ABSTRACT
This study aimed to evaluate the safety and efficacy of oral administration of Ashwagandha root extract (ARE) in cats subjected to different types of stress. A randomized, double-blind, placebo-controlled veterinary clinical trial was conducted with sixteen healthy pet cats. Each cat was randomly assigned to receive ARE (15 mg/kg body weight) or a placebo and were subjected to four types of stress over a period of one month. No detrimental changes were observed in ALP, ALT, AST, glucose, creatinine, and BUN levels on day 30 with ARE with all values within the physiological range. Additionally, in the ARE-treated group, the protein (p < 0.05) and albumin (p < 0.01) levels were increased significantly, indicating overall improved liver function. Post 30 days ARE treatment, significant modulation of malondialdehyde (MDA) (p < 0.001), superoxide dismutase (SOD) (p < 0.001), glutathione (GSH) (p < 0.01) and catalase (p < 0.001) levels were observed with ARE compared to placebo control group. After 30 days ARE treatment, serum cortisol levels significantly decreased (p < 0.001), indicating strong anti-stress effects. There was a significant decrease in cytokine levels (TNF-α, IFN-γ & IL-10), NFκB and Nrf-2 levels in ARE treated group indicating potent anti-inflammatory effects. Overall, these promising results demonstrate that ARE possesses adaptogenic and anti-inflammatory properties and is safe in felines.
1. Introduction
Over the last decades, the use of companion animals has increased significantly primarily due to significantly improved living standards and changes in population structure (an increase in the number of single and elderly people) (Enders-Slegers and Hediger Citation2019). According to American Veterinary Medical Association (2022), in the USA, cat ownership increased from 25% in 2016 to 26% in 2020 and then to 29% in 2022 (Applebaum et al. Citation2023; Short Citation2022). Based on data from World Atlas, the countries with the highest number of pet cats worldwide include the USA, China, Russia, UK, Brazil, and France (Foreman-Worsley et al. Citation2021; Martens et al. Citation2019; Aegerter et al. Citation2017). Pets living with humans have to undergo a variety of adaptive changes and often go through stress absent in wildlife (Behnke et al. Citation2021).
Stress is a biological response of the body caused by extraordinary circumstances defined as a stressor threatening its natural homeostasis (Laugero and Moberg Citation2000; Karatsoreos and McEwen Citation2011; Amat et al. Citation2016; Yaribeygi et al. Citation2017). Pets experience stress due to physical factors (e.g. infection, haemorrhage) or psychological factors (e.g. restraint and perceived threat). The stress response is activated as soon as the animal senses an actual or potential threat to its biological wellbeing (Yang et al. Citation2022). This stress response involves the activation of hypothalamus-pituitary-adrenal and sympathetic-adrenal medulla axes, resulting in the modulation of a variety of physiological functions and behaviours (Desborough Citation2000; Umamaheswaran et al. Citation2018; Hueston and Deak Citation2014). Prolonged stress can lead to oxidative stress induced cellular and tissue damage, triggering a series of pathological processes giving rise to chronic problems (McMichael Citation2007). Malondialdehyde (MDA) is the oxidative product of lipid peroxidation and indicates increase in reactive oxygen species (ROS) (Todorova et al. Citation2005). Further, physiological antioxidant enzymes like superoxide dismutase (SOD), catalase and reduced glutathione (GSH) undergo disturbance in excessive oxidative stress situation (Ighodaro and Akinloye Citation2018).
The stress response may also lead to suppression of immune system function by sustained high levels of cortisol hormone which modulates cytokines like interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α) and interleukin-10 (IL-10) (Minamoto et al. Citation2015; Contreras et al. Citation2021; Smith et al. Citation1999). Oxidative stress is integral to the pathological stress response. The relationship between oxidative stress and disease progression is intricate and can make or break the overall progression of disease (McMichael Citation2007). Moreover, oxidative stress can trigger inflammatory responses via the imbalance of two important transcription factors including the nuclear factor kappa light chain enhancer of activated B cells (NFκB) and nuclear factor erythroid 2-related factor 2 (Nrf-2) signalling pathways (Tian et al. Citation2017; Bellezza et al. Citation2018). Hence, targeting oxidative stress can be a plausible strategy to mitigate stress.
Lately, there has been a growing awareness of the impact of stress on cats and herbal supplements with adaptogenic properties may prove beneficial (Sayed et al. Citation2019a). Health supplements with stress reducing effects might play a crucial role in preserving both the physical and psychological equilibrium in cats (Rutherfurd-Markwick et al. Citation2013). Ashwagandha (Withania somnifera L. Dunal) is a well-known herb with well documented effects on improving stress adaptation responses in humans and animals (Bhattacharya and Muruganandam Citation2003).
Ashwagandha root extract (ARE) is known to promote general health by its adaptogenic, increasing resistance to illnesses, and improving the overall mental well-being (Bharti et al. Citation2016; Choudhary et al. Citation2017). It is rich in withanolides like withaferin A which is known to possess promising pharmacological effects like anti-pancreatitis, anti-neurodegeneration, leptin sensitization, and hepatoprotective (Tiruveedi et al. Citation2018; Kumar et al. Citation2021; Lee et al. Citation2016; Jadeja et al. Citation2015). These withanolides are primarily responsible for the antioxidant effects of ARE. Therefore, this randomized double blind veterinary trial was conducted to investigate the pharmacological effects of ARE on feline stress induced changes. The cats were subjected to four different types of stress (separation induced stress, noise induced stress, restrain induced stress and travel & feed delay induced stress) over a period of one month and sample were collected after 15 and 30 days of ARE treatment. The hematological profile, markers of liver and kidney function were investigated. Further, the effects of ARE on critical biomarkers of oxidative stress (MDA, SOD, catalase and GSH), stress (cortisol), inflammation (IFN-γ, TNF-α, IL-10) and transcription factors (NFκB, Nrf-2) were studied over a period of one month.
2. Materials and methods
2.1. Chemicals, kits and reagents
All the chemicals and reagents used in the study were of analytical grade unless stated otherwise and were procured from Sigma Aldrich, USA. The enzyme linked immunosorbent assay (ELISA) kits for Feline cortisol (Cat. No. CK-bio-20728), Feline Nrf-2 (Cat. No. CK-bio-28668), Feline NFκB (Cat. No. CK-bio-28649) and IL-10 were procured from Shanghai Coon Koon Biotech Co., Ltd., China. Feline IFN-γ ELISA kit (Cat. No. EF2RB) was procured from Invitrogen Inc. USA. The high concentration Ashwagandha root extract (ARE) KSM-66 (containing ≥5% withanolides) was provided as a gift sample by Ixoreal BioMed Inc., Los Angeles, California, USA.
2.2. Study animals
Sixteen Persian cats (either sex) aged 1–3 years without any sign of stress and anxiety were enrolled in the study. The cats were all considered healthy based on complete physical examinations. Cats were fed with feline diet by Whiskas® (Tuna flavour) providing every essential nutrient to the animals. Cats were excluded from participating if they were being treated with other psychoactive medications, homeopathic remedies, pheromonal products, supplements, or a special diet to control anxiety. Other reasons for exclusion were pregnancy, lactation, concurrent participation in any other clinical study, and any other condition or situation which could disturb the conduct of the study. The study was approved by the Institutional Animal Ethics Committee (IAEC) at the College of Veterinary Science, P.V.N.R. Telangana Veterinary University, Hyderabad (30/25/C.V.Sc, Hyderabad, dated 2/7/2022) as per the guidelines of Committee for Control and Supervision of Experiments on Animals (CCSEA), New Delhi, India. The animals were under regular observation by an experienced veterinarian.
2.3. Sample size, randomization and trial interventions
The study was a randomized, placebo-controlled, double-blind veterinary clinical trial. The enrolled cats were randomly assigned to 1 of the 2 groups (Placebo and ARE) in a 1:1 randomization ratio with 8 cats in the placebo group (15 mg/kg, oral), and the other 8 cats in the ARE group (15 mg/kg, oral). The dose was selected based on available literature (Kaur et al. Citation2022). Randomization was performed using an automated random number generation method, which was pre-determined for the site. The placebo (starch) was identical to the investigational product in appearance, colour, odour, taste, and packaging. The ARE and placebo formulations were packed into identical containers and labelled equivalently to ensure blinding. All cats were assessed at day 0, week 2 (day 15), and week 4 (day 30). The provides schematic of the study design and the timeline of treatments and sampling protocol.
2.4. Stress induction
The stress in cats was induced in a systematic way with a specific stress schedule for each week as enlisted in . During week 1, the cats were subjected to separation and attendant induced stress, which consisted of three subtypes (separation, relocation and unfamiliar caretakers) and were performed every alternative day. During week 2, the cats were subjected to noise-induced stress which consisted of three subtypes (firecrackers, banging the door and booming sound) of noise stress carried out every alternative day. During week 3, the cats were subjected to restrain induced stress, which also consisted of three subtypes (manipulation, dark room and grabbling & hold) and were executed every alternative day. During the last week of the study, cats underwent travel and feed modification related stress which also consisted of four subtypes (car transport, cage transfer, feed delay and feed deprivation), performed every alternative day.
Table 1. The schedule of different types of stress induced in cats.
2.5. Blood sample collection and blood profile parameters
Blood was collected from the left side of the cephalic vein unilaterally under adequate restrain. The blood was transferred into two separate tubes for hematology (in heparinized tubes) and serum separation. Serum samples were separated from the blood by centrifugation at 4,000 rpm for 15 min and were stored at −80°C until further analysis. The blood collection was performed in the morning before feeding the cats. Total erythrocyte count (TEC), total leucocyte count (TLC), mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH), platelets and haemoglobin (Hb) were assessed from the blood samples of the cats using a hematology analyser. TEC and TLC were expressed in cells/L, and haemoglobin was expressed in g/dL.
2.6. Measurement of serum biochemical parameters
To estimate the levels of important biomarkers of liver and kidney health status, key markers were estimated in the serum. The liver health was estimated based on the levels of total protein (g/dL), albumin (g/dL), globulin (g/dL), alkaline phosphatase (ALP) (IU/L), alanine aminotransferase (ALT) (IU/L), aspartate aminotransferase (AST) (IU/L). On the other hand, kidney function was evaluated based on blood urea nitrogen (BUN) (mg/dL), and creatinine (mg/dL) levels using commercially manufactured kits (ERBA, Mannheim, Germany). The estimation of glucose (mg/dL) was performed to evaluate the effect of interventions on glucose homeostasis.
2.7. Measurement of markers of oxidative stress
The antioxidant markers such as MDA, SOD, GSH and catalase were estimated in the serum during the study. The levels of MDA were measured as per the thiobarbituric acid reactive substrate (TBARS) method in accordance with earlier described methods (De Leon and Borges Citation2020; Renushe et al. Citation2022). The SOD measurements were based on pyrogallol method and whereas catalase was measured based on hydrogen peroxide decomposition method and were expressed in IU/mL (Chelpuri et al. Citation2022). The levels of GSH were estimated by using Ellman’s reagent as per an earlier described protocol and were expressed in µM/mL (Chelpuri et al. Citation2022; Rahman et al. Citation2006).
2.8. Evaluation of cortisol and inflammatory cytokines
Cortisol is a very important hormone involved in physiological stress response. Serum cortisol was assessed using sandwich ELISA method with precoated plates. Briefly, the samples were incubated in antibody precoated plates followed by washing with wash buffer. Next the samples were incubated with HRP conjugated secondary antibody followed by washing. Next the substrate solution was added for colour development, the reaction was stopped using the stop solution and the absorbance was measured at 450 nm. The concentration of the hormones in the samples was determined based on optical density and measured with the standard curve. Serum cortisol was expressed in ng/mL. On the other side, two important pro-inflammatory cytokines IFN-γ and TNF-α were measured (pg/mL) and an anti-inflammatory cytokine IL-10 (pg/mL) were also assessed in the serum samples by ELISA. The method was followed based on the instructions of the manufacturer. The sample absorbance was measured at 450 nm and the quantification was performed based on the respective standard curve.
2.9. Evaluation of levels of important transcription factors
To evaluate the effect of the treatment interventions, two critically important transcription factors NFκB and Nrf-2 were studied by ELISA. The experimental protocol was followed as the instructions of the manufacturer.
2.10. Statistical analysis
Data were presented as mean ± SD. All the pharmacological effects between the groups were evaluated by two-way ANOVA followed by Bonferroni’s Posthoc analysis. The results were analysed in GraphPad Prism version 5.0 and p < 0.05 was considered as statistically significant.
3. Results
3.1. Effect of ARE on hematological profile of cats under stress
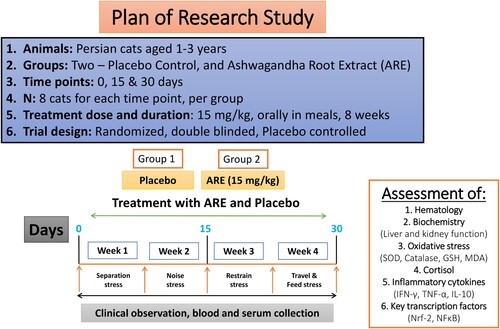
Hematological profile provides macro-insights into the gross effects that a treatment intervention may have under stress conditions. The levels of important hematological parameters, including RBC, WBC, MCV, MCH, platelet count and haemoglobin were measured. At day 15 and day 30, the RBC count was significantly higher (p < 0.001 vs placebo at both time points, A) in ARE treated group cats compared to the placebo group. However, the levels were within the physiological range. The TLC was also significantly increased at day 15 (p < 0.01, B) and day 30 (p < 0.001, B) in the ARE treated group compared to the placebo control group. In case of MCV (C), MCH (C) and platelet count (D), no significant changes were observed with ARE treatment compared to the placebo control. Interestingly, Hb levels were significantly higher on day 15 (p < 0.001, F) and day 30 (p < 0.001, F) in ARE treated group compared to the placebo control group.
Figure 2. Effect of ARE interventions on important blood parameters, (A) Red blood cell count (RBC), (B) White blood cell count (WBC), (C) Mean corpuscular volume (MCV), (D) Mean corpuscular hemoglobin (MCH), (E) Platelet count, (F) Hemoglobin (Hb). Data n = 8; statistically analysed by Mean ± SEM. **Significantly different from placebo group at p < 0.01, ***Significantly different from placebo group at p < 0.001. ARE: Ashwagandha root extract.
3.2. Effect of ARE on biochemical markers of liver and kidney function in cats under stress
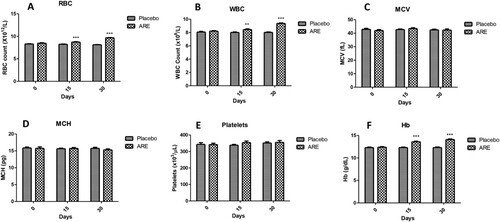
In order to assess the effects of ARE intervention on the liver and kidney function of cats under stress, the levels of ALT, AST, ALP, protein, albumin, globulin, creatinine and BUN were measured. The liver function marker ALT was found to be significantly reduced at day 30 (p < 0.05, A) compared to the placebo control group. AST levels showed a trend of decline in the ARE treated group of cats, however the levels were physiological range without any significant change (B). Similarly, the ALP levels were observed to be slightly decreasing in the stressed cats treated with ARE compared to placebo control group cats, but the changes were non-significant and within physiological range (C). Interestingly, at day 30 the protein levels in serum were significantly higher in the ARE treated group (p < 0.05, D) compared to the placebo control group. Further, the levels of albumin were also found to be significantly enhanced in the ARE treated group after 30 days of treatment (p < 0.01, A) compared to the placebo control group, indicating beneficial effects on liver physiology. Globulin levels were also evaluated (B), but remained within physiological range throughout the duration of the study in both the groups.
Figure 3. Effect of ARE treatment on the biochemical markers of liver function. (A) ALT, (B) AST, (C) ALP, (D) Protein. Data n = 8; statistically analysed by Mean ± SEM. *Significantly different from placebo group at p < 0.05. ALT: Alanine transaminase, ALP: Alkaline phosphatase, ARE: Ashwagandha root extract, AST: Aspartate aminotransferase.
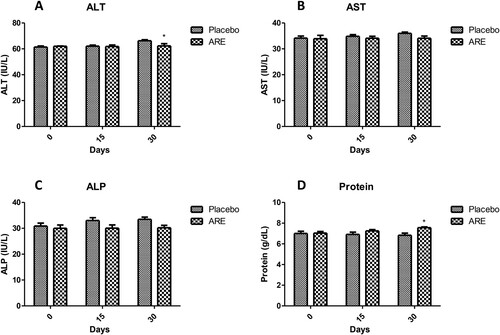
Figure 4. Effect of ARE treatment on important serum biochemical markers. (A) Serum albumin, (B) Serum globulin, (C) Serum creatinine, (D) Blood urea nitrogen (BUN), (E) Glucose. Data n = 8; statistically analysed by Mean ± SEM. **Significantly different from placebo group at p < 0.01, ***Significantly different from placebo group at p < 0.001. ARE: Ashwagandha root extract.
On the other side, creatinine levels were found to be significantly reduced in ARE treated cats (p < 0.01, C) after 30 days compared to the placebo control group. However, the levels of BUN, another marker of kidney function, remained unchanged in ARE treated group and were similar to the placebo control group (D). Interestingly, glucose levels were significantly higher in the placebo control group cats compared to the ARE treated group showing ARE treated cats had substantially lower level of stress (p < 0.001 vs placebo at both 15 and 30 days; E).
3.3. Effect of ARE on markers of oxidative stress in cats under stress
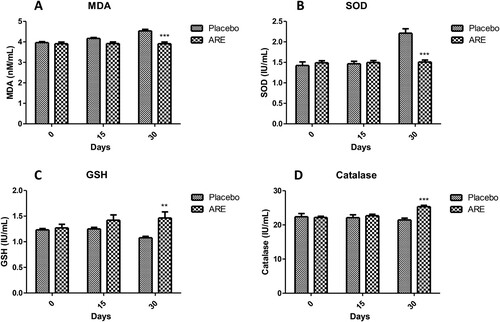
Oxidative stress is an important physiological process triggered during stress. The levels of important markers of oxidative stress like MDA, SOD, GSH and catalase were evaluated to assess the impact of ARE intervention. The levels of MDA were observed to be significantly lower in the ARE treated cats at day 30 (p < 0.001, A) when compared to the placebo control cats, which exhibited substantially higher levels. SOD is an important antioxidant defence related enzyme and was found to be markedly lower in the ARE treated group at day 30 (p < 0.001, B) when compared to the placebo control group. GSH is a very important antioxidant defence biomolecule and its levels were found to be significantly increased in the ARE treated group cats after 30 days (p < 0.01, C) compared to the lower levels observed in the placebo control group. Further, catalase is critical for peroxide radicals and its levels were found to be significantly higher in the ARE treated group cats (p < 0.001, D) compared to the placebo control group. These results suggest that ARE exhibits strong antioxidant effects to reduce the harmful effects of stress.
Figure 5. Effect of ARE treatment on oxidative stress markers. (A) Malondialdehyde (MDA), (B) Superoxide dismutase (SOD), (C) Glutathione (GSH), (D) Catalase. Data n = 8; statistically analysed by Mean ± SEM. **Significantly different from placebo group at p < 0.01, ***Significantly different from placebo group at p < 0.001. ARE: Ashwagandha root extract.
3.4. ARE intervention reduces stress by decrease in cortisol
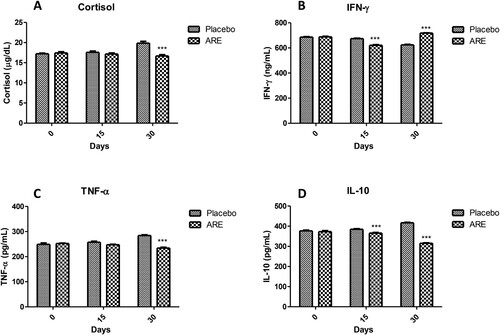
The levels of cortisol were measured to evaluate the pharmacological effects of ARE on cortisol levels. Interestingly, the levels of cortisol were significantly high in the placebo control group, and 30 days ARE intervention provided a remarkable reduction (p < 0.001, A).
Figure 6. Effect of ARE treatment on cortisol and cytokines levels. (A) Cortisol, (B) Interferon-γ (IFN-γ), (C) Tumor necrosis factor-α (TNF-α), (D) Interleukin-10 (IL-10). Data n = 8; statistically analysed by Mean ± SEM. ***Significantly different from placebo group at p < 0.001. ARE: Ashwagandha root extract.
3.5. Effect of ARE intervention on inflammation markers in cats under stress
Levels of three important cytokines were measured including two pro-inflammatory (IFN-γ, TNF-α) and one anti-inflammatory (IL-10) cytokine. It was observed that the levels of IFN-γ were significantly high in the placebo control group whereas ARE treated group showed significantly lower levels on day 15 (p < 0.001 vs placebo, B) and day 30 (p < 0.001 vs placebo, B). Similarly, the levels of another pro-inflammatory cytokine TNF-α were found to be significantly reduced on day 30 in the ARE treated group compared to the placebo control group (p < 0.001 vs placebo, C). On the other side, the levels of IL-10 were also found to be significantly lower on day 15 (p < 0.001 vs placebo, D) and day 30 (p < 0.001 vs placebo, D) in ARE treated group as compared to the placebo control group.
3.6. Effect of ARE intervention on important transcription factors involved in inflammation
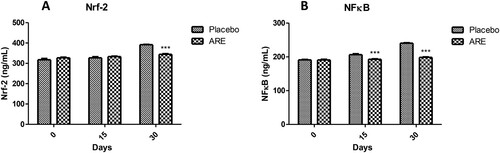
Inflammation in response to stress is controlled by various transcription factors and two of the important transcription factors are Nrf-2 and NFκB. The levels of Nrf-2 were within a similar range in both the placebo control and ARE treatment group till 15 days. Interestingly, the levels of Nrf-2 were significantly higher in the placebo control group on day 30 (A) indicating stress induced inflammation activation. Further, the levels of Nrf-2 were markedly lower in the ARE treated group on day 30 as compared to the placebo control group (p < 0.001 vs placebo, A). NFκB is known as the master regulator of inflammation and its levels were found to be significantly high on day 15 as well as day 30 in the placebo control group (B). On the other side, the levels of NFκB were significantly lower in the ARE treated group cats on both the time points (p < 0.001 vs placebo on day 15 & 30, B). These results indicate that ARE can significantly ameliorate stress in cats by reduction of key inflammation associated transcription factors, Nrf-2 and NFκB.
Figure 7. Effect of ARE treatment on important transcription factors viz; Nrf-2 and NFκB. (A) Nrf-2, (B) NFκB. Data n = 8; statistically analysed by Mean ± SEM. ARE: Ashwagandha root extract; Nrf-2: nuclear factor erythroid 2-related factor 2 (Nrf-2); NFκB: nuclear factor kappa light chain enhancer of activated B cells. Data n = 8; statistically analysed by Mean ± SEM. ***Significantly different from placebo group at p < 0.001. ARE: Ashwagandha root extract.
4. Discussion
Pet cats have gained immense interest worldwide owing to their small size, pretty looks and docile nature. Increasing human contacts have great advantages for the overall wellbeing of the animals however, it comes with adaptation to the human environment (González-Ramírez and Landero-Hernández Citation2021). Human environment noise, restrain, feed and travel may lead to acute or chronic stress in pet cats (Behnke et al. Citation2021; Amat et al. Citation2016; Stella et al. Citation2013). Dietary supplementation of adaptogenic herbs may alleviate the stress induced physiological changes in cats. The study aimed to investigate the safety and efficacy of oral administration of ARE in healthy cats which were subjected to four types of stress viz; separation induced stress, noise induced stress, restrain induced stress and travel & feed delay induced stress over a period of one month. The study was a randomized, double blinded veterinary clinical trial. To evaluate the anti-stress effects, various parameters, including hematological profiles, biochemical markers, antioxidant indicators, serum cortisol levels, and anti-inflammatory responses were assessed. The analysis of various parameters shed light on the adaptogenic potential of ARE in cats. Oral administration of 15 mg/kg body weight of ARE exhibited promising results in alleviating the physiological signs of stress in cats, with no adverse events. This study provided insights into the potential use of ARE for stress-related issues in felines.
Hematology provides details about the gross impact of different pharmacological interventions (Spada et al. Citation2016). A drastic decline in the hematological parameters can be a sign of toxicity as seen in case of cancer chemotherapy (Shahid Citation2016). The effects of ARE on hematological parameters, including total RBC, WBC, and haemoglobin concentration, are consistent with the findings of previous studies. For instance, Priyanka et al. observed comparable increases in hematological parameters in horses treated with ARE (Priyanka et al. Citation2020). This reflects the potential of ARE to enhance the hematopoiesis, thus, overall hematological homeostasis. Moreover, the immune system relies on a healthy balance of blood cells to effectively respond to stress and maintain an overall healthy well-being. Therefore, the observed effects of ARE on hematological parameters highlight its promising effects as an adaptogen to improve the immune response.
Liver and kidneys are two of the most important organs playing a crucial role in a plethora of biological processes. Under stress situations, the liver and kidney functions may be compromised culminating in the form of altered biochemical profile (Chida et al. Citation2006; Coppolino et al. Citation2019). The biochemical findings of this study show no detrimental effects of ARE and concur with the available literature. No pathological impact on liver enzymes (ALT, AST, ALP), kidney function parameters (creatinine and BUN) were observed with ARE intervention. In fact, some of the parameters indicated an overall improved liver and kidney function. Comparable findings were also evident in earlier published studies conducted on healthy human volunteers (Verma et al. Citation2021). A significant increase in protein and albumin were observed with ARE, which sheds light on its potential beneficial impact on protein metabolism. Increased albumin and globulin demonstrate ARE potential effect in adaptogenic response, crucial in managing stress-induced physiological changes. Interestingly, glucose levels were also reduced by ARE intervention, providing rationale for its use as an adaptogen for reducing stress.
Antioxidants defence is an important protective physiological barrier, assisting the physiological response against ROS (Halliwell Citation1999). Some of the important antioxidant enzymes are SOD, catalase and glutathione which neutralize the harmful free radicals which are potentially toxic to cells and tissues. Studies have illustrated the potential of ARE to improve defence mechanisms by enhancing antioxidant systems (Bhattacharya et al. Citation2000; Ahmed et al. Citation2018). Interestingly, ARE intervention reduced lipid peroxidation marker MDA and reduced the overall stress induced oxidative stress as evident by the modulation of the levels of SOD, GSH and catalase. These results indicate the potential role of withanolides present in ARE. Withaferin A is one of the most active Ashwagandha withanolides and has been shown to have potent antioxidant effects against pathological oxidative stress in multiple preclinical models (Tiruveedi et al. Citation2018; Sayed et al. Citation2019b; Peddakkulappagari et al. Citation2019; Tekula et al. Citation2018).
Cortisol is an extremely important stress hormone that plays critical role in orchestrating the physiological stress response (Katsu and Baker Citation2021). An increase in cortisol is seen in a variety of pathological acute and chronic stress conditions. Cortisol plays an integral role in the control of metabolic activity whereas pathological levels of inflammation and oxidative stress may lead to significant disturbance. Persistent increase in cortisol is a trigger for the development of obesity (Hewagalamulage et al. Citation2016). This study demonstrated a significant reduction in serum cortisol levels among ARE treated cats, while the placebo group experienced a significant increase over the study period. Concurrently, a study conducted by Chandrasekhar et al. on humans also reported the reduction in cortisol levels with ashwagandha supplementation, accompanied by an improvement in stress and anxiety-related parameters (Chandrasekhar et al. Citation2012). Additionally, findings reported by Abedon et al. and Wankhede et al. also reflected the potential of ashwagandha to modulate cortisol secretion and effectively manage the physiological response to stress in rats (Abedon et al. Citation2008; Wankhede et al. Citation2015). These findings suggest that ARE might exert its stress-reducing effects through its influence on the hypothalamic–pituitary–adrenal (HPA) axis, thereby, regulating the production and release of cortisol.
Inflammatory response due to stress can lead to a pathological cascade of events that may trigger acute and chronic tissue damage (Liu et al. Citation2017). Some of the most important cytokines which are involved in chronic inflammation and stress are IFN-γ and TNF-α. Their upregulation has been associated with increased stress and may lead to disease pathology (Kim and Maes Citation2003). On the other side, IL-10 is an anti-inflammatory cytokine whose levels tend to decrease during higher inflammatory state (Hu et al. Citation2014). IFN-γ and TNF-α are pro-inflammatory cytokines and their reduction observed with ARE reflects the anti-inflammatory property of the ARE. Further, modulation of IL-10 levels indicates the overall reduction in inflammation. This is supported by a previous study which proved a similar reduction (Sikandan et al. Citation2018). Moreover, there was reduction of NFκB, and Nrf-2 levels by ARE intervention in cats under stress. These transcription factors are crucial in regulating immune responses, particularly in managing inflammation and handling oxidative stress (Prasad Citation2016; Saleem et al. Citation2020; Tharakan et al. Citation2021). Interestingly, these findings highlight the potential of ashwagandha in augmenting antioxidant mechanisms and in attenuating stress-related inflammation. Furthermore, the study illustrates the impact of ARE on inflammation and oxidative stress, thus aiding the body in managing stress-related challenges. In future, it would be interesting to study the effect of ARE on the neurological parameters and neurochemistry behind the effects on stress related modulation in cats.
5. Conclusion
To conclude, this study adds to the knowledge that ARE has potential hemopoietic, anti-stress, antioxidant, adaptogenic, and immunostimulant properties against feline stress. The supplement was demonstrated to be safe and effective in cats, with no adverse event during the study period. However, further investigation with a larger sample size and dose variability would provide more insights into the adaptogenic, antioxidant, and anti-inflammatory effects of ARE in cats.
Conflicts of interest
The authors declare that there are no conflicts of interest pertaining to this research work.
Ethical approval
The study was approved by the Institutional Animal Ethics Committee (IAEC) at the College of Veterinary Science, P.V.N.R. Telangana Veterinary University, Hyderabad (30/25/C.V.Sc, Hyderabad, dated 2/7/2022) as per the guidelines of Committee for Control and Supervision of Experiments on Animals (CCSEA), New Delhi, India.
Acknowledgements
The authors would like to thank the Associate Dean, College of Veterinary Science for the support. The authors are thankful to Ixoreal Biomed. Inc., Los Angeles, CA, USA for providing the gift samples of high concentration Ashwagandha root extract (KSM66).
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Abedon B, Auddy B, Hazra J, Mitra A, Ghosal S. 2008. A standardized Withania somnifera extract significantly reduces stress-related parameters in chronically stressed humans: a double-blind, randomized, placebo-controlled study. Jana. 11:50–56.
- Aegerter J, Fouracre D, Smith GC. 2017. A first estimate of the structure and density of the populations of pet cats and dogs across Great Britain. PLoS One. 12:e0174709. doi:10.1371/journal.pone.0174709.
- Ahmed W, Mofed D, Zekri A-R, El-Sayed N, Rahouma M, Sabet S. 2018. Antioxidant activity and apoptotic induction as mechanisms of action of Withania somnifera (Ashwagandha) against a hepatocellular carcinoma cell line. J Int Med Res. 46:1358–1369. doi:10.1177/0300060517752022.
- Amat M, Camps T, Manteca X. 2016. Stress in owned cats: behavioural changes and welfare implications. J Feline Med Surg. 18:577–586. doi:10.1177/1098612X15590867.
- Applebaum JW, Peek CW, Zsembik BA. 2023. Examining U.S. pet ownership using the general social survey. Soc Sci J. 60:110–119. doi:10.1080/03623319.2020.1728507.
- Behnke AC, Vitale KR, Udell MA. 2021. The effect of owner presence and scent on stress resilience in cats. Appl Anim Behav Sci. 243:105444. doi:10.1016/j.applanim.2021.105444.
- Bellezza I, Giambanco I, Minelli A, Donato R. 2018. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochimica et Biophysica Acta (BBA) – Molecular Cell Research. 1865:721–733. doi:10.1016/j.bbamcr.2018.02.010.
- Bharti VK, Malik JK, Gupta RC. 2016. Ashwagandha: multiple health benefits. Nutraceuticals. 717–733.
- Bhattacharya S, Bhattacharya A, Sairam K, Ghosal S. 2000. Anxiolytic-antidepressant activity of Withania somnifera glycowithanolides: an experimental study. Phytomedicine. 7:463–469. doi:10.1016/S0944-7113(00)80030-6.
- Bhattacharya S, Muruganandam A. 2003. Adaptogenic activity of Withania somnifera: an experimental study using a rat model of chronic stress. Pharmacol Biochem Behav. 75:547–555. doi:10.1016/S0091-3057(03)00110-2.
- Chandrasekhar K, Kapoor J, Anishetty S. 2012. A prospective, randomized double-blind, placebo-controlled study of safety and efficacy of a high-concentration full-spectrum extract of ashwagandha root in reducing stress and anxiety in adults. Indian J Psychol Med. 34:255–262. doi:10.4103/0253-7176.106022.
- Chelpuri Y, Pabbathi S, Alla GR, Yadala RK, Kamishetti M, Banothu AK, Boinepally R, Bharani KK, Khurana A. 2022. Tropolone derivative hinokitiol ameliorates cerulein-induced acute pancreatitis in mice. Int Immunopharmacol. 109:108915. doi:10.1016/j.intimp.2022.108915.
- Chida Y, Sudo N, Kubo C. 2006. Does stress exacerbate liver diseases? J Gastroenterol Hepatol. 21:202–208. doi:10.1111/j.1440-1746.2006.04110.x.
- Choudhary D, Bhattacharyya S, Bose S. 2017. Efficacy and safety of Ashwagandha (Withania somnifera (L.) dunal) root extract in improving memory and cognitive functions. J Diet Suppl. 14:599–612. doi:10.1080/19390211.2017.1284970.
- Contreras ET, Vanderstichel R, Hovenga C, Lappin MR. 2021. Evaluation of hair and nail cortisol concentrations and associations with behavioral, physical, and environmental indicators of chronic stress in cats. J Vet Intern Med. 35:2662–2672. doi:10.1111/jvim.16283.
- Coppolino G, Leonardi G, Andreucci M, Bolignano D. 2019. Oxidative stress and kidney function: a brief update. Curr Pharm Des. 24:4794–4799. doi:10.2174/1381612825666190112165206.
- De Leon JAD, Borges CR. 2020. Evaluation of oxidative stress in biological samples using the thiobarbituric acid reactive substances assay. JoVE (Journal of Visualized Experiments). e61122.
- Desborough J. 2000. The stress response to trauma and surgery. Br J Anaesth. 85:109–117. doi:10.1093/bja/85.1.109.
- Enders-Slegers M-J, Hediger K. 2019. Pet ownership and human–animal interaction in an aging population: rewards and challenges. Anthrozoös. 32:255–265. doi:10.1080/08927936.2019.1569907.
- Foreman-Worsley R, Finka LR, Ward SJ, Farnworth MJ. 2021. Indoors or outdoors? An international exploration of owner demographics and decision making associated with lifestyle of pet cats. Animals (Basel). 11:253. doi:10.3390/ani11020253.
- González-Ramírez MT, Landero-Hernández R. 2021. Pet–human relationships: dogs versus cats. Animals (Basel). 11:2745. doi:10.3390/ani11092745.
- Halliwell B. 1999. Antioxidant defence mechanisms: from the beginning to the end (of the beginning). Free Radical Res. 31:261–272. doi:10.1080/10715769900300841.
- Hewagalamulage SD, Lee T, Clarke I, Henry B. 2016. Stress, cortisol, and obesity: a role for cortisol responsiveness in identifying individuals prone to obesity. Domest Anim Endocrinol. 56:S112–S120. doi:10.1016/j.domaniend.2016.03.004.
- Hu D, Wan L, Chen M, Caudle Y, LeSage G, Li Q, Yin D. 2014. Essential role of IL-10/STAT3 in chronic stress-induced immune suppression. Brain Behav Immun. 36:118–127. doi:10.1016/j.bbi.2013.10.016.
- Hueston CM, Deak T. 2014. The inflamed axis: the interaction between stress, hormones, and the expression of inflammatory-related genes within key structures comprising the hypothalamic–pituitary–adrenal axis. Physiol Behav. 124:77–91. doi:10.1016/j.physbeh.2013.10.035.
- Ighodaro O, Akinloye O. 2018. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defence grid. Alexandria Journal of Medicine. 54:287–293. doi:10.1016/j.ajme.2017.09.001.
- Jadeja RN, Urrunaga NH, Dash S, Khurana S, Saxena NK. 2015. Withaferin-A reduces acetaminophen-induced liver injury in mice. Biochem Pharmacol. 97:122–132. doi:10.1016/j.bcp.2015.07.024.
- Karatsoreos IN, McEwen BS. 2011. Psychobiological allostasis: resistance, resilience and vulnerability. Trends Cogn Sci. 15:576–584.
- Katsu Y, Baker ME. 2021. Cortisol, Handbook of hormones. Elsevier; 947–949.
- Kaur J, Seshadri S, Golla KH, Sampara P. 2022. Efficacy and safety of standardized ashwagandha (Withania somnifera) root extract on reducing stress and anxiety in domestic dogs: a randomized controlled trial. J Vet Behav. 51:8–15. doi:10.1016/j.jveb.2022.03.002.
- Kim Y-K, Maes M. 2003. The role of the cytokine network in psychological stress. Acta Neuropsychiatr. 15:148–155. doi:10.1034/j.1601-5215.2003.00026.x.
- Kumar S, Phaneuf D, Julien J-P. 2021. Withaferin-A treatment alleviates TAR DNA-binding protein-43 pathology and improves cognitive function in a mouse model of FTLD. Neurotherapeutics. 18:286–296. doi:10.1007/s13311-020-00952-0.
- Laugero KD, Moberg GP. 2000. Energetic response to repeated restraint stress in rapidly growing mice. American Journal of Physiology-Endocrinology and Metabolism. 279:E33–E43. doi:10.1152/ajpendo.2000.279.1.E33.
- Lee J, Liu J, Feng X, Salazar Hernández MA, Mucka P, Ibi D, Choi JW, Ozcan U. 2016. Withaferin A is a leptin sensitizer with strong antidiabetic properties in mice. Nat Med. 22:1023–1032. doi:10.1038/nm.4145.
- Liu Y-Z, Wang Y-X, Jiang C-L. 2017. Inflammation: the common pathway of stress-related diseases. Front Hum Neurosci. 11:316. doi:10.3389/fnhum.2017.00316.
- Martens P, Su B, Deblomme S. 2019. The ecological paw print of companion dogs and cats. BioScience. 69:467–474. doi:10.1093/biosci/biz044.
- McMichael MA. 2007. Oxidative stress, antioxidants, and assessment of oxidative stress in dogs and cats. J Am Vet Med Assoc. 231:714–720. doi:10.2460/javma.231.5.714.
- Minamoto Y, Otoni CC, Steelman SM, Büyükleblebici O, Steiner JM, Jergens AE, Suchodolski JS. 2015. Alteration of the fecal microbiota and serum metabolite profiles in dogs with idiopathic inflammatory bowel disease. Gut Microbes. 6:33–47. doi:10.1080/19490976.2014.997612.
- Peddakkulappagari CS, Saifi MA, Khurana A, Anchi P, Singh M, Godugu C. 2019. Withaferin A ameliorates renal injury due to its potent effect on inflammatory signaling. Biofactors. 45:750–762. doi:10.1002/biof.1534.
- Prasad KN. 2016. Simultaneous activation of Nrf2 and elevation of antioxidant compounds for reducing oxidative stress and chronic inflammation in human Alzheimer's disease. Mech Ageing Dev. 153:41–47. doi:10.1016/j.mad.2016.01.002.
- Priyanka G, Anil Kumar B, Lakshman M, Manvitha V, Kumar BK. 2020. Adaptogenic and immunomodulatory activity of Ashwagandha root extract: An experimental study in an equine model. Front Vet Sci. 7:541112. doi:10.3389/fvets.2020.541112.
- Rahman I, Kode A, Biswas SK. 2006. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc. 1:3159–3165. doi:10.1038/nprot.2006.378.
- Renushe AP, Banothu AK, Bharani KK, Mekala L, Kumar JM, Neeradi D, Hanuman DDV, Gadige A, Khurana A. 2022. Vincamine, an active constituent of Vinca rosea ameliorates experimentally induced acute lung injury in Swiss albino mice through modulation of Nrf-2/NF-κB signaling cascade. Int Immunopharmacol. 108:108773. doi:10.1016/j.intimp.2022.108773.
- Rutherfurd-Markwick K, Hendriks W, Morel P, Thomas D. 2013. The potential for enhancement of immunity in cats by dietary supplementation. Vet Immunol Immunopathol. 152:333–340. doi:10.1016/j.vetimm.2013.01.007.
- Saleem S, Muhammad G, Hussain MA, Altaf M, Bukhari SNA. 2020. Withania somnifera L.: insights into the phytochemical profile, therapeutic potential, clinical trials, and future prospective. Iran J Basic Med Sci. 23:1501.
- Sayed N, Khurana A, Godugu C. 2019a. Pharmaceutical perspective on the translational hurdles of phytoconstituents and strategies to overcome. J Drug Deliv Sci Technol. 53:101201. doi:10.1016/j.jddst.2019.101201.
- Sayed N, Khurana A, Saifi MA, Singh M, Godugu C. 2019b. Withaferin A reverses bile duct ligation-induced liver fibrosis by modulating extracellular matrix deposition: role of LOXL2/Snail1, vimentin, and NFκB signaling. Biofactors. 45:959–974. doi:10.1002/biof.1546.
- Shahid S. 2016. Review of hematological indices of cancer patients receiving combined chemotherapy & radiotherapy or receiving radiotherapy alone. Crit Rev Oncol Hematol. 105:145–155. doi:10.1016/j.critrevonc.2016.06.001.
- Short I. 2022. AVMA news. 2:2–4.
- Sikandan A, Shinomiya T, Nagahara Y. 2018. Ashwagandha root extract exerts anti-inflammatory effects in HaCaT cells by inhibiting the MAPK/NF-κB pathways and by regulating cytokines. Int J Mol Med. 42:425–434.
- Smith J, Allen S, Quandt J. 1999. Changes in cortisol concentration in response to stress and postoperative pain in client-owned cats and correlation with objective clinical variables. Am J Vet Res. 60:432–436. doi:10.2460/ajvr.1999.60.04.432.
- Spada E, Proverbio D, Baggiani L, Canzi I, Perego R. 2016. Hematological reference values for stray colony cats of northern Italy. Comp Clin Path. 25:903–910. doi:10.1007/s00580-016-2280-7.
- Stella J, Croney C, Buffington T. 2013. Effects of stressors on the behavior and physiology of domestic cats. Appl Anim Behav Sci. 143:157–163. doi:10.1016/j.applanim.2012.10.014.
- Tekula S, Khurana A, Anchi P, Godugu C. 2018. Withaferin-A attenuates multiple low doses of Streptozotocin (MLD-STZ) induced type 1 diabetes. Biomed Pharmacother. 106:1428–1440. doi:10.1016/j.biopha.2018.07.090.
- Tharakan A, Shukla H, Benny IR, Tharakan M, George L, Koshy S. 2021. Immunomodulatory effect of Withania somnifera (Ashwagandha) extract—a randomized, double-blind, placebo controlled trial with an open label extension on healthy participants. J Clin Med. 10:3644. doi:10.3390/jcm10163644.
- Tian T, Wang Z, Zhang J. 2017. Pathomechanisms of oxidative stress in inflammatory bowel disease and potential antioxidant therapies. Oxid Med Cell Longevity. 2017:4535194.
- Tiruveedi VL, Bale S, Khurana A, Godugu C. 2018. Withaferin A, a novel compound of Indian ginseng (Withania somnifera), ameliorates Cerulein-induced acute pancreatitis: possible role of oxidative stress and inflammation. Phytother Res. 32:2586–2596. doi:10.1002/ptr.6200.
- Todorova I, Simeonova G, Kyuchukova D, Dinev D, Gadjeva V. 2005. Reference values of oxidative stress parameters (MDA, SOD, CAT) in dogs and cats. Comp Clin Path. 13:190–194. doi:10.1007/s00580-005-0547-5.
- Umamaheswaran S, Dasari SK, Yang P, Lutgendorf SK, Sood AK. 2018. Stress, inflammation, and eicosanoids: an emerging perspective. Cancer Metastasis Rev. 37:203–211. doi:10.1007/s10555-018-9741-1.
- Verma N, Gupta SK, Tiwari S, Mishra AK. 2021. Safety of ashwagandha root extract: a randomized, placebo-controlled, study in healthy volunteers. Complement Ther Med. 57:102642. doi:10.1016/j.ctim.2020.102642.
- Wankhede S, Langade D, Joshi K, Sinha SR, Bhattacharyya S. 2015. Examining the effect of Withania somnifera supplementation on muscle strength and recovery: a randomized controlled trial. J Int Soc Sports Nutr. 12:43. doi:10.1186/s12970-015-0104-9.
- Yang K, Deng X, Jian S, Zhang M, Wen C, Xin Z, Zhang L, Tong A, Ye S, Liao P. 2022. Gallic acid alleviates gut dysfunction and boosts immune and antioxidant activities in puppies under environmental stress based on microbiome–metabolomics analysis. Front Immunol. 12:813890. doi:10.3389/fimmu.2021.813890.
- Yaribeygi H, Panahi Y, Sahraei H, Johnston TP, Sahebkar A. 2017. The impact of stress on body function: A review. EXCLI J. 16:1057.