (). Therefore, we found that 100 nM CNP pre-IVM for 6 h could effectively maintain meiotic arrest of yak oocytes.
Table 2. Effect of C-type natriuretic peptide (CNP) pre-IVM on germinal vesicle rate of yak oocytes.
3.2. CNP treatment improves maturation and embryo development of yak oocyte
To investigate the effect of CNP treatments before IVM on the maturation and embryo development of yak oocytes, immature yak COCs were cultured in pre-IVM medium with 0, 50, 100 or 200 nM CNP for 6 h, followed by 24, 26 or 28 h conventional IVM. The result showed that the nuclear maturation (MII) rate (79.42 ± 0.58%) was greater (P < .05) when yak oocytes were cultured in pre-IVM medium with 100 nM CNP for 6 h followed by 28 h conventional IVM than the other treatments of CNP concentrations and IVM duration (). After IVF with Jersey spermatozoa and IVC of embryos, cleavage rate (63.76 ± 0.36%) and blastocyst rate (32.97 ± 0.36%) were also greater (P < .05) in this treatment than other treatments ().
Table 3. Maturation rate for 24–28 h IVM after 6 h pre-IVM with C-type natriuretic peptide (CNP).
Table 4. Effects of C-type natriuretic peptide (CNP) concentration in pre-IVM for 6 h on embryo development.
3.3. CNP treatment maintains TZP density of yak oocyte
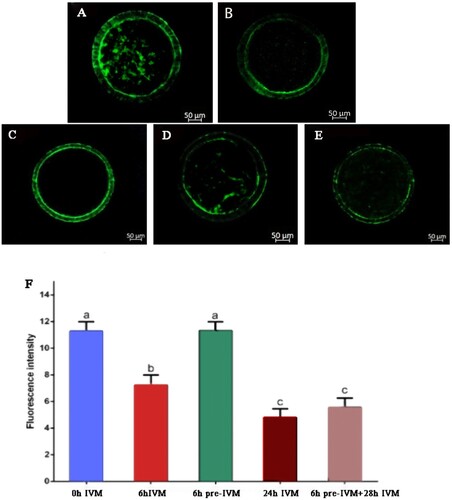
The TZP density of yak oocytes was evaluated with Phalloidin-FITC staining after oocyte collection (control, 0 h IVM), 6 h IVM, 6 h pre-IVM, 24 h IVM, and 6 h pre-IVM plus 28 h standard IVM. Data showed that the density of TZPs decreased after 6 h standard IVM (P < .05), whereas, after 6 h pre-IVM, COCs presented the same density of TZPs (P > .05) compared to the freshly isolated oocytes (0 h IVM) group. There was a decrease in TZP density at the end of 24 h IVM and 6 h pre-IVM plus 28 h IVM, compared to what occurred with 0 h IVM, 6 h IVM and 6 h pre-IVM (P < .05), but there was not significant difference in TZP density between 24 h standard IVM and 6 h pre-IVM plus 28 h standard IVM though it was slightly greater in 6 h pre-IVM plus 28 h standard IVM group ().
Figure 1. Effect of C-type natriuretic peptide treatment on transzonal projection population of yak oocyte. Phalloidin-FITC average fluorescence intensity in the zona area of COCs: (A) after recovery (0 h IVM), (B) 6 h of conventional IVM (6 h IVM), (C) 6 h pre-IVM with 100 nM CNP (6 h pre-IVM), (D) 24 h conventional IVM (24 h IVM), and (E) 6 h pre-IVM with 100 nM CNP followed by 28 h IVM (6 h pre-IVM + 28 h IVM). (F) Average pixel intensity in the region between the oocyte and cumulus cells was quantified with Image J. At least 10 COCs per group were assessed in four replicates. Each bar represents mean ± standard error and analysed with one-way ANOVA followed by Tukey’s multiple-comparison test. Values with different superscripts (a, b, and c) are significantly different (P < .05).
3.4. CNP treatment reduces ROS abundance and increases GSH content
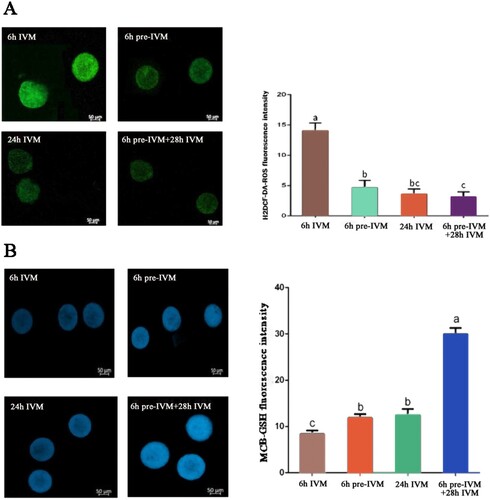
Intra-oocyte ROS level and intra-oocyte GSH content were measured after 6 h IVM, 6 h pre-IVM, 24 h IVM, and 6 h pre-IVM plus 28 h standard IVM. The results showed that 6 h pre-IVM resulted in lesser ROS level than 6 h IVM, 24 h IVM, and 6 h pre-IVM plus 28 h standard IVM (P < .05). 6 h pre-IVM plus 28 h standard IVM produced similar ROS level to 24 h IVM (P < .05, (A)). In contrast, 6 h pre-IVM resulted in greater GSH content than 6 h IVM (P < .05). 6 h pre-IVM plus 28 h standard IVM produced greater GSH than 24 h IVM (P < .05, (B)).
Figure 2. Effect of C-type natriuretic peptide treatment on GSH and ROS levels of yak oocyte. (A) H2DCF-DA-ROS average fluorescence intensity per oocyte after 6 h of conventional IVM (6 h IVM), 6 h pre-IVM with 100 nM CNP (6 h pre-IVM), 24 h conventional IVM (24 h IVM), and 6 h pre-IVM with 100 nM CNP followed by 28 h IVM (6 h pre-IVM + 28 h IVM). (B) MCB-GSH average fluorescence intensity per oocyte following IVM. A total of 30 oocytes were assessed per group (3 replicates). Each bar represents mean ± standard error and analysed with one-way ANOVA followed by Tukey’s multiple-comparison test. Values with different superscripts (a, b, and c) are significantly different (P < .01).
3.5. Effect of pre-IVM with CNP on gene expression of oocytes
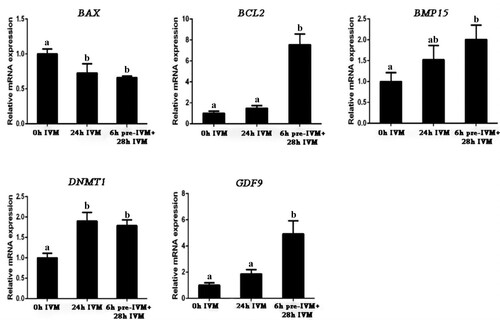
Gene expression levels in oocytes and embryos were evaluated by RT–qPCR. Compared to uncultured oocytes (0 h IVM), both standard IVM 24 h and 6 h pre-IVM plus 28 h standard IVM downregulated the expression of BAX in oocytes (P < .05), but upregulated expression of DNMT1 (P < .05). Two-step IVM upregulated expression of BCL2 and GDF9 (P < .05). The expression of BAX, DNMT1 and BMP15 were not different between 24 h standard IVM and 6 h pre-IVM plus 28 h standard IVM (P > .05, ).
Figure 3. Effect of C-type natriuretic peptide (CNP) pre-treatment on gene expression of yak oocyte. After yak oocytes were in vitro matured 0 h (0 h IVM), 24 h conventional IVM (24 h IVM), and 6 h pre-IVM with 100 nM CNP followed by 28 h IVM (6 h pre-IVM + 28 h IVM), the expression levels of BAX, BCL2, DNMT1, GDF9 and BMP15 were detected by RT-qPCR and were presented relative to GAPDH as the mean ± standard error. Values with different superscripts (a, and b) are significantly different (P < .05; one-way ANOVA followed by Tukey’s multiple-comparison test). n = 10 per each group.
4. Discussion
Pre-IVM with CNP delays GVBD, but CNP inhibits oocyte meiotic resumption in a dose-dependent manner, a time-dependent inhibitory effect with species-variation. Pre-IVM with 200 nM CNP sustains meiotic arrest for 6 h in cattle (Zhang et al. Citation2017b; Xi et al. Citation2018; Soto-Heras et al. Citation2019b) and juvenile goat (Soto-Heras et al. Citation2019a), 4 h in sheep (Zhang et al. Citation2018). Pre-IVM with 100 and 50 nM sustains meiotic arrest for 4 h in adult goat (Zhang et al. Citation2015) and 24 h in mouse (Wei et al. Citation2017), respectively. It is reported that CNP binding to its receptor NPR2 in CCs plays a functional role in maintaining the arrest of meiosis (Zhang et al. Citation2010; Xi et al. Citation2018; Xi et al. Citation2021). NPR2 mRNA is significantly decreased during IVM of COCs and promotes oocyte meiotic resumption (Yang et al. Citation2016). CNP pre-treatment can delay the decrease of in NPR2 expression, hence sustaining meiotic arrest (Zhang et al. Citation2015). Oestradiol can further prolong CNP meiotic arrest by promoting NPR2 in COCs (Zhang et al. Citation2011; Xi et al. Citation2018; Soto-Heras et al. Citation2019a). Similar to these previous studies in other species, we found that 100 nM CNP sustains meiotic arrest for 6 h, however, after 6 h of culture, the GV oocyte rates in all groups were significantly lower than the rate in freshly isolated oocytes in yaks.
After comparison of the maturation rates of yak oocytes pre-IVM with various concentrations of CNP (0, 50, 100, or 200 nM) for 6 h followed by 24, 26, or 28 h conventional IVM and cleavage rate and blastocyst formation after IVF and IVC, we established a two-step method for yak oocyte maturation, i.e. yak COCs were first meiotically arrested by 100 nM CNP in pre-maturation medium for 6 h and then matured in conventional maturation medium for 28 h. The results showed that the maturation rate was significantly increased compared with that of the control group. After in vitro fertilization with spermatozoa of Jersey, the cleavage rate and blastocyst rate in the two-step group were significantly higher than those in the control group. These results were similar to those observed in other animal species (Zhang et al. Citation2015; Wei et al. Citation2017; Zhang et al. Citation2017a, Citation2017b; Zhang et al. Citation2018; Soto-Heras et al. Citation2019a, Citation2019b; Jia and Wang Citation2020) and human (Sanchez et al. Citation2019), which indicate that CNP treatment before IVM enhances maturation of yak oocytes and developmental competence of their crossbred embryos.
Communication between CCs and the oocyte is partly mediated by TZPs (Matzuk et al. Citation2002). In IVM of growing oocytes from domestic species, the number of TZPs significantly decreased (Makita and Miyano Citation2014; Soto-Heras et al. Citation2019a; Yin et al. Citation2020). In the present study, there was a progressive decrease in TZP density during IVM of yak oocytes. However, after 6 h of culture, pre-IVM with CNP maintained the density of TZPs but conventional IVM significantly decreased the density of TZPs compared with freshly isolated oocytes, although it was not different at the end of IVM between the two IVM systems. This indicated that CNP prevented the decrease TZPs during IVM. This is possibly beneficial to oocyte maturation and embryo development, as TZPs allow the transfer of mRNA and metabolic molecules essential for oocyte maturation (Russell et al. Citation2016; Yin et al. Citation2020), although there was no significant difference in the density of TZPs at the end of IVM between the conventional IVM and two-step IVM in the present study. In mouse (Romero et al. Citation2016) and human COCs (Sánchez et al. Citation2017), it also has been reported that supplementation during the pre-IVM phase with CNP can result in maintenance of TZPs. However, there is an increase in TZPs after 6 h of culture in either pre-IVM or IVM medium in bovine COCs (Soto-Heras et al. Citation2019b).
ROS-induced oxidative stress impairs oocyte maturation and embryo development (Rodríguez-Nuevo et al. Citation2022; Zhang et al. Citation2022), whereas GSH is a significant non-enzymatic antioxidant for the oocyte. Treatment with CNP before IVM resulted in lesser ROS after IVM in bovine and goat oocytes compared with that in the control groups (Soto-Heras et al. Citation2019a; Jia et al. Citation2021). In yak oocytes, the increase in GSH levels and decreased ROS content were associated with oocyte maturation and subsequent development (Xiong et al. Citation2018). In the present study, we observed that the two-step IVM method significantly decreased ROS accumulation and significantly increased intra-oocyte GSH levels compared to conventional IVM. These results provided evidence that treatment of yak oocytes with CNP before IVM improved antioxidant defence mechanisms, thus, enhanced developmental competence of yak oocytes.
Oocyte-secreted factors, GDF9 and BMP15, have been shown to regulate growth, differentiation, and function of granulosa and thecal cells during follicular development playing a vital role in oocyte development, ovulation, fertilization, and embryonic competence (Sanfins et al. Citation2018; Morikawa et al. Citation2021). Postnatal growth of mammalian oocytes is accompanied by a progressive gain of DNA methylation. DNMT1 is one of the most important DNMTs that play essential functions for maintenance or de novo methylation processes. The expression level of DNMT1 is significantly higher in the matured yak oocytes (Pan et al. Citation2019). The downregulation of expression of BAX and upregulation of BCL2 in oocytes during IVM improve oocyte developmental competence (Pan et al. Citation2015; Veshkini et al. Citation2018). In the present study, we observed that the two-step IVM method significantly increased mRNA expression levels of GDF9, BMP15 and BCL2 in yak oocytes compared to conventional IVM. The expression of DNMT1 after IVM was significantly upregulated compared with the fresh oocytes. To our knowledge, this is the first time that pre-treatment with CNP before IVM is reported to increase BCL2 expression in oocytes after IVM. All these changes after pre-treatment with CNP may enhance oocyte development competence.
5. Conclusion
In conclusion, CNP could temporarily maintain the meiotic arrest of yak oocytes cultured in vitro. Pre-IVM with CNP maintained TZP density, decreased ROS accumulation and increased GSH content in yak oocyte. A two-step maturation strategy was established for yak oocytes, in which temporary meiotic arrest for 6 h by 100 nM CNP followed by conventional IVM for 28 h enhances the maturation of yak oocytes and developmental competence of crossbred embryos derived from IVF between yak oocytes and cattle spermatozoa. This may have important applications in in vitro production of yak crossbred embryos.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Blaschka C, Sánchez-Guijo A, Zimmer B, Stöhr J, Kotarski F, Grothmann H, Hartmann MF, Wudy SA, Wrenzycki C. 2019. Temporal expression pattern of steroid-metabolizing enzymes in bovine COC during in vitro maturation employing different gonadotropin concentrations. Theriogenology. 131:182–192.
- Blondin P, Coenen K, Guilbault LA, Sirard MA. 1997. In vitro production of bovine embryos: developmental competence is acquired before maturation. Theriogenology. 47:1061–1075.
- Cao XK, Cheng J, Huang YZ, Wang XG, Ma YL, Peng SJ, Chaogetu B, Zhuoma Z, Chen H. 2019. Growth performance and meat quality evaluations in three-way cross cattle developed for the Tibetan Plateau and their molecular understanding by integrative Omics analysis. J Agric Food Chem. 67:541–550.
- Edwards R. 1965. Maturation in vitro of mouse, sheep, cow, pig, rhesus monkey and human ovarian oocytes. Nature. 208:349–351.
- Hidaka T, Fukumoto Y, Yamamoto S, Ogata Y, Horiuchi T. 2018. Variations in bovine embryo production between individual donors for OPU-IVF are closely related to glutathione concentrations in oocytes during in vitro maturation. Theriogenology. 113:176–182.
- Jia Z, Wang X. 2020. Effects of C-type natriuretic peptide on meiotic arrest and developmental competence of bovine oocyte derived from small and medium follicles. Sci Rep. 10:18213.
- Jia Z, Yang X, Liu K. 2021. Treatment of cattle oocytes with C-type natriuretic peptide before in vitro maturation enhances oocyte mitochondrial function. Anim Reprod Sci. 225:106685.
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
- Makita M, Miyano T. 2014. Steroid hormones promote bovine oocyte growth and connection with granulosa cells. Theriogenology. 82:605–612.
- Matzuk MM, Burns KH, Viveiros MM, Eppig JJ. 2002. Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science. 296:2178–2180.
- Morikawa R, Lee J, Miyano T. 2021. Effects of oocyte-derived growth factors on the growth of porcine oocytes and oocyte-cumulus cell complexes in vitro. J Reprod Dev. 67:273–281.
- Pan Y, Cui Y, Baloch AR, Fan J, He J, Li G, Zheng H, Zhang Y, Lv P, Yu S. 2015. Insulinlike growth factor I improves yak (Bos grunniens) spermatozoa motility and the oocyte cleavage rate by modulating the expression of Bax and Bcl-2. Theriogenology. 84:756–762.
- Pan Y, Wang M, Baloch AR, Zhang Q, Wang J, Ma R, Xu G, Kashif J, Wang L, Fan J, et al. 2019. FGF10 enhances yak oocyte fertilization competence and subsequent blastocyst quality and regulates the levels of CD9, CD81, DNMT1, and DNMT3B. J Cell Physiol. 234:17677–17689.
- Rodríguez-Nuevo A, Torres-Sanchez A, Duran JM, De Guirior C, Martínez-Zamora MA, Böke E. 2022. Oocytes maintain ROS-free mitochondrial metabolism by suppressing complex I. Nature. 607:756–761.
- Romero S, Sánchez F, Lolicato F, Van Ranst H, Smitz J. 2016. Immature oocytes from unprimed juvenile mice become a valuable source for embryo production when using C-type natriuretic peptide as essential component of culture medium. Biol Reprod. 95:64.
- Russell DL, Gilchrist RB, Brown HM, Thompson JG. 2016. Bidirectional communication between cumulus cells and the oocyte: old hands and new players? Theriogenology. 86:62–68.
- Sanchez F, Le AH, Ho VNA, Romero S, Van Ranst H, De Vos M, Gilchrist RB, Ho TM, Vuong LN, Smitz J. 2019. Biphasic in vitro maturation (CAPA-IVM) specifically improves the developmental capacity of oocytes from small antral follicles. J Assist Reprod Genet. 36:2135–2144.
- Sánchez F, Lolicato F, Romero S, De Vos M, Van Ranst H, Verheyen G, Anckaert E, Smitz JEJ. 2017. An improved IVM method for cumulus-oocyte complexes from small follicles in polycystic ovary syndrome patients enhances oocyte competence and embryo yield. Hum Reprod. 32:2056–2068.
- Sanfins A, Rodrigues P, Albertini DF. 2018. GDF-9 and BMP-15 direct the follicle symphony. J Assist Reprod Genet. 35:1741–1750.
- Silva BR, Silva JRV. 2022. Mechanisms of action of non-enzymatic antioxidants to control oxidative stress during in vitro follicle growth, oocyte maturation, and embryo development. Anim Reprod Sci. 249:107186.
- Soto-Heras S, Menéndez-Blanco I, Catalá MG, Izquierdo D, Thompson JG, Paramio MT. 2019a. Biphasic in vitro maturation with C-type natriuretic peptide enhances the developmental competence of juvenile-goat oocytes. PLoS One. 14:e0221663.
- Soto-Heras S, Paramio MT, Thompson JG. 2019b. Effect of pre-maturation with C-type natriuretic peptide and 3-isobutyl-1-methylxanthine on cumulus-oocyte communication and oocyte developmental competence in cattle. Anim Reprod Sci. 202:49–57.
- Strączyńska P, Papis K, Morawiec E, Czerwiński M, Gajewski Z, Olejek A, Bednarska-Czerwińska A. 2022. Signaling mechanisms and their regulation during in vivo or in vitro maturation of mammalian oocytes. Reprod Biol Endocrinol. 20:37.
- Veshkini A, Mohammadi-Sangcheshmeh A, Ghanem N, Abazari-Kia AH, Mottaghi E, Kamaledini R, Deldar H, Ozturk I, Gastal EL. 2018. Oocyte maturation with royal jelly increases embryo development and reduces apoptosis in goats. Anim Reprod. 15:124–134.
- Wei Q, Zhou C, Yuan M, Miao Y, Zhao X, Ma B. 2017. Effect of C-type natriuretic peptide on maturation and developmental competence of immature mouse oocytes in vitro. Reprod Fertil Dev. 29:319–324.
- Wiener G, Han JL, Long RJ. 2003. The yak. 2nd ed. Bangkok: RAP.
- Xi G, An L, Jia Z, Tan K, Zhang J, Wang Z, Zhang C, Miao K, Wu Z, Tian J. 2018. Natriuretic peptide receptor 2 (NPR2) localized in bovine oocyte underlies a unique mechanism for C-type natriuretic peptide (CNP)-induced meiotic arrest. Theriogenology. 106:198–209.
- Xi G, An L, Wang W, Hao J, Yang Q, Ma L, Lu J, Wang Y, Wang W, Zhao W, et al. 2021. The mRNA-destabilizing protein tristetraprolin targets “meiosis arrester” Nppc mRNA in mammalian preovulatory follicles. Proc Natl Acad Sci USA. 118:e2018345118.
- Xia W, Osorio JS, Yang Y, Liu D, Jiang MF. 2018. Short communication: characterization of gene expression profiles related to yak milk protein synthesis during the lactation cycle. J Dairy Sci. 101:11150–11158.
- Xiong X, Lan D, Li J, Lin Y, Zi X. 2018. Effects of zinc supplementation during in vitro maturation on meiotic maturation of oocytes and developmental capacity in yak. Biol Trace Elem Res. 185:89–97.
- Xiong X, Yang M, Yu H, Hu Y, Yang L, Zhu Y, Fei X, Pan B, Xiong Y, Fu W, et al. 2022. MicroRNA-342-3p regulates yak oocyte meiotic maturation by targeting DNA methyltransferase 1. Reprod Domest Anim. 57:761–770.
- Xiong XR, Lan DL, Li J, Yin S, Xiong Y, Zi XD. 2019b. Identification of differential abundances of mRNA transcript in cumulus cells and CCND1 associated with yak oocyte developmental competence. Anim Reprod Sci. 208:106135.
- Xiong XR, Li J, Zi XD, Lan DL, He SM, Wu JB. 2019a. Establishment of new model for cattle-yak efficient production. Chin J Vet Sci. 39:1007–1013. Chinese.
- Yang L, Wei Q, Li W, Xi Q, Zhao X, Ma B. 2016. NPR2 is involved in FSH-mediated mouse oocyte meiotic resumption. J Ovarian Res. 9:6.
- Yang RF, Xiong XR, Zi XD. 2019. Effect of cysteine, insulin-like growth factor-1 and epidermis growth factor during in vitro oocyte maturation and in vitro culture of yak-cattle crossbred embryos. J Appl Anim Res. 47:463–466.
- Yin C, Liu J, Chang Z, He B, Yang Y, Zhao R. 2020. Heat exposure impairs porcine oocyte quality with suppressed actin expression in cumulus cells and disrupted F-actin formation in transzonal projections. J Anim Sci Biotechnol. 11:71.
- Zhang J, Wei Q, Cai J, Zhao X, Ma B. 2015. Effect of C-type natriuretic peptide on maturation and developmental competence of goat oocytes matured in vitro. PLoS One. 10:e0132318.
- Zhang M, Su YQ, Sugiura K, Wigglesworth K, Xia G, Eppig JJ. 2011. Estradiol promotes and maintains cumulus cell expression of natriuretic peptide receptor 2 (NPR2) and meiotic arrest in mouse oocytes in vitro. Endocrinology. 152:4377–4385.
- Zhang M, Su YQ, Sugiura K, Xia G, Eppig JJ. 2010. Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science. 330:366–369.
- Zhang T, Fan X, Li R, Zhang C, Zhang J. 2018. Effects of pre-incubation with C-type natriuretic peptide on nuclear maturation, mitochondrial behavior, and developmental competence of sheep oocytes. Biochem Biophys Res Commun. 497:200–206.
- Zhang T, Wang L, Pan Y, He H, Wang J, Zhao T, Ding T, Wang Y, Zhao L, Han X, et al. 2022. Effect of rapamycin treatment on oocyte in vitro maturation and embryonic development after parthenogenesis in yaks. Theriogenology. 193:128–135.
- Zhang T, Zhang C, Fan X, Li R, Zhang J. 2017b. Effect of C-type natriuretic peptide pretreatment on in vitro bovine oocyte maturation. In Vitro Cell Dev Biol Anim. 53:199–206.
- Zhang Y, Wang H, Liu W, Yang Y, Wang X, Zhang Z, Guo Q, Wang C, Xia G. 2017a. Natriuretic peptides improve the developmental competence of in vitro cultured porcine oocytes. Reprod Biol Endocrinol. 15:41.
- Zi XD. 2003. Reproduction in female yaks (Bos grunniens) and opportunities for improvement. Theriogenology. 59:1303–1312.
- Zi XD, Liu S, Xia W, Xiong XR, Luo B. 2018. Transcriptional profiles of crossbred embryos derived from yak oocytes in vitro fertilized with cattle sperm. Sci Rep. 8:11571.
