ABSTRACT
The potential benefits of β-carotene, a leading provitamin A carotenoid, have been meticulously explored in various livestock species, propelling a realm of extensive research in this domain. Despite of not being considered as an essential micronutrient for these animals, its supplementation has gained substantial attention within the agricultural industry due to its capacity to enhance animal well-being, productivity, and the quality of the derived products. In pursuit of a comprehensive understanding, this review endeavours to juxtapose the effects of β-carotene supplementation in poultry, swine, and cattle. In doing so, we delve into a diverse range of physiological responses, intricate metabolic pathways, and the profound influence of β-carotene on pivotal aspects such as immune response, antioxidant status, and reproduction across these three important livestock species. Recognizing diverse reactions to β-carotene supplementation across species is pivotal for refining animal production and welfare standards. This review sheds light on the complex interplay between β-carotene and livestock physiology, contributing to a holistic understanding of how this provitamin A carotenoid can optimize animal health, productivity, and the sustainability of the livestock industry. Furthermore, it underscores the significance of tailoring nutritional strategies to the specific needs of various animal species, ultimately benefiting both producers and consumers.
Introduction
Found in plants, algae, and also available synthetically, β-carotene is a natural pigment that acts as a provitamin, potentially transforming into vitamin A (retinol) in mammals and birds (Takaichi Citation2011; Bogacz-Radomska and Harasym Citation2018; Akram et al. Citation2021). This multifunctional compound, widely recognized for its vibrant orange-red hue, plays an important role in the biological processes of various organisms (Johra et al. Citation2020). Moreover, β-carotene’s significance extends beyond its role as a precursor to vitamin A. It is increasingly being recognized for its immunomodulatory properties, which contribute in the regulation of the immune system, and its antioxidant properties, which protect cells from oxidative stress mitigating the harmful effects of free radicals (Anjani et al. Citation2022).
Mammals and birds typically lack the biological pathways necessary for endogenous carotenoid synthesis, leading them to acquire carotenoids from their diet (Maoka Citation2020). As a result, the predominant avenue for β-carotene uptake in poultry, swine and cattle is through ingestion. While β-carotene is naturally found in variable quantity in plant-based feedstuffs, the utilization of synthesized forms is also prevalent, encompassing an array of feed additives (EFSA Citation2012). However, it is worth noting that, unlike vitamin A, β-carotene may not be essential for birds and mammals, as they can survive without consuming this pro-vitamin A carotenoid when retinol is present in sufficient amounts (Green and Fascetti Citation2016).
In livestock sector, β-carotene is known to have positive effects on health and certain physiological functions (Lopez-Flores et al. Citation2020; Nabi et al. Citation2020). When utilized as a dietary supplement in domestic fowl, swine, and cattle from synthetic sources, this provitamin A compound has shown promising results. For instance, in poultry, β-carotene supplementation has been associated with improved immune function, leading to reduced susceptibility to common infections and diseases (Calislar Citation2019; Nabi et al. Citation2020). Similarly, in swine and cattle, β-carotene has shown remarkable benefits for overall well-being (Riley et al. Citation2023). Studies have revealed that its supplementation can enhance reproductive performance in sows and cows, leading to improved litter sizes and calving rates (Coffey and Britt Citation1993; Iwanska and Strusinska Citation1997; Khemarach et al. Citation2021). Furthermore, in cattle, β-carotene has been found to positively influence milk production and composition, benefiting both dairy farmers and consumers (Conboy Stephenson et al. Citation2021; Mary et al. Citation2021).
While the positive effects of β-carotene supplementation are evident across different livestock species (Green and Fascetti Citation2016), it is crucial to acknowledge the variations in response to this compound among these species (Shastak and Pelletier Citation2023). Factors such as the animal’s age, breed, and existing nutritional status can influence the efficacy of β-carotene in conferring health benefits. Additionally, environmental factors, such as exposure to stress and dietary composition, may also modulate the impact of β-carotene supplementation (Haskell Citation2012; Green and Fascetti Citation2016).
This review delves into the effects of β-carotene supplementation in poultry, swine, and cattle, highlighting the similarities and differences in response to this provitamin A compound. Specifically, the paper will concentrate on the following key facets:
Absorption and metabolism;
Immune response;
Antioxidant capacity;
Reproduction and fertility;
Future perspectives.
Through a comprehensive examination of these five key subjects, this review endeavours to offer deeper insights into the potential benefits associated with incorporating β-carotene as a nutritional strategy to bolster animal health and boost productivity. Additionally, it seeks to pinpoint specific areas that warrant more extensive research, ultimately driving the progress of our understanding within this field.
Absorption and metabolism
Before evaluating the effects of β-carotene supplementation, it is essential to understand its absorption and metabolism in each species. Poultry, swine, and cattle differ in their gastrointestinal anatomy and physiology, which affects β-carotene bioavailability. Variations in enzyme activity and tissue distribution also contribute significantly to the variability in β-carotene conversion to vitamin A among different species (Devery and Milborrow Citation1994).
Poultry possess a unique proximal digestive system characterized by a crop, proventriculus, and gizzard, which aids in the efficient processing of grains and seeds (Pan and Yu Citation2014). In contrast, cattle possess a four-chambered foregut, including the rumen, reticulum, omasum, and abomasum, facilitating the breakdown of fibrous plant material through fermentation (Soltis et al. Citation2023). Swine, on the other hand, feature a simple, single-chambered stomach well-suited for digesting a varied diet, encompassing both plant material and animal proteins (Vukmirovic et al. Citation2017). Due to the differing complexities of their digestive systems, poultry, cattle, and swine exhibit variations in β-carotene utilization.
The variability of β-carotene degradation by microorganisms in the rumen has been documented in previous studies, with reported values reaching as high as 55% (Noziere et al. Citation2006). However, the influence of microflora in the avian crop and scarce species residing in the proventriculus and gizzard of poultry, as well as the stomach of swine, on β-carotene degradation remains uncertain (Gabriel et al. Citation2006; Flahou et al. Citation2018; Shang et al. Citation2018). It is possible that their impact on β-carotene degradation is negligible compared to that observed in the rumen.
Retinaldehyde (retinal) can be synthesized from a lipophilic molecule of β-carotene, which is absorbed through passive diffusion and subsequently cleaved by oxygenases in the intestinal mucosal cells, suggesting its potential for forming vitamin A (Hynd Citation2019). In most vertebrates, including swine and cattle, β-carotene 15,15′-monooxygenase (BCM1) plays a crucial role in facilitating the synthesis of vitamin A (Nagao et al. Citation2000). BCM1 facilitates the oxidative cleavage of β-carotene at its central double bond, leading to the production of two molecules of retinal from one molecule of β-carotene (Kim et al. Citation2011). These retinal molecules can subsequently undergo further conversions to produce other forms of vitamin A, such as retinol and retinoic acid (Kowatz et al. Citation2013). A high-fat diet and vitamin A deficiency are found to increase BCM1 activity in enterocytes, whereas flavonoids have been shown to decrease its activity (van Vliet et al. Citation1996; Nagao et al. Citation2000). Additionally, there is another enzyme called β-carotene 9′, 10′-monooxygenase (BCM2), which exclusively catalyzes the asymmetric oxidative cleavage of β-carotene at the 9,10′ double bond (Kiefer et al. Citation2001). While both of these oxygenases exhibit activity in the small intestine of mammals such as swine and cattle, in the case of domestic fowl, the conversion of β-carotene is primarily attributed to the BCM1 enzyme (Sklan Citation1983; Jlali et al. Citation2012; Calislar Citation2019). Furthermore, the presence of the BCM1 enzyme in the liver of mammals (Nagao Citation2004) and the BCM2 enzyme in the liver of chickens (Gao et al. Citation2016) suggests a potential mechanism for the conversion of β-carotene that remains unconverted in the enterocytes.
Mammals typically exhibit greater absorption of β-carotene, whereas birds and fish have a higher efficiency in absorbing oxygenated xanthophylls, such as lutein and zeaxanthin (Schiedt et al. Citation1985). Chickens, for instance, are generally recognized to have lower capacities for absorbing and accumulating carotenes (Na et al. Citation2004; Green and Fascetti Citation2016). Contrary to what might be expected, the conversion of β-carotene to retinol by BCM1 in poultry appears to be better (ranging from 2–6 parts β-carotene to 1 part retinol) when compared to swine (7-40:1) and cattle (6-8:1) (Green and Fascetti Citation2016). Differences in oxygenase activity and conversion rates vary not only between monogastric and ruminant species but also within ruminants, as shown by Yang and Tume (Citation1993). Their study revealed that sheep intestines displayed higher BCM1 enzyme activity compared to cow or goat intestines. This difference in enzyme activity possibly contributes to the observation that among ruminants, bovines tend to accumulate higher concentrations of carotenoids, particularly β-carotene. This phenomenon might be attributed to potentially lower vitamin A synthesis efficiencies in enterocytes (Noziere et al. Citation2006). Furthermore, a notable variation in conversion rates exists among various breeds of dairy cows. For instance, when comparing Jersey to Holstein cows, it becomes evident that Jersey cows exhibit a heightened capacity to assimilate β-carotene in comparison to Holstein cattle (Qui Citation2020). Moreover, the conversion ratio of β-carotene to retinol differs between these two breeds. This inherent breed-based disparity subsequently exerts an influence on the concentrations of β-carotene and retinol present in milk. The intricate breed-specific variations, along with various influencing factors such as the animal’s physiological characteristics, the composition of the feed matrix, feed processing methods, levels and types of dietary fats, the presence of diverse carotenoids, the intake of dietary β-carotene, and the overall vitamin A status, collectively contribute to a high level of complexity (EFSA Citation2012). This complexity poses significant challenges when attempting to standardize the estimation of the conversion of β-carotene to vitamin A, making it extremely difficult to practically implement such standardization in cattle, swine, and domestic fowl. Notably, the average conversion rate of β-carotene in swine is generally regarded as the lowest among livestock, likely attributed to the relatively lower activity of oxygenase enzymes involved in its conversion (Shastak and Pelletier Citation2023). Schweigert et al. (Citation1995) observed a conversion rate of just 4% from radiolabeled 14C β-carotene to labelled retinol in pigs in their study.
The portion of β-carotene that remains unconverted into vitamin A can access the bloodstream, utilizing lipoproteins as the means of transportation (Parker Citation1996). Among both mammals and birds, the liver serves as a principal organ for the accumulation of substantial β-carotene quantities, followed by adipose tissue, kidneys, skin, and lungs (Shete and Quadro Citation2013; Moreno et al. Citation2016; Nogareda et al. Citation2016; von Lintig et al. Citation2020).
It is worth noting that there are currently two types of commercial β-carotene sources available in the market: pro-vitamin A β-carotene and oxidized non-provitamin A β-carotene (Riley et al. Citation2023). The latter is typically produced from the former through a reaction with oxygen. As a result of this reaction, β-carotene loses its pro-vitamin A activity and cannot be converted to vitamin A in the intestinal mucosa or liver of animals (Elefson et al. Citation2023). Therefore, it is challenging to directly compare the effects of both types of β-carotene in the metabolism of mammals and birds. This review paper primarily focuses on exploring and comparing the physiological effects of pro-vitamin A β-carotene.
In conclusion, the bioavailability and utilization of β-carotene differ significantly among species due to variations in gastrointestinal anatomy, enzyme activity, and tissue distribution. The synthesis of vitamin A from β-carotene involves crucial enzymes like BCM1 and BCM2, but conversion rates vary widely between species, with swine generally exhibiting the lowest efficiency among livestock and poultry due to lower oxygenase enzyme activity. These factors underscore the need for species-specific considerations in evaluating the effects of β-carotene supplementation.
Immune response
The immune system plays a pivotal role in upholding animal well-being and countering instances of infectious diseases (Düpjan and Dawkins Citation2022). The capacity of β-carotene to bolster immune activity through its influence on various immune cells has been established (Roe and Fuller Citation1993; Hughes Citation1999; Chew and Park Citation2004; Imamura et al. Citation2006). When it comes to β-carotene supplementation, the responses observed in poultry, swine, and cattle have been diverse, encompassing aspects like antibody production, cytokine profiles, and the overall state of immune competence (Daniel et al. Citation1990; Hoskinson et al. Citation1992; Chew and Park Citation2004; Calislar Citation2019; Nabi et al. Citation2020).
Assessing the impacts of β-carotene on immune function proves to be a complex task, primarily due to the ambiguity surrounding whether these effects stem from β-carotene in isolation or result from the conversion of β-carotene to vitamin A. This challenge arises from the intricate determination of the quantity of ingested carotene that successfully reaches the bloodstream and undergoes metabolism. A fundamental requirement for such evaluations involves providing the subjects, whether avian or mammalian, with adequate levels of supplemental vitamin A (Spiegler et al. Citation2012). This step is crucial as it plays a significant role in diminishing the conversion of carotenes into vitamin A within the intestinal mucosa (Green and Fascetti Citation2016). Moreover, the utilization of β-carotene-oxygenase blockers or radioisotope-labelled β-carotene for investigating its precise molecular mechanisms is infrequent, intricate, and associated with substantial expenses (Schweigert et al. Citation1995).
The mechanisms of action underlying the effects of β-carotene supplementation on various aspects of immune function have been elucidated through insightful research. In a study involving domestic fowl, it was demonstrated that β-carotene supplementation led to a significant increase in the expression of CC chemokine receptor-9 and polymeric immunoglobulin receptor in the ileal tissue. Additionally, there was an observed enhancement in the expression of pIgR in the jejunal tissue. This supplementation was also correlated with improved mRNA expression levels of mucin-2, zonula occludens-1, zonula occludens-2, as well as occludins in the ileal tissues (Hui et al. Citation2020). These molecular alterations induced by β-carotene supplementation potentially contribute to a strengthened intestinal barrier function and enhanced mucosal immunity in poultry, thereby positively impacting their overall immune system robustness. In swine, the addition of β-carotene supplements was observed to decrease the expression of interleukin-5 (IL-5) in the jejunal region and interleukin-15 (IL-15) in the mesenteric lymph nodes, as demonstrated by Lo Verso et al. (Citation2020). These changes in cytokine expression following β-carotene supplementation suggest a potential modulatory effect on the local immune response in the intestinal tract of swine. In cattle, β-carotene supplementation has been shown to yield an augmented population of peripheral blood leukocytes (Otomaru et al. Citation2018). This phenomenon can partly be attributed to the cellular uptake of β-carotene by subcellular organelles within blood lymphocytes and neutrophils (Chew et al. Citation2000). Remarkably, this internalization of β-carotene has also been associated with a reduction in DNA damage within these specific cell types (Lee et al. Citation2011). Additionally, the supplementation has been reported to trigger an increase in the count of T helper cells, a vital subset of the immune system, as documented by Alexander et al. (Citation1985). These observed effects, including the increased leukocyte population, reduced DNA damage, and enhanced T helper cell count due to β-carotene supplementation, collectively contribute to bolstering the immune response in animals.
In the realm of poultry research, the influence of β-carotene on immune function has generally yielded inconsistent outcomes. A meticulously crafted investigation carried out by Tengerdy et al. (Citation1990) delved into the repercussions of β-carotene supplementation (at a dose of 50 mg/kg of feed), along with vitamin E (at 300 mg/kg of feed) and vitamin A (at 60,000 IU/kg of feed), both individually and in various combinations. This exploration centred on disease resistance and humoral immune response within broiler chickens afflicted by Escherichia coli infection, commencing from 1-day post-hatch. The actual infection challenge was administered at the 3-week mark, wherein virulent E. coli cells were introduced through the post-thoracic air sac. The duration of the study was 4 weeks. The efficacy of β-carotene in isolation was not similar to that of either of the vitamins in safeguarding the broiler chickens against E. coli infection. Likewise, it did not yield any noteworthy augmentation of humoral immunity, including the production of agglutinating antibodies. Interestingly, when combined with vitamin E, β-carotene exhibited a substantial increase in disease resistance, concurrently mitigating hepatomegaly resulting from the E. coli infection. Strikingly, β-carotene’s effectiveness was akin to that of both vitamin A and E in preventing weight loss and ameliorating hepatomegaly due to endotoxicosis, as a result of its potential anti-inflammatory properties.
At the same time, Haq et al. (Citation1996) found that the supplementation of β-carotene (at a concentration of 0.04% in the diet) to broiler breeder hens vaccinated against Newcastle disease virus did not exhibit any noticeable impact on the neonatal immunity of their offspring for a span of up to 3 weeks post-hatch (P > 0.05). However, a different outcome emerged when a combination of supplementary vitamin E and β-carotene was administered to breeder birds. This combination was observed to enhance the function of lymphocytes in day-old chicks (P < 0.05), as assessed through the measurement of their blastogenic response.
In contrast, a more recent study conducted by Wang et al. (Citation2023) explored the effects of supplementing egg-laying breeding hens’ diets with β-carotene at a concentration of 120 mg/kg feed. This supplementation resulted in a significant enhancement (P < 0.05) of body weight gain in their offspring at 21 days after hatching. The group whose progenitors were subjected to β-carotene supplementation displayed significant elevations in Serum Hepatocyte Growth Factor levels on days 7, 14, 21, and 42, alongside with heightened leptin levels specifically on day 14 (P < 0.05). The observed elevation of Hepatocyte Growth Factor is noteworthy due to its established role in the regulation of various immune cell functions, encompassing tasks such as differentiation, maturation, cytokine production, cellular migration, adhesion, and T cell effector activities (Sagi and Hieronymus Citation2018). Leptin, identified as a key mediator, plays a crucial role in orchestrating the immune response due to alterations in overall nutritional status (Kiernan and MacIver, Citation2021).
Additionally, Karadas et al. (Citation2016) observed that the administration of β-carotene at a dosage of 10 mg/kg feed led to a significant elevation in antibody titres (P < 0.05) following Newcastle disease virus vaccination in broilers that were 14 days old. This effect was not observed in the groups that received supplementation of vitamin E or organic selenium, highlighting the unique impact of β-carotene on the antibody response in this study.
The immunomodulatory impact of ß-carotene on the immune system of developing and mature birds has been substantiated through investigations conducted by Cucco et al. (Citation2006) and Cucco et al. (Citation2007). These studies were carried out on indigenous avian species within the pheasant family, further reinforcing the positive influence of ß-carotene on avian immune function.
In porcine studies, the supplementation of β-carotene has been demonstrated to have a positive impact on immune system dynamics, particularly in the enhancement of antibody production and the promotion of lymphocyte proliferation (Zomborszky-Kovacs et al. Citation2000; Lang et al. Citation2020). The research conducted by Zomborszky-Kovacs et al. (Citation2000) focused on piglets, where β-carotene supplementation at a dosage of 88 mg/kg of feed was initiated seven days prior to the weaning process. This supplementation regimen continued for an additional two weeks post-weaning. Notably, the introduction of pro-vitamin A carotenoid resulted in a statistically significant decrease in neutrophil count and a concurrent statistically significant increase in lymphocyte count (P < 0.05), as compared to the non-supplemented control group. While alterations in the counts of white blood cells warrant careful consideration, an elevation in lymphocyte number coupled with a reduction in neutrophil count may signify a favourable shift towards a more adaptive and potentially heightened immune response. This interpretation aligns with the observed positive influence of β-carotene-enriched feed on piglet weight gain (P < 0.05) in comparison to the control group.
Lang et al. (Citation2020) conducted an experiment involving piglets to assess the effects of dietary β-carotene supplementation. Piglets were assigned to three groups: a low-supplementation group receiving 40 mg of β-carotene per kg of feed, a high-supplementation group receiving 80 mg of β-carotene per kg of feed, and a control group with no supplementation. The experimental period lasted for 10 days, spanning from day 14 to day 24 after birth, with the piglets being weaned on day 21. Analysis of the serum samples on d 24 revealed noteworthy immunological and cytokine variations among the groups. In the high β-carotene supplementation group, both IgA and IL-10 levels in the serum exhibited a statistically significant increase compared to the control group (P < 0.05). On the other hand, the serum levels of IgM, TNF-α, and IL-6 in the β-carotene groups were notably lower than those in the control group (P < 0.05). By taking into consideration the outcomes demonstrating increased average daily gain and average daily feed intake in the high β-carotene group in comparison to the control group (P < 0.05), it can be inferred that the alterations observed in IgA, IgM, TNF-α, and IL-6 levels collectively contribute positively to the overall well-being of the animals. This suggests a potential beneficial effect of high β-carotene supplementation on the growth and health of the piglets.
Similar positive effects on immune function in swine were observed in relation to oxidized β-carotene, as indicated in a recent study by Jun et al. (Citation2021). However, the research conducted by Elefson et al. (Citation2023) demonstrated inconclusive evidence regarding the influence of oxidized β-carotene on the immune status of sows and their offspring.
Considerable research has been conducted on the influence of β-carotene on the immune response in cattle. The findings from these studies also indicate favourable effects on immunity, similar to observations in poultry and swine (Kaewlamun et al. Citation2011). For example, Michal et al. (Citation1994) conducted a study wherein Holstein cows were allocated into three groups: an unsupplemented control group and two groups supplemented daily with either 300 mg or 600 mg of β-carotene, starting four weeks prior to their predicted calving dates. Blood samples were collected at various time points relative to calving: at weeks –4, –2, –1, 0 (within 24 h after calving), 1, 2, and 4. These samples were used to isolate lymphocytes and neutrophils. The researchers observed that blood lymphocyte proliferation in response to specific mitogens like concanavalin A, phytohemagglutinin, and pokeweed mitogen was notably higher (P < 0.05) during the peripartum period in cows that received β-carotene supplementation, compared to those in the unsupplemented control group. Furthermore, enhanced phagocytic activity of blood neutrophils was particularly evident in the group fed with 300 mg of β-carotene during the first week post calving. The capacity of blood neutrophils to perform intracellular killing was also heightened in the β-carotene supplemented group at week 0. Additionally, cows supplemented with β-carotene exhibited increased iodine uptake and nitroblue tetrazolium reduction by blood neutrophils (P < 0.05). Based on their findings, the researchers concluded that incorporating β-carotene into the diet could lead to elevated concentrations of β-carotene in the blood around the time of calving. This, in turn, appears to enhance the cows’ defense mechanisms by reinforcing the functions of lymphocytes and phagocytes. Based on their research, Daniel et al. (Citation1990, Citation1991) proposed that β-carotene may provide a level of safeguarding to the mammary gland against infections by potentially augmenting intracellular bactericidal activity within phagocytes.
In a recent scientific investigation, Otomaru et al. (Citation2020) conducted a comprehensive assessment of the impact of β-carotene supplementation on the peripheral blood leukocyte population in Japanese Black calves. The study involved the oral administration of 20 mg/d of β-carotene to a designated group of calves from 2 to 8 weeks of age, while a control group received no supplementation. Notably, the analysis revealed a significant increase in the CD4+ cell count within the group receiving β-carotene, particularly evident at the 4-week mark (P < 0.05). These findings provide robust evidence supporting the influence of β-carotene supplementation on the distribution of peripheral blood leukocytes among Japanese Black calves.
In conclusion, the intricate relationship between β-carotene supplementation and immune function varies across various animal species. While the impacts are diverse and complex, both positive and inconsistent outcomes are evident in poultry, swine, and cattle studies (). Notably, recent research underscores the potential anti-inflammatory properties of β-carotene, and its role in enhancing antibody production, lymphocyte proliferation, and immune cell distribution, contributing to the overall understanding of its immunomodulatory effects across different animals.
Table 1. Effects of β-carotene supplementation on immune parameters.
Antioxidant capacity
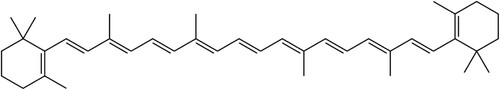
Beyond its role as a provitamin A source, β-carotene demonstrates remarkable antioxidant capabilities, thereby offering cell and tissue protection against oxidative stress. The seminal work by Burton and Ingold in Citation1984, published in the journal Science, provided the initial substantiation that β-carotene pertains to an erstwhile undiscovered category of biological antioxidants. Notably, its proficient radical-trapping antioxidant attributes manifest prominently at partial oxygen pressures considerably below 150 torr, which corresponds to the oxygen pressure in ambient air. Importantly, these lower oxygen partial pressures are prevalent within the majority of tissues under physiological circumstances. β-carotene’s isoprenoid structure, marked by cyclization at both ends and a conjugated system of alternating double and single bonds (), facilitates the efficient reduction of reactive oxidative species like excited state oxygen (Dutta et al. Citation2005). This unique feature prevents immediate degradation and allows the molecule’s delocalized electrons to smoothly dissipate energy differences through interactions with the solvent, ultimately safeguarding surrounding biomolecules (Dickman Citation2019). In mammals, there is evidence suggesting that dietary β-carotene could potentially be taken up by mitochondria and play a role in safeguarding mitochondrial DNA and cytochrome c against oxidative damage often linked to the aging process (Liu and Ames Citation2005).
β-carotene, with its established antioxidant properties, is believed to exert a significant influence in mitigating redox imbalance on cells and tissues across both mammalian and avian species (Marounek and Pebriansyah Citation2018; Black et al. Citation2020). In a study carried out on laying hens, the effects of β-carotene supplementation were investigated. For a period of 6 weeks, the laying hens were provided with feed containing 100 mg of β-carotene per kg (Lee et al. Citation2010). The results revealed a notable reduction in lipid peroxidation in the skin (P < 0.05) when compared to the control group, which did not receive β-carotene supplementation. This reduction was quantified through measurements of 2-thiobarbituric acid and malonaldehyde levels, established markers of oxidative stress.
Not only synthetic sources of β-carotene may exhibit favourable effects on immunity and antioxidative capacity in poultry, but also dietary components rich in β-carotene, such as Indigofera zollingeriana have demonstrated potential in diminishing malondialdehyde levels (P < 0.05) in the thigh muscles of broiler chickens (Santi et al. Citation2015). While the observed effect has been attributed to the β-carotene present in the top leaf meal, it raises inquiries about the possible contribution of other compounds with antioxidative properties in the plant.
Similarly, within the context of swine physiology, the potential antioxidative effects of β-carotene have garnered scientific interest. It is noteworthy that pigs possess a finite enzymatic capacity for the conversion of β-carotene to vitamin A (Shastak and Pelletier Citation2023). This particular metabolic characteristic underscores the significance of β-carotene as a dietary antioxidant in their health (Li et al. Citation2021). Notably, research involving the supplementation of pregnant sows with β-carotene, administered at levels of 30 or 90 mg/kg of feed from the 90th day of gestation until parturition, generated significant effects. Specifically, heightened activity of the blood serum glutathione peroxidase (P < 0.05), a well-established antioxidative enzyme, was documented (Yuan et al. Citation2020). Furthermore, the supplementation exhibited a noteworthy effect on serum nitric oxide levels (P < 0.05), a recognized chain-breaking antioxidant in the context of lipid oxidation initiated by free radicals (Hummel et al. Citation2006).
Ruminants, however, exhibit a distinct digestive physiology that influences the effects of β-carotene on antioxidative capacity. β-carotene’s conversion to vitamin A primarily occurs in the small intestine of non-ruminant animals (Nagao et al. Citation2000). In ruminants, the microbial fermentation occurring in the forestomachs can degrade a significant portion of β-carotene before absorption, limiting its availability for antioxidative purposes (Noziere et al. Citation2006). Nevertheless, studies have indicated that β-carotene can still contribute to antioxidative defense mechanisms in ruminants by influencing the composition of rumen microbiota and even microbiota in milk as well as promoting the synthesis of certain beneficial compounds such VFA (Spears and Weiss Citation2008; Qui Citation2020; Mary et al. Citation2021). The direct impact of β-carotene on ruminant antioxidative capacity, however, might be more subdued compared to monogastric animals.
In a study conducted by Khemarach et al. (Citation2021), lactating Holstein dairy cows were subjected to a dietary intervention where they were provided with either a placebo or a daily dose of 400 mg of β-carotene for a duration of 160 days. Regular collection of milk samples occurred at ten-day intervals over the course of the experiment. The group of cows supplemented with pro-vitamin A carotenoid exhibited notably elevated levels of superoxide dismutase and glutathione peroxidase activities compared to the non-supplemented group (P < 0.05). These observed enzymatic enhancements suggest a potential mitigation of oxidative stress in the dairy cows receiving β-carotene supplementation. It is important to highlight that the direct influence of ß-carotene on the enhancement of antioxidative enzymes within mammals is a rather improbable scenario. Instead, its effects are more likely to be mediated through the modulation of gene expression. This modulation can occur either by directly impacting the genes responsible for encoding these enzymes or by being converted into vitamin A, which can subsequently transform into its active form, retinoic acid, thereby initiating the observed impact on antioxidative enzymes (Shastak et al. Citation2023).
To summarize, the unique antioxidant capabilities of β-carotene go beyond its provitamin A role, thanks to its distinctive isoprenoid structure. This structure enables effective reduction of oxidative species, safeguarding biomolecules. Across various species, from laying hens to dairy cows, β-carotene supplementation consistently associates with reduced oxidative stress markers and increased enzymatic activities, highlighting its significant role in supporting antioxidative mechanisms.
Reproduction and fertility
Reproductive performance is vital for sustainable livestock production. β-carotene has been associated with improved reproductive outcomes, including increased fertility rates, enhanced sperm quality, and healthier offspring (Hemken and Bremel Citation1982; Brief and Chew Citation1985; Coffey and Britt Citation1993; Almbro et al. Citation2011; Nabi et al. Citation2020; Khemarach et al. Citation2021; Ma et al. Citation2021; Wang et al. Citation2023). Nevertheless, the effects of β-carotene on reproduction appear to be species-specific, and further investigation is needed to elucidate underlying mechanisms.
Data regarding the impacts of β-carotene on poultry reproduction is notably limited, possibly attributed to multiple factors. Primarily, poultry are acknowledged to possess comparatively diminished capacities for both absorbing and accumulating carotenes in comparison to mammals (Na et al. Citation2004; Green and Fascetti Citation2016). Additionally, the potential positive influence on fertility, embryo vitality, and egg quality traits might be constrained due to the extremely low transfer of β-carotene to eggs. In general, the deposition of β-carotene in egg yolk accounts up to 1% (Hammershoj et al. Citation2010). Furthermore, alternative carotenoids such as canthaxanthin could exert a more significant role in reproductive processes among poultry (Rosa et al. Citation2012). This discrepancy could be partly attributed to the considerably higher absorption and deposition of canthaxanthin in egg yolk compared to β-carotene, with a ratio of 40 to 1 (Grashorn Citation2016).
In comparison to domestic fowl, there exists a substantial body of research dedicated to investigating the impact of β-carotene on swine reproductive performance. Numerous studies have been conducted, yielding mixed results. Some studies have suggested enhancements in reproductive outcomes, including heightened litter size and increased piglet survivability (Brief and Chew Citation1985; Coffey and Britt Citation1993; Kostoglou et al. Citation2000), while others have found no significant effects (Tokach et al. Citation1994; Stender et al. Citation1999). A similar trend is observable in the case of oxidized β-carotene (Chen et al. Citation2021; Elefson et al. Citation2023). This variability in outcomes can be attributed to a range of factors, such as differing conversion rates of normal β-carotene to vitamin A, potential independent effects on reproduction, variable bioavailability, the method and frequency of administration (be it through injection or dietary supplementation), and varying dosage levels (Shastak and Pelletier, Citation2023).
Research findings have highlighted the potential benefits of supplementary β-carotene in reducing embryonic mortality and enhancing litter size at birth (Brief and Chew Citation1985; Kostoglou et al. Citation2000). Nevertheless, the precise mechanism through which β-carotene exerts these effects on embryo survival remains unclear. One hypothesis suggests that either vitamin A or β-carotene might impact the uterine environment by influencing the glandular or surface epithelium. These components are responsible for producing crucial secretions necessary for embryonic development (Coffey and Britt Citation1993). At the same time, experiments involving gilts injected with β-carotene demonstrated heightened levels of uterine-specific proteins on the 15th day of pregnancy (Chew et al. Citation1982). Trials conducted ex vivo have further indicated that β-carotene can stimulate the production of progesterone in dispersed luteal cells sourced from pigs (Talavera and Chew Citation1986), as well as enhance luteal steroidogenesis by bovine luteal cells (Arikan and Rodway Citation2000). Given the pivotal role of progesterone in sustaining pregnancy and encouraging uterine protein secretion in swine, these findings provide deeper insights into potential underlying mechanisms (Chew et al. Citation2001; Ziecik et al. Citation2011; Geisert et al. Citation2014). Interestingly, analyses have revealed significant absorption of β-carotene across different subcellular fractions of granulosa and luteal cells, as well as within the endometrium of pigs (Chew et al. Citation1994). These observations bolster the hypothesis of β-carotene’s involvement in these specific tissues. Additionally, strong evidence supports the idea that intact β-carotene can cross the placental barrier from the maternal bloodstream to the fetus in various mammalian species (Spiegler et al. Citation2012). This suggests a potential direct impact of this pro-vitamin A carotenoid on embryonic development. β-carotene’s positive impact on sow reproductive organs and its involvement in maintaining reproductive hormone balance likely underlie these effects.
In the realm of bovine biology, the influence of β-carotene on reproductive performance remains a subject demanding further comprehensive investigation. Plausible evidence suggests that β-carotene could potentially exert beneficial impacts on fertility and reproductive success in cattle. Indeed, there have been reports indicating that β-carotene may play a distinct role from vitamin A in influencing the reproductive performance of dairy cows (Schweigert Citation2003; Kawashima et al. Citation2009). A recent study by Lee et al. (Citation2021) endeavoured to elucidate this phenomenon by administering a daily supplement of 400 mg of β-carotene. The supplementation commenced four weeks prior to embryo transfer and persisted for four weeks thereafter, specifically targeting beef cows exhibiting diminished fertility. The outcome of this intervention was noteworthy, revealing a conspicuous elevation in plasma β-carotene concentration (P < 0.001) and a marked improvement in conception rates (P = 0.024) when compared to the control group without β-carotene supplementation. This observation is in agreement with the findings of Khemarach et al. (Citation2021), who provided 400 mg/head/day of β-carotene administration in a 160-day experiment. The timeline of this study was meticulously synchronized with the initiation of the first ovulation, denoted as day 0. Throughout the extensive duration of this experimental exploration, the group receiving pro-vitamin A carotenoid supplementation consistently exhibited a significantly higher (P < 0.05) prevalence of pregnancies relative to the control group without β-carotene supplementation. Statistical analysis employing Cox’s proportional hazards regression model underscored a compelling 44% augmented likelihood of pregnancy in cows benefiting from β-carotene supplementation. These findings collectively underscore the potential of β-carotene as a catalyst for improved reproductive outcomes in cattle.
Bhatnagar et al. (Citation2020) administered a daily oral dose of 500 mg of β-carotene to dairy cows experiencing fertility issues over a span of 60 days. The cows’ estrus signs were diligently monitored twice a day, and artificial insemination was performed when they exhibited clear indications of standing estrus. The results revealed a notable disparity in conception rates between the cows supplemented with β-carotene (64%) and those in the non-supplemented control group (38%). This suggests a potential positive impact of β-carotene supplementation on fertility enhancement in dairy cows.
As its effects on swine, the enhancement of reproductive function in cattle through β-carotene supplementation is likely attributed to its influence on luteal steroidogenesis, as suggested by previous research (Arikan and Rodway Citation2000). However, analogous to the swine studies, the impact of β-carotene on reproductive performance in cattle exhibits variability. Several investigations have yielded inconclusive results, reporting no discernible effect (Wang et al. Citation1988; Kaewlamun et al. Citation2011), while others have corroborated the findings of the aforementioned studies (Akar and Gazioglu Citation2006; Kawashima et al. Citation2009).
In summary, β-carotene’s influence on reproductive outcomes varies across species, with stronger evidence suggesting positive effects in swine and cattle (). While limited data exists for poultry, factors such as absorption rates and egg transfer hinder its impact. In swine, β-carotene supplementation may improve embryo survival and hormonal balance, potentially through uterine effects. Cattle studies show enhanced conception rates with β-carotene supplementation, but discrepancies exist among different investigations. Overall, further research is required to uncover the underlying mechanisms and species-specific effects of β-carotene on reproductive performance.
Table 2. Impact of β-carotene supplementation on reproductive outcomes.
Future perspectives
As the livestock industry undergoes continuous advancements, the significance of β-carotene supplementation warrants comprehensive exploration, fuelled by the ever-growing demand for sustainable and optimized animal production practices. The intricate role of β-carotene in enhancing animal health and performance necessitates a deeper understanding of its multifaceted mechanisms across diverse livestock species. To achieve this, further investigation is imperative to comprehend not only the molecular intricacies but also the broader physiological implications of β-carotene’s interactions within the complex metabolic networks of livestock.
Delving into the differences of gene expression patterns, metabolic pathways, and the synergistic interactions with other essential nutrients is crucial for elucidating the multifaceted impact of β-carotene on animal well-being and performance (van Helden et al. Citation2011; Wei et al. Citation2020). It is not only about understanding how β-carotene is metabolized within the animal’s body, but also about uncovering the signalling pathways and potential epigenetic modifications that may contribute to its broader effects on growth, immune function, and overall resilience (Kim et al. Citation2019).
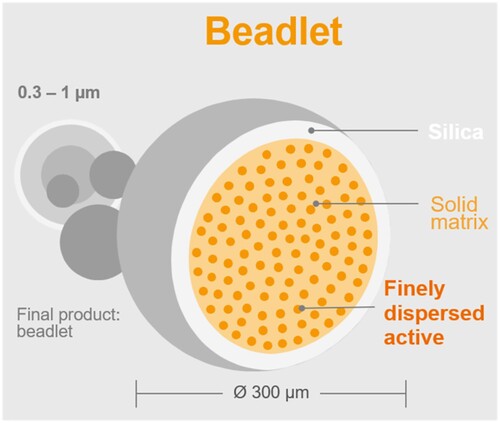
In addition, the development of innovative strategies for delivering β-carotene should ensure precision and efficiency, and these strategies are suitable for interdisciplinary collaborations (Molteni et al. Citation2022). Leveraging encapsulation techniques () and nanotechnology-driven delivery mechanisms presents an avenue to heighten the bioavailability and stability of β-carotene, ensuring its targeted delivery to the intended biological sites, particularly in ruminant nutrition contexts (Shankaranarayanan et al. Citation2018). Exploring these cutting-edge approaches not only advances the field of animal nutrition but also contributes to the evolving landscape of nanotechnology applications in agriculture.
Exploring the utilization of β-carotene-enriched dietary components, such as yellow corn, alfalfa meal, or pasture grasses, offers a prospective avenue for enhancing provitamin A content within livestock diets while maintaining ecological sustainability. However, a notable challenge lies in the considerable variation of β-carotene content and its accessibility in crops like corn, pasture grasses, and alfalfa, coupled with their limited storage stability (Park et al. Citation1983; Zurak et al. Citation2021). As technological advancements continue to unfold, addressing these challenges might involve innovative solutions such as genetic engineering to enhance the natural production of β-carotene within these feed sources.
Although livestock grazing remains a viable strategy to increase their ß-carotene intake, the increasing trend towards confined housing systems, exemplified by figures as high as 64% in the USA, underscores the need for adaptable strategies (Arnott et al. Citation2017). This shifting paradigm prompts researchers and practitioners to explore novel ways of incorporating β-carotene supplementation within confined systems, such as optimized feed formulations with synthetic sources and novel delivery mechanisms tailored to these environments (EFSA Citation2012).
In light of the evolving livestock industry and the demand for sustainable practices, a comprehensive investigation into β-carotene supplementation’s significance becomes crucial. Understanding its diverse mechanisms across species is vital for enhancing animal health and performance, necessitating both molecular insights and broader physiological implications. Furthermore, innovative strategies like nanotechnology-driven delivery methods offer potential precision, while exploring β-carotene-enriched dietary components holds promise for ecological enhancement. Adapting to changing livestock environments through optimized formulations and synthetic sources highlights the need for adaptable strategies in confined housing systems.
Conclusion
In conclusion, the cross-species perspective on β-carotene supplementation in poultry, swine, and cattle underscores the multifaceted nature of its effects on various aspects of animal health and performance. While the effects of β-carotene are evident in immune response, antioxidant capacity, and reproductive function, the intricacies of its mechanisms and outcomes vary significantly among species due to differences in metabolism, physiology, and digestive anatomy. The absorption and metabolism of β-carotene are influenced by the unique gastrointestinal anatomy and enzymatic activity of each species. This complexity leads to variations in the conversion of β-carotene to vitamin A, with swine generally exhibiting lower efficiency in this conversion process. The presence of different enzymes such as BCM1 and BCM2 further contributes to these differences and emphasizes the need for species-specific considerations. Understanding β-carotene’s effects demands exploring intricate genetic expression patterns, metabolic pathways, and synergistic interactions with essential nutrients. Investigating signalling pathways and potential epigenetic modifications adds depth to comprehension. As livestock practices evolve, harnessing β-carotene’s potential requires interdisciplinary collaboration, technological advancements, and adaptable strategies. Navigating its multifaceted mechanisms and species-specific impacts enriches our understanding and offers pathways for enhanced animal health and performance in a changing industry.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Akar Y, Gazioglu A. 2006. Relationship between vitamin A and β-carotene levels during the postpartum period and fertility parameters in cows with and without retained placenta. Bull Vet Inst Pulawy. 50:93–96.
- Akordor FY, Stone JB, Walton JS, Leslie KE, Buchanan-Smith JG. 1986. Reproductive performance of lactating Holstein cows fed supplemental β-carotene. J Dairy Sci. 69(8):2173–2178. doi:10.3168/jds.S0022-0302(86)80650-6.
- Akram S, Mushtaq M, Waheed A. 2021. β-carotene: beyond provitamin A. In: Mushtaq M, Anwar F, editors. A centum of valuable plant bioactives. Amsterdam: Academic Press; p. 1–31.
- Alexander M, Newmark H, Miller RG. 1985. Oral beta-carotene can increase the number of OKT4+ cells in human blood. Immunol Lett. 9:221–224.
- Almbro M, Dowling DK, Simmons LW. 2011. Effects of vitamin E and β-carotene on sperm competitiveness. Ecol Lett. 14:891–895. doi:10.1111/j.1461-0248.2011.01653.x.
- Anjani G, Ayustaningwarno F, Eviana R. 2022. Critical review on the immunomodulatory activities of carrot’s β-carotene and other bioactive compounds. J Funct Foods. 99:105303. doi:10.1016/j.jff.2022.105303.
- Aréchiga CF, Staples CR, McDowell LR, Hansen PJ. 1998. Effects of timed insemination and supplemental β-carotene on reproduction and milk yield of dairy cows under heat stress. J Dairy Sci. 81(2):390–402. doi:10.3168/jds.S0022-0302(98)75589-4.
- Arikan S, Rodway RG. 2000. Effect of cyclodextrin-encapsulated β-carotene on progesterone production by bovine luteal cells. Anim Reprod Sci. 64(3-4):149–160. doi:10.1016/s0378-4320(00)00202-5.
- Arnott G, Ferris CP, NE O. 2017. Review: welfare of dairy cows in continuously housed and pasture-based production systems. Animal. 11(2):261–273. doi:10.1017/S1751731116001336.
- Ascarelli I, Edelman Z, Rosenberg M, Folman Y. 1985. Effect of dietary carotene on fertility of high-yielding dairy cows. Anim Prod. 40(2):195–207. doi:10.1017/S0003356100025307.
- Ay SS, Kaya D, Kucukaslan I, Agaoglu AR, Emre B, Handler J, Findik M, Aslan S. 2012b. Beneficial effects of beta-carotene injections prior to treatment with PGF2α on the fertility of postpartum dairy cows. Rev Med Vet (Toulouse). 163:387–392.
- Ay SS, Küçükaslan I, Kaya D, Aslan S. 2012a. The change in luteal blood flow and luteal size after β-carotene and GnRH injections in early pregnant dairy cows. Kafkas Univ Vet Fak. 18(6):1035–1041.
- Bhatnagar PC, Choudhary JL, Bhardwaj B, Sharma CSDK. 2020. Synergic effect of β-carotene in reproductive functioning of dairy cows. J Entomol Zool. 8(1):700–702.
- Black HS, Boehm F, Edge R, Truscott TG. 2020. The benefits and risks of certain dietary carotenoids that exhibit both anti- and pro-oxidative mechanisms-A. Comprehensive review. Antioxidants (Basel). 9(3):264. doi:10.3390/antiox9030264.
- Bogacz-Radomska L, Harasym J. 2018. β-carotene – properties and production methods. Food Qual Saf. 2(2):69–74. doi:10.1093/fqsafe/fyy004.
- Brief S, Chew BP. 1985. Effects of vitamin A and β-carotene on reproductive performance in gilts. J Anim Sci. 60:998–1004.
- Burton GW, Ingold KU. 1984. β-carotene: an unusual type of lipid antioxidant. Science. 224:569–573. doi:10.1126/science.6710156.
- Çalişlar S. 2019. The important of β-carotene on poultry nutrition. Selcuk J Agric Food Sci. 33:252–259.
- Chen J, Chen J, Zhang Y, Lv Y, Qiao H, Tian M, Cheng L, Chen F, Zhang S, Guan W. 2021. Effects of maternal supplementation with fully oxidised β-carotene on the reproductive performance and immune response of sows, as well as the growth performance of nursing piglets. Br J Nutr. 125(1):62–70. doi:10.1017/S0007114520002652.
- Chew BP. 1996. Importance of antioxidant vitamins in immunity and health in animals. Anim Feed Sci Technol. 59:103–114. doi:10.1016/0377-8401(95)00891-8.
- Chew BP, Park JS. 2004. Carotenoid action on the immune response. J Nutr. 134(1):257S–261S. doi:10.1093/jn/134.1.257S.
- Chew BP, Park JS, Weng BC, Wong TS, Hayek MG, Reinhart GA. 2000. Dietary beta-carotene absorption by blood plasma and leukocytes in domestic cats. J Nutr. 130(9):2322–2325. doi:10.1093/jn/130.9.2322.
- Chew BP, Rasmussen H, Pubols MH, Preston RL. 1982. Effect of vitamin A and β-carotene on plasma progesterone and uterine protein secretion in gilts. Theriogenology. 18:643–654.
- Chew BP, Szenci O, Wong TS, Gilliam VL, Hoppe PP, Coehlo MB. 1994. Uptake of β-carotene by plasma, follicular fluid, granulosa cells, luteal cells and endometrium in pigs after administrating injectable β-carotene (abstr). J Anim Sci. 72:100.
- Chew BP, Weng BB, Kim HW, Wong TS, Park JS, Lepine AJ. 2001. Uptake of β-carotene by ovarian and uterine tissues and effects on steroidogenesis during the estrous cycle in cats. Am J Vet Res. 62(7):1063–1067. doi:10.2460/ajvr.2001.62.1063.
- Coffey MT, Britt JH. 1989. Effect of injecting β-carotene (BC) or vitamin A (Vit A) on reproductive performance of sows. J Anim Sci. 69(Suppl 1):360.
- Coffey MT, Britt JH. 1993. Enhancement of sow reproductive performance by β-carotene or vitamin A. J Anim Sci. 71:1198–1202.
- Conboy Stephenson R, Ross RP, Stanton C. 2021. Carotenoids in milk and the potential for dairy based functional foods. Foods. 10(6):1263. doi:10.3390/foods10061263.
- Cucco M, Guasco B, Malacarne G, Ottonelli R. 2006. Effects of beta-carotene supplementation on chick growth, immune status and behaviour in the grey partridge, perdix perdix. Behav Processes. 73(3):325–332. doi:10.1016/j.beproc.2006.08.002.
- Cucco M, Guasco B, Malacarne G, Ottonelli R. 2007. Effects of beta-carotene on adult immune condition and antibacterial activity in the eggs of the grey partridge, perdix perdix. Comp Biochem Physiol A Mol Integr Physiol. 147(4):1038–1046. doi:10.1016/j.cbpa.2007.03.014.
- Damron BL, Goodson SR, Harms RH, Janky DM, Wilson HR. 1984. Beta-carotene supplementation of laying hen diets. Br Poult Sci. 25(3):349–352. doi:10.1080/00071668408454875.
- Daniel LR, Chew BP, Tanaka TS, Tjoelker LW. 1990. Beta-carotene and vitamin A effects on bovine phagocyte function in vitro during the peripartum period. J Dairy Sci. 74(1):124–131. doi:10.3168/jds.S0022-0302(91)78152-6.
- Daniel LR, Chew BP, Tanaka TS, Tjoelker LW. 1991. In vitro effects of beta-carotene and vitamin A on peripartum bovine peripheral blood mononuclear cell proliferation. J Dairy Sci. 74(3):911–915. doi:10.3168/jds.S0022-0302(91)78240-4.
- de Ondarza MB, Wilson JW, Engstrom M. 2009. Case study: effect of supplemental β-carotene on yield of milk and milk components and on reproduction of dairy cows. Prof Anim Sci. 25:510–516. doi:10.15232/S1080-7446(15)30742-7.
- Devery J, Milborrow BV. 1994. Beta-carotene-15,15'-dioxygenase (EC 1.13.11.21) isolation reaction mechanism and an improved assay procedure. Br J Nutr. 72(3):397–414. doi:10.1079/bjn19940042.
- Dickman JJ. 2019. Antioxidants and beta-carotene: a general overview, a research history, and modern scholarship. ChemistryThesis. 17:1–22. https://pillars.taylor.edu/chemistry-student/17.
- Düpjan S, Dawkins MS. 2022. Animal welfare and resistance to disease: interaction of affective states and the immune system. Front Vet Sci. 9:929805. doi:10.3389/fvets.2022.929805.
- Dutta D, Chaudhuri UR, Chakraborty R. 2005. Structure, health benefits, antioxidant property and processing and storage of carotenoids. African J Biotechnol. 4(13 spec. iss.):1510–1520. doi:10.4314/ajfand.v4i13.71773.
- EFSA (European Food Safety Authority). 2012. Scientific opinion on the safety and efficacy of beta-carotene as a feed additive for all animal species and categories. EFSA Journal. 10(6):2737.
- Elefson SK, Ross JW, Rademacher CJ, Greiner LL. 2023. Evaluation of oxidized beta-carotene on sow and piglet immune systems, sow reproductive performance, and piglet growth. J Anim Sci. 101:skad066. doi:10.1093/jas/skad066.
- Flahou B, Rossi M, Bakker J, Langermans JA, Heuvelman E, Solnick JV, Martin ME, O’Rourke J, Ngoan LD, Hoa NX, et al. 2018. Evidence for a primate origin of zoonotic helicobacter suis colonizing domesticated pigs. ISME J. 12(1):77–86. doi:10.1038/ismej.2017.145.
- Gabriel I, Lessire M, Mallet S, Guillot J. 2006. Microflora of the digestive tract: critical factors and consequences for poultry. Worlds Poult Sci J. 62(3):499–511. doi:10.1017/S0043933906001115.
- Gao YY, Ji J, Sun JL, Xu BL, Wang LH, Bi CK, Z Y. 2016. Xanthophyll supplementation regulates carotenoid and retinoid metabolism in hens and chicks. Poult Sci. 95(3):541–549. doi:10.3382/ps/pev335.
- Geisert RD, Lucy MC, Whyte JJ, Ross JW, Mathew DJ. 2014. Cytokines from the pig conceptus: roles in conceptus development in pigs. J Anim Sci Biotechnol. 5(1):51. doi:10.1186/2049-1891-5-51.
- Gossen N, Hoedemaker M. 2005. Effect of beta-carotin serum concentration on the reproductive performance in dairy cows. Berl Münchener Tierärztliche Wochenschr. 118:326–333.
- Grashorn M. 2016. Feed additives for influencing chicken meat and egg yolk color. In: Carle R, Schweigert RM, editor. Handbook on natural pigments in food and beverages. Sawston, UK: Woodhead Publishing; p. 283–302.
- Green AS, Fascetti AJ. 2016. Meeting the vitamin A requirement: the efficacy and importance of β-carotene in animal species. Sci World J. 2016:7393620. doi:10.1155/2016/7393620.
- Haliloglu S, Baspinar N, Serpek B, Erdem H, Bulut Z. 2002. Vitamin A and beta-carotene levels in plasma, corpus luteum and follicular fluid of cyclic and pregnant cattle. Reprod Domest Anim. 37:96–99. doi:10.1046/J.1439-0531.2002.00338.X.
- Hammershoj M, Kidmose U, Steenfeldt S. 2010. Deposition of carotenoids in egg yolk by short-term supplement of coloured carrot (Daucas carota) varieties as forage material for egg-laying hens. J Sci Food Agric. 90:1163–1171.
- Haq AU, Bailey CA, Chinnah A. 1996. Effect of beta-carotene, canthaxanthin, lutein, and vitamin E on neonatal immunity of chicks when supplemented in the broiler breeder diets. Poult Sci. 75(9):1092–1097. doi:10.3382/ps.0751092.
- Haskell MJ. 2012. The challenge to reach nutritional adequacy for vitamin A: β-carotene bioavailability and conversion–evidence in humans. Am J Clin Nutr. 96(5):1193S–1203S. doi:10.3945/ajcn.112.034850.
- Hemken RW, Bremel DH. 1982. Possible role of beta-carotene in improving fertility in dairy cattle. J Dairy Sci. 65(7):1069–1073. doi:10.3168/jds.S0022-0302(82)82314-X.
- Hoskinson CD, Chew BP, Wong TS. 1992. Effects of injectable β-carotene and vitamin A on lymphocyte proliferation and polymorphonuclear neutrophil function in piglets. Biol Neonate. 62:325–336.
- Hughes DA. 1999. Effects of carotenoids on human immune function. Proc Nutr Soc. 58(3):713–718. doi:10.1017/s0029665199000932.
- Hui J, Li L, Li R, Wu M, Yang Y, Wang J, Fan Y, Zheng X. 2020. Effects of supplementation with β-carotene on the growth performance and intestinal mucosal barriers in layer-type cockerels. Anim Sci J. 91(1):e13344. doi:10.1111/asj.13344.
- Hummel SG, Fischer AJ, Martin SM, Schafer FQ, Buettner GR. 2006. Nitric oxide as a cellular antioxidant: a little goes a long way. Free Radic Biol Med. 40(3):501–506. doi:10.1016/j.freeradbiomed.2005.08.047.
- Hurley WL, Doane RM. 1989. Recent developments in the roles of vitamins and minerals in reproduction. J. Dairy Sci. 72:784–804. doi:10.3168/JDS.S0022-0302(89)79170-0.
- Hynd PI. 2019. Digestion in the mono-gastric animal. In: Hynd PI, editor. Animal nutrition: from theory to practice. Clayton South, Australia: CSIRO Publishing; p. 42–63.
- Imamura T, Bando N, Yamanishi R. 2006. Beta-carotene modulates the immunological function of RAW264, a murine macrophage cell line, by enhancing the level of intracellular glutathione. Biosci Biotechnol Biochem. 70(9):2112–2120. doi:10.1271/bbb.60056.
- Ishida M, Nishijima Y, Ikeda S, Yoshitani K, Obata A, Sugie Y, Aoki Y, Yamaji T, Fujita M, Nakatsuji Y, Kume S. 2018. Effects of supplemental β-carotene on colostral immunoglobulin and plasma β-carotene and immunoglobulin in Japanese black cows. Anim Sci J. 89(8):1102–1106. doi:10.1111/asj.13032.
- Iwańska S, Strusińska D. 1997. The effect of beta-carotene and vitamins A, D3 and E on some reproductive parameters in cows. Acta Vet Hung. 45(1):95–107.
- Jlali M, Graulet B, Chauveau-Duriot B, Chabault M, Godet E, Leroux S, Praud C, Le Bihan-Duval E, Duclos MJ, Berri C. 2012. A mutation in the promoter of the chicken β,β-carotene 15,15'-monooxygenase 1 gene alters xanthophyll metabolism through a selective effect on its mRNA abundance in the breast muscle. J Anim Sci. 90(12):4280–4288. doi:10.2527/jas.2012-5240.
- Johra FT, Bepari AK, Bristy AT, Reza HM. 2020. A mechanistic review of β-carotene, lutein, and zeaxanthin in eye health and disease. Antioxidants (Basel). 9(11):1046. doi:10.3390/antiox9111046.
- Jun C, Jiaming C, Yinzhi Z, Yantao L, Hanzhen Q, Min T, Lin C, Fang C, Shihai Z, Wutai G. 2021. Effects of maternal supplementation with fully oxidized β-carotene on the reproductive performance and immune response of sows, as well as the growth performance of nursing piglets. Br J Nutr. 125:62–70. doi:10.1017/S0007114520002652.
- Kaewlamun W, Okouyi M, Humblot P, Techakumphu M, Ponter AA. 2011. Does supplementing dairy cows with β-carotene during the dry period affect postpartum ovarian activity, progesterone, and cervical and uterine involution? Theriogenology. 75(6):1029–1038. doi:10.1016/j.theriogenology.2010.11.010.
- Karadas F, Erdogan S, Kor D, Oto G, Uluman M. 2016. The effects of different types of antioxidants (Se, vitamin E and carotenoids) in broiler diets on the growth performance, skin pigmentation and liver and plasma antioxidant concentrations. Rev Bras Cienc Avic. 18:101–116.
- Kawashima C, Kida K, Schweigert FJ, Miyamoto A. 2009. Relationship between plasma beta-carotene concentrations during the peripartum period and ovulation in the first follicular wave postpartum in dairy cows. Anim Reprod Sci. 111(1):105–111. doi:10.1016/j.anireprosci.2008.02.008.
- Kawashima C, Nagashima S, Sawada K, Schweigert FJ, Miyamoto A, Kida K. 2010. Effect of β-carotene supply during close-up dry period on the onset of first postpartum luteal activity in dairy cows. Reprod Domest Anim. 45(6):e282–e287. doi:10.1111/j.1439-0531.2009.01558.x.
- Khemarach S, Yammuen-Art S, Punyapornwithaya V, Nithithanasilp S, Jaipolsaen N, Sangsritavong S. 2021. Improved reproductive performance achieved in tropical dairy cows by dietary beta-carotene supplementation. Sci Rep. 11(1):23171. doi:10.1038/s41598-021-02655-8.
- Kiefer C, Hessel S, Lampert JM, Vogt K, Lederer MO, Breithaupt DE, von Lintig J. 2001. Identification and characterization of a mammalian enzyme catalyzing the asymmetric oxidative cleavage of provitamin A. J Biol Chem. 276:14110–14116.
- Kiernan K, MacIver NJ. 2021. The role of the adipokine leptin in immune cell function in health and disease. Front Immunol. 11:622468. doi:10.3389/fimmu.2020.622468.
- Kim D, Kim Y, Kim Y. 2019. Effects of β-carotene on expression of selected MicroRNAs, histone acetylation, and DNA methylation in colon cancer stem cells. J Cancer Prev. 24(4):224–232. doi:10.15430/JCP.2019.24.4.224.
- Kim YK, Wassef L, Chung S, Jiang H, Wyss A, Blaner WS, Quadro L. 2011. β-Carotene and its cleavage enzyme β-carotene-15,15'-oxygenase (CMOI) affect retinoid metabolism in developing tissues. FASEB J. 25(5):1641–1652. doi:10.1096/fj.10-175448.
- Kostoglou P, Kyriakis S, Papasteriadis A, Roumpies N, Alexopoulos C, Saoulidis K. 2000. Effect of β-carotene on health status and performance of sows and their litters. J Anim Physiol Anim Nutr. 83:150–157. doi:10.1046/j.1439-0396.2000.00263.x.
- Kowatz T, Babino D, Kiser P, Palczewski K, von Lintig J. 2013. Characterization of human β,β-carotene-15,15'-monooxygenase (BCMO1) as a soluble monomeric enzyme. Arch Biochem Biophys. 539(2):214–222. doi:10.1016/j.abb.2013.05.007.
- Krammer G, Aurich J. 2010. Effect of intramuscularly administered β-carotene on reproductive performance in sows. Berl Munch Tierarztl Wochenschr. 123:496–499. doi:10.2376/0005-9366-123-496.
- Lang W, Gong H, Li R, Wu M, Chu Q, Wang T, Zheng X. 2020. Effects of supplemental β-carotene on growth performance, immune function and intestinal mucin in weaned piglets. Res Sq. doi:10.21203/rs.3.rs-18500/v1.
- Lee C, Lee B, Na J, An G. 2010. Carotenoid accumulation and their antioxidant activity in spent laying hens as affected by polarity and feeding period. Anim Biosci. 23(6):799–805. doi:10.5713/ajas.2010.90296.
- Lee HJ, Park YK, Kang MH. 2011. The effect of carrot juice, beta-carotene supplementation on lymphocyte DNA damage, erythrocyte antioxidant enzymes and plasma lipid profiles in Korean smoker. Nutr Res Pract. 5:540–547.
- Lee SH, Yang YR, Cheon HY, Shin NH, Lee JW, Bong SH, Hwangbo S, Kong IK, Shin MK. 2021. Effects of hydrogenated fat-spray-coated β-carotene supplement on plasma β-carotene concentration and conception rate after embryo transfer in hanwoo beef cows. Animal. 15(12):100407. doi:10.1016/j.animal.2021.100407.
- Li Q, Yang S, Chen F, Guan W, Zhang S. 2021. Nutritional strategies to alleviate oxidative stress in sows. Anim Nutr. 9:60–73. doi:10.1016/j.aninu.2021.10.006.
- Liu J, Ames BN. 2005. Reducing mitochondrial decay with mitochondrial nutrients to delay and treat cognitive dysfunction, Alzheimer’s disease, and Parkinson’s disease. Nutr Neurosci. 8:67–89.
- Lopez-Flores NM, Meza-Herrera CA, Perez-Marin C, Blache D, Arellano-Rodríguez G, Zuñiga-Garcia S, Navarrete-Molina C, Peña CG, Rosales-Nieto CA, Veliz-Deras FG. 2020. Precision betacarotene supplementation enhanced ovarian function and the LH release pattern in yearling crossbred anestrous goats. Animals (Basel). 10(4):659. doi:10.3390/ani10040659.
- Lo Verso L, Matte JJ, Lapointe J, Talbot G, Bissonnette N, Blais M, Guay F, Lessard M. 2020. Impact of birth weight and neonatal nutritional interventions with micronutrients and bovine colostrum on the development of piglet immune response during the peri-weaning period. Vet Immunol Immunopathol. 226:110072. doi:10.1016/j.vetimm.2020.
- Ma D, Han P, Song M, Zhang H, Shen W, Huang G, Zhao M, Sun Q, Zhao Y, Min L. 2021. β-carotene rescues busulfan disrupted spermatogenesis through elevation in testicular antioxidant capability. Front Pharmacol. 12:593953. doi:10.3389/fphar.2021.593953.
- Maoka T. 2020. Carotenoids as natural functional pigments. J Nat Med. 74:1–16. doi:10.1007/s11418-019-01364-x.
- Marounek M, Pebriansyah A. 2018. Use of carotenoids in feed mixtures for poultry: a review. Agric Trop Subtrop. 51(3):107–111. doi:10.2478/ats-2018-0011.
- Mary AEP, Artavia Mora JI, Ronda Borzone PA, Richards SE, Kies AK. 2021. Vitamin E and beta-carotene status of dairy cows: a survey of plasma levels and supplementation practices. Animal. 15(8):100303. doi:10.1016/j.animal.2021.
- Michal JJ, Heirman LR, Wong TS, Chew BP, Frigg M, Volker L. 1994. Modulatory effects of dietary beta-carotene on blood and mammary leukocyte function in periparturient dairy cows. J Dairy Sci. 77(5):1408–1421. doi:10.3168/jds.S0022-0302(94)77079-X.
- Molteni C, La Motta C, Valoppi F. 2022. Improving the bioaccessibility and bioavailability of carotenoids by means of nanostructured delivery systems: A comprehensive review. Antioxidants. 11(10):1931. doi:10.3390/antiox11101931.
- Moreno JA, Díaz-Gómez J, Nogareda C, Angulo E, Sandmann G, Portero-Otin M, Serrano JC, Twyman RM, Capell T, Zhu C, Christou P. 2016. The distribution of carotenoids in hens fed on biofortified maize is influenced by feed composition, absorption, resource allocation and storage. Sci Rep. 6:35346. doi:10.1038/srep35346.
- Na J-C, Song J-Y, Lee B-D, Lee S-J, Lee C-Y, An G-H. 2004. Effect of polarity on absorption and accumulation of carotenoids by laying hens. Anim Feed Sci Technol. 117(3):305–315. doi:10.1016/j.anifeedsci.2004.08.012.
- Nabi F, Arain MA, Rajput N, Alagawany M, Soomro J, Umer M, Soomro F, Wang Z, Ye R, Liu J. 2020. Health benefits of carotenoids and potential application in poultry industry: a review. J Anim Physiol Anim Nutr. 104:1809–1818.
- Nagao A. 2004. Oxidative conversion of carotenoids to retinoids and other products. J Nutr. 134(1):237S–240S. doi:10.1093/jn/134.1.237S.
- Nagao A, Maeda M, Lim BP, Kobayashi H, Terao J. 2000. Inhibition of beta-carotene-15,15'-dioxygenase activity by dietary flavonoids. J Nutr Biochem. 11(6):348–355. doi:10.1016/s0955-2863(00)00090-5.
- Nogareda C, Moreno JA, Angulo E, Sandmann G, Portero M, Capell T, Zhu C, Christou P. 2016. Carotenoid-enriched transgenic corn delivers bioavailable carotenoids to poultry and protects them against coccidiosis. Plant Biotechnol J. 14(1):160–168. doi:10.1111/pbi.12369.
- Noziere P, Graulet B, Lucas A, Martin B, Grolier P, Doreau M. 2006. Carotenoids for ruminants: from forages to dairy products. Anim Feed Sci Technol. 131:418–450. doi:10.1016/j.anifeedsci.2006.06.018.
- Oliveira RC, Guerreiro BM, Morais Junior NN, Araujo RL, Pereira RAN, Pereira MN. 2015. Supplementation of prepartum dairy cows with β-carotene. J Dairy Sci. 98(10):7419.
- Otomaru K, Ogawa R, Oishi S, Iwamoto Y, Hong H, Nagai K, Hyakutake K, Kubota C, Kaneshige T. 2018. Effect of beta-carotene supplementation on the serum oxidative stress biomarker and antibody titer against live bovine respiratory syncytial virus vaccination in Japanese black calves. Vet Sci. 5(4):102. doi:10.3390/vetsci5040102.
- Otomaru K, Ogawa R, Oishi S, Iwamoto Y, Ishikawa S, Nagai K. 2020. Effect of beta-carotene supplementation on the peripheral blood leukocyte population in Japanese black calves. J Nutr Sci Vitaminol (Tokyo). 66(4):381–385. doi:10.3177/jnsv.66.381.
- Pan D, Yu Z. 2014. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes. 5(1):108–119. doi:10.4161/gmic.26945.
- Park YW, Anderson MJ, Walters JL, Mahoney AW. 1983. Effects of processing methods and agronomic variables on carotene contents in forages and predicting carotene in alfalfa hay with near-infrared-reflectance spectroscopy. J Dairy Sci. 66:235–245.
- Parker RS. 1996. Absorption, metabolism, and transport of carotenoids. FASEB J. 10(5):542–551. doi:10.1096/fasebj.10.5.8621054.
- Pasquariello R, Anipchenko P, Pennarossa G, Crociati M, Zerani M, Brevini TA, Gandolfi F, Maranesi M. 2022. Carotenoids in female and male reproduction. Phytochemistry. 204:113459. doi:10.1016/j.phytochem.2022.113459.
- Qui ND. 2020. Assessment of milk and gut microbiota of lactating Jersey cows in relation to milk quality and animal health management [PhD Thesis], Okayama University, Japan.
- Rakes AH, Owens MP, Britt JH, Whitlow LW. 1985. Effects of adding beta-carotene to rations of lactating cows consuming different forages. J Dairy Sci. 68(7):1732–1737. doi:10.3168/jds.S0022-0302(85)81019-5.
- Reid-McWhinney SL. 1991. The immunoenhancing effects of dietary ß-carotene and canthaxanthin in chicks [PhD Thesis], Texas A&M University, USA.
- Riley WW, Nickerson JG, Mogg TJ, Burton GW. 2023. Oxidized β-carotene is a novel phytochemical immune modulator that supports animal health and performance for antibiotic-free production. Animals (Basel). 13(2):289. doi:10.3390/ani13020289.
- Roe DA, Fuller CJ. 1993. Carotenoids and immune function. In: Klurfeld DM, editor. Nutrition and immunology. Human nutrition. Boston, MA: Springer; p. 229–230.
- Rosa AP, Scher A, Sorbara JO, Boemo LS, Forgiarini J, Londero A. 2012. Effects of canthaxanthin on the productive and reproductive performance of broiler breeders. Poult Sci. 91(3):660–666. doi:10.3382/ps.2011-01582.
- Sagi Z, Hieronymus T. 2018. The impact of the epithelial–mesenchymal transition regulator hepatocyte growth factor receptor/met on skin immunity by modulating langerhans cell migration. Front Immunol. 9:517. doi:10.3389/fimmu.2018.00517.
- Sales JN, Dias LM, Viveiros AT, Pereira MN, Souza JC. 2008. Embryo production and quality of Holstein heifers and cows supplemented with beta-carotene and tocopherol. Anim Reprod Sci. 106(1-2):77–89. doi:10.1016/j.anireprosci.2007.04.001.
- Santi MA, Sumiati S, Abdullahet L. 2015. Cholesterol and malondialdehyde contents of broiler-chicken meat supplemented with indigofera zolingeriana top leaf meal. Media Peternakan. 38(3):163–168.
- Schiedt K, Leuenberger FJ, Vecchi M, Glinz E. 1985. Absorption, retention and metabolic transformations of carotenoids in rainbow trout, salmon and chicken. Pure Appl Chem. 57(5):685–692. doi:10.1351/pac198557050685.
- Schweigert FJ. 2003. Research note: changes in the concentration of beta-carotene, alpha-tocopherol and retinol in the bovine corpus luteum during the ovarian cycle. Arch Tierernahr. 57:307–310.
- Schweigert FJ, Krieger K, Schnurrbusch U, Schams D, Gropp J. 2002. Effect of dietary beta-carotene on the early embryonic development and uterine fluid composition of gilts. J Anim Physiol Anim Nutr. 86(7):265–272. doi:10.1046/j.1439-0396.2002.00384.x.
- Schweigert FJ, Rosival I, Rambeck WA, Gropp J. 1995. Plasma transport and tissue distribution of [14C] beta-carotene and [3H]retinol administered orally to pigs. Int J Vitam Nutr Res. 65(2):95–100.
- Shang Y, Kumar S, Oakley B, Kim WK. 2018. Chicken gut microbiota: importance and detection technology. Front Vet Sci. 5:254. doi:10.3389/fvets.2018.00254.
- Shankaranarayanan J, Arunkanth K, Dinesh KC. 2018. Beta carotene -therapeutic potential and strategies to enhance its bioavailability. Nutri Food Sci Int J. 7(4):555716. doi:10.19080/NFSIJ.2018.07.555716.
- Shastak Y, Gordillo A, Pelletier W. 2023. The relationship between vitamin A status and oxidative stress in animal production. J Appl Anim Res. 51(1):546–553. doi:10.1080/09712119.2023.2239319.
- Shastak Y, Pelletier W. 2023. Vitamin A supply in swine production: a review of current science and practical considerations. Appl Anim. Sci. 39(5):289–305.
- Shete V, Quadro L. 2013. Mammalian metabolism of β-carotene: gaps in knowledge. Nutrients. 5(12):4849–4868. doi:10.3390/nu5124849.
- Sklan D. 1983. Carotene-cleavage activity in chick intestinal mucosa cytosol: association with a high-molecular-weight lipid-protein aggregate fraction and partial characterization of the activity. Br J Nutr. 50:417.
- Soltis MP, Moorey SE, Egert-McLean AM, Voy BH, Shepherd EA, Myer PR. 2023. Rumen biogeographical regions and microbiome variation. Microorganisms. 11(3):747. doi:10.3390/microorganisms11030747.
- Sordillo LM, Scott NL. 1994. Alternative approaches for the prevention and treatment of mastitis. Bov Proceed. 27:54–60.
- Spears JW, Weiss WP. 2008. Role of antioxidants and trace elements in health and immunity of transition dairy cows. Vet J. 176(1):70–76. doi:10.1016/j.tvjl.2007.12.015.
- Spiegler E, Kim YK, Wassef L, Shete V, Quadro L. 2012. Maternal-fetal transfer and metabolism of vitamin A and its precursor β-carotene in the developing tissues. Biochim Biophys Acta. 1821(1):88–98. doi:10.1016/j.bbalip.2011.05.003.
- Stender D, Irvin R, Baas TJ. 1999. Effect of beta-carotene on reproductive performance in swine. ASL-R1553. Ames: Iowa State University. https://www.extension.iastate.edu/pages/ansci/swinereports/asl-1553.pdf.
- Takaichi S. 2011. Carotenoids in algae: distributions, biosyntheses and functions. Mar Drugs. 9(6):1101–1118. doi:10.3390/md9061101.
- Talavera F, Chew BP. 1986. Retinol, retinoic acid and β-carotene stimulate steroidogenesis in porcine corpora lutea in vitro (abstr). J Dairy Sci. 69:351–352.
- Tengerdy RP, Lancetera NG, Nockels CF. 1990. The effects of ß-carotene on disease protection and humoral immunity in the chicken. Avian Dis. 34:848–854.
- Tjoelker LW, Chew BP, Tanaka TS, Daniel LR. 1988. Bovine vitamin A and beta-carotene intake and lactational status. 1. Responsiveness of peripheral blood polymorphonuclear leukocytes to vitamin A and beta-carotene challenge in vitro. J Dairy Sci. 71:3112–3119.
- Tjoelker LW, Chew BP, Tanaka TS, Daniel LR. 1990. Effect of dietary vitamin A and beta-carotene on polymorphonuclear leukocyte and lymphocyte function in dairy cows during the early dry period. J Dairy Sci. 73(4):1017–1022. doi:10.3168/jds.S0022-0302(90)78760-7.
- Tokach MD, Goodband RD, Nelssen JL. 1994. Influence of a single injection of beta-carotene and or vitamin A at weaning on subsequent reproductive performance of sows. Manhattan: Proc Kansas State University Swine Day; p. 11.
- Trojacanec S, Bobos S, Pajic M. 2012. Influence of beta-carotene and vitamin A supplementation on the ovarian activity of dairy cows with chronic fertility impairment. Vet Arch. 82:567–575.
- van Helden YGJ, Godschalk RWL, Swarts HJM, Hollman PCH, van Schooten FJ, Keijer J. 2011. Beta-carotene affects gene expression in lungs of male and female Bcmo1 −/− mice in opposite directions. Cell Mol Life Sci. 68:489–504. doi:10.1007/s00018-010-0461-0.
- van Vliet T, van Vlissingen MF, van Schaik F, van den Berg H. 1996. Beta-Carotene absorption and cleavage in rats is affected by the vitamin A concentration of the diet. J Nutr. 126(2):499–508. doi:10.1093/jn/126.2.499.
- von Lintig J, Moon J, Lee J, Ramkumar S. 2020. Carotenoid metabolism at the intestinal barrier. Biochim Biophys Acta Mol Cell Biol Lipids. 1865(11):158580. doi:10.1016/j.bbalip.2019.158580.
- Vukmirović D, Čolović R, Rakita S, Brlek T, Đuragić O, Solà-Oriol D. 2017. Importance of feed structure (particle size) and feed form (mash vs. pellets) in pig nutrition – A review. Anim Feed Sci Technol. 233:133–144. doi:10.1016/j.anifeedsci.2017.06.016.
- Wang G, Wan D, Wang T, Gong H, Tao Y, Zhang S, Lan H, Li S, Wu M, Zheng X, Sun P. 2023. Effects of β-carotene supplementation in the diet of laying breeder hens on the growth performance and liver development of offspring chicks. Anim Biotech. doi:10.1080/10495398.2023.2218416.
- Wang JY, Owen FG, Larson LL. 1988. Effect of beta-carotene supplementation on reproductive performance of lactating Holstein cows. J Dairy Sci. 71(1):181–186. doi:10.3168/jds.S0022-0302(88)79540-5.
- Wei C, Tan X, Liu G, Wan F, Zhao H, Zhang C, You W, Liu X, Zhang X, Jin Q. 2020. β-carotene as a dietary factor affecting expression of genes connected with carotenoid, vitamin A and lipid metabolism in the subcutaneous and omental adipose tissue of beef cattle. J Anim Feed Sci. 29:11–18. doi:10.22358/jafs/117866/2020.
- Yang Y, Tume RK. 1993. A comparison of beta-carotene-splitting activity isolated from intestinal mucosa of pasture-grazed sheep, goats and cattle. Biochem Mol Biol Int. 30:209–217.
- Yuan X, Yan J, Hu R, Li Y, Wang Y, Chen H, Hou DX, He J, Wu S. 2020. Modulation of gut microbiota and oxidative status by β-carotene in late pregnant sows. Front Nutr. 7:612875. doi:10.3389/fnut.2020.612875.
- Ziecik AJ, Waclawik A, Kaczmarek MM, Blitek A, Jalali BM, Andronowska A. 2011. Mechanisms for the establishment of pregnancy in the pig. Reprod Domest Anim. 46(Suppl 3):31–41. doi:10.1111/j.1439-0531.2011.01843.x.
- Zomborszky-Kovács M, Bárdos L, Bíró H, Tuboly S, Wolf-Táskai E, Tóth A, Soós P. 2000. Effect of beta-carotene and nucleotide base supplementation on blood composition and immune response in weaned pigs. Acta Vet Hung. 48(3):301–311. doi:10.1556/AVet.48.2000.3.7.
- Zurak D, Grbeša D, Duvnjak M, Kiš G, Međimurec T, Kljak K. 2021. Carotenoid content and bioaccessibility in commercial maize hybrids. Agriculture. 11(7):586. doi:10.3390/agriculture11070586.