 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This research was conducted to determine the effects of Andrographis paniculate extract on hybrid catfish. The fish were fed 0, 0.2, 0.4 and 0.6 g/kg extract to supplement their diets for 90 days. The highest weigh gain, average daily growth, specific growth rate, feed efficiency and protein efficiency ratio, as well as the lowest feed conversion rate, were found in the group fed 0.6 g/kg. RBC were at their highest in all treatment groups, while the highest levels of total WBC and haematocrit percentage were observed in the group fed 0.6 g/kg. ALT and triglyceride were at their lowest levels in all treatment groups, while the highest amounts of glucose were found in all treatment groups. The highest levels of high-density lipoprotein and the lowest cholesterol were observed in fish fed 0.6 g/kg. Lysozyme activity was initially observed at a significant difference on days 60–90, the highest activity was found in the group fed 0.6 g/kg. After bacterial challenge test, lysozyme activity and WBC were significantly increased in all treatment groups, the lowest cumulative mortality was found in the group fed 0.6 g/kg. These results suggest that 0.6 g/kg of the extract has positive effects on hybrid catfish.
Introduction
Hybrid catfish are produced from female Asian catfish, Clarias microcephalus, and male African catfish, C. gariepinus. The culturing of this fish is rapidly gaining in popularity in Southeast Asia due to its rapid growth, resistance to disease, possibility for high stocking density, hypoxia tolerance and excellent quality of its meat. This hybrid is an important aquaculture and economically important worldwide (Ponjarat et al. Citation2019; Chaivichoo et al. Citation2020; Zakaria et al. Citation2022). Historically, hybrid catfish have been in high demand in Southeast Asian countries such as Thailand, the Philippines, Indonesia, Malaysia and Cambodia. In 2022, the production of this hybrid catfish in Thailand reached an estimated volume exceeding 100,000 tonnes, encompassing both natural capture and inland culture, and contributed to a total value surpassing 150 million USD (Fisheries statistics group of Thailand Citation2022).
However, when there is a demand for high productivity, fish aquarists often use a high-density farming model, which can make fish stressed, grow slowly, become easily infected with disease and eventually die which in turn causes huge economic losses (Ndashe et al. Citation2023). In preliminary problem-solving, farmers use chemicals and antibiotics to prevent and treat infections, which, in the early stages of treatment, can be quite effective (Harikrishnan et al. Citation2011; Kamaraj et al. Citation2018). Prolonged use of antibiotics can lead to the progression of antibiotic-resistant pathogens. Also, chemicals left as residues in the fish are dangerous for human consumption and cause harmful bioaccumulation impacts (Hidayat et al. Citation2018). Nowadays, several plants or their by-products are being used instead of antibiotics; these are regarded as being safer since they contain many organic bioactive components such as phenolic, polyphenolic, alkaloid, quinone and essential oils (Chakraborty et al. Citation2014; Tan et al. Citation2018; Nowosa et al. Citation2023). Panase et al. (Citation2018a). For example, it has been shown that the extract of Euphorbia hirta can have an effect on growth parameters, hematological indices and organosomatic indices on hybrid catfish Clarias macrocephalus × C. gariepinus. Moreover, with regards to hybrid catfish, Clarias macrocephalus × C. gariepinus, Houttuynia cordata extract was shown to promote growth and hematological indices (Panase et al. Citation2018b). In addition, Apium graveolens plant extract sprayed on feed, was shown to improve growth performance, serum biochemical indices and lysozyme activity indices of Labeo chrysophekadion (Sutthi et al. Citation2020). The medicinal herb Andrographis paniculate, which is tropical and sub-tropical Asia, Southeast Asia and India and has a greatly bitter taste, is used to cure liver disorders, children's bowel complaints, colic discomfort, the common cold and upper respiratory infections (Jayakumar et al. Citation2013; Hossain et al. Citation2014). The aerial part of A. paniculata is commonly used in Chinese medicine where it is believed to cool and relieve internal heat, inflammation, pain and against cancer as a form of detoxification (Gupta et al. Citation2017; Suriyo et al. Citation2021; Valdiani et al. Citation2022). The major bioactive compounds of A. paniculata are andrographolide deoxyandrographolide, neoandrographolide, 14-deoxy-11,12-didehydroandrographide and isoandrographolide respectively (Valdiani et al. Citation2017). When their food is supplemented with (A. paniculata) aqueous methanolic extract at a dose of 2%, Pangasianodon hypopthalmus can show improved growth indices, immunity and prevention of Aeromonas hydrophila infection, lysozyme activity, total white blood cell, red blood cell, haemoglobin content and haematocrit (Maiti et al. Citation2023). However, the effect of A. paniculate on hybrid catfish has not yet been reported.
Therefore, the objective of this study was to investigate A. paniculata extract's effectiveness in supplementing growth performance, biochemical indices, hematological indices, organosomatic indices, innate immunity and disease resistance against A. hydrophila in hybrid catfish, C. macrocephalus × C. garipinus.
Materials and methods
Preparation of herbal extract
Fresh Andrographis paniculata plants were obtained from the University of Phayao, Thailand. Fresh A. paniculata was gathered and rinsed thoroughly in water before air drying. The plants were dried for 96 h in an oven at 37°C. The dried herbs were finely ground (0.9 mm) and the plant powder was soaked in ethanol (1:2 ratio). 250 g of plant powder was combined with 2000 ml of 95% ethanol and stored at room temperature for 96 h. The mixture was then filtered twice (Whatman filter paper No. 1), before being evaporated to dry it out (below 40°C) using a rotary evaporator. The extract was stored in a test tube and wrapped in foil and then was carefully preserved in the refrigerator.
Phytochemical screening
The standard procedures described by Edeoga et al. (Citation2005) were employed in this investigation, and alkaloids were detected using the Dragendorff and Hager's technique. The Liebermann-Burchard's test was used to evaluate the presence of cardiac glycosides, steroids and terpenoids. The Molisch, Keller-Killiani and Kedde's tests were used to identify normal sugar, deoxy sugar and unsaturated lactone rings, respectively. Using a Molisch's test, it was discovered that sugar was present at the intersection of two liquid layers because of the appearance of a purple tint.
A sodium picrate technique was used to test for cyanogenic glycosides throughout the cyanogenic glycosides screening process. Saponins screening, a frothing test, was used to test for saponins. Lead acetate was used to test for tannins and pseudo tannins during the tannin screening process. A ferric chloride technique was employed to test for the presence of phenolic chemicals. A Shinoda's test was used to detect flavonoids. Finally, anthraquinones were detected using a modified Borntrager's approach ().
Table 1. Preliminary phytochemical screening methods for Andrographis paniculata.
Experimental fish and the conditions for acclimatization
Hybrid catfish (Clarias macrocephalus × C. gariepinus) were bought from a fish farm in Phayao Province Thailand. The experimental fish were transported to the lab and acclimatized for two weeks in a cement pond (2 × 3 × 0.5 m) containing 3000 L of water with a natural photoperiod and a weekly continuous aeration and water recirculation system. The following water quality parameters were monitored during the acclimation: the temperature was held constant at 26.3 ± 1.23°C, the dissolved oxygen (DO) content was held constant at 7.7 ± 0.52 mg/l, and the pH was held constant at 7.6 ± 0.35 throughout the experiment. The experimental fish were fed with commercial fish feed at a rate of 7% of their body weight per day (30% crude protein) twice daily (08.00; 17.00) without A. paniculata extract.
Supplemented diet preparation
Throughout the experiment, a commercially available fish diet containing 30% crude protein (fat 4%, moisture 12% and fibre 8%) was employed. Treatment 1 was not sprayed with A. paniculata extract; treatment 2, 3 and 4 were sprayed and completely combined in a drum mixer in the ratio of 200, 400 and 600 mg/kg, respectively. For all groups, the pellets (2 mm in diameter) were coated with a 4% agar solution at a concentration of 10 ml/kg and air dried again. They were then stored at room temperature in separate sterile containers for seven days, and the feed was quickly devoured by the experimental fish.
Experimental design
360 healthy fish with an average body weight of 10.5 ± 0.44 g and a mean total length of 5.08 ± 0.52 cm was placed in 12 net cages (1 × 2 × 0.8 m; mesh size, 2.5 mm2) in four triplicate groups after acclimatization. All groups were placed in the same earthen pond, with a stocking density of 30 fish per net cage. All groups were fed a diet tailored to their needs at 7% of their body weight twice a day. Water quality was measured even though all net cages were placed in the same earthen pond. The observed water quality parameters were as follows: water temperature fluctuated between 27.8–29.6°C, dissolved oxygen 6.3–7.82 mg/L 1, pH 7.23–8.79 and total dissolved solids 0.21–0.52 g/L.
Growth performance and survival rate
At 15-day intervals, all the fish in each net cage were weighed to adjust feed volume, and evaluate growth parameters such as weight gain (WG), average daily gain (ADG), specific growth rate (SGR), feed conversion rate (FCR), protein efficiency ratio (PER) and survival rate (SR), which were determined using the following equations (Bagenal Citation1978):
Study of hematological indices
Nine fish/groups (three fish/replications) were chosen at random, and they were given a 3-minute anesthesia with MS-222 1: 4000 in dechlorinated water (Harikrishnan et al. Citation2010). On days 30, 60 and 90 of the experimental periods, blood samples were taken from the caudal vein (0.8 ml) using syringes with 26G needles that contained blood in K3 EDTA tubes. A blood sample was diluted 1:300 in a 0.85% NaCl solution to determine the total red blood cell levels (RBC 109 mm3), and the haematocrit percentage (%Hct) of the blood samples was measured using the microcentrifuge method. A blood sample was diluted 1:50 in a 2% solution of acetic acid to assess the total white blood cell level (WBC 107 mm3), RBC and WBC were manually counted in a Neubauer chamber (Rehulka Citation2003).
Serum biochemistry study
Serum biochemistries were investigated on days 30, 60 and 90 of the feeding trial. Following the evaluation of growth indices and survival, nine fish from each group (3 fish each replication) were chosen at random for blood collection. Non-heparinized syringes were used to draw blood samples (0.8 ml/fish) from the fishes’ caudal veins. Blood samples were put into micro-centrifuge tubes and allowed to clot at room temperature for 4 h before being used to collect serum. After this, they were put into a centrifuge at 5000 rpm for 15 min at 25 degrees Celsius, and the supernatants were kept in sterile serum tubes at −20 degrees Celsius until required (not for longer than 7 days). The serum samples were frozen and brought to the Chiang Mai Veterinary Laboratory Centre Limited Partnership in Chiang Mai, Thailand, for analysis. Serum indices, activities of the alanine transaminase (ALT), aspartate transaminase (AST), creatinine, alkaline phosphatase, triglycerides, glucose, cholesterol, total protein, albumin, globulin, low density lipoprotein, high-density lipoprotein were evaluated using an analytical chemistry analyzer (P400 and PC400, HORIBA, Japan).
Serum lysozyme activity
Lysozyme activity was checked every 30 days, nine fish from each group were randomly selected for serum preparation. Lysozyme activity was measured using the turbidimetric method (Koskela et al. Citation2004) with some modifications. The maximum activity was discovered in a 0.05 M phosphate buffer, pH 6.0, in which the substrate, lyophilized Micrococcus lysodeikticus, was suspended (3.0 mg/ml). The bacterial solution was then mixed with 25 l of serum from each sample. At 25 degrees Celsius, the absorbance rate was measured at 450 nm (Multimode Reader LB 942) at 30 s intervals (total measurement duration: 3 min). The findings were expressed as unit/ml, which was calculated using one unit of lysozyme activity and defined as 0.001 per minute of absorbance rate before being lowered.
Organosomatic indices
At the end of the experiment, three fish from each net cage (replicate) were selected randomly and anesthetized with MS-222 1:4000 in dechlorinated water for 3 min (Harikrishnan et al. Citation2010). The visceral organs were separated so that the liver, spleen, kidney and intestine could be used to calculate the hepatosomatic index (HSI, %), spleenosomatic index (SSI %), Renosomatic index (RSI %) and intestinosomatic index (ISI%), respectively. Calculations were computed as follows (Ronald and Bruce Citation1990; Hadidi et al. Citation2008):
Bacterial culture and its LD50 determination in hybrid catfish, Clarias macrocephalus × C. gariepinus
Aeromonas hydrophila was procured from the Faculty of Fisheries, Kasetsart University (Thailand), and bacterial preparation for the challenge test on the A. hydrophila was done by culturing the sample at 25°C in tryptic soy broth (TSB) for 24 h. The culture was centrifuged at 3000 rpm for 10 min. The supernatants were discarded, and the suspension was held in phosphate-buffered saline (PBS, pH 7). The bacterial suspension was adjusted to an optical density (OD) of 600 nm to correspond to approximately 109 CFU/ml for inducing fish infection as follows by Wangkahart (Citation2018).
The virulence and pathogenicity were tested using LD50 before the challenge test on Clarias macrocephalus × C. gariepinus, 109 CFU/ml were found. To study the bacterial resistance to A. hydrophila, in the Clarias macrocephalus × C. gariepinus, 120 fish (10 fish per replication) from each group, were used. After 90 days of feeding trials, the four groups were injected intraperitoneally with 1.5 ml of 109 CFU/ml. The cumulative mortality rates (%) for each group were monitored for 14 days after the bacterial injection.
Hematological and serum lysozyme activity of hybrid catfish after A. hydrophila challenged test
Hematological indices such as red blood cell (RBC), white blood cell (WBC), haematocrit (Htc) and serum lysozyme activity were determined in the surviving fish after day 14, post-injection. Three fish from each group were randomly selected for blood and serum preparation using the method above mentioned.
Statistical analysis
The normality and homogeneity of variance were tested before the analysis. A one-way analysis of variance (ANOVA) was used to analyze the data, followed by a Tukey's test at a significance level of 95% (p < 0.05). SPSS software version 25 for Windows (SPSS Inc., Chicago, USA) was used for statistical analyses. The data were all given as mean ± SD.
Results
Phytochemical analysis
The results revealed that phytochemical constituents such as coumarins, tannins, terpenoids, lactones, steroids and sugars were found in the ethanolic extract of A. paniculata but flavonoids, alkaloids, saponins, cardiac glycosides, cyanogenic glycosides and anthraquinones were not found in this study ().
Table 2. Preliminary phytochemical constituents of 95% ethanol extract from Andrographis paniculata extract.
Growth indices and survival evaluation
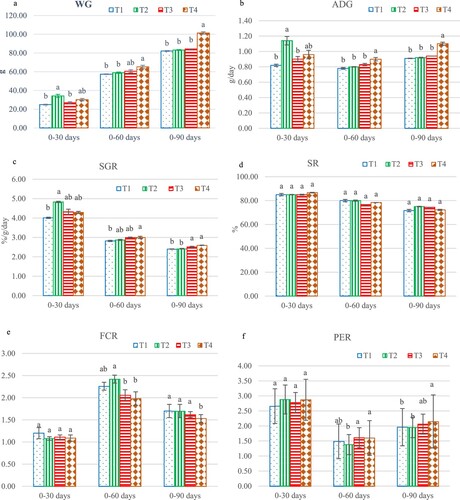
The growth indices of C. macrocephalus × C. gariepinus showed that weight gain (WG) was significantly different (p < 0.05). At day 30 the highest WG was found in group fed 0.2 g/kg (T2) at 34.45 ± 1.97 g when compared to control group at 24.86 ± 1.60 g (p < 0.05), but on days 60 and 90, the group fed 0.6 g/kg (T4) was found to have the highest WG at 24.03 ± 2.48 g compared to other dietary groups ((a)). The average daily gain (ADG) was significantly different (p < 0.05) from days 30, 60 and 90. On day 30, the group fed T2 had the highest ADG at 0.43 ± 0.01 g/day, 1.60 ± 0.16 g/day respectively, but on days 60 and 90 the group fed T4 had the highest ADG compared to the control group ((b)). The specific growth rate (SGR) had a significant difference on day 30 of the experiment, the highest value and the lowest value were found in groups fed T4 and T1 at 4.83% ± 1.22%/g/day, 4.01% ± 1.35%/g/day, respectively, and there was also a significant difference on days 60 and 90. The higher values were found in groups fed T4 (2.60% ± 1.01%/g/day) and T3 (1.64% ± 0.14%/g/day), but the lower value was found in group fed T1 ((c)). Nevertheless, there were no significant differences (p > 0:05) in survival rate between treatment groups, with T2 charting the highest value at 77% ± 2.35% ((d)). The feed conversion rate (FCR) was significantly different on days 60 and 90 of the experiment. On day 60, the highest FCR was found in the group fed T2 (1.08 ± 0.13), but on day 90, the lowest level was found in the group fed T4 at 2.69 ± 0.29 ((e)). Protein efficiency ratio (PER) showed a significant difference on days 60 and 90. Day 60 the fish in T4 (1.24 ± 0.07) and T3 (1.34 ± 0.011) groups showed the higher value when compared to T2 group, but on day 90, the highest was found in groups T4 at 1.21 ± 0.12 ((f)).
Figure 1. Growth indices such as weight gain: WG (a), average daily gain: ADG (b), specific growth rate: SGR (c), survival rate: SR (d), feed conversion rate: FCR (e) and protein efficiency ratio: PER (f) of hybrid catfish, Clarias macrocephalus × C. gariepinus, which were fed different levels of Andrographis paniculata. Different letters indicate significant differences (p < 0.05). T1 (0.00 g/kg), T2 (0.2 g/kg), T3 (0.4 g/kg) and T4 (0.6 g/kg).
Hematological indices
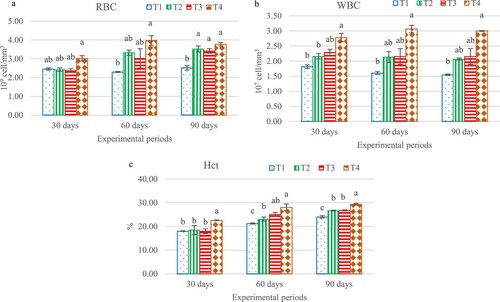
In the first sampling on day 30 of the experiment, RBC (total red blood) in T4 exhibited higher value (3.02 × 109 /mm3) than the other groups. On day 60, T4 shows significantly different (P < 0.05) when compared to the control group. Meanwhile, the highest level was found in all the treatment groups. On day 90, the highest RBC was found in all treatment groups when compared to the control group. T4 showed the highest level (3.77 ± 0.16), followed by groups T2 (3.51 ± 0.27), T3 (3.41 ± 0.20) and T1 (2.71 ± 0.16 109 cell/mm3), respectively (a). Besides, WBC (total white blood cell) shows similar trends on days 30, 60 and 90 of the experimental periods and it was significantly different. The highest level was found in the T4 group, followed by groups T3, T2 and T1, on day 90 at 2.96 ± 0.21, 2.21 ± 0.44, 2.16 ± 0.04 and 1.55 ± 0.04 107 cell/mm3 respectively ((b)). Haematocrit percentage (Htc), on day 30, the highest percentage was found in T4 group whilst other three groups were not significantly different to each other. Moreover, T4 group was found to be consecutive increasing and were significantly (P < 0.05) higher as compared to the other groups, the highest level was found in the T4 groups and lowest on the control group on day 90 at 28.66 ± 0.47 and 24.00% ± 0.88% ((c)).
Figure 2. The hematological indices of hybrid catfish, Clarias macrocephalus × C. gariepinus, which were fed different levels of Andrographis paniculata extract for 30, 60, and 90 days: total white blood cells, WBC (a); total red blood cells, RBC (b); haematocrit, Hct (c); Significant differences (p < 0.05) are indicated by different letters.
Serum and biochemical indices
Biochemical indices such as alanine transaminase (ALT), aspartate transaminase (AST), creatinine, glucose, cholesterol, LDL, HDL, total protein, triglyceride, albumin, globulin and alkaline phosphatase (ALP) were investigated in this study. The results showed that all parameters were shown to be significantly different (p < 0.05) except for AST, creatinine, ALP, total protein, albumin, globulin and LDL. The parameters were significantly different, ALT, glucose and HDL were clearly shown on days 60 and 90 of the experiment, but triglyceride was significantly different on days 30, 60 and 90, while cholesterol was only significantly different on day 90 ((a–l)).
Figure 3. Effect of different levels of Andrographis paniculata extract on activities of for analysis. Serum indices, activities of the alanine transaminase ALT (a), aspartate transaminase AST (b), creatinine (c), Alkaline phosphatase (d), glucose (e), total protein (f), albumin (g), globulin (h), Triglycerides (i), cholesterol (j), low density Lipoprotein, LDL (k) and high-density Lipoprotein, HDL (l) in hybrid catfish, Clarias macrocephalus × C. gariepinus. Values are mean ± SD and different letters indicate difference (p < 0.05). T1 (0.00 g/kg), T2 (0.2 g/kg), T3 (0.4 g/kg) and T4 (0.6 g/kg).
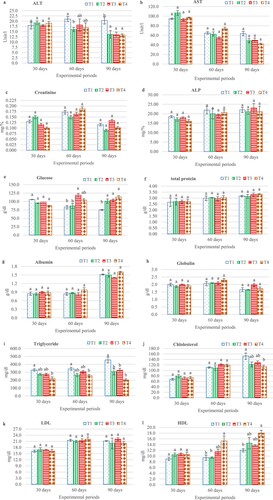
The ALT shows its highest value in T1 and its lowest in T2 on day 60. However, on day 90, the highest ALT was found in T1 (20.33 ± 2.88 unit/l) whilst the significantly lowest ALT was found in all treatment groups, but lowest level found in T4 at 3.33 ± 1.04 unit/l ((a)). Serum glucose levels started to become significantly different on days 60–90, the highest glucose amount was observed in T3, but the lowest level was found in T1 and T2 on day 60, while on day 90, the highest glucose level was exhibited in all treatment groups when compared to the control group, the highest and lowest levels were 113.25 ± 8.00 and 74.83 ± 2.12 g/dl, respectively ((e)). Surprisingly, it was shown that serum triglyceride can be reduced by inclusion of the extract on days 30, 60 and 90, this parameter showed similar results, the T4 group showed a significantly lower level but the highest level was found in T1; however, the lowest level was found in all treatment groups on day 90 (T4, T2 and T3 were 202.50 ± 26.07, 307 ± 71.55 and 325 ± 41.08 mg/dl, respectively) when compared to the T1 group (454.50 ± 51.61 mg/dl) ((i)). Serum cholesterol level was revealed to be significantly different on day 90 of the experiment, the lowest and highest levels were observed in the T4 and T1 groups, at 113.00 ± 9.92 and 151.83 ± 20.05 mg/dl respectively ((j)). Serum HDL levels showed a similar trend to that of glucose, this parameter was shown, initially, to be different on days 60–90, the highest level was found in T4 and the lowest HDL was found in T1 and T2 on day 60, but the highest HDL was observed on day 90 in the T4 group followed by: T3, T2 and T1 were 18.00 ± 1.41, 13.67 ± 0.57, 14.50 ± 0.57 and 12.00 ± 1.00 mg/dl respectively ((l)).
Lysozyme activity
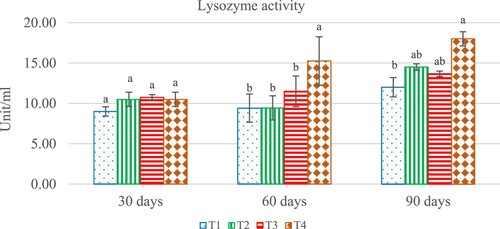
Serum lysozyme activity in all treatment groups was significantly different (P < 0.05) and shown to be higher than in the control group (T1), as observed on days 60 and 90. On day 60, the highest activity was found in T4 group when compared to other groups. On day 90, serum lysozyme activity in the T4 group (18.00 ± 0.88 Unit/ml) was consistently higher and significantly (P < 0.05) different from the values in the T2 and T1 groups at 14.50 ± 0.04 and 12.00 ± 1.20 unit/ml ().
Figure 4. Effect of different levels of Andrographis paniculata extract on activities of for analysis lysozyme activity (m) in hybrid catfish, Clarias macrocephalus × C. gariepinus. Values are mean ± SD and different letters indicate difference (p < 0.05). T1 (0.00 g/kg), T2 (0.2 g k), T3 (0.4 g /kg) and T4 (0.6 g/kg).
Organosomatic indices
Organosomatic indices derived from the liver, spleen, kidney and intestine after the end of the experiment (90 days), in all groups, were not shown to be significantly different (P > 0.05). While the trends of their indices showed that the T4 group had the highest hepatosomatic index (1.93% ± 0.09%), the control group had the lowest (1.74% ± 0.12%). Spleenosomatic index was at its highest and lowest levels in the groups T2 (2.20% ± 0.03%) and T1 (1.80% ± 0.01%), respectively. In addition, the kidney index was found to be highest in group T4 (0.55% ± 0.02%) while the lowest was found in group T1 (0.47% ± 0.04%). Similarly, with regards to intestinosomatic index, the T2 group had the highest value at 2.20% ± 0.09%, followed by groups T3, T4 and T1 as 2.12% ± 1.01% and 1.96% ± 1.22%, respectively ().
Table 3. Organosomatic indices of hybrid catfish, Clarias macrocephalus × C. gariepinus were fed with different concentrations of Andrographis paniculata extract for 90 days.
Pathogenic challenge test
The observed cumulative mortality after A. hydrophila injections at 109 CFU/ml for 14 days was its highest in the T1 group at about 60% when compared with the other groups, and mortality was found on the first 4 days of the experimental period. During the pathogenic challenge study, we observed gasping, weak movement, rotting fins and the darkening of the skin. Cumulative mortality was recorded at 50%, 40% and 30% in T3, T2 and T4, respectively (). Hematological indices such as RBC, WBC and haematocrit percentage (Htc) were evaluated after challenge with A. hydrophila for 14 days, the results showed the same trend; all the treatment groups were significantly different (P < 0.05) from those in the control group. The highest and lowest groups were found in T4 and T1 groups, respectively, Lysozyme activity in fish that survived after the A. hydrophila injection was at 109 CFU/ml. The highest and lowest groups was found in the T4 group and T1 group that received A. paniculata extract at 25.50 ± 0.88 and 20.75 ± 1.2 Unit/ml, respectively ().
Figure 5. Cumulative mortality (%) of hybrid catfish, Clarias macrocephalus × C. gariepinus, fed with the four different concentrations of Andrographis paniculata extract against Aeromonas hydrophila for 14 days. T1 (0.00 g /kg), T2 (0.2 g/ kg), T3 (0.4 g/ kg) and T4 (0.6 g /kg).
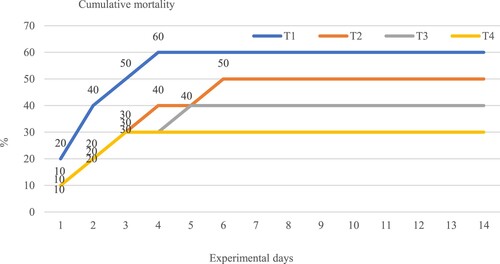
Table 4. Blood parameters and lysozyme activity of hybrid catfish, Clarias macrocephalus × C. gariepinus after Aeromonas hydrophila injections at 109 CFU/ml for 14 days.
Discussion
The using of A. paniculata extract in the diet of fish used in this experiment appeared to be significantly effective in terms of their growth performance, haematology, serum biochemical indices and lysozyme activity, in both the pre-challenge and post-challenge, A. hydrophilla injection, period. Present findings showed that a 95% ethanolic solution of A. paniculata extract significantly improved growth performance in the T4 group (0.6 g/kg). This may have been due to the existence of bioactive compounds in A. paniculata, such as andrographolide, deoxyandrographolide, neoandrographolide, 14-deoxy-11,12-didehydroandrographide, isoandrographolide, coumarins, tannins, phenolics, terpenoids, lactones, steroids and sugars etc., that have been found in this plant species (Rajalakshmi and Cathrine Citation2016; Nagajothi et al. Citation2018; Naomi et al. Citation2022). According to Maiti et al. (Citation2023) reported that Pangasianodon hypopthalmus could improve weight gain, specific growth rate and feed conversion ratio after fed 2% A. paniculata leaf extract. Similarly, Basha et al. (Citation2013) revealed that Labeo rohita fed an andrographolide diet improved WG, SGR, protein efficiency ratio and decreased feed conversion ratio. In addition, Pagrus major fed with medicinal herbs increased WG, FE and survival rates above those found in the control group (Ji et al. Citation2007). Moreover, Mishra et al. (Citation2023) reported that andrographolide can stimulate growth performance in Cyprinus carpio due to an increase in its ability to use of protein, energy, digestion and better absorption of nutrients as a result of its inclusion in their diets. It has also been shown that A. paniculata can reduce the growth rate of various pathogenic bacteria in Penaeus monodon, increasing their survival chances due to this herb’s effectiveness in improving a fish's ability to digest and absorb nutrients and stimulate humoral immunity by enhancing the microbial killing activity of red blood cells and cell phagocytosis (Citarasu et al. Citation2003). Meanwhile, Immanuel et al. (Citation2009) reported that Nile tilapia fed Cynodon dactylon, Aegle marmelos, Withania somnifera and Zingiber officinale can stimulate WG, SGR, FER and reduce FCR. Besides, Shi et al. (Citation2020) reported that an increase in WG, SGR, ADG and reduced FCR may be due to increased levels of andrographolide in the diet, which can lead to better absorption of nutrients in the intestines of Monopterus albus. According to Abdel-Tawwab et al. (Citation2010), the use of green tea, Camellia sinensis at a level 0.5 g/kg of feed can enhance growth performance due to the palatability or attractiveness of the diet, which in turn causes increased feed intake and enhanced growth performance in Nile tilapia. Moreover, various other plant extracts have been shown to improve growth performance in fish, such as Euphorbia hirta plant leaf extract supplemental diet of 300 mg/kg, which has a positive effect on hybrid catfish, improving their growth performance, survival rate and feed utilization (Panase et al. Citation2018a). This may be because bioactive substances can improve growth performance by promoting the synthesis of digestive enzymes, bile, mucus and as such can be effective as feed additives in that they increase feed intake, ingestion, the villi length and goblet cell numbers in the intestine (Rudy et al. Citation2018; Wang et al. Citation2018; Shi et al. Citation2020: Xu et al. Citation2020; Abdel-Latif et al. Citation2022). According to Baba et al. (Citation2016), Avena sativa extract can be used to improve WG and SGR and decrease FCR in Cyprinus carpio because the metabolites of plants can stimulate growth and immunity via innate adaptive immune responses and can trigger immune cell activity, enhance phagocytosis and enhance the secretion of inflammatory markers to resist various pathogens in fish. Although we do not investigate the effect of A. paniculate on digestive enzyme, we found that Zingiber officinalis and Cyanodon dactylon supplementations could be increased SGR and decreased FCR in Macrobrachium rosenbergii, because of an increase in digestive enzyme secretion that can result in improvements in digestibility, stimulating the appetite and increasing food consumption and efficiencies (El-Desouky et al. Citation2012). Furthermore, Ghosal et al. (Citation2020) revealed that Basella alba, Tribulus terrestris, Mucuna pruriens and Asparagus racemosus can stimulate growth performance in Nile tilapia as it helps the fish to control reproduction and enhances growth and innate immunity because, all four plant extracts contained phytoconstituents such as saponins, alkaloids and tannins that have been reported to act as feed intake deterrents and digestive enzyme inhibitors in Nile tilapia. Importantly though, Peng et al. (Citation2021) revealed that condensed tannins did not impact on growth performance, but rather enhanced animal health by improving the intestinal microbial ecosystem in Lateolabrax japonicus. Meanwhile, Astragalus membranaceus extract can be used to improve growth performance in Pangasianodon hypophthalmus due to the presence of phenolic acids and flavonoids that can increase digestive enzyme activities, voluntary feeding intake, feed efficiency and improve protein retention (Abdel-Latif et al. Citation2022). Besides, Ahmadifar et al. (Citation2021) reported that polyphenols have affected the growth of gene expression in Huso huso, and this may be because tannins help in activating growth hormones (GH) and insulin-like growth factor-I (IGF-I) genes, which are known to be important genes for growth.
Hematological indicators often alter in response to stress, illness and supplemental diet circumstances; however, RBC, WBC and Htc are mostly impacted by dietary treatments (Reverter et al. Citation2014; Hassaan et al. Citation2019). In addition, erythrocytes and haemoglobin are essential in the transfer of oxygen and carbon dioxide (Nya and Austin Citation2009). The results of our study showed that all groups fed with A. paniculata extract had significantly higher RBC, WBC and Hct than the control group, especially, the group were fed 0.6 g/kg A. paniculata extract had the highest values. Based on present findings, exhibited that varying physiological properties could enhance oxygen volume and anti-infection ability, thus enhancing fish growth indices and health statuses (Taufek et al. Citation2016). According to Maiti et al. (Citation2021) reported that A. paniculata leaf extract could increase RBC in Pangasianodon hypopthalmus due to its iron content, iron being a substance that can produce red blood cells. Besides, Velichkova et al. (Citation2019) reported that increases in RBC in Cyprinus carpio may be because of mineral and iron constituents that can stimulate the excitability of muscles and blood coagulation. However, Aloe vera extract can also increase the RBC and Htc of C. carpio after an A. hydrophila infection. In addition, polysaccharides and iron in herbal extracts have also been associated with increased erythropoiesis and increased Htc levels (Alishahi et al. Citation2010). In addition, Binaii et al. (Citation2014) reported that Urtica dioica extract can improve RBC and Hct in Huso huso due to the presence of vitamins and minerals from the extract that aid by increasing haematopoiesis. Furthermore, C. carpio fed with Aegle marmelos extract, haematology was increased due to iron, which induces erythropoiesis and lymphopoiesis, resulting in an increase in RBC, Hct and WBC respectively.
Biochemical indices indicators are commonly used to assess the health status during supplementation of herbal substances to animals, including fish (Ngugi et al. Citation2015; Abdel-Tawwab et al. Citation2018). Present results indicated that the liver enzymes were not affected by the extract. In addition, A. paniculata leaf extract exhibited positive effect on liver function because ALT in all treatment groups showed significantly lower whilst AST and ALP were not significantly different when compared to control group. These enzymes were released into the blood circulation at high volumes indicated that the animals were facing some form of stressor that was causing their livers to be stressed, they were becoming damaged, hepatocyte injury, liver necrosis and livers impaired (Liu et al. Citation2007; Li et al. Citation2022). Similarly, Labeo chrysophekadion fed leaf ethanolic extract of Apium graveolens, growth performance was positive effect but not affected to ALT and AST (Sutthi et al. Citation2020). Similar results were observed in Oreochmomis niloticus fed with some herbal extracts, ALP could be significantly decreased (Ghosal et al. Citation2020). In contrary, Abdel-Tawwab et al. (Citation2018) reported that serum AST, ALT, ALP, urea and creatinine of African catfish fed diets containing different levels of clove basil leaf extract were significantly increased with increasing concentrations. Since glucose molecules play a significant role in animal bioenergetics, which is transferred to ATP synthesis by cellular respiration processes (Lucas Citation1996). In this study, Serum glucose was significantly increased on days 60 and 90. According to Saeidi et al. (Citation2017) revealed that serum glucose of rainbow trout (Oncorhynchus mykiss) was increased after being fed stinging nettle (Urtica dioica). Glucose levels are indicators of stress levels, higher glucose levels are generally maintained in fish due to glycogen breakdown in the liver, after which the glucose molecules are converted into energy via glycogenolysis as part of the cellular respiration process (Vijayan et al. Citation1997). According to Lin et al. (Citation2016), polyphenolic compounds can be improved the catalytic activity of glucose phosphorylation; what is more, natural plant extracts, for example eugenol, can affect glucose metabolization. This result is similar to Velichkova et al. (Citation2020) reported that serum glucose of Oncorhynchus mykiss was significantly increased after fed with supplement of 1% Acorus calamus extract. Besides, glucose level was not significantly affected by some herbal extract supplementations, for examples, Clarias gariepinus fingerlings after fed with Aloe vera polysaccharides at low pH, glucose level was not significantly different when compared to control diet Gabriel et al. (Citation2019) and in Nile tilapia fed seaweed extract supplementation (Ashour et al. Citation2020).
In this study, cholesterol and triglyceride levels were decreased in all groups fed with the extract and showed their lower levels in group T4. Similarly, dietary supplementation with 0.50–4.00 g/kg Ginkgo biloba extract revealed that effectively decreased serum cholesterol and triglyceride levels in Carassius auratus (Sun Citation2008) and hybrid grouper (Tan et al. Citation2018). In addition, serum cholesterol of Clarias gariepinus has reduced after fed with Ocimum gratissimum leaf extract (Abdel-Tawwab et al. Citation2018). Cholesterol levels in serum can be significantly decreased by diet, enzyme activities and hepatic activities. Moreover, cholesterol levels can be changed by the sexual cycle of fish because cholesterol is the precursor of the five major classes of steroid hormones (Berg et al. Citation2002). Meanwhile, the difference in cholesterol of serum may be the existence of plant sterols such as stigmasterol, campesterol and phenolic compounds (Frémont et al. Citation2000). According to Binaii et al. (Citation2014), Urtica dioica extract can decrease serum cholesterol and triglyceride levels in Huso huso due to the presence of stigmasterol, campesterol and phenol compounds. Beyond this, Chickens fed a Carvacrol Lowers diet can show a decrease in serum triglycerides, due to the steroids and phenolics that may affect lipid synthesis rather than cholesterol synthesis (Lee et al. Citation2003). This revealed that A. paniculata extract supplementation had hypolipidemic effect in fish. The levels of HDL in serum are important parameters of lipid metabolism in animals and the lipid-lowering effect in serum is beneficial to health Adler and Holub (Citation1997). Our study indicated that the LDL levels in all experimental groups were not significantly different because the extract had no effect on this serum parameter. On the other hand, the HDL level of all groups fed with A. paniculata extract was affected, especially on day 90 where it was shown to be significantly different when compared the control group, and an even higher level was observed in the T4 group. Similarly, Ginkgo biloba extract could effectively increase serum HDL content in hybrid grouper (Tan et al. Citation2018). This may be due to bioactive compounds, such as minerals and iron, stimulate HDL activity by transporting cholesterol from the blood to the liver.
Lysozyme activity serves as a primary defence, providing humoral-specific immunity to set up cellular defence mechanisms. In addition, lysozyme is found in the serum and mucus of fish (Ellis Citation1999; Panase et al. Citation2017). Various research in innate immune system, the researchers had revealed lysozyme activity used to evaluate immune status in finfish, for example, Harikrishnan et al. (Citation2010) lysozyme activity in goldfish (Carassius auratus) was used to evaluate the effects of immunostimulants obtained by herbal plants. Similarly, Pratheepa and Sukumaran (Citation2014) also used serum lysozyme activity to evaluate immune status in Cyprinus carpio after fed Euphorbia hirta plant leaf extract. This study had indicated that all the extract-treated groups had different lysozyme activity levels at day 60 and that the difference was very clearly seen on day 90; group T4 had the highest values compared to other groups. Agreement with the findings of Maiti et al. (Citation2023) reported that P. hypopthalmus fed with A. paniculata leaf extract could increase serum lysozyme activity due to the bioactive compounds of this herb, which could stimulate the non-specific immune system response naturally occurring in fish. In addition, it improves cellular defence mechanisms and has the ability to destroy the cell walls of some pathogens. According to Basha et al. (Citation2013), feeding the andrographolide in Labeo rohita can increase lysozyme activity due to the andrographolide stimulating an increase in the mechanism of a non-specific immune response. In addition, it breaks down the cell walls of both gram-negative and positive gram-positive bacteria. All the above reports show the positive effect of medicinal plant extract on lysozyme activity, which corresponds to our study by the increased serum lysozyme activity.
Organosomatic indices such as hepatosomatic index (HSI), spleen somatic index (SSI), kidney index (KI) and intestinal somatic index (ISI) were used to evaluate the results of this study. This is because the indices have effects, though changes in organosomatic size and are influenced by various factors such as water quality, stocking density, feed or supplemental diets and stress (Akani and Daka Citation2015; Kareem et al. Citation2016). Present study indicated that the organosomatic indices in all experimental groups were not significantly different (P > 0.05). This like the findings of Shi et al. (Citation2020) hepatosomatic and viscerosomatic indices of Monopterus albus were not significantly changed after fed with 75–300 mg/kg andrographolide for eight weeks. In addition, Oreochromis niloticus fed an Aloe vera supplement showed VI, FI and KI were not significantly different Gabriel et al. (Citation2015). Meanwhile, (Bahabadi et al. Citation2014) reported that feeding Oncorhynchus mykiss with Achillea millefolium extract had no effect on HSI, ISI and SSI. Moreover, some organosomatic indices of red hybrid tilapia, Oreochromis mossambicus × Oreochromis niloticus were not changed after fed with papaya leaf extract inclusion (Somdare et al. Citation2023). According to our research, dietary treatments had no adverse effects on organosomatic indices of hybrid catfish. The reason the organs have value is not different because of the bioactive compound of this herb's lack of negative effect and safety on organosomatic indices in fish.
Pathogenic test
Present study showed that all groups that were fed with A. paniculate extract supplement showed a lower cumulative mortality rate when compared with the control group. After the 90-day feeding trial, experimental fish from each group were injected with an A. hydrophila suspension at 109 CFU/ml for 14 days. Mortality in all these groups was found only in the first 4 days of the experiment and the highest cumulative mortality rate was observed in the control group, while the lowest cumulative mortality rate was found in group T4. This agrees with the findings of Maiti et al. (Citation2023), where it was reported that P. hypopthalmus fed with A. paniculata extract showed a reduction in cumulative mortality rate when infected with A. hydrophila. In addition, Basha et al. (Citation2013) have demonstrated that andrographolide can resist A. hydrophila in Labeo rohita because andrographolide stimulates non-specific immune response mechanisms. Thus, all the extracted groups had better body readiness than the control group for resistance to A. hydrophila. Our study indicates that all groups that were fed with A. paniculata extract after injection with A. hydrophila for 14 days had higher values of hematological indices when compared to the control group. Similar findings were reported in African catfish, Clarias gariepinus were fed with 5–15 g/kg Ocimum gratissimum leaf extract for 12 weeks and then were further exposed to Listeria monocytogenes for 14 days, RBC, WBC, Htc and haemoglobin were significantly increased a in a dose-dependent manner (Abdel-Tawwab et al. Citation2018). Besides, Catla-catla was exposed to Aeromonas hydrophila and A. veronii at 105 cfu/ml, haemoglobin, erythrocyte and leukocyte count were increased (Palanikani et al. Citation2018). Similarly, Palanikani et al. (Citation2020) showed that A. paniculate extract can control A. hydrophila in Labeo rohita, which may have minerals and iron that can produce red blood cells and increase oxygen transportation throughout the body of the fish, resulting in an increase in RBC and increased levels of WBC as a result of increased phagocytic activity.
All groups that were fed A. paniculata extract after injection with A. hydrophila for 14 days had higher lysozyme activity values than the control group. Similar results were found by Maiti et al. (Citation2023) who reported that supplementation with A. paniculata leaf extract could improve lysozyme activity on P. hypopthalmus after being infected with A. hydrophila. Moreover, Basha et al. (Citation2013) reported that supplementation with andrographolide was able to increase the activity of lysozyme on Labeo rohita after infection with A. hydrophila. This was because, prior to infection, all the groups of fish that received the extract had non-specific immune system stimulation, and when infected, all groups receiving extracts had better body readiness than the control groups. In addition, Palanikani et al. (Citation2020) reported that the ability of A. paniculata extract to increase the phagocytic index (monocyte, neutrophil and lymphocyte) and act as immune cells by engulfing and destroying the pathogen; these are vital components of the non-specific immune system.
Conclusion
The results obtained from our study demonstrated that hybrid catfish, C. macrocephalus x C. gariepinus fed with ethanolic extract of A. paniculata for three months can improve the growth performance, feed utilization, efficiency of protein utilization, hematological indices and serum biochemical indices. Besides, A.pamiculata extract can increase the proper functioning of the immune system, as well as improve lysozyme activity and A. hydrophila resistance. Moreover, A. paniculata extract can be introduced into feed without impacting liver, kidney, spleen and intestine indices. The appropriate concentration to use is suggested by our research as 0.6 g/kg, because it is safe and no adverse effects were discerned that would prevent the production of healthy fish for human consumption.
Compliance with ethical standards
The procedures for this research, concerning animal care and experimentation, were approved by the Committee for the Institution of Animal Care at the University of Phayao, Thailand (ID: 1-030-65).
CRediT authorship contribution statement
Chitra Ear: Conceptualization, Investigation, Formal analysis, writing original draft, Writing-review and editing. Sontaya Sookying: Data curation, Methodology, Formal analysis, Supervision. Korntip Kannika: Data curation, Formal analysis, Methodology, Supervision. Chatmongkon Suwannapoom: Conceptualization, Methodology, Supervision Paiboon Panase: Data curation, Investigation. Resources, Visualization, Visualization, Writing-review and editing, Validation, writing review and editing, Conceptualization, Funding acquisitions, Supervision, Project administration.
Acknowledgements
We would like to thank The Thailand Science Research and Innovation Fund and the University of Phayao, for providing facilities for the present research. Moreover, the authors would like to thank all the staff and students in the fisheries programme for their help with data collection.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Abdel-Latif HM, Ahmed HA, Shukry M, Chaklader MR, Saleh RM, Khallaf MA. 2022. Astragalus membranaceus extract (AME) enhances growth, digestive enzymes, antioxidant capacity, and immunity of Pangasianodon hypophthalmus juveniles. Fishes. 7(6):319. doi:10.3390/fishes7060319.
- Abdel-Tawwab M, Adeshina I, Jenyo-Oni A, Ajani EK, Emikpe BO. 2018. Growth, physiological, antioxidants, and immune response of African catfish, Clarias gariepinus (B.), to dietary clove basil, Ocimum gratissimum, leaf extract and its susceptibility to listeria monocytogenes infection. Fish Shellfish Immunol. 78:346–354. doi:10.1016/j.fsi.2018.04.057.
- Abdel-Tawwab M, Ahmad MH, Seden ME, Sakr SF. 2010. Use of green tea, camellia sinensis in practical diet for growth and protection of Nile tilapia, Oreochromis niloticus (L.), against Aeromonas hydrophila infection. J World Aquac Soc. 41(s2):203–213. doi:10.1111/j.1749-7345.2010.00360.x.
- Adler AJ, Holub BJ. 1997. Effect of garlic and fish-oil supplementation on serum lipid and lipoprotein concentrations in hypercholesterolemic men. Am J Clin Nutr. 65(2):445–450. doi:10.1093/ajcn/65.2.445.
- Ahmadifar E, Fallah HP, Yousefi M, Dawood MAO, Hoseinifar SH, Adineh H, Yilmaz S, Paolucci M, Doan HV. 2021. The gene regulatory roles of herbal extracts on the growth, immune system, and reproduction of fish. Animals (Basel). 11:2167. doi:10.3390/ani11082167.
- Akani NP, Daka ER. 2015. Evaluation of weight changes, condition factor and organosomatic indices of Clarias gariepinus exposed to sub-lethal concentrations of an oilfield wastewater. Curr Stud Comp Educ Sci Technol. 2:338–354.
- Alishahi M, Ranjbar MM, Ghorbanpour M, Peyghan R, Mesbah M, Razi JM. 2010. Effects of dietary Aloe vera on some specific and nonspecific immunity in the common carp (Cyprinus carpio). Int J Vet Res. 4:189–195. doi:10.22059/IJVM.2010.21352.
- Ashour M, Mabrouk MM, Ayoub HF, El-Feky MMMM, Zaki SZ, Hoseinifar SH Jr, Rossi W, Van Doan H, El-Haroun E, Goda AMA-S. 2020. Effect of dietary seaweed extract supplementation on growth, feed utilization, hematological indices, and non-specific immunity of Nile tilapia, Oreochromis niloticus challenged with Aeromonas hydrophila. J Appl Phycol. 32:3467–3479. doi:10.1007/s10811-020-02178-1.
- Baba E, Acar U, Ontas C, Kesbic OS, Yilmaz S. 2016. The use of Avena sativa extract against Aeromonas hydrophila and its effect on growth performance, hematological and immunological parameters in common carp (Cyprinus carpio). Ital J Anim Sci. 15:325–333. doi:10.1080/1828051X.2016.1185977.
- Bagenal T. 1978. Methods for assessment of fish production in fresh waters-3. Oxford: Blackwell Scientific Publication. doi:10.1002/iroh.19690540313.
- Bahabadi MN, Banaee M, Taghiyan M, Haghi BN. 2014. Effects of dietary administration of yarrow extract on growth performance and blood biochemical parameters of rainbow trout (Oncorhynchus mykiss). Int J Aquat Biol. 2:275–285. doi:10.22034/ijab.v2i5.138.
- Basha KA, Raman RP, Prasad KP, Kumar K, Nilavan E, Kumar S. 2013. Effect of dietary supplemented andrographolide on growth, non-specific immune parameters and resistance against Aeromonas hydrophila in Labeo rohita (Hamilton). Fish Shellfish Immunol. 35(5):1433–1441. doi:10.1016/j.fsi.2013.08.005.
- Berg JM, Tymoczk JL, Stryer L. 2002. Biochemistry. New York: W. H. Freeman and Company.
- Binaii M, Ghiasi M, Vahid-Farabi SM, Pourgholam R, Fazli H, Safari R, Alavi SE, Taghavi MJ, Bankehsaz Z. 2014. Biochemical and hemato-immunological parameters in juvenile beluga (Huso huso) following the diet supplemented with nettle (Urtica dioica). Fish Shellfish Immunol. 36(1):46–51. doi:10.1016/j.fsi.2013.10.001.
- Chaivichoo P, Koonawootrittriron S, Chatchaiphan S, Srimai W, Na-Nakorn U. 2020. Genetic components of growth traits of the hybrid between north African catfish (Clarias gariepinus Burchell, 1822) and bighead catfish (C. macrocephalus günther, 1864). Aquaculture. 521:735082. doi:10.1016/j.aquaculture.2020.735082.
- Chakraborty SB, Horn P, Hancz C. 2014. Application of phytochemicals as growth promoters and endocrine modulators in fish culture. Rev Aquacult. 6:1–19. doi:10.1111/raq.12021.
- Citarasu T, Venkatramalingam K, Micheal Babu M, Raja Jeya Sekar R, Petermarian M. 2003. Influence of the antibacterial herbs, solanum trilobatum, A. paniculata and psoralea corylifolia on the survival, growth and bacterial load of penaeus monodon post larvae. Aquac Int. 11(6):581–595. doi:10.1023/B:AQUI.0000013322.53358.53.
- Edeoga HO, Okwu DE, Mbaebie BO. 2005. Phytochemical constituents of some Nigerian medicinal plants. Afr J Biotechnol. 4(7):685–688. doi:10.5897/AJB2005.000-3127.
- El-Desouky H, El-Asely A, Shaheen AA, Abbass A. 2012. Effects of Zingiber officinalis and Cyanodon dactylon on the growth performance and immune parameters of Macrobrachium rosenbergii. J Fish Mar Sci. 4(3):301–307. doi:10.5829/idosi.wjfms.2012.04.03.62120.
- Ellis AE. 1999. Immunity to bacteria in fish. Fish Shellfish Immunol. 9(4):291–308. doi:10.1006/fsim.1998.0192.
- Fishery statistics group of Thailand. 2022. Fisheries statistics of Thailand 2022. Department of Fisheries. Ministry of Agriculture and Cooperative. Thailand. No. 12/2023.
- Frémont L, Gozzelino MT, Linard A. 2000. Response of plasma lipids to dietary cholesterol and wine polyphenols in rats fed polyunsaturated fat diets. Lipids. 35(9):991–999. doi:10.1007/s11745-000-0610-2.
- Gabriel NN, Qiang J, He J, Ma XY, Kpundeh MD, Xu P. 2015. Dietary Aloe vera supplementation on growth performance, some haemato-biochemical parameters and disease resistance against Streptococcus iniae in tilapia (GIFT). Fish Shellfish Immunol. 15:1–11. doi:10.1016/j.fsi.2015.03.002.
- Gabriel NN, Wilhelm MR, Habte-Tsion HM, Chimwamurombe P, Omoregie E, Iipinge LN, Shimooshili K. 2019. Effect of dietary Aloe vera polysaccharides supplementation on growth performance, feed utilization, hemato-biochemical parameters, and survival at low pH in African catfish (Clarias gariepinus) fingerlings. Int Aquat Res. 11:57–72. doi:10.1007/s40071-019-0219-8.
- Ghosal I, Mukherjee D, Chakraborty SB. 2020. The effects of four plant extracts on growth, sex reversal, immunological and haemato-biochemical parameters in Nile tilapia, Oreochmomis niloticus (linnaeus, 1758). Aquac Res. 52(2):559–576. doi:10.1111/are.14914.
- Gupta S, Mishra KP, Ganju L. 2017. Broad-spectrum antiviral properties of andrographolide. Arch Virol. 162(3):611–623. doi:10.1007/s00705-016-3166-3.
- Hadidi S, Gavin WG, Timothy JW, Jeffrey TS, Gregory DW. 2008. Spleen size predicts resistance of rainbow trout to Flavobacterium psychrophilum challenge. J Immunol. 180(6):4156–4165. doi:10.4049/jimmunol.180.6.4156.
- Harikrishnan R, Balasundaram C, Heo MS. 2010. Herbal supplementation diets on hematology and innate immunity in goldfish against Aeromonas hydrophila. Fish Shellfish Immunol. 28:354–361. doi:10.1016/j.fsi.2009.11.013.
- Harikrishnan R, Kim JS, Kim MC, Balasundaram C, Heo MS. 2011. Lactuca indica extract as feed additive enhances immunological parameters and disease resistance in Epinephelus bruneus to Streptococcus iniae. Aquaculture. 318(12):43–47. doi:10.1016/j.aquaculture.2011.04.049.
- Hartanti D, Cahyani AN. 2020. Plant cyanogenic glycosides: an overview. Farmasains J Farm Il Kes. 5(2):1–6. doi:10.22219/farmasains.v5i1.10047.
- Hassaan MS, EL Nagar AG, Salim HS, Fitzsimmons K, El-Haroun ER. 2019. Nutritional mitigation of winter thermal stress in Nile tilapia by propolis-extract: associated indicators of nutritional status, physiological responses and transcriptional response of delta-9-desaturase gene. Aquaculture. 511:734256. doi:10.1016/j.aquaculture.2019.734256.
- Hidayat Y, Fuad F, Nurhidayati M. 2018. Implementation of economic democracy principle in Islamic banking policies through financial services authority (FSA) in Indonesia. At-Taradhi: J Stu Eko. 8(2):132–154. doi:10.18592/at-taradhi.v8i2.1996.
- Hossain MS, Urbi Z, Sule A, Hafizur Rahman KM. 2014. Andrographis paniculata (Burm. f.) wall. ex nees: a review of ethnobotany, phytochemistry, and pharmacology. Sci World J. 2014:274905. doi:10.1155/2014/274905.
- Immanuel G, Uma RP, Iyapparaj P, Citarasu T, Punitha PSM, Michael BM, Palayesam A. 2009. Dietary medicinal plant extracts improve growth, immune activity and survival of tilapia Oreochromis mossambicus. J Fish Biol. 74(7):1462–1475. doi:10.1111/j.1095-8649.2009.02212.x.
- Jayakumar T, Hsieh CY, Lee JJ, Sheu JR. 2013. Experimental and clinical pharmacology of Andrographis paniculata and its major bioactive phytoconstituent andrographolide. Evid Base Compl Alternative Med. 2013:1–16. doi:10.1155/2013/846740.
- Ji SC, Takaoka O, Jeong GS, Lee SW, Ishimaru K, Seoka M, Takii K. 2007. Dietary medicinal herbs improve growth and some non-specific immunity of red sea bream Pagrus major. Fish Sci. 73(1):63–69. doi:10.1111/j.1444-2906.2007.01302.
- Kamaraj C, Deepak P, Balasubramani G, Karthi S, Arul D, Aiswarya D, Amutha V, Vimalkumar E, Mathivanan D, Suseem SR, et al. 2018. Target and non-target toxicity of fern extracts against mosquito vectors and beneficial aquatic organisms. Ecotoxicol Environ Saf. 161:221–230. doi:10.1016/j.ecoenv.2018.05.062.
- Kareem ZH, Abdelhadi YM, Christianus A, Karim M, Romano N. 2016. Effects of some dietary crude plant extracts on the growth and gonadal maturity of Nile tilapia (Oreochromis niloticus) and their resistance to Streptococcus agalactiae infection. Fish Physiol Biochem. 42(2):757–769. doi:10.1007/s10695-015-0173-3.
- Koskela J, Rahkonen R, Pasternack M, Knuutinen H. 2004. Effect of immunization with two commercial vaccines on feed intake, growth, and lysozyme activity in European whitefish (Coregonus lavaretus L.). Aquaculture. 234(1-4):41–50. http://doi.org/10.1016/j.aquaculture.2003.11.036.
- Lee KW, Everts H, Kapperst HJ, Yeom KH, Beynen AC. 2003. Dietary carvacrol lowers body weight gain but improves feed conversion in female broiler chickens. J Appl Poult Res. 12(4):394–399. doi:10.1093/japr/12.4.394.
- Li X, Qin C, Fang Z, Sun X, Shi H, Wang Q, Zhao H. 2022. Replacing dietary fish meal with defatted black soldier fly (Hermetia illucens) larvae meal affected growth, digestive physiology and muscle quality of tongue sole (Cynoglossus semilaevis). Front Physiol. 13:855957. doi:10.3389/fphys.2022.855957.
- Lin D, Xiao M, Zhao J, Li Z, Xing B, Li X, Kong M, Li L, Zhang Q, Liu Y, et al. 2016. An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules. 21(10):1374. doi:10.3390/molecules21101374.
- Liu S, Zang X, Liu B, Zhang X, Arunakumara KKU, Zhang X, Liang B. 2007. Effect of growth hormone transgenic synechocystis on growth, feed efficiency, muscle composition, haematology and histology of turbot (Scophthalmus maximus L.). Aquac Res. 38:1283–1292. doi:10.1111/j.1365-2109.2007.01796.x.
- Lucas A, Watson JJ. 1996. Physical concepts of bioenergetics. Bioenergetics of aquatic animals. English edition. London: Taylor and Francis. doi:10.1201/9781482295313.
- Maiti S, Saha S, Jara P, Chowdhury A, Khatua S, Ghosh TK. 2023. Effect of dietary Andrographis paniculata leaf extract on growth, immunity, and disease resistance against Aeromonas hydrophila in Pangasianodon hypopthalmus. J Appl Aquac. 35(2):305–329. doi:10.1080/10454438.2021.1959861.
- Mishra A, Shah BR, Roy K, Abdelsalam EEE, Piačková V, Shaik HA, Dvořák P, Velíšek J, Agbeko KFK, Mráz J. 2023. Andrographolide loaded pickering emulsion: A bioactive component for improved growth, digestibility, and haematological properties in cultured common carp Cyprinus carpio. Aquaculture. 562:738810. doi:10.1016/j.aquaculture.2022.738810.
- Nagajothi S, Mekala P, Raja A, Raja MJ, Senthilkumar P. 2018. Andrographis paniculata: qualitative and quantitative phytochemical analysis. J Pharmacogn Phytochem. 7(4):1251–1253. doi:10.3390/molecules181012192.
- Naomi R, Bahari H, Ong ZY, Keong YY, Embong H, Rajandram R, Teoh SH, Othman F, Hasham R, Yin KB, et al. 2022. Mechanisms of natural extracts of Andrographis paniculata that target lipid-dependent cancer pathways: A view from the signaling pathway. Int J Mol Sci. 23(11):5972. doi:10.3390/ijms23115972.
- Ndashe K, Hang’ombe BM, Changula K, Yabe J, Samutela MT, Songe MM, Kefi AS, Njobvu Chilufya L, Sukkel M. 2023. An assessment of the risk factors associated with disease outbreaks across tilapia farms in central and southern Zambia. Fishes. 8:49. doi:10.3390/fishes8010049.
- Ngugi CC, Oyoo-Okoth E, Mugo-Bundi J, Orina PS, Chemoiwa EJ, Aloo PA. 2015. Effects of dietary administration of stinging nettle (Urtica dioica) on the growth performance, biochemical, hematological and immunological parameters in juvenile and adult Victoria labeo (Labeo victorianus) challenged with Aeromonas hydrophila. Fish Shellfish Immunol. 44:533–541.
- Nowosa J, Jasiński S, Arciuch-Rutkowska M, Abdel-Latif HMR, Wróbel M, Mikiewicz M, Zielonka Ł, Kotsyumbas IY, Muzyka VP, Brezvyn OM, et al. 2023. Effects of Bee pollen on growth performance, intestinal microbiota and histomorphometry in African catfish. Animals (Basel). 13:132. doi:10.3390/ani13010132.
- Nya EJ, Austin B. 2009. Use of garlic, Allium sativum, to control Aeromonas hydrophila infection in rainbow trout, Oncorhynchus mykiss (Walbaum). J Fish Dis. 32(11):963–970. doi:10.1111/j.1365-2761.2009.01100.x.
- Palanikani R, Chanthini KMP, Soranam R, Thanigaivel A, Karthi S, Senthil-Nathan S, Murugesan AG. 2020. Efficacy of Andrographis paniculata supplements induce a non-specific immune system against the pathogenicity of Aeromonas hydrophila infection in Indian major carp (Labeo rohita). Environ Sci Pollut Res. 27:23420–23436. doi:10.1007/s11356-019-05957-7.
- Palanikani R, Soranam R, Chanthini KMP. 2018. Pathogenicity and control of Aeromonas hydrophila and A. veronii in Indian major carps (Catla-catla) by the effect of herbal supplement of Andrographis paniculata (Lamiales: Acanthaceae). Inter J Fish Aqu Stud. 8(3):361–370.
- Panase P, Kamee B, Moungmor S, Tipdacho P, Matidtor J, Sutthi N. 2018a. Effects of Euphorbia hirta plant leaf extract on growth performance, hematological and organosomatic indices of hybrid catfish, Clarias macrocephalus × C. gariepinus. Fish Sci. 84(6):1025–1036. doi:10.1007/s12562-018-1234-1.
- Panase P, Khuangbun L, Suphason T, Tipdacho P. 2018b. Evaluation of Houttuynia cordata Thunb leaf extract on growth performance, feed utilization, and hematological indices of hybrid catfish (Clarias macrocephalus × Clarias gariepinus). Comp Clin Path. 27:947–958. doi:10.1007/s00580-018-2686-5.
- Panase P, Saenphet S, Saenphet K. 2017. Visceral and serum lysozyme activities in some freshwater fish (three catfish and two carps). Comp Clin Pathol. 26:169–173. doi:10.1007/s00580-016-2362-6.
- Pant DR, Pant ND, Saru DB, Narayan Yadav UN, Khanal PD. 2017. Phytochemical screening and study of antioxidant, antimicrobial, antidiabetic, anti-inflammatory and analgesic activities of extracts from stem wood of Pterocarpus marsupium Roxburgh. J Intercult Ethnopharmacol. 6(2):1–176. doi:10.5455/jice.20170403094055.
- Peng K, Zhao H, Wang G, Chen B, Mo W, Huang Y. 2021. Effect of condensed tannins on growth performance, intestinal immune capacity and bacterial microbiomes of Lateolabrax japonicus. Aquac Res. 52(11):5321–5331. doi:10.1111/are.15402.
- Ponjarat J, Singchat W, Monkheang P, Suntronpong A, Tawichasri P, Sillapaprayoon S, Ogawa S, Muangmai N, Baicharoen S, Peyachoknagul S, et al. 2019. Evidence of dramatic sterility in F1 male hybrid catfish [male Clarias gariepinus (Burchell, 1822) × female C. macrocephalus (Günther, 1864)] resulting from the failure of homologous chromosome pairing in meiosis I. Aquaculture. 505:84–91. doi:10.1016/j.aquaculture.2019.02.035.
- Pratheepa V, Sukumaran N. 2014. Effect of Euphorbia hirta plant leaf extract on immunostimulant response of Aeromonas hydrophila infected Cyprinus carpio. PeerJ. 2:e671. doi: 10.7717/peerj.671.
- Rajalakshmi V, Cathrine L. 2016. Phytochemical screening and antimicrobial activity of ethanolic extract of Andrographis paniculata. J Pharmacogn Phytochem. 5(2):175–177. doi:10.4103/0250-474X.57294.
- Rehulka J. 2003. Haematological analyses in rainbow trout Oncorhynchus mykiss affected by viral haemorrhagic septicaemia (VHS). Dis Aquat Org. 56(3):185–193. doi:10.3354/dao056185.
- Reverter M, Bontemps N, Lecchini D, Banaigs B, Sasal P. 2014. Use of plant extracts in fish aquaculture as an alternative to chemotherapy: current status and future perspectives. Aquaculture. 433:50–61. doi:10.1016/j.aquaculture.2014.05.048.
- Ronald WG, Bruce AB. 1990. Organosomatic indices and an autopsy-based assessment as indicators of health condition of fish. J Am Fish Soc. 8:93–108. doi:10.4236/ijg.2012.33049.
- Rudy AN, Meylianawati OFA, Yanti PS, Esti HH. 2018. The effects of dietary Eleutherine bulbosa on the growth, leukocyte profile, and digestive enzyme activity of the striped catfish Pangasianodon hypophthalmus. Nusant Biosci. 10:46–51.
- Saeidi AMR, Adel M, Caipang CMA, Dawood MAO. 2017. Immunological responses and disease resistance of rainbow trout (Oncorhynchus mykiss) juveniles following dietary administration of stinging nettle (Urtica dioica). Fish Shellfish Immunol. 71:230–238. doi:10.1016/j.fsi.2017.10.016.
- Shaikh JR, Patil MK. 2020. Qualitative tests for preliminary phytochemical screening: An overview. Int J Chem Stud. 8(2):603–608. doi:10.22271/chemi.2020.v8.i2i.8834.
- Shi Y, Zhong L, Liu Y, Zhang J, Lv Z, Li Y, Hu Y. 2020. Effects of dietary andrographolide levels on growth performance, antioxidant capacity, intestinal immune function and microbioma of rice field eel (Monopterus albus). Animals (Basel). 10 (10):1744. doi:10.3390/ani10101744.
- Somdare PO, Hamid NKA, Kari ZA. 2023. Effect of papaya leaf extract inclusion on growth performance and haematological parameters of red hybrid tilapia, Oreochromis mossambicus × Oreochromis niloticus fed diets formulated with hermetia meal and azolla. Agric Rep. 2:29–45.
- Sun H. 2008. Effects of Ginkgo biloba leaf extract on blood biochemical indexes and antioxidant activity in Carassius auratus. China Feed. 13:36–37.
- Suriyo T, Chotirat S, Rangkadilok N, Pholphana N, Satayavivad J. 2021. Interactive efects of andrographis paniculata extracts and cancer chemotherapeutic 5-fluorouracil on cytochrome P450s expression in human hepatocellular carcinoma HepG2 cells. J Herb Med. 26:100421. doi:10.1016/j.hermed.2021.100421.
- Sutthi N, Panase A, Chitmanat C, Sookying S, Ratworawong K, Panase P. 2020. Effects of dietary leaf ethanolic extract of Apium graveolens L. on growth performance, serum biochemical indices, bacterial resistance and lysozyme activity in Labeo chrysophekadion (Bleeker, 1849). Aquac Rep. 18:100551. doi:10.1016/j.aqrep.2020.100551.
- Tan X, Sun Z, Zhou C, Huang Z, Tan L, Xun P, Huang Q, Lin H, Ye C, Wang A. 2018. Effects of dietary dandelion extract on intestinal morphology, antioxidant status, immune function and physical barrier function of juvenile golden pompano Trachinotus ovatus. Fish Shellfish Immunol. 73:197–206. doi:10.1016/j.fsi.2017.12.020.
- Taufek NM, Aspani F, Muin H, Raji AA, Razak SA, Alias Z. 2016. The effect of dietary cricket meal (Gryllus bimaculatus) on growth performance, antioxidant enzyme activities, and haematological response of African catfish (Clarias gariepinus). Fish Physiol Biochem. 42(4):1143–1155. doi:10.1007/s10695-016-0204-8.
- Valdiani A, Ofoghi H, Akbarizare M, Talei D. 2022. Andrographis paniculata extract as an immunity modulator against cancer via telomerase inhibition. 3 Biotech. 12:319. doi:10.1007/s13205-022-03373-2.
- Valdiani A, Talei D, Lattoo SK, Ortiz R, Rasmussen SK, Batley J, Rafi MY, Maziah M, Sabu KK, Abiri R, et al. 2017. Genoproteomics-assisted improvement of Andrographis paniculata: toward a promising molecular and conventional breeding platform for autogenous plants affecting the pharmaceutical industry. Critic Rev Biotechnol. 37(6):803–816. doi:10.1080/07388551.2016.1260525.
- Velichkova K, Sirakov I, Stoyanova S. 2020. Growth efficiency, biochemical blood parameters and meat quality of rainbow trout (Oncorhynchus mykiss W.), fed with supplement of sweet flag extract (Acorus calamus L.). Bulg J Agric Sci. 26(Suppl. 1):180–185.
- Velichkova K, Sirakov I, Stoyanova S, Zhelyazkov G, Staykov Y, Slavov T. 2019. Effect of Acorus calamus L. extract on growth performance and blood parameters of common carp (Cyprinus carpio L.) cultivated in a recirculation system. J Cent Eur Agric. 20(2):585–591. doi:10.5513/JCEA01/20.2.2544.
- Vijayan MM, Pereira C, Forsyth RB, Kennedy CJ, Iwama GK. 1997. Handling stress does not affect the expression of hepatic heat shock protein 70 and conjugation enzymes in rainbow trout treated with β-naphthoflavone. Life Sci. 61(2):117–127. doi:10.1016/s0024-3205(97)00366-4.
- Wang CY, Li ZB, Sun YZ, Chen Q, Li WJ, Huang YC, Lu J. 2018. Effects of Chinese herbal medicines mixture on growth performance digestive enzyme activity immune response of juvenile Japanese seabass, Lateolabrax japonicus. Aquac Nutr. 24(2):683–693. doi:10.1111/anu.12597.
- Wangkahart E. 2018. Effect of Aeromonas hydrophila infection on hematological value in hybrid catfish (Clarias macrocephalus × C. gariepinus) and sensitivity test to antibiotic drugs. SWU Sci J. 34(1):151–166. http://ejournals.swu.ac.th/index.php/ssj.
- Xu A, Shang-Guan J, Li Z, Gao Z, Huang Y, Chen Q. 2020. Effects of garlic powder on feeding attraction activity, growth and digestive enzyme activities of Japanese seabass, Lateolabrax japonicus. Aquacult Nutr. 26(2):390–399. doi:10.1111/anu.13001.
- Zakaria MK, Kari ZA, Van Doan H, Kabir MA, Che Harun H, Mohamad Sukri SA, Goh KW, Wee W, Khoo MI, Wei LS. 2022. Fermented soybean meal (FSBM) in African catfish (Clarias gariepinus) diets: effects on growth performance, fish gut microbiota analysis, blood haematology, and liver morphology. Life. 12(11):1851. doi:10.3390/life12111851.
