ABSTRACT
The effects of Aronia melanocarpa (AM) dietary supplementation on production performance, meat and egg quality, yolk volatile substances and antioxidant capacity of laying hens. A total of 480 Roman brown laying hens aged 25 wks were randomly divided into control, LAM, MAM and HAM groups and fed 0%, 1%, 4% and 7% AM, respectively. The results showed that AM dietary supplementation significantly increased the eggshell strength, significantly reduced 24 h dripping loss of breast and thigh muscles, and increased pH and 48 h dripping loss of breast and thigh muscles. Cooking loss was significantly lower only in the HAM group for breast muscle. The MAM group was able to significantly increase albumen height and Haugh units. Esters gradually decreased with the increase in AM content, and the addition of AM led to a decrease in alcoholic compounds but increased the contents of alkenes, alkanes, ketones, and acids. The dietary AM supplementation significantly reduces the MDA content of serum, liver, ovary, chest muscle, thigh muscle and yolk and increases the activities of GSH-Px, T-SOD and T-AOC. In summary, dietary AM supplementation could improve antioxidant capacity and expression of related genes, thereby improving meat and egg quality.
Highlights
Aronia melanocarpa can improve the meat and egg quality of laying hens by enhancing their antioxidant capacity without affecting their production performance.
1. Introduction
With the improvement in living standards, consumers’ requirements for taste and quality of food have also increased. For the poultry industry, high-speed growth and cost savings are not the only pursuits; improving the quality of meat and eggs is also an urgent need for massive industrialization (Alnahhas et al. Citation2023). In today's intensive production conditions, many factors in feeding and management cause stress on livestock and poultry, increasing the free radicals in their body. Excessive free radicals can thus make the antioxidant systems in livestock and poultry unable to maintain the redox balance in the body, resulting in a state of oxidative stress, known to have adverse effects on their health and meat quality after slaughter (Wang et al. Citation2020a, Citation2022; Amevor et al. Citation2022). On the one hand, it reduces the immunity of livestock and poultry, inducing various diseases, and the use of excessive drugs (such as antibiotics) to treat sick animals may affect the safety of meat and egg products due to the absorption and long-term retention by livestock, having a certain impact on the environment due to the antibiotic components in their excreta, and affecting potentially the human health after ingestion (Sidor et al. Citation2021; Mlaga et al. Citation2022). On the other hand, oxidative stress causes a decline in meat and egg quality after slaughter and shortens its shelf life (Abd et al. Citation2023). During normal conditions, there is a complex antioxidant defence system scavenging reactive oxygen species (ROS) and maintaining the redox state of different cells. This antioxidant defence system consists of antioxidant enzymes, such as superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX). It has been reported that polyphenols can reduce ROS and increase the content of antioxidant enzymes, thus improving the body's antioxidant capacity. Plant extracts such as hot red pepper powder, Moringa oleifera, Essential oils (Abd et al. Citation2022) and Withania somnifera (Salem et al. Citation2022) possess biological functions such as antibacterial, immune regulation, antioxidant and growth promoting. They have a positive impact on animal production performance and health, mainly manifested in growth promoting and antioxidant effects, having thus the potential to replace antibiotic growth promoters in poultry feed. Aronia melanocarpa (AM) is also known as the wild berry or ageless berry, Rosaceae and is native to North America (Sidor et al. Citation2021; Negreanu-Pirjol et al. Citation2023). In recent years, the planting area for AM has been increased, and most of the planting areas are in the northeast of China, which is the convenient environment. Current research on AM has primarily focused on its nutrient-rich fruits, which are rich in sugars, proteins, procyanidins, anthocyanins, flavonoids and polyphenols. These components exhibit potent potential for scavenging free radicals (McDougall et al. Citation2016). The fruits of AM have been found to show multiple bioactivities potentially beneficial to human health, including antidiabetic, anti-infective, antineoplastic and antioxidant activities, as well as heart, liver and neuroprotective effects (Ren et al. Citation2022). Due to the sour, astringent, and bitter almond tastes of raw fruit, it has limited application as a fruit juice or in the juice industry but only used in mixed juices. China's current national food safety standards revealed that AM has become a food-grade product after a safety risk assessment. Therefore, AM is recently being used as a new feed additive. The aim of this work was to study the effects of AM as a feed supplement on production performance, meat and egg quality, volatile substances in yolk and the antioxidant capacity of laying hens, providing a theoretical basis for the effect of AM on laying hens.
2. Materials and methods
2.1. Plant material and animal preparation
The fresh fruits of AM were provided by the Mingqian Green Egg Breeding Professional Cooperative in Liaoyuan City, Jilin Province and dried out under sunlight. Before feeding, the animal room was fumigated and sterilized with formaldehyde and potassium permanganate. A total of 240 25-week-old Roman brown laying hens with similar body weight and normal development were selected and randomly assigned into four groups with three replicates in each group, 20 in each replicate. All groups were fed with diet as described in Table S1. The proximate nutrient component of AM is shown in Table S2. The control group was fed with basal diet and the experimental groups were received the same diet further supplemented with different levels of AM (1%, 4% and 7% dried fruit powder of AM, control, LAM, MAM and HAM, respectively). The hens were accommodated in wire cages measuring 45 × 45 × 50 cm, with three hens allocated per cage. The trial lasted for 9 weeks from August to September 2019. All hens were fed three times daily at 7:00 am, 11:00 am and 5:00 pm. Laying hens had ad libitum access to drinking water during the whole study period. The house temperature was maintained at 24 °C throughout the experiment with 17 h of light and 7 h of darkness. Two chickens were selected from each replicate, with six chickens collected from each group. six hens per experimental group were euthanized by CO2 asphyxiation and necropsied. A blood sample was then collected from the wing vein and centrifuged at 4000 rpm for 30 min. Whole blood was collected from the vein for separating serum, liver, ovary, breast muscle and thigh muscle were stored at −80 °C for subsequent tests.
2.2. Determination of organ index
After 9 weeks of the experiment, six hens were selected from each experimental group, the livers, ovaries and guts were dissected, washed with normal saline, and weighed to calculate the organ index = organ weight/body weight.
2.3. Determination of production performance
During the experiment, the number of eggs laid and egg weight of each group each week were recorded, the feed intake of each group was measured, and the feed conversion rate (FCR), egg rate, egg weight and average daily feed intake (ADFI) were calculated.
2.4. Determination of meat quality
The breast and thigh muscles with the same size and shape were sampled and stored in a 4 °C refrigerator. The pH values of the breast and thigh muscles were measured, 24, 48, 72, 96, 120, 144 and 168 h after slaughter, with a portable pH meter that the probe was inserted into at a depth of about 1 cm. The same part of the left breast and thigh muscles with the same size and shape were sampled and stored at 4 °C for 24 h. Then, the fat and sarcolemma on the surface were removed and respectively rest until they can be completely covered with the whole cuvette, and a colorimeter (SC-80C, Beijing, China) was used to measure brightness (L*), redness (a*) and yellowness (b*). The same part of the right breast and thigh were collected to remove the fat and fascia attached to the surface of the meat sample which was trimmed into L × B × H = 5 cm × 3 cm × 3 cm meat slice, weighed and recorded it as O1. The end of the meat sample was hook up with a thin iron wire, and placed it in an inflatable food bag, with the muscle fibre kept in direction vertical and downward. By refraining from directly handling the food bag, its opening was securely fastened and then suspended on the refrigerator's cooling shelf for 24 h. After that, the meat sample was taken out, the juice on its surface was gently wiped off with clean filter paper, and then weighed it as O2, measure it continuously at an interval of 24–144 h, and calculate the drip loss in the muscle was calculated according to the following formula: Drip loss (%) = [(Ol-O2)/O1] × 100%. Another size L × B × H = 2 cm × 2 cm × 3 cm of meat sample was taken, and its weight was denoted as P1 after removing subcutaneous fat and fascia. The thermometer was inserted into the centre of the meat sample was wrapped the meat sample with a plastic bag, with the mouth of the bag kept upward, and put it in an 85 °C constant temperature water bath until the heart temperature in the muscle reaches 70 °C. Then, it was immediately removed, cool down at room temperature, the juice on the surface of the meat piece was blotted with filter paper, and then weighed it again and recorded it as P2. The cooking loss was thus calculated according to the following formula: Cooking loss (%) = (P1-P2) /P1 × 100%. The breast muscles and thigh muscles of uniform size in the same part were refrigerated at 4 °C for 24 h. The surface connective tissue and fat were removed, and the meat samples were put into plastic bags and heated in a constant temperature water bath at 85 °C for 30–40 min. When the internal temperature of the meat sample reached 70 °C, it was removed and allowed to cool at room temperature for 30 min before sampling with a sampler. Ten meat pillars were taken from each meat sample, and their shear force values of the meat pillars were measured by the muscle tenderness meter (C-LM3B digital explicit Muscle tenderness meter, Jilin Agricultural University). The measured values were recorded and the average value was considered, with N as the unit.
2.5. Determination of egg quality
After 9 weeks of the experiment, five eggs were randomly collected from each replicate, with 15 eggs from each group undergoing quality testing. Briefly, the egg shape index, Haugh unit, eggshell strength and eggshell thickness were detected. The egg shape index (the long diameter/short diameter of the egg) was measured by using a vernier calipe, the eggshell strength was measured by using an eggshell strength tester (Model EFG-050, Robotmation, Japan), an eggshell thickness measuring instrument (Model ETG-1061, Robotmation, Japan) was used to detect egg shell thickness, the colorimetric card was used to measure egg yolk colour, while a multifunctional egg quality detector (EMT-5200, Robotmation, Japan) was used to detect Haugh unit and albumen height.
2.6. Determination of volatile substances in cooked egg yolk
3 eggs were randomly selected from each group, boiled in the boiling water for 10 min and then put in cool water. After cooling down, the yolk was collected and mixed. On Agilent 7890B/ 5977A/7693 Automatic injector series Gas Chromatographs (Agilent Technologies, Palo Alto, CA), A DB-WAX capillary column (30 m × 0.25 mm i.d × 0.25 mm film thickness, Agilent Technologies, Palo Alto, CA) was used to determine volatile flavour compounds in egg yolk by GC-MS. 10.00 g of the samples of each group were accurately weighed and placed in a P&T sample bottle. Nitrogen is added to one end of the sample bottle, and the other end is connected to an adsorption tube containing Tenax TA adsorbent (activated until there are no prior chromatographic peaks). The samples were kept at 60 °C, and the nitrogen purged flow rate was 50 mL/min, and the adsorption lasted for 40 min. Then the volatiles was directly desorbed in the GC injection port and desorbed at 250 °C for 5 min using the splitless injection at 250 °C of an inlet temperature, and 260 °C of MS detector. The pressure of carrier gas was ultra-high purity helium at 7.07 kPa. The programme temperature used started at 40 °C for 2 min, followed by 230 °C at 5 °C/min, and a 10 min hold at 230 °C. Mass spectra were recorded at an ionization voltage of 70 eV in the electron-impact mode and temperature 250 °C, with 30–500 m/z of scan range and 0.69 s of scan time. The qualification of the volatile flavour compounds was analysed by comparing the mass spectra with NIST 11 mass-spectral library.
2.7. Determination of antioxidant parameters
After 9 weeks of the experiment, the hen's liver, ovary, breast muscle, thigh muscle and yolk collected were homogenized in phosphate buffered saline, and then centrifuged at 4 °C at 800 g for 20 min to obtain 10% tissue homogenate. The supernatants were used to measure the total protein concentration and oxidation parameters, while serum was used to detect oxidation parameters. Total protein concentration, total superoxide dismutase (T-SOD, A001-1), glutathione peroxidase (GPX, A005-1), total antioxidant capacity (T-AOC, A015-1) and malondialdehyde (MDA, A003-1) concentrations according to manufacturer's instructions and kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) by biochemical methods. The content of MDA was measured with the thiobarbituric acid method, while the content of GPX was measured with the dithiodinitrobenzoic acid method (Li et al. Citation2023). The T-AOC was measured by chemical colorimetry method, and the activity of T-SOD was measured by the xanthine oxidase method (Ding et al. Citation2022).
2.8. RNA isolation and quantitative real-Time PCR (qRT-PCR)
A part of the liver and ovary were immediately removed and stored at −80 °C. Total RNA was then extracted by the Trizol method. The quality and concentration were determined by agarose gel electrophoresis and nucleic acid quantification, respectively. The latter involved using a nucleic acid quantitative analyser (Smart Spec Plus BIO-RAD, Hercules, California, USA). cDNAs were synthesized by using Prime Script RT reagent Kit with gDNA Eraser from total RNA (Sparkjade Shandong, China) and used to quantify gene expression levels with PCR amplification kit and genes specific primers (Table S3) for SOD1, GPX1 and NQO1 in liver and ovary tissue samples. The PCR amplification was performed in triplicate, and the relative quantification of gene expression was analysed according to the 2−ΔΔCt method.
2.9. Statistical analysis
The data were presented as Mean ± SD using IBM SPSS Statistics 26 software, while Graphpad Prism 9 was used to draw the column charts. One-way analysis of variance (ANOVA) was conducted to assess significant differences, followed by post hoc testing using the Duncan method. Results were considered statistically significant at P < 0.05. The Spearman correlation coefficient was used for correlation statistics, *represents significant difference, ** represents extremely significant difference, and *** represents extremely significant difference.
3. Results
3.1. Effects of feed supplemented with AM on organ indices of laying hens
The effects of AM on the organ indices of laying hens are shown in . The results showed that compared with the control group, the AM groups had similar liver, ovary, and intestinal indices of laying hens (P > 0.05).
Table 1. Effects of feed supplemented with AM on organ index of laying hens.
3.2. Effects of feed supplemented with AM on production performance of laying hens
The effects of AM on the production performance of laying hens are shown in . Compared with the control group, the addition of AM significantly increased egg weight at the first week and decreased feed conversion ratio (FCR) at the first and second weeks (P < 0.05). At the same time, the average daily feed intake (ADFI) was not significantly different among all groups (P > 0.05). Interestingly, the egg rate was lower in MAM group on week 1 and in all AM groups on week 2 compared to controls (also lower in LAM on week 3). On week 7, also significant differences are shown (P > 0.05).
Table 2. Effects of feed supplemented with AM on production performance of laying hens.
3.3. Effects of feed supplemented with AM on meat quality of laying hens
3.3.1. Effects of feed supplemented with AM on pH of laying hens
The effects of AM on the muscle pH of laying hens are shown in . The pH of the breast muscle and thigh muscle in each group of AM was compared and analysed at 24, 48, 72, 96, 120, 144 and 168 h. The overall changes in the pH of breast and thigh muscles at each test time showed an initial decreasing trend (P < 0.05), followed by an increase.
Table 3. Effects of feed supplemented with AM on pH of laying hens.
3.3.2. Effects of feed supplemented with AM on muscle colour and shear force of laying hens
As shown in . Compared with the control group, the brightness (L*), redness (a*) and yellowness (b*) of the breast muscles in all AM groups significantly decreased (P < 0.05). Compared with the control group, the brightness of thigh muscles in the LAM and MAM groups decreased (P < 0.05). Compared with the control group, the redness of thigh muscles in all AM groups increased but was significantly increased only in the MAM group (P < 0.05). Interestingly, the yellowness decreased in all groups compared with the control group, except in the HAM group, wherein there was a significant increase (P < 0.05). Compared with the control group, there was no significant difference in the shear force of the breast and thigh muscles in all AM groups (P < 0.05).
Table 4. Effects of feed supplemented with AM on muscle colour and shear force of laying hens.
3.3.3. Effects of feed supplemented with AM on muscle dripping and cooking loss of laying hens
The muscle dripping and cooking loss of laying hens supplemented with AM in their feed are shown in . Compared with the control group, the drip loss of breast and thigh muscles in all AM groups significantly reduced at 24 h (P < 0.05). However, at 48, 72, 96, 120 and 144 h in all AM groups, the drip loss of breast and thigh muscles showed an increasing trend compared with the control group (P < 0.05). Concerning the cooking loss, the breast muscle in the HAM group was lower than that in the control group (P < 0.05), the thigh muscle in all AM groups were lower than that in the control group (P < 0.05).
Table 5. Effects of feed supplemented with AM on muscle dripping and cooking loss.
3.4. Effects of feed supplemented with AM on egg quality of laying hens
The effects of AM on the egg quality of laying hens are shown in . Compared with the control group, all AM groups showed improved eggshell strength, with only the MAM group showing increased albumen height and Haugh unit (P < 0.05), but had no significant effect on the eggshell thickness and yolk colour (P > 0.05). The MAM group can significantly improve the egg shape index compared to the control group (P < 0.05). 3.5 Effects of Feed Supplemented with AM on Volatile Substances in Yolk of Laying Hens.
Table 6. Effects of feed supplemented with AM on egg quality of laying hens.
The effects of AM on volatile substances in yolk are shown in and . A total of 41 volatile substances were detected in the yolk of four kinds of eggs. All substances were divided into 7 classes, which included 13 esters, 6 alcohols, 7 alkenes, 6 alkanes, 2 ketones, 3 acids and 8 heterocyclic compounds. Esters gradually decreased with the increase in AM content, and the addition of AM led to a decrease in alcoholic compounds but increased the contents of alkenes, alkanes, ketones and acids.
Figure 1. GC-MS total ion current chromatograms of egg yolks. Abbreviations: a: control group, b: LAM group, c: MAM group, d: HAM group.
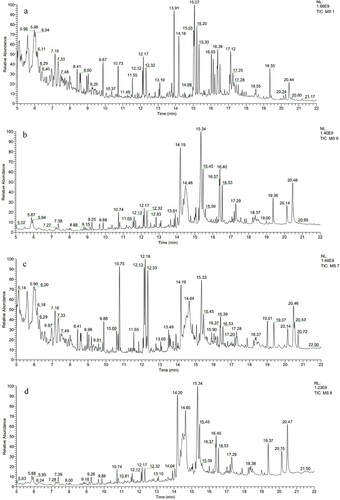
Table 7. Effects of feed supplemented with AM on volatile substances in yolk of laying hens.
3.6. Effects of feed supplemented with AM on antioxidant parameters of laying hens
The effects of AM on the antioxidant capacity of laying hens are shown in . Compared to the control group, all AM groups showed significant reductions in MDA content in both serum and yolk, as well as increases in T-SOD, GPX and T-AOC levels (P < 0.05). Additionally, compared to the control group, LAM and MAM groups exhibited significant decreases in MDA content in liver and thigh muscles (P < 0.05), along with increased levels of T-SOD, GPX and T-AOC (P < 0.05), while HAM group had no significant effect. Interestingly, only the LAM group significantly increased T-AOC content (P < 0.05) in breast muscles without any notable impact on other indicators (P > 0.05).
Table 8. Effects of feed supplemented with AM on antioxidant parameters of laying hens.
3.7. Effects of feed supplemented with AM on antioxidant gene expression of laying hens
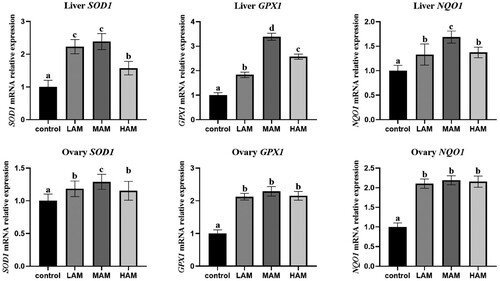
The effects of AM on the antioxidant gene expression of laying hens are shown in . In terms of the liver, compared with the control group, all AM groups significantly increased the SOD1, GPX1 and NQO1 gene expressions (P < 0.05). In the aspect of the ovary, compared with the control group, all AM groups significantly increased SOD1, GPX1 and NQO1 gene expressions (P < 0.05).
Figure 2. Effects of AM on Liver and ovary antioxidant gene expression of laying hens. Note:a,b,c,d Different superscripts within a row indicate a significant difference (P < 0.05). Abbreviations: SOD1, Superoxide Dismutase 1, GPX1, Glutathione peroxidase 1, NQO1, NADPH: Quinone Oxidoreductase 1. Values are expressed as mean ± standard deviation (n = 6). Data are the mean of six replicates. Control: Basal diet, LAM: 1% of Aronia melanocarpa plus diet, MAM: 4% of Aronia melanocarpa plus diet, HAM: 7% of Aronia melanocarpa plus diet.
3.8. Correlation analysis of antioxidant genes in ovarian tissue with meat quality and egg quality
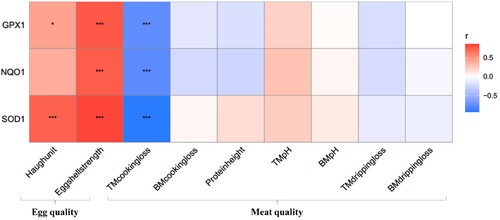
The results of correlation analysis showed that GPX1 and SOD1 were positively correlated with Haugh unit and eggshell strength (P < 0.05), and negatively correlated with thigh muscle cooking loss (P < 0.001). NQO1 was positively correlated with eggshell strength and negatively correlated with thigh muscle cooking loss (P < 0.001) ().
Figure 3. Correlation analysis of antioxidant genes in ovarian tissue with meat quality and egg quality. Abbreviations: GPX1, Glutathione peroxidase 1, NQO1, NADPH: Quinone Oxidoreductase 1, SOD1, Superoxide Dismutase 1. BM: Breast muscle, TM: Thigh muscle. Red: Positive correlation, Blue: Negative correlation. Values are expressed as mean ± standard deviation (n = 6). Data are the mean of six replicates. *P < 0.05, **P < 0.01, ***P < 0.001.
4. Discussion
Eggs are popular among consumers because they are rich in protein, lipids, amino acids and minerals. To meet the increasing consumption of eggs, several chicken farms have sprung up around the world (Lee et al. Citation2023). For commercial laying hens, the laying rate is as high as 95% from 200 days of age to the peak laying period (Wood et al. Citation2021). However, the continuous ovulation of laying hens in the peak laying period is easy to cause oxidative stress, which leads to the reduction in laying rate and egg laying cycle (Zmrhal et al. Citation2023). Several studies have found that adding antioxidants to diets can improve the antioxidant capacity of laying hens and reduce oxidative stress (Erinle and Adewole Citation2022; Mnisi et al. Citation2022; Vlaicu et al. Citation2022; Oni et al. Citation2023). AM is a natural fruit with a high polyphenol content than most berries, such as blueberries (Kulling and Rawel Citation2008). Although it shows a high antioxidant effect, it needs extensive processes to remove their sour taste before it can be used for consumption (Hatefi et al. Citation2021). This study was thus conducted to investigate the effects of dietary AM supplementation on production performance, meat and egg quality, yolk volatiles and antioxidant capacity of laying hens during the peak laying period following a certain experimental and theoretical basis for the use of AM as a potential new feed additive.
It has been shown that when the ovaries are subjected to oxidative stress, the egg production of laying hens decreases. Therefore, the level of egg production is closely related to the ovarian function of laying hens (Korver Citation2023). However, some studies have found that different antioxidant substances could improve the laying rate of laying hens (Frizzell et al. Citation2017; Gao et al. Citation2020; Chen et al. Citation2021), which was consistent with the results of this study revealing that AM can improve the laying rate of laying hens in the first week due to its richness of antioxidant components, such as polyphenols that have been reported by many literatures (Gao et al. Citation2020).
The oxidation degree of meat quality is closely related to the health of laying hens. Studies have indicated that oxidative stress can result in the deterioration of meat quality in laying hens. The oxidation of meat leads to a decline in quality, causing odour, discoloration, and loss of essential nutrients, thereby impacting the health of laying hens (Katemala et al. Citation2021). The pH can not only directly express the acidity of muscle but also reflect the speed of glycogenesis in the body of slaughtered animals (Bist et al. Citation2023). It has a vital impact on meat quality, and hence, it is one of the important indicators for the determination of meat quality. Some studies have revealed that muscles with high pH had higher water-holding capacity (Alnahhas et al. Citation2017; Semwogerere et al. Citation2018, Citation2019; Toomer et al. Citation2019). The effect of pH on the water-holding capacity of meat is thus essential due to the electrostatic charge effect of proteins. When the electrostatic charge decreases, the proteins condense and the distance between myofibrils decreases, which reduces the attraction to water and water-holding capacity. Some studies showed that the pH value of meat after slaughter firstly declined and then increased. The lower the pH value, the closer it was to the isoelectric point of the protein, indicating the lowered water-holding capacity and dark meat colour, which suggested an obvious correlation between water power and pH value (Aristides et al. Citation2018; Leishman et al. Citation2021). Our results also found that dietary AM supplementation could increase the muscle pH and muscle system water-holding capacity, as well as reduce the muscle drip loss in laying hens. This suggests that AM can slow down the glycogenesis, extend the preservation time and increase the juiciness and taste of muscles.
The meat colour is one of the important indicators reflecting the quality of muscle and is the main factor affecting consumers’ acceptance of meat. The brightness (L*) value reflects the reflective degree of the meat surface. When the brightness (L*) value of the meat colour is below 55, it indicates that the meat quality is normal and stable (Da Silva et al. Citation2017; Shaviklo et al. Citation2021). When it reaches 60, the flesh is pale, and the ability to hold water is poor. The redness (a*) value indicates the redness of the meat. The higher the redness (a*) value is within a certain range, the higher the degree of freshness and tenderness of the meat. The yellowness (b*) value indicates the antioxidant capacity of meat. The higher the yellowness (b*) value, the poorer the antioxidant capacity. It was found that the MAM group significantly increased redness (a*) and significantly decreased brightness (L*) and yellowness (b*) compared with other groups, thereby improving the tenderness and antioxidant capacity of the chest and thigh muscles. Concerning the shearing force, it is one of the evaluation methods of meat tenderness (Muhammad et al. Citation2021). The results of this study showed that there was no significant difference in breast tenderness (shear force) between the AM and control groups.
The egg shape index has a great influence on hatchability. The egg shape index of MAM group was significantly higher than that of other groups. The pigment deposition in egg yolk largely depends on the amount and type of carotenoids (Elkin and Harvatine Citation2023). Since the poultry cannot synthesize these pigment substances themselves, they need to be ingested from the diet. AM does not contain carotenoids. Therefore, there was no significant change in yolk colour. Interestingly, egg yolks in the HAM group were lower in colour, probably due to a decrease in the amount of corn added (Fletcher et al. Citation1985). The Haugh unit and albumen height can reflect the freshness of eggs, and their height can reflect the viscosity of egg protein and the quality of protein (Zheng et al. Citation2020). This experimental study showed that compared with the control group, the Haugh unit and albumen height of the MAM group was significantly improved, indicating that the freshness was higher. The eggshell thickness is also an important part of egg quality measurement, an index to reflect the anti-breakage rate of eggs (Myers and Ruxton Citation2023), and thus has a very significant impact on eggshell damage rate. This study found that the diet supplemented with AM did not have a significant effect on the eggshell thickness, but the eggshell strength of the MAM group was significantly higher than that of other groups. This is consistent with the findings of Obianwuna et al. (Citation2022), who showed that the addition of antioxidants to feed can improve eggshell strength.
Eggs are one of the foods that provide proteins for humans. Therefore, changes in egg flavour can not only reflect the freshness of food materials but also affect consumers’ choices (Xiang et al. Citation2022a). The content and type of volatile substances in eggs directly affect the flavour of eggs (Harlina et al. Citation2018). It has been found that egg production is related to four volatile substances, namely alkanes, aromatics, alcohols and aldehydes (Kobayashi et al. Citation2019). In particular, when the content of alkanes is high, egg production is relatively high (Xiang et al. Citation2022b). This study showed that the content of alkanes in egg yolks of hens fed with AM was relatively high, as well as the content of ketones and acids. This may be due to the high content of tannin in AM (Qin et al. Citation2012). The lipid oxidation process is also established to be connected with alcohol formation. Alcohols and acids arise from the reduction and oxidation of their corresponding aldehydes (Wang et al. Citation2020b). AM can reduce the content of alcohols and increase the content of acids, and the content of aldehydes increases significantly. This is also consistent with the previous results of increasing egg production and antioxidant indicators.
Numerous studies have shown that when oxidative stress is triggered, the accumulation of intracellular ROS causes lipid peroxidation, which can be monitored by MDA content. The study found that AM prevented alcohol-induced chronic liver injury by regulating the Nrf2 signalling pathway in C57BL/6 mice and reduced the content of MDA (Zhao et al. Citation2021). The present study found that the MDA content in laying hens during the peak laying period could also be reduced by adding AM. Antioxidant enzymes are known to protect the antioxidant system. AM polysaccharides have revealed to alleviate inflammation and oxidative stress damage of brain tissue in aging mice by regulating the Nrf2/HO-1 signal pathway and also improve the contents of T-SOD, GPX and T-AOC (Song et al. Citation2019). In this study, the contents of T-SOD, GPX and T-AOC in laying hens during the peak laying period were also be increased by adding AM. Specifically, the antioxidant effect in serum and ovary was the best, which might be due to the continuous ovulation of laying hens during the peak laying period, resulting in the production of a large amount of ROS in the ovary of laying hens. Other studies have found that in the aging mouse model induced by D-galactose, AM has an additive effect on the increase of antioxidant enzymes (SOD1, GPX1, CAT) gene expression in liver and the reduction of lipid peroxidation in serum, liver and kidney (Wang et al. Citation2020b). It was found in this study that gene expression in liver and ovary of SOD1, GPX1 and NQO1 in laying hens during the peak laying period was increased by adding AM. The correlation analysis showed that GPX1 was positively correlated with eggshell strength and Haugh unit (P < 0.05). This could mean that in eggs, there is a positive association between GPX1 activity and the strength of the shell and the quality of the egg white. This may be because GPX1 plays an active role in defending against oxidative stress, helping to protect the structural integrity of the eggshell and egg white quality. NQO1 was positively correlated with eggshell strength and negatively correlated with cooking loss of thigh muscle (P < 0.001). This could mean that NQO1 plays an active role in maintaining eggshell strength, and may also have a role in reducing muscle cooking loss, which may be related to the function of NQO1 in antioxidant and cellular metabolism regulation. SOD1 was positively correlated with eggshell strength and Haugh unit, and negatively correlated with cooking loss of thigh muscle (P < 0.001). This suggests that SOD1 plays an important role in maintaining eggshell strength and egg white quality, and may reduce the muscle cooking loss by reducing the effects of oxidative stress on muscle.
5. Conclusions
In conclusion, the present study demonstrates that dietary supplementation with Aronia melanocarpa (AM) enhances the meat and egg quality of laying hens during the peak laying period. Through the determination of volatile substances in egg yolk, it was found that adding AM to feed could increase the content of olefins, alkanes, ketones and acids, reduce alcohol and enrich the flavour of egg yolk. The correlation analysis can reveal that AM can enhance the antioxidant capacity of laying hens by increasing antioxidant gene expression in ovarian tissue, thereby improving meat quality and egg quality. Further research could explore the specific mechanisms of action of these enzymes in egg quality formation and determine how to improve the egg quality by regulating their activity. AM can thus be added to the feed as a diet containing antioxidant function. The results of the study suggest that the inclusion of 4% AM was able to better improve the meat and egg quality and antioxidant capacity of laying hens.
Author contributions
Bo Jing: Data curation, Writing – original draft, Writing – review & editing, Investigation, Resources, Data curation. Hui Song: Conceptualization, Methodology, Supervision. Haoyuan Wu and Zhenhua Liu: Software. Hongmei Shang and Yuting Li: Funding acquisition.
supplementary_figure_ion_concentration
Download Zip (5.8 MB)Supplementary_Table
Download MS Word (23.1 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The results and analyses presented in this paper are freely available upon request.
Additional information
Funding
References
- Abd EM, de Oliveira MC, Attia YA, Kamal M, Almohmadi NH, Youssef IM, Khalifa NE, Moustafa M, Al-Shehri M, Taha AE. 2023. The efficacy of polyphenols as an antioxidant agent: an updated review. Int J Biol Macromol. 250:126525. doi:10.1016/j.ijbiomac.2023.126525.
- Abd EM, El-Saadony MT, Saad AM, Salem HM, Ashry NM, Abo GM, Shukry M, Swelum AA, Taha AE, El-Tahan AM, et al. 2022. Essential oils and their nanoemulsions as green alternatives to antibiotics in poultry nutrition: a comprehensive review. Poult Sci. 101(2):101584. doi:10.1016/j.psj.2021.101584.
- Alnahhas N, Berri C, Chabault-Dhuit M, Bourin M, Arnould C, Le Bihan-Duval E. 2017. Combined effect of divergent selection for breast muscle ultimate pH and dietary amino acids on chicken performance, physical activity and meat quality. Animal. 11(2):335–344. doi:10.1017/S1751731116001580.
- Alnahhas N, Pouliot E, Saucier L. 2023. The hypoxia-inducible factor 1 pathway plays a critical role in the development of breast muscle myopathies in broiler chickens: a comprehensive review. Front Physiol. 14:1260987. doi:10.3389/fphys.2023.1260987.
- Amevor FK, Cui Z, Ning Z, Shu G, Du X, Jin N, Deng X, Xu D, Tian Y, Zhang Y, et al. 2022. Dietary quercetin and vitamin E supplementation modulates the reproductive performance and antioxidant capacity of aged male breeder chickens. Poult Sci. 101(6):101851. doi:10.1016/j.psj.2022.101851.
- Aristides L, Venancio EJ, Alfieri AA, Otonel R, Frank WJ, Oba A. 2018. Carcass characteristics and meat quality of broilers fed with different levels of Saccharomyces cerevisiae fermentation product. Poult Sci. 97(9):3337–3342. doi:10.3382/ps/pey174.
- Bist RB, Subedi S, Chai L, Yang X. 2023. Ammonia emissions, impacts, and mitigation strategies for poultry production: A critical review. J Environ Manage. 328:116919. doi:10.1016/j.jenvman.2022.116919.
- Chen F, Zhang H, Du E, Jin F, Zheng C, Fan Q, Zhao N, Guo W, Zhang W, Huang S, et al. 2021. Effects of magnolol on egg production, egg quality, antioxidant capacity, and intestinal health of laying hens in the late phase of the laying cycle. Poult Sci. 100(2):835–843. doi:10.1016/j.psj.2020.10.047.
- Da Silva DCF, de Arruda AMV, Gonçalves AA. 2017. Quality characteristics of broiler chicken meat from free-range and industrial poultry system for the consumers. J Food Sci Technol. 54(7):1818–1826. doi:10.1007/s13197-017-2612-x.
- Ding X, Cai C, Jia R, Bai S, Zeng Q, Mao X, Xu S, Zhang K, Wang J. 2022. Dietary resveratrol improved production performance, egg quality, and intestinal health of laying hens under oxidative stress. Poult Sci. 101(6):101886. doi:10.1016/j.psj.2022.101886.
- Elkin RG, Harvatine KJ. 2023. A review of recent studies on the enrichment of eggs and poultry meat with omega-3 polyunsaturated fatty acids: novel findings and unanswered questions. Poult Sci. 102(10):102938.
- Erinle TJ, Adewole DI. 2022. Fruit pomaces—their nutrient and bioactive components, effects on growth and health of poultry species, and possible optimization techniques. Anim Nutr. 9:357–377. doi:10.1016/j.aninu.2021.11.011.
- Fletcher DL, Papa CM, Halloran HR, Burdick D. 1985. Utilization and yolk coloring capability of dietary Xanthophylls from yellow corn, corn gluten meal, alfalfa, and coastal Bermudagrass. Poult Sci. 64(8):1458–1463.
- Frizzell KM, Lynch E, Rathgeber BM, Dixon WT, Putman CT, Jendral MJ. 2017. Effect of housing environment on laying hen meat quality: assessing pectoralis major pH, colour and tenderness in three strains of 80-81 week-old layers housed in conventional and furnished cages. Br Poult Sci. 58(1):50–58. doi:10.1080/00071668.2016.1236364.
- Gao S, Li R, Heng N, Chen Y, Wang L, Li Z, Guo Y, Sheng X, Wang X, Xing K, et al. 2020. Effects of dietary supplementation of natural astaxanthin from Haematococcus pluvialis on antioxidant capacity, lipid metabolism, and accumulation in the egg yolk of laying hens. Poult Sci. 99(11):5874–5882. doi:10.1016/j.psj.2020.08.029.
- Harlina PW, Ma M, Shahzad R, Gouda MM, Qiu N. 2018. Effect of clove extract on lipid oxidation, antioxidant activity, volatile compounds and fatty acid composition of salted duck eggs. J Food Sci Technol. 55(12):4719–4734. doi:10.1007/s13197-018-3367-8.
- Hatefi A, Zare Shahneh A, Ansari Pirsaraie Z, Alizadeh AM, Atashnak MP, Masoudi R, Pio F. 2021. The stimulation and inhibition of beta-2 adrenergic receptor on the inflammatory responses of ovary and immune system in the aged laying hens. Bmc Vet Res. 17(1):195. doi:10.1186/s12917-021-02892-z.
- Katemala S, Molee A, Thumanu K, Yongsawatdigul J. 2021. Meat quality and Raman spectroscopic characterization of Korat hybrid chicken obtained from various rearing periods. Poult Sci. 100(2):1248–1261.
- Kobayashi Y, Nojima Y, Sakamoto T, Iwabuchi K, Nakazato T, Bono H, Toyoda A, Fujiyama A, Kanost MR, Tabunoki H. 2019. Comparative analysis of seven types of superoxide dismutases for their ability to respond to oxidative stress in Bombyx mori. Sci Rep. 9(1):2170. doi:10.1038/s41598-018-38384-8.
- Korver DR. 2023. Review: current challenges in poultry nutrition, health, and welfare. Animal. 17:100755. doi:10.1016/j.animal.2023.100755.
- Kulling SE, Rawel HM. 2008. Chokeberry (Aronia melanocarpa) – A review on the characteristic components and potential health effects. Planta Med. 74(13):1625–1634. doi:10.1055/s-0028-1088306.
- Lee C, Lee J, Eor JY, Kwak M, Huh CS, Kim Y. 2023. Effect of consumption of animal products on the gut microbiome composition and gut health. Food Sci Anim Resour. 43(5):723–750. doi:10.5851/kosfa.2023.e44.
- Leishman EM, Ellis J, van Staaveren N, Barbut S, Vanderhout RJ, Osborne VR, Wood BJ, Harlander-Matauschek A, Baes CF. 2021. Meta-analysis to predict the effects of temperature stress on meat quality of poultry. Poult Sci. 100(11):101471.
- Li H, Hou Y, Hu J, Li J, Liang Y, Lu Y, Liu X. 2023. Dietary naringin supplementation on hepatic yolk precursors formation and antioxidant capacity of three-yellow breeder hens during the late laying period. Poult Sci. 102(5):102605. doi:10.1016/j.psj.2023.102605.
- McDougall GJ, Austin C, Van Schayk E, Martin P. 2016. Salal (Gaultheria shallon) and Aronia (Aronia melanocarpa) fruits from Orkney: phenolic content, composition and effect of wine-making. Food Chem. 205:239–247. doi:10.1016/j.foodchem.2016.03.025.
- Mlaga KG, Attivi K, Agboka K, Osseyi E, Tona K. 2022. The long-term effects of dietary replacement of fish meal with black soldier fly (Hermetia illucens). Larvae on nutritional content and eggshell quality in layer chickens. J World’s Poult Res. 12(3):181–191. doi:10.36380/jwpr.2022.21.
- Mnisi CM, Mhlongo G, Manyeula F. 2022. Fruit pomaces as functional ingredients in poultry nutrition: A review. Front Anim Sci. 3:883988. doi:10.3389/fanim.2022.883988.
- Muhammad AI, Mohamed DAA, Chwen LT, Akit H, Samsudin AA. 2021. Effect of sodium selenite, selenium yeast, and bacterial enriched protein on chicken egg yolk color, antioxidant profiles, and oxidative stability. Foods. 10(4):871. doi:10.3390/foods10040871.
- Myers M, Ruxton CHS. 2023. Eggs: healthy or risky? A review of evidence from high quality studies on hen’s eggs. Nutrients. 15(12):2657. doi:10.3390/nu15122657.
- Negreanu-Pirjol B, Oprea OC, Negreanu-Pirjol T, Roncea FN, Prelipcean A, Craciunescu O, Iosageanu A, Artem V, Ranca A, Motelica L, et al. 2023. Health benefits of antioxidant bioactive compounds in the fruits and leaves of Lonicera caerulea L. and Aronia melanocarpa (Michx.) Elliot. Antioxidants (Basel). 12(4):951. doi:10.3390/antiox12040951.
- Obianwuna UE, Oleforuh-Okoleh VU, Wang J, Zhang H, Qi G, Qiu K, Wu S. 2022. Natural products of plants and animal origin improve albumen quality of chicken eggs. Front Nutr. 9:875270. doi:10.3389/fnut.2022.875270.
- Oni AI, Adeleye OO, Adebowale TO, Oke OE. 2023. The role of phytogenic feed additives in stress mitigation in broiler chickens. J Anim Physiol Anim Nutr (Berl). 108(1):81–98. doi:10.1111/jpn.13869.
- Qin G, Tao S, Cao Y, Wu J, Zhang H, Huang W, Zhang S. 2012. Evaluation of the volatile profile of 33 Pyrus ussuriensis cultivars by HS-SPME with GC–MS. Food Chem. 134(4):2367–2382. doi:10.1016/j.foodchem.2012.04.053.
- Ren Y, Frank T, Meyer G, Lei J, Grebenc JR, Slaughter R, Gao YG, Kinghorn AD. 2022. Potential benefits of black chokeberry (Aronia melanocarpa) fruits and their constituents in improving human health. Molecules. 27(22):7823. doi:10.3390/molecules27227823.
- Salem HM, El-Saadony MT, Abd El-Mageed TA, Soliman SM, Khafaga AF, Saad AM, Swelum AA, Korma SA, Gonçalves Lima CM, Selim S, et al. 2022. Promising prospective effects of Withania somnifera on broiler performance and carcass characteristics: A comprehensive review. Front Vet Sci. 9:918961. doi:10.3389/fvets.2022.918961.
- Semwogerere F, Neethling J, Muchenje V, Hoffman LC. 2018. Effects of production systems on the carcass and meat quality characteristics of spent laying hens. Poult Sci. 97(6):1990–1997. doi:10.3382/ps/pey074.
- Semwogerere F, Neethling J, Muchenje V, Hoffman LC. 2019. Meat quality, fatty acid profile, and sensory attributes of spent laying hens fed expeller press canola meal or a conventional diet. Poult Sci. 98(9):3557–3570. doi:10.3382/ps/pez092.
- Shaviklo AR, Alizadeh-Ghamsari AH, Hosseini SA. 2021. Sensory attributes and meat quality of broiler chickens fed with mealworm (Tenebrio molitor). J Food Sci Technol. 58(12):4587–4597. doi:10.1007/s13197-020-04946-w.
- Sidor A, Drożdżyńska A, Brzozowska A, Gramza-Michałowska A. 2021. The effect of plant additives on the stability of polyphenols in dried black chokeberry (Aronia melanocarpa) fruit. Foods. 10(1):44. doi:10.3390/foods10010044.
- Song E, Park H, Kim H. 2019. Additive effect of walnut and chokeberry on regulation of antioxidant enzyme gene expression and attenuation of lipid peroxidation in d-galactose-induced aging-mouse model. Nutr Res. 70:60–69.
- Toomer OT, Livingston ML, Wall B, Sanders E, Vu TC, Malheiros RD, Livingston KA, Carvalho LV, Ferket PR. 2019. Meat quality and sensory attributes of meat produced from broiler chickens fed a high oleic peanut diet. Poult Sci. 98(10):5188–5197.
- Vlaicu PA, Untea AE, Turcu RP, Panaite TD, Saracila M. 2022. Rosehip (Rosa canina L.) meal as a natural antioxidant on lipid and protein quality and shelf-life of polyunsaturated fatty acids enriched eggs. Antioxidants (Basel). 11(10):1948. doi:10.3390/antiox11101948.
- Wang J, Jia R, Celi P, Ding X, Bai S, Zeng Q, Mao X, Xu S, Zhang K. 2020a. Green tea polyphenol epigallocatechin-3-gallate improves the antioxidant capacity of eggs. Food Funct. 11(1):534–543. doi:10.1039/C9FO02157D.
- Wang X, Liu Y, Zhao H, Wu Y, Liu C, Duan G, Wang Y, Liu T, Huang P, Li Y, et al. 2022. Effects of dietary ramie powder at various levels on the production performance, serum biochemical indices, antioxidative capacity, and intestinal development of laying hens. Front Physiol. 12:823734. doi:10.3389/fphys.2021.823734.
- Wang Z, Liu Y, Zhao X, Liu S, Liu Y, Wang D. 2020b. Aronia melanocarpa prevents alcohol-induced chronic liver injury via regulation of Nrf2 signaling in C57BL/6 mice. Oxid Med Cell Longev. 2020:1–13. doi:10.1155/2020/4054520.
- Wood PL, Muir W, Christmann U, Gibbons P, Hancock CL, Poole CM, Emery AL, Poovey JR, Hagg C, Scarborough JH, et al. 2021. Lipidomics of the chicken egg yolk: high-resolution mass spectrometric characterization of nutritional lipid families. Poult Sci. 100(2):887–899. doi:10.1016/j.psj.2020.11.020.
- Xiang X, Hu G, Jin Y, Jin G, Ma M. 2022a. Nondestructive characterization gender of chicken eggs by odor using SPME/GC-MS coupled with chemometrics. Poult Sci. 101(3):101619.
- Xiang XL, Hu G, Jin YG, Jin GF, Ma MH. 2022b. Nondestructive characterization gender of chicken eggs by odor using SPME/GC-MS coupled with chemometrics. Poult Sci. 101(3):101619. doi:10.1016/j.psj.2021.101619.
- Zhao Y, Liu X, Zheng Y, Liu W, Ding C. 2021. Aronia melanocarpa polysaccharide ameliorates inflammation and aging in mice by modulating the AMPK/SIRT1/NF-κB signaling pathway and gut microbiota. Sci Rep. 11(1):20558. doi:10.1038/s41598-021-00071-6.
- Zheng YW, Zhao LH, Wei YM, Ma QG, Ji C, Zhang JY. 2020. Effects of main cereal type and feed form on production performance, egg quality and egg sanitary indices of laying hens. Br Poult Sci. 61(2):164–168. doi:10.1080/00071668.2019.1704685.
- Zmrhal V, Svoradova A, Venusova E, Slama P. 2023. The influence of heat stress on chicken immune system and mitigation of negative impacts by baicalin and baicalein. Animals (Basel). 13(16):2564. doi:10.3390/ani13162564.
