Abstract
In the Miocene sandstones of the Nankang Formation in north-eastern Taiwan, different varieties of the trace fossil Ophiomorpha are abundant. In certain beds, a peculiar reworking of vertical Ophiomorpha shafts was observed. This reworking consists of an inner, lined tube positioned in the centre of the shaft. The shaft is lined by thin walls with small knobs and is distinctly different from the shafts of the Ophiomorpha nodosa mazes found in the same beds which have thick walls and large knobs. Because both outer and the inner tube walls were constructed by small sub-pellets, a diagenetic origin can be ruled out. The presence of sub-pellets further indicates that the inner tubes were also constructed by crustaceans and not commensal organisms such as worms or fish. The abundance of these vertical shafts suggests that they were constructed for a specific purpose, and the similarities in sub-pellets indicate that they likely were constructed by different generations of the same crustacean species. Because brooding chambers were not observed and are rare among extant marine crustaceans, we suggest that the vertical shafts were constructed to encourage juvenile shrimp to resettle in the parental burrows after they had completed their pelagic larval stages.
Introduction
Background and aim
Along the north-east coast of Taiwan, Oligocene to Miocene sediments deposited in littoral to shallow marine environments have been uplifted and tilted by tectonic processes related to the collision of a volcanic arc on the Philippine Sea Plate and the Eurasian continental plate (Suppe, Citation1984). The intense weathering and erosion caused by waves and torrential rains have resulted in a rugged coastline and sparse vegetation cover, providing detailed exposure of sedimentary and biogenic structures. These sandstones have recorded a set of tectonic and eustatic sea level changes, allowing a large number of different environmental settings to be studied. The individual beds of these strata often show characteristic ichnofossil assemblages representing different recurrent environments that are repeated several times in the succession as the sea level fell and rose. What makes these sandstones particularly interesting is the fact that many of the beds are dominated by a single ichnospecies. For example, in the Yehliu Member of the Taliao Formation, some beds are completely dominated by Schaubcylindrichnus, while overlying or underlying beds are dominated by Ophiomorpha (Löwemark & Nara, Citation2013).
In certain beds of the Nankang Formation, typical mazes of Ophiomorpha nodosa are common. However, in between these Ophiomorpha mazes, a distinctly different kind of Ophiomorpha with considerably thinner and less knobby walls can be found. These thin-walled Ophiomorpha often display a peculiar thin-walled inner tube positioned in the centre of the Ophiomorpha shafts. In contrast, the shafts extending from the thick-walled, knobby Ophiomorpha mazes rarely display this kind of inner tubes.
The aim of this study was to test whether these inner tubes are the result of diagenetic processes, if they were an integral part of the original Ophiomorpha burrow system, or if they represent a secondary reworking of the tubes by another organism. Furthermore, if the inner tubes can be shown to be the work of an organism, we aim to address the possible behaviours that may have resulted in the formation of these inner tubes, and the environmental significance of the presence of these inner burrows.
Geological setting
Taiwan is situated at the eastern boundary of the Eurasian continent and owes its steep topography to the collision between the volcanic arc on the Philippine Sea plate, and the continental margin of the Eurasian plate (Suppe, Citation1984). As a result of this collision, two north–south-oriented mountain ranges have formed, the eastern representing the volcanic arc of the Philippine Sea plate, and the western range representing metamorphosed and uplifted sediment from the Eurasian passive margin. Because of the north-westward motion of the Philippine Sea plate, the collision between the two plates is oblique. This means that while full collision is taking place in the central parts, an incipient collision can be found in the southern parts of the island, and in the north, where the collision started, it is now in a post-collisional, collapsing phase (Shyu, Sieh, Chen, & Liu, Citation2005; Suppe, Citation1984). During the Penglai orogeny, the strata comprising Taiwan island were uplifted and deformed (Teng et al., Citation1991), resulting in imbricated thrust faults along the north-east coast (Ho, Citation1986). The collision in combination with the opening of the Okinawa trough has resulted in uplifting, faulting and folding of the several thousand metres thick Cenozoic sediments that were deposited in a half graben system that developed along the Eurasian continental margin as a consequence of the opening of the South China Sea (Chen, Citation2005).
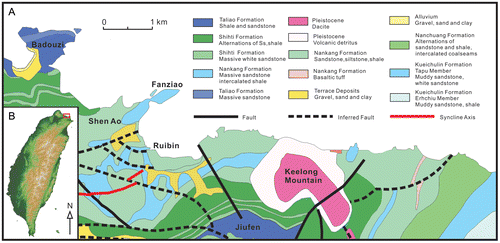
The Miocene strata in Taiwan consist of three main depositional cycles, each containing a coal-bearing and a marine unit deposited on the passive margin of the Eurasian continent. The study area is located on the north-east coast in strata belonging to the Nankang Formation (Figure ), which is encased between the Shitih and the Nanchuang Formations, extending NE–SW through Taiwan (Ho, Citation1986; Huang, Citation1990). Mostly, the Nankang Formation consists of paralic to shallow marine deposits characterised by strong NW–SE facies changes. It is generally recognised that there are two transgressive–regressive cycles in middle Miocene to upper Miocene. The Nankang Formation is deposited during the transgressive period, while the underlying Shitih Formation and the overlying Nanchuang Formation were deposited during regressive periods (Chou, Citation1970; Hong & Wang, Citation1988; Huang, Citation1990). However, more detailed sequence stratigraphy studies on the north-east coast have revealed that there are seven smaller transgressive–regressive cycles within the two major Miocene cycles (Yu & Teng, Citation1996).
Figure 1. (A) Geological map showing distribution of the Nankang Formation along the north-east Coast of Taiwan. Most observations were made at Fanziao promontory and on a few small islets just next to the Ruibin settlement. (B) The inset map shows the location of study area in north-eastern Taiwan.
The Miocene age (12–17 Ma) of the Nankang Formation has been constrained by planktonic foraminifers and calcareous nannoplankton; biozone boundaries of N8/N9 and NN4/NN5 are located in the middle part of the Nankang Formation and can be correlated with the standard outcrops at Chuhuangkeng which contains a continuous fossils record (Huang, Cheng, & Huang, Citation1979).
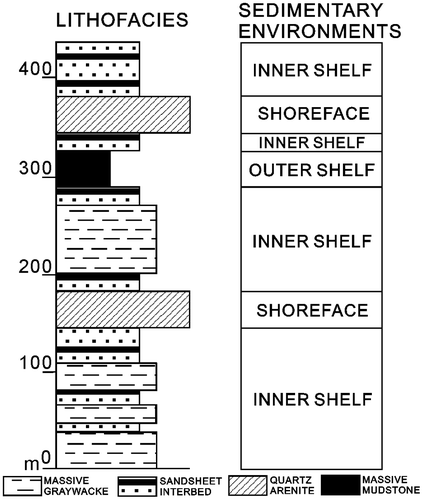
The traditional section of Nangkang Formation exposed at north-east coast has been well studied (Ho, Hsu, Jen, & Fong, Citation1964; Huang, Citation1955; Wang, Citation1953; Yen & Chen, Citation1953; Yu & Teng, Citation1996). The total thickness of the formation is about 500 m; it can be roughly divided into three parts (Figure ). Sandstone with abundant sea urchin and mollusca fossils make up the lower part, a 50 m thick shale and a 29-m-thick tuff constitute the middle part, and the upper sandstone contains several beds rich in Operculina and mollusca fossils (Huang et al., Citation1979).
Figure 2. Simplified stratigraphic column of the Nangang Formation, which consists of about one and a half transgressive–regressive cycle. The sedimentary environments of the Nankang Formation fluctuated between shoreface and wave-dominated outer shelf (Modified from Yu & Teng, Citation1999).
Ophiomorpha and burrows of thalassinidean shrimp
The trace fossil Ophiomorpha consists of a tunnel system where the walls have been reinforced with pellets, giving the external wall a knobby appearance, while the inner wall is typically smooth. The burrows range from short vertical or inclined tubes to complex mazes or 3D boxworks of interconnected shafts and tunnels. Some workers have related variations in the 3D architecture of the burrow system to external factors such as substrate and flow regimes (Bromley, Citation1996; Frey, Howard, & Pryor, Citation1978). In contrast, Miller and Curran (Citation2001) found no significant difference in burrow characteristics when comparing different facies in intertidal settings. Rather, the producers of Ophiomorpha tended to avoid inhospitable environments than to adapt their burrows to a harsher environment. They further noticed that wall pellet packing and arrangement varied a lot between different burrow systems, but also within the same system.
Crustacean burrow systems may fill many ethological functions, and different parts of one system may actually represent quite different behaviours. For example, different parts of the burrow system has been attributed to a number of different behaviours such as deposit feeding (Bird & Poore, Citation1999; Griffis & Chavez, Citation1988; Miller & Curran, Citation2001), shelter (Griffis & Chavez, Citation1988; Grow, Citation1981), storage of plant material for later use or for gardening of microbes (Dworschak, Citation2004; Dworschak, Koller, & Abed-Navandi, Citation2006; Frey & Howard, Citation1975; Gibert & Ekdale, Citation2010), simple turnover chambers (Bromley, Citation1996), or for reproduction purposes (Forbes, Citation1973; Griffis & Chavez, Citation1988). However, the burrow systems of crustaceans are also often used and modified by commensal organisms (Gibert, Netto, Tognoli, & Grangeiro, Citation2006; Karplus, Szlep, & Tsurnamal, Citation1974; Scheibling et al., Citation1990). Callianassa major is probably the best known modern analogue of Ophiomorpha producing animals, but other groups of crustaceans such as Upogebia or Axius are also known to produce burrow systems with knobby walls. The animal secretes mucus threads that are used to produce pellets of sediment grains (Dworschak, Citation1998) which are used to reinforce the walls in substrates where the cohesive forces of the sediment are too weak to keep the tunnels open.
Material and methods
Observations on stratigraphy and trace fossils were primarily made at Fanziao promontory and on a few small islets by the Ruibin settlement (Figure ). The varied topography caused by the combination of coastal erosion and several fracture systems allowed observations on both bedding plane and cross-sections of the 3D burrow systems to be made on several hundred specimens. Diameters and wall thicknesses of outer and inner tubes were registered using a digital Vernier caliper. A number of burrows containing inner tubes were sampled for thin section preparation and XRD analysis in order to allow differences in the composition of substrate and wall material to be assessed.
Results
Description of the Fanziao section
The total length of Fanziao section is approximately 35 m, made up by massive sandstone with one interbedded thin mudstone layer (Figure ). Sandstones are poorly sorted, and the grain size change is not significant. However, the sand–mud ratio shows distinct variations, with upwards increasing sand content from the muddier bottom part of Fanziao section to the middle part, which is characterised by muddy sandstones. The muddy mid-part is overlain by relatively sand-rich beds, with an upward increasing sand–mud ratio. The top of the sand-rich section is again overlaid by relative mud-rich sandstones. Most of the sedimentary structure have been obliterated due to heavy bioturbation, only swaley cross-stratifications is present sporadically within sandstones where they are sandwiched by bioturbated layers. The thickness of these layers is about 20 cm each, and they sometimes form quasi-planar beddings, with obvious erosional bases visible at the bottom of the structures. Another dominant sedimentary structure within sandstones is mud laminations. These are only centimetre thick and sometimes appear along with swaley cross-stratification. Through the frequency of mud-rich layers, the relative amount of mud content within the sandstone could be estimated. Lenticular sandstone beds are found at the bottom and the top of Fanziao section intercalated within the muddy sandstone.
Figure 3. Stratigraphic column of Fanziao section showing a storm wave-dominated sedimentary environment that fluctuated between lower shoreface and inner offshore. The stratigraphic cycles are reflected in variations in the sand–mud ratio, as well as in the trace fossil assemblage.
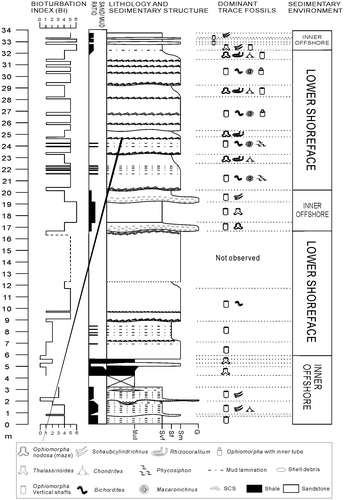
The bioturbation index of sandstones jumps from 1 to 5 in upper thick sandstone section due to interlacing of swaley cross-stratification layers and bioturbated layers. The bioturbation index of lenticular sandstone beds is low (0–1). In contrast, the bioturbation index of muddy sandstone is usually high, especially so for the muddy bed in the middle part of the section. In addition to the four Ophiomorpha types described below, the sandstones at Fanziao contain numerous Bichordites and Macaronichnus in certain beds, and Rhizocorallium is common in the layers where O. nodosa is abundant. Chondrites and Planolites were occasionally encountered, and a few structures that could possibly be attributed to Piscichnus waitemata were seen. Schaubcylindrichnus and Thalassinoides were found only within the muddy sandstones and shales. The ichnofacies therefore is interpreted as varying between the Skolithos ichnofacies and Cruziana ichnofacies. Interestingly, despite the high abundance of crustacean burrows, only few stingray feeding pits (Piscichnus waitemata) were observed, in stark contrast to the underlying Taliao Formation where feeding pits of stingray were found to be targeted directly to the shafts of Ophiomorpha burrow systems (Löwemark, Citation2015).
Three key beds were identified; these contain concentrated shell debris and are located at 2, 17 and 20 m in the column. The key beds display wavy bedding with erosional bases. The bed at 2 m showed loading structures with a vertical extent approaching 50 cm. The two beds at 17 and 20 m of the column consist of thick sandstones containing accumulations of complete sea urchin fossils. The two beds are separated by a fully bioturbated layer. These key beds allow us to correlate the Fanziao column to the lower part of Nangang Formation column described in earlier studies (Yu, Citation1997; Yu & Teng, Citation1996).
Ophiomorpha morphologies
In the Nankang Formation, at least four distinct morphotypes of Ophiomorpha-like burrows can be distinguished: (A) knobby, thick-walled mazes; (B) thin, straight tubes with regular branches; (C) dense, dendritically branching tube systems; and (D) thin-walled, L-shaped shafts (Figure ).
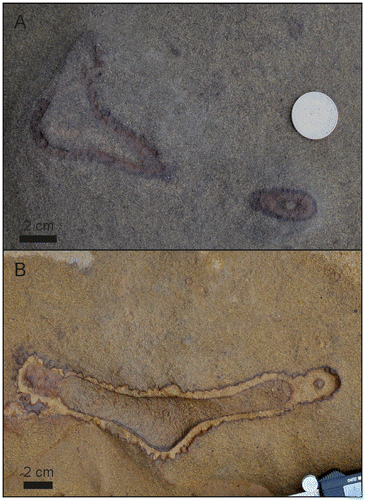
Figure 4. Photographs of the four morphotypes of Ophiomorpha distinguished at Fanziao. (A) Bedding plane view of O. nodosa which produces extensive mazes of thickly lined knobby tubes. (B) Bedding plane view of the thin, straight segments with widely spaced regular branches. (C) Bedding plane view of densely branching, dendritic tube systems. (D) Cross-section view of L-shaped shaft with inner tube. (E) 3D-reconstruction of the four types of Ophiomorpha.
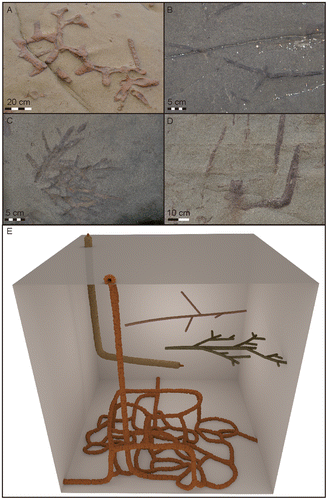
The tube systems of morphotype A closely correspond to O. nodosa. Walls are generally smooth on the inside and mammillated by coarse pellets on the outside. The tubes are typically arranged into horizontal mazes following certain layers, rather than 3D boxworks. Different layers of the maze may be connected with other horizontal mazes at deeper levels through vertical or inclined shafts. The knobby shafts of O. nodosa are often vertical in the upper part, but become curved, approaching the horizontal, in the lower part where they connect to the maze. Vertical shafts may show large vertical extent, and the junctions between shafts and maze typically show swellings that may indicate that they were used as turning chambers. Although the maze system is somewhat similar to O. irregulaire (Bromley & Pedersen, Citation2008), wall lining and pellet shape do not correspond to O. irregulaire. In most specimen, the wall thickness and pellet size is uniform around the tube, no spiky pellets or thinner walls on the burrow floor, as described for O. irregulaire (Lopez-Cabrera & Olivero, Citation2014), were observed. Furthermore, vertical shafts are abundant on bedding plane views, in contrast to O. irregulaire where vertical shafts are rare (Boyd, McIlroy, Herringshaw, & Leaman, Citation2012). The uniform wall thickness and pellet size in the observed Ophiomorpha also sets it aside from O. rudis which typically display smooth walls in the horizontal sections (Riahi, Uchman, Stow, Soussi, & Ben Ismail Lattrache, Citation2014; Uchman, Citation2009). Although O. nodosa occur abundantly in layers where the other three morphotypes are common, examples of connections between the different systems are dubious. The different morphotypes sometimes cross-cut each other, but a reworking of larger Ophiomorpha by smaller, as seen in e.g. Miocene estuarine sands from Delaware, USA (Miller & Curran, Citation2001; Miller, Curran, & Martino, Citation1998), was not observed. Inner tubes, as described in detail below for morphotype D, were only seen in a few isolated instances.
Morphotype B consists of long, almost straight segments of lined tubes. From these straight segments, sparse branches diverge at about 60 degrees from the main tube. The branching is often paired and does not display the pronounced swellings at the junctions typical for Ophiomorpha. The tube diameter typically lies around 1 cm, and the walls have a distinct lining that at least in some cases seem to be constructed by discrete pellets, giving the exterior a mammillated appearance. Morphotype B was primarily observed in bedding plane view, but on surfaces with high numbers of straight segments, high numbers of horizontal cuts of vertical, lined tubes of the same diameter and morphology could be observed. This suggests that the Morphotype B was connected to the sediment surface at regular intervals.
Morphotype C is similar to the straight segments described above, but displays a considerably denser, dendritic branching pattern, and typically has a slightly larger diameter (1–2 cm) and coarser pellets in the wall lining.
Although both morphotype B and C occur abundantly in the same beds as O. nodosa and regularly cross-cut O. nodosa, no indisputable evidence of any interconnection was found.
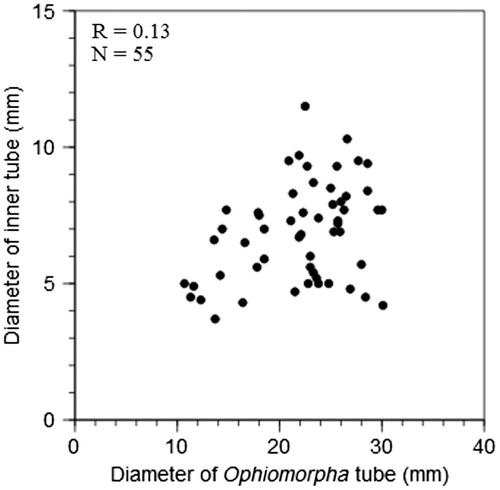
The fourth morphotype consists of a lined tube that extends for a considerable vertical distance before abruptly turning horizontal, giving that tube system an overall L-shaped appearance (Figure ). Horizontal parts are rarely observed even in areas where vertical shafts are common, suggesting that the horizontal part is short and not part of an extensive maze as in the adjacent O. nodosa. Both lining and pellets are considerably less pronounced compared to O. nodosa. The tube diameter is slightly smaller than for O. nodosa, typically ranging between 2 and 3 cm. In the vertical shafts, signs of an inner tube are common. This inner tube is lined by a thin knobby wall similar in colour to the larger outer wall. A comparison of paired samples of Ophiomorpha vertical shaft diameters and the diameters of the inner tubes from the same samples reveals a weak correlation between the two (Figure ). This weak correlation indicates that the size of the organism producing the inner tube is largely independent of the diameter of the vertical shafts.
Figure 5. Different aspects of the vertical shafts containing inner tubes. (A) Vertical shaft with inner tube. Where the shaft abruptly turns horizontal the inner tube expands to fill the entire burrow. The continuation of the system into deeper levels visible in this specimen was rarely seen in other specimen. (B) Close-up showing the wall of the Ophiomorpha shaft and a distinct inner tube. (C) Bedding plane view of Ophiomorpha with a distinct inner tube lined by material of the similar material as the material of the outer wall. (D) The rare occurrence of twin inner tubes in a single shaft (bedding plane view).

Figure 6. Scatter plot showing the relationship between outer tube diameter of Ophiomorpha and the diameter of the secondary inner tubes. The low correlation coefficient of 0.13 calculated through linear regression suggests that the diameter of the inner tube is largely independent of the diameter of the vertical shafts.
In a few instances, thin-walled vertical shafts with inner tubes partly overlap or intergrades to the thick-walled tube systems of O. nodosa systems were seen (Figure ).
Thin section analysis
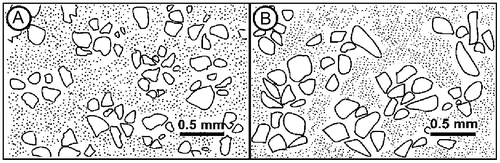
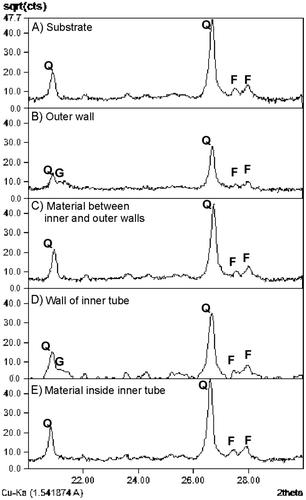
Results from the thin section analysis show that the grains are sub-rounded to angular, well sorted and that the grains are dominated by quartz, feldspar and glauconite (Figure ). XRD measurements of the wall lining show that the matrix primarily consists of goethite, while in the surrounding substrate and in the tube fill, the matrix is a mixture of clay minerals (Figure ). The particles vary in size from silt to medium sand, but most grains are of fine sand size. There is little difference in grain size distribution between surrounding substrate, wall material and tube fill material, but while the surrounding substrate and the tube fill are grain supported, the wall material is matrix supported. In the wall material, matrix makes up about 60–65% of the material. In both the inner and outer tube walls, sub-pellets sensu Frey et al. (Citation1978) could be observed. However, there is some difference in the arrangement of the sub-pellets. In the outer wall, the sub-pellets appear to be slightly larger than the sub-pellets from the inner tube. The outer wall sub-pellets are also more uniform in size and appear better organised, while the inner wall sub-pellets are less distinct and vary more in size and outline (Figure ).
Figure 8. Thin sections of Ophiomorpha with inner tube. (i) Idealised cross-section of Ophiomorpha showing the positions of the studied components. (A) Ambient substrate outside of the outer wall. (B) Outer wall and boundary to ambient substrate. (C) Material between inner and outer wall. (D) Wall of the inner tube. (E) Material inside inner tube. Q = Quartz, F = Feldspar, G = Goethite, Gl = Glauconite. Note the presence of goethite as a major matrix mineral exclusively in both the inner and outer walls, while glauconite is absent in both the inner and outer walls. The matrix of the substrate and burrow fill consists of mixtures of clay minerals as matrix. It is evident that both walls are matrix supported, whereas the substrate and burrow fills are grain supported.
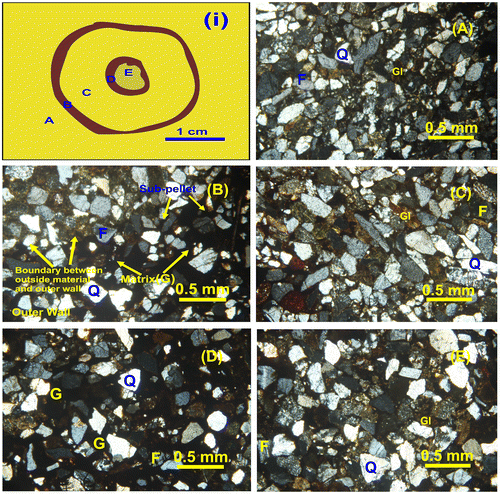
Figure 9. XRD analysis of the different components of the Ophiomorpha shaft and inner tube. The material of ambient substrate (A) and the tube fills (C and E) contains quartz and feldspars, while the wall material of both outer (B) and inner (D) tubes contain goethite. Q = Quartz, F = Feldspar, G = Goethite. Result is shown on 2θ between 20° and 30°.
Discussion
The nature of the inner tube
We initially proposed three potential explanations for the occurrence of the inner tube, a purely diagenetic origin, an integral part of the original burrow system, or a secondary reworking of the initial tube. The diagenetic origin can be dismissed as the thin section analysis clearly demonstrates that both the outer and inner walls are constructed by a large number of sub-pellets (Figure ). Furthermore, while the material in the walls is matrix supported, the sediment in the surrounding substrate and the fill of both the main shaft and the inner tube are grain supported. Diagenesis may change the matrix, or sometimes even partly alter grains (Galloway, Citation1974), but no known diagenetic processes can rearrange the grains into sub-pellets. We therefore dismiss diagenesis as cause for the inner tubes, although diagenetic processes undoubtedly have altered the matrix and thus enhancing the visibility of the inner tube’s wall material. The inner tube is also distinctly different from the draft fill channels described from some callianassid burrows (cf. Seilacher, Citation2007). The idea that the inner tube should be an integral part of the burrow system gains some support from the fact that restricted apertures are often observed in the tube systems of modern crustaceans that construct Ophiomorpha-like burrows (e.g. Frey & Howard, Citation1975; Swinbanks & Luternauer, Citation1987). However, the length of the inner tubes observed in the Nankang Formation reach several tens of cm, making it highly unlikely that the same producer that constructed the original tube with an inner diameter of 2–3 cm should be able to maintain a much thinner tube in which it hardly could fit. Studies on modern crustaceans have shown that they need to spend more than 20% of their time maintaining the burrow system (Gagnon, Beaudin, Silverberg, & Mauviel, Citation2013; Stamhuis, Reede-Dekker, van Etten, de Wiljes, & Videler, Citation1996). The pelleted nature of the inner wall clearly indicates that the inner tubes were actively maintained. It therefore seems unlikely that the inner tube should have been constructed by the larger producer, as it would have had no possibility of maintaining the upper reaches of the inner tube.
Consequently, the most probably explanation for the presence of the inner tube is that it was constructed inside the original, larger Ophiomorpha shaft by another producer. However, a number of distinct behaviours resulting in an inner tube produced by a secondary inhabitant can be conceived. The inner tube could be the result of commensal organisms occupying the same burrow system, it could represent the invasion by another species, or the reoccupation of the burrow by other organisms after the original producer died or moved on. The construction of new burrows in an older system has also been documented as the response to erosional events (Howard, Citation1978). Another possibility includes the construction of brooding chambers, or burrows constructed to attract colonisation by juveniles after their pelagic larval stages.
Ethological explanations for the inner tube
Commensalism seems unlikely in our case since the vertical shaft has been restricted by the construction of the inner tube, effectively blocking it for usage by its original producer, thus rather akin to an invasion or hostile takeover rather than commensalism. Furthermore, because the inner tube wall was constructed by sub-pellets of similar size and shape as the outer wall, fish or vermiform co-habitants are effectively ruled out.
Similar inner tubes were described by Pollard, Goldring, and Buck (Citation1993) and tentatively interpreted to have been constructed by relocated animals, possibly of a different species. However, the inner tubes described in their study differ in several important ways. First, the material filling the Ophiomorpha consisted of muddy coarse silt, distinctly different from wall material. In the described burrows, the grain size is similar in wall and fill, although walls were matrix supported while ambient sediment was clast supported. Second, Pollard et al. (Citation1993) makes no mention of a lined wall for the inner shaft. In the studied material, there is a clear tube wall that appears to be pelleted in a way similar to the outer wall. If the inner tubes represent invasions or reoccupation of abandoned burrows by other shrimp species, then the question arises why almost exclusively in the vertical shafts, and not in the curved shafts of O. nodosa? The high number of vertical shafts found to contain inner tubes suggests that they were constructed for a specific purpose. A secondary reoccupation or invasion of Ophiomorpha burrows from Pleistocene shallow subtidal or tidal flat environments in North Carolina was rejected by Curran (Citation1976) because of similarities in wall lining and tunnel arrangement. In analogue, it seems unlikely that secondary or commensal organisms would be responsible for the reburrowing of the Ophiomorpha observed in the Nangang Formation.
Crustacean burrows may fill two functions in the reproductive cycle: egg incubation (brooding) and recruiting juveniles returning after their planktonic larval phase (Atkinson, Citation1988). Brooding chambers constructed by the adults are common among insects and land living arthropods, but examples from marine crustaceans are rare (Genise, et al., Citation2008). Only a small number of fossil structures related to crustacean burrow systems have been interpreted as brooding chambers. In Pleistocene coastal deposits, burrows of a considerably smaller diameter were seen to branch off from bulbous chambers that occurred in close association with O. nodosa (Curran, Citation1976; Curran & Frey, Citation1977), and similar traces from Miocene strata in Uruguay were similarly interpreted as brooding chambers (Verde & Martínez, Citation2004). Another example of presumed brooding chambers was described from a Campanian age omission surface in Israel. These chambers contain a number of small pits on the floor that presumably held the eggs (Lewy & Goldring, Citation2006). To our knowledge, no unequivocal brooding chambers have been described for modern marine crustacean that build knobby-walled tube systems, but Forbes (Citation1973) showed that the larvae of Callianassa kraussi live their two larval stages in parent burrows and are actually unable to swim. They therefore metamorphosed within the parents burrow and excavated small new burrows directly into the wall of the parent burrow. Moreover, in certain freshwater shrimp, the brooding and first juvenile stages take place within the parents’ burrow system, from which the juvenile later construct miniscule burrows radiating out from the main burrow (Suter, Citation1977).
In the studied material, no chambers similar to the ones described by Curran (Citation1976), Verde and Martínez (Citation2004), or Lewy and Goldring (Citation2006) were observed, and no burrows were seen to excavate from the tube wall of the main Ophiomorpha burrows. A brood chamber origin of the inner tubes therefore seems unlikely.
This only leaves the hypothesis that the vertical shafts with inner tubes represent structures built by the parental crustaceans in order to facilitate the settlement of juveniles after their pelagic larval stages. If this is the case, then no brooding chambers would be expected. Many species of ghost shrimp disperse their larvae in the open water where they may migrate shorter or longer distances before returning to the shelf waters for reproduction in later stages of their life cycles (Tamaki et al., Citation2010, Citation2013). In some species, the juveniles have been seen to return to the adult’s burrow system where they produce a set of smaller side burrows within the adult system before producing their own system (Kornienko, Citation2013; Shimoda & Tamaki, Citation2004; Tamaki, et al., Citation1992). For example, burrows by Upogebia affinis showed smaller burrows made by juvenile shrimp extending from enlarged chambers. These were interpreted to be excavations by young shrimp. Because the larvae of U. affinis are free-swimming carnivores, the interpretation is that they likely returned after their larval stage to occupy the parent burrows (Frey & Howard, Citation1975).
The relatively high abundance of vertical shafts with L-shaped lower parts and the scarcity of observations on horizontal parts of the burrow system suggest that the system primarily consists of vertical shafts with short horizontal components, in stark contrast to O. nodosa where the maze systems constitute most of the burrow system. The fact that the thin-walled L-shaped shafts are abundant and have short horizontal extensions suggests that they were constructed for a specific purpose. Because any sign of brooding chambers is absent, we propose that the purpose of the vertical shafts was to provide optimal conditions for juvenile shrimp after they finished their pelagic larval stages. The relatively thin wall of the vertical shaft, in comparison with O. nodosa, could indicate that the structure was in use over a much shorter time period than the shafts of O. nodosa, consistent with having been constructed for the specific purpose of attracting juvenile shrimp. This hypothesis gains support from the observation that the sub-pellets of the outer and inner wall appears to have been constructed in a similar way, suggesting that they were produced by the same species. This kind of behaviour where the adult crustacean constructs a burrow in order to attract juveniles has actually been observed in O. puerelis (Gibert et al., Citation2006). Although morphologically very different from the observed inner tubes, this could be considered a modern analogue for the observed recolonisation of the vertical shafts.
It has been shown for modern thalassinid shrimp that substrate plays a significant role in determining the burrow morphology (Griffis & Chavez, Citation1988). The same species produce different morphologies in different substrates. It consequently seems unlikely that the same species would produce different types of burrows in the same substrate, unless the burrow systems filled entirely different purposes. The burrows with inner tube therefore likely are either constructed by another species or constructed for a specific purpose. The similarities in the construction and the possible connections between the shafts with inner tubes and O. nodosa mazes indicate that these two burrows may be part of the same system, but fill two different purposes: the mazes are feeding structures (cf. Bromley, Citation1996) while the vertical shafts are built to attract recolonisation by juveniles.
Diagenetic alteration of the tube material
The outer and inner walls thus appear to have been constructed by the same organism, but why is the wall material cemented by goethite, a mineral rare in the substrate matrix and in the tube fills? Several reasons can be put forward to explain the accumulation of goethite (FeOOH) as a major matrix mineral in both the walls. A simple argument would be attributed to selective uptake of iron rich compounds from deposits in cracks and fissures due to the input of Fe-rich waters by the trace maker (Ter & Buckeridge, Citation2012). Another possibility would be that the trace maker was perhaps feeding on the microbes that had been cultivated in the walls of the burrow (Uchman, Citation2009). Studies on extant Thalassinidean ghost shrimp have revealed slightly reducing conditions in the wall material related to the organic material in the mucus (Bird, et al., Citation2000). Microbial growth accelerates the oxidation of Fe2+ to Fe3+ by acting as a catalyst (Weber et al., Citation2012), as the microbes require Fe2+ for obtaining their energy at depths where the availability of organic carbon is minimal. This implies that the overall environment in the walls was oxidising, with the available oxidising pore waters giving rise to iron bearing hydroxides and oxyhydroxide minerals such as goethite. Goethite deposition can also be supported by the presence of glauconite in certain parts of the trace fossil outside of the walls. This indicates that there was an abundance of iron-rich clays which degraded to give rise to glauconite and probably goethite because of the overall oxidising conditions and also due to abundance of iron (McConchie, et al., Citation1979). Glauconite is formed under reducing conditions, while the presence of goethite in the walls suggests an oxidising environment in the walls. Consequently, glauconite is primarily absent from the walls.
Paleoenvironmental setting and significance
Although there is no significant change in lithology in the Fanziao section, cyclic changes were observed in sand–mud ratio and trace fossil assemblage. Through these observations, the sedimentary environment of these kinds of massive sandstones traditionally described as offshore transition zone (Yu, Citation1997; Yu & Teng, Citation1996) can be separated into two sub-sedimentary environments: lower shoreface and inner offshore. The lower shoreface begins at the lower limit of the fair-weather wave base (Reinson, Citation1984) and the inner offshore lays beneath it, above the storm wave base, thus both are affected by storm wave process. Here, the differences in trace fossil suites are used to draw the boundary between them following Pemberton et al. (Citation2001, Citation2012).
The inner offshore facies is made up by muddy sandstone and shale intercalated by lenticular coarser grain sandstone beds. Higher mud content represents more distal environments further away from the sediment source. The lenticular sandstone beds are usually interpreted as tempestites (Huang et al., Citation1979; Yu & Teng, Citation1996) where the bioturbation is less intense due to rapid deposit rate (MacEachern & Bann, Citation2008) and contain only vertical dwelling traces such as Ophiomorpha, representing opportunistic colonisation of the sandy substrate following storms (Pemberton & MacEachern, Citation1995; Pemberton, et al., Citation1992). During fair-weather periods, mud and sand were constantly disturbed by animals, forming intensely bioturbated layers with overprinted burrows where it is difficult to identify all the trace fossils (Figure ). For example, at 18 m in the column, a fully bioturbated bed lays right on top of a thick storm bed of cumulated fossils, only Thalassinoides and vertical shafts of Ophiomorpha can be identified, indicating a subsequent calm period. The trace fossil suite of the inner offshore muddy sandstones contains Thalassinoides, Schaubcylindrichnus, Chondrites and vertical Ophiomorpha shafts. Schaubcylindrichnus burrows were found only in these muddy sandstones, which help to divide sedimentary environment.
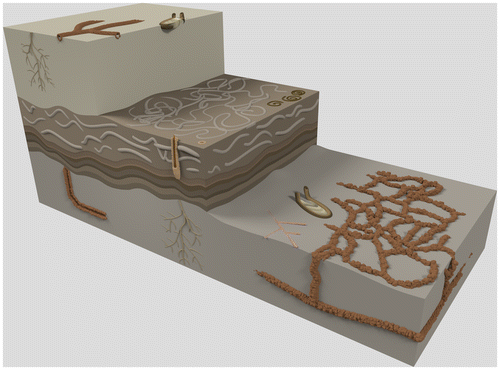
Figure 11. A 3D ichnofabric model of the lower shoreface environment in the Fanziao section. A transition layer of post-storm deposits (middle) is encased by two fair-weather layers. Biogenic features of the fair-weather beds are dominated by Cruziana ichnofacies, while in contrast, the ichnofacies of the post-storm bed is more Skolithos like. In the fair-weather deposit (bottom and top beds), the trace fossil suite is made up of O. nodosa, Rhizocorallium, Chondrites and two additional types of thinner Ophiomorpha, indicating Cruziana ichnofacies. The middle layer represents post-storm deposit and can be sub-divided into two parts: the lower part consists of swaley cross-stratification with rare vertical Ophiomorpha shafts, while the upper part is fully bioturbated by Bichordites and Macaronichnus. This suggests that these trace fossils represent an opportunistic colonisation of the substrate and that the colonising window of Bichordites and Macaronichnus is shorter than for Ophiomorpha. Consequently, a succession from swaley cross-stratification, Bichordites and Macaronichnus burrows to Ophiomorpha burrows can be expected. If fair-weather conditions extend long enough, an assemblage typical of the Cruziana ichnofacies will develop (top layer). In reality, the middle layer that represents frequent or severe storm-dominated conditions (Pemberton et al., Citation2001) may be much thicker than the beds with well-developed Cruziana facies and actually make up most of the massive sandstones in the Fanziao section. Either the time between two storms is too short for a biological assemblage to form, or the storms were so strong that the original substrate was swashed away. Evidence of frequent storm events are seen in the recurring superimposed succession of storm deposits that cut off the tops of the trace fossils.
The lower shoreface is made up by thick massive sandstones, displaying swaley cross-stratifications characterised by strongly erosional amalgamated bed sets and intensively burrowed beds followed by swaley cross-stratifications. This results in rapid bioturbation index shifts from 1 to 5. Low bioturbation index occurs in the layers with swaley cross-stratifications containing few vertical dwelling trace fossils. Beds with high bioturbation index sit right on top of the swaley cross-stratifications and extend until the next bed with swaley cross-stratification. Disparate trace fossil suites were observed in the different layers within the massive sandstones. These characteristics represent frequent periodic storm deposits, where the storms tended to destroy fair-weather benthic communities (Frey, Pemberton, & Saunders, Citation1990; Pemberton, Citation1992; Pemberton & Robert, Citation1984). Different trace fossil suites emplaced during storm periods and fair-weather periods, respectively, allow the deposits to be separated. Fair-weather period deposits consist of O. nodosa, vertical Ophiomorpha shafts, Rhizocorallium and Chondrites, indicating an impoverished Cruziana ichnofacies. Sometimes wavy remnants are inserted in the amalgamated sandstone beds. Storm period deposits consist of vertical Ophiomorpha shafts, Bichordites, Macaronichnus and Phycosiphon, representing the Skolithos ichnofacies. Within the Macaronichnus–Ophiomorpha ichnofabric, Macaronichnus burrows probably represent an opportunistic behaviour (Curran, Citation1985; Pollard et al., Citation1993). In this environment, Bichordites is also an opportunistic trace fossil similar to Macaronichnus, colonising the layers right above the swaley cross-stratification. Moreover, the morphology of Ophiomorpha tends to switch from horizontal O. nodosa to vertical shafts. The shift in orientation being a response as energy levels increased (Howard & Frey, Citation1975).
The peculiar Ophiomorpha with inner tubes were found within storm layers in the lower shoreface environment where they coexist with Bichordites and Macaronichnus, but are located in the upper part of the layer.
We speculate that the occurrence of the vertical Ophiomorpha shafts with inner tubes found in the storm beds is related to the need to provide additional possibilities for the juveniles after the storms had temporarily destroyed the original opening of the O. nodosa maze system.
Conclusions
Based on detailed field observation on the morphology of the different types of Ophiomorpha, and on thin section and XRD analysis of tube walls and tube fill materials, several facts can be established:
| • | The presence of pellets in the wall of the inner tube effectively rules out a diagenetic origin of the inner tube. | ||||
| • | The inner tubes are constructed by similar sub-pellets as the outer tubes, suggesting that both walls were constructed by similar or related organisms. | ||||
| • | The abundance of the vertical, L-shaped shafts suggest that they were constructed for a specific purpose. | ||||
Based on these facts, we propose that the vertical shafts were constructed by the adult crustaceans in order to facilitate the resettlement of juvenile shrimp returning to the sea floor after finishing their pelagic larval stages. The inner tubes therefore represent a phase of tube construction by the juveniles before they were large enough to roam the maze system together with the adult crustaceans.
Acknowledgements
Wen-Shan Chen and Chi-Yu Lee (National Taiwan University), Huei-Fen Chen (National Taiwan Ocean University) and Cai-Run Yu (Chinese Culture University) are thanked for assistance with the mineralogical evaluations. Jorge Villegas Martín and one anonymous reviewer are cordially thanked for their constructive comments.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Atkinson, R. J. A. (1988). Physiological ecology of burrowing decapods. In A. A. Fincham & P. S. Rainbow (Eds.), Aspects of decapod crustacean biology (pp. 201–226). London: Zoological Society of London.
- Bird, F. L., Boon, P. I., & Nichols, P. D. (2000). Physicochemical and microbial properties of burrows of the deposit-feeding Thalassinidean ghost shrimp Biffarius arenosus (Decapoda: Callianassidae). Estuarine, Coastal and Shelf Science, 51, 279–291. doi:10.1006/ecss.2000.0676 10.1006/ecss.2000.0676
- Bird, F. L., & Poore, G. C. B. (1999). Functional burrow morphology of Biffarius arenosus (Decapoda: Callianassidae) from southern Australia. Marine Biology, 134, 77–87. doi:10.1007/s002270050526
- Boyd, C., McIlroy, D., Herringshaw, L. G., & Leaman, M. (2012). The recognition of ophiomorpha irregulaire on the basis of pellet morphology: Restudy of material from the type locality. Ichnos, 19, 185–189. doi:10.1080/10420940.2012.734880
- Bromley, R. G. (1996). Trace fossils: Biology, taphonomy and applications. London: Chapman and Hall. 361 pp.10.1007/978-1-4899-2875-7
- Bromley, R. G., & Pedersen, G. K. (2008). Ophiomorpha irregulaire, Mesozoic trace fossil that is either well understood but rare in outcrop or poorly understood but common in core. Palaeogeography, Palaeoclimatology, Palaeoecology, 270, 295–298. doi:10.1016/j.palaeo.2008.07.017 10.1016/j.palaeo.2008.07.017
- Chen, W.-S. (2005). Characteristic trace fossils from shoreface to offshore environments of an Oligocene succession, Northeastern Taiwan. TAO, 16, 1097–1120.
- Chou, J. T. (1970). A stratigraphy and sedimentary analysis of the Miocene in northern Taiwan. Petroleum Geology of Taiwan, 7, 145–189.
- Curran, H. A. (1976). A trace fossil brood structure of probable callianassid origin. Journal of Paleontology, 50, 249–259.
- Curran, H. A. (1985). The trace fossil assemblage of a Cretaceous nearshore environment: Englishtown formation of delaware, U.S.A. In K. A. Curran (Ed.), Biogenic structures: Their use in interpreting depositional environments. Society of Economic Paleontologists and Mineralogists, Special Publication (pp. 264–276).10.2110/pec.85.35
- Curran, H. A., & Frey, R. W. (1977). Pleistocene trace fossils from North Carolina (USA), and their Holocene analogues. In T. P. Crimes & J. C. Harper (Eds.), Trace fossils (Vol. 2, pp. 139–162). Seel House Press.
- Dworschak, P. C. (1998). The role of tegumental glands in burrow construction by two mediterranean callianassid shrimp. Senkenbergiana maritima, 28, 143–149.
- Dworschak, P. C. (2004). Biology of Mediterranean and Caribbean Thalassinidea (Decapoda). In A. Tamaki (Ed.), Proceedings of the Symposium on “Ecology of large bioturbators in tidal flats and shallow sublittoral sediments – from individual behavior to their role as ecosystem engineers” (pp. 15–22). Nagasaki: Nagasaki University.
- Dworschak, P. C., Koller, H., & Abed-Navandi, D. (2006). Corallianassalongiventris and Pestarella tyrrhena (Crustacea, Thalassinidea,Callianassidae). Marine Biology, 148, 1369–1382.10.1007/s00227-005-0161-8
- Forbes, A. T. (1973). An unusual abbreviated larval life in the estuarine burrowing prawn Callianassa kraussi (Crustacea: Decapoda: Thalassinidea). Marine Biology, 22, 361–365. doi:10.1007/BF00391395
- Frey, R. W., & Howard, J. D. (1975). Endobenthic adaptations of juvenile thalassinidean shrimp. Bulletin of the Geological Society of Denmark, 24, 283–297.
- Frey, R. W., Howard, J. D., & Pryor, W. A. (1978). Ophiomorpha: Its morphologic, taxonomic, and environmental significance. Palaeogeography, Palaeoclimatology, Palaeoecology, 23, 199–229.10.1016/0031-0182(78)90094-9
- Frey, R. W., Pemberton, S. G., & Saunders, T. D. A. (1990). Ichnofacies and bathymetry: A passive relationship. Journal of Paleontology, 64, 155–158.
- Gagnon, J.-M., Beaudin, L., Silverberg, N., & Mauviel, A. (2013). Mesocosm and in situ observations of the burrowing shrimp Calocaris templemani (Decapoda: Thalassinidea) and its bioturbation activities in soft sediments of the Laurentian Trough. Marine Biology, 160, 2687–2697. doi:10.1007/s00227-013-2262-0
- Galloway, W. E. (1974). Deposition and diagenetic alteration of sandstone in Northeast Pacific Arc-Related basins: Implications for graywacke genesis. Geological Society of America Bulletin, 85, 379–390. doi:10.1130/0016-7606(1974)85<379:dadaos>2.0.co;2 10.1130/0016-7606(1974)85<379:DADAOS>2.0.CO;2
- Genise, J. F., Bedatou, E., & Melchor, R. N. (2008). Terrestrial crustacean breeding trace fossils from the Cretaceous of Patagonia (Argentina): Palaeobiological and evolutionary significance. Palaeogeography, Palaeoclimatology, Palaeoecology, 264, 128–139. doi:10.1016/j.palaeo.2008.04.004 10.1016/j.palaeo.2008.04.004
- Gibert, J. M. D., & Ekdale, A. A. (2010). Paleobiology of the crustacean trace fossil Spongeliomorpha iberica in the Miocene of Southeastern Spain. Acta Palaeontologica Polonica, 55, 733–740. doi:10.4202/app.2010.0010
- Gibert, J. M. d., Netto, R. G., Tognoli, F. M. W., & Grangeiro, M. E. (2006). Commensal worm traces and possible juvenile thalassinidean burrows associated with Ophiomorpha nodosa, Pleistocene, southern Brazil. Palaeogeography, Palaeoclimatology, Palaeoecology, 230, 70–84.10.1016/j.palaeo.2005.07.008
- Griffis, R. B., & Chavez, F. L. (1988). Effects of sediment type on burrows of Callianassa californiensis Dana and C. gigas Dana. Journal of Experimental Marine Biology and Ecology, 117, 239–253. doi:10.1016/0022-0981(88)90060-3 10.1016/0022-0981(88)90060-3
- Grow, L. (1981). Burrowing behaviour in the crayfish, Cambarus diogenes diogenes girard. Animal Behaviour, 29, 351–356. doi:10.1016/S0003-3472(81)80094-2 10.1016/S0003-3472(81)80094-2
- Ho, C. S. (1986). Introduction to the geology of Taiwan: Explanatory text of the geologic map of Taiwan (p. 163). Taipei: Central Geologic Survey, MOEA.
- Ho, C. S., Hsu, M. Y., Jen, L. S., & Fong, G. S. (1964). Geology and coal resources of the northern coastal area of Taiwan. Bulletin of the Geological Survey of Taiwan, 15, 1–23.
- Hong, E., & Wang, Y. (1988). Basin analysis of the upper Miocene-lower Pliocene series in northwestern foothills of Taiwan. Ti-Chih, 8, 1–20.
- Howard, J. D. (1978). Sedimentology and trace fossil. In P. B. Basan (Ed.), Trace fossil concepts (pp. 11–42). Oklahoma City: Sepm Short Course, SEPM.
- Howard, J. D., & Frey, R. W. (1975). Estuaries of the Georgia coast, U.S.A.: Sedimentology and biology. II. Regional animal-sediment characteristics of Georgia estuaries. Senckenbergiana Maritima, 7, 33–103.
- Huang, C. K. (1955). Gold-copper deposits in the Chinkuashih mine, Taiwan, with special reference to the mineralogy. Acta geologica Taiwanica, 7, 1–20.
- Huang, C. Y. (1990). Upper Miocene stratigraphy and the horizon of post- Lushanian fauna turnover in Northeastern Taiwan. Proceedings of the Geological Society of China, 33, 303–328.
- Huang, C. Y., Cheng, Y. M., & Huang, T. (1979). Preliminary biostratigraphic study of the Nankang Formation in the Shuinantung section, northern Taiwan. Ti-Chih (In Chinese), 2, 1–11.
- Karplus, I., Szlep, R., & Tsurnamal, M. (1974). The burrows of alpheid shrimp associated with gobiid fish in the northern Red Sea. Marine Biology, 24, 259–268. doi:10.1007/BF00391901
- Kornienko, E. S. (2013). Burrowing shrimp of the infraorders Gebiidea and Axiidea (Crustacea: Decapoda). Russian Journal of Marine Biology, 39, 1–14. doi:10.1134/S1063074013010033
- Lewy, Z., & Goldring, R. (2006). Campanian crustacean burrow system from Israel with brood and nursery chambers representing communal organization. Palaeontology, 49, 133–140. doi:10.1111/j.1475-4983.2005.00532.x
- Lopez-Cabrera, M. I., & Olivero, E. B. (2014). Ophiomorpha irregulaire and associated trace fossils from the upper cretaceous of Patagonia, Argentina: Palaeogeographical and ethologicalsignificance. Spanish Journal of Palaeontology, 29, 33–44.
- Löwemark, L. (2015). Evidence for targeted elasmobranch predation on thalassinidean shrimp in the Miocene Taliao sandstone formation, NE Taiwan. Lethaia, 48, 227–234. doi:10.1111/let.12101
- Löwemark, L., & Nara, M. (2013). Morphological variability of the trace fossil Schaubcylindrichnus coronus as a response to environmental forcing. 1; Palaeontologica electronica, 16, 1; 5A 14p.
- MacEachern, J. A., & Bann, K. L. (2008) The role of ichnology in refining shallow marine facies models. In G. Hampson, R. Steel, P. Burgress, & R. Dalrymple (Eds.), Recent advances in models of siliciclastic shallow-marine stratigraphy (pp. 73–116). SEPM Special Publication.
- McConchie, D. M., Ward, J. B., Mccann, V. H., & Lewis, D. W. (1979). A Mössbauer investigation of glauconite and its geological significance. Clays and Clay Minerals, 27, 339–348.10.1346/CCMN
- Miller, M. F., & Curran, H. A. (2001). Behavioral plasticity of modern and Cenozoic burrowing thalassinidean shrimp. Palaeogeography, Palaeoclimatology, Palaeoecology, 166, 219–236.10.1016/S0031-0182(00)00210-8
- Miller, M. F., Curran, H. A., & Martino, R. L. (1998). Ophiomorpha nodosa in estuarine sands of the lower Miocene Calvert formation at the Pollack Farm Site, Delaware. In B. R. N. (Ed.), Geology and paleontology of the lower Miocene Pollack Farm Fossil Site, Delaware (pp. 41–46). Delaware: Delaware Geological Survey.
- Pemberton, G. S., Spila, M., Pulham, A. J., Saunders, T., MacEachern, J. A., Robbins, D., & Sinclair, I. K. (2001). Ichnology and sedimentology of shallow to marginal marine systems: Ben Nevis & Avalon Reservoirs, Jeanne d’Arc Basin, Short Course Notes 15. St. John’s: Geological Association of Canada, 343 pp.
- Pemberton, S. G. (Ed.). (1992). Applications of ichnology to petroleum exploration. Calgary: SEPM – Society for Sedimentary Geology.
- Pemberton, S. G., & MacEachern, J. A. (1995). The ichnological signature of storm deposits: The use of trace fossils in event stratigraphy. In C. E. Brett (Ed.), Paleontological event horizons – Ecological and evolutionary implications (pp. 73–109). New York, NY: Columbia University Press.
- Pemberton, S. G., MacEachern, J. A., Dashtgard, S. E., Bann, K. L., Gingras, M. K., & Zonneveld, J.-P. (2012). Chapter 19 – Shorefaces. In K. Dirk & G. B. Richard (Eds.), Developments in sedimentology (pp. 563–603). Amsterdam: Elsevier.
- Pemberton, S. G., MacEachern, J. A., & Ranger, M. J. (1992). Ichnology and event stratigraphy: The use of trace fossils in recognizing tempestites. In S. G. Pemberton (Ed.), Application of Ichnology to Petroleum Exploration. A Core Workshop. SEPM Core Workshop Notes (pp. 85–117). Calgary.10.2110/cor.92.17
- Pemberton, S. G. F., & Robert, W. (1984). Quantitative methods in ichnology: Spatial distribution among populations. Lethaia, 17, 33–49.10.1111/let.1984.17.issue-1
- Pollard, J. E., Goldring, R., & Buck, S. G. (1993). Ichnofabrics containing Ophiomorpha: significance in shallow-water facies interpretation. Journal of the Geological Society, London, 150, 149–164.10.1144/gsjgs.150.1.0149
- Reinson, G. E. (1984). Barrier-island and associated strand-plain systems. In R. G. Walker (Ed.), Facies models (pp. 119–140). Geoscience Canada, Reprint Series 1. Geological Society of Canada, St. John’s.
- Riahi, S., Uchman, A., Stow, D., Soussi, M., & Ben Ismail Lattrache, K. (2014). Deep-sea trace fossils of the Oligocene–Miocene Numidian Formation, northern Tunisia. Palaeogeography, Palaeoclimatology, Palaeoecology, 414, 155–177. doi:10.1016/j.palaeo.2014.08.010 10.1016/j.palaeo.2014.08.010
- Scheibling, R., Evans, T., Mulvay, P., Lebel, T., Williamson, D., & Holland, S. (1990). Commensalism between an epizoic limpet, Patelloida nigrosulcata, and its gastropod hosts, Haliotis roei and Patella laticostata, on intertidal platforms off Perth, Western Australia. Marine and Freshwater Research, 41, 647–655. doi:10.1071/MF9900647 10.1071/MF9900647
- Seilacher, A. (2007). Trace fossil analysis. Berlin: Springer.
- Shimoda, K., & Tamaki, A. (2004). Burrow morphology of the ghost shrimp Nihonotrypaea petalura (Decapoda: Thalassinidea: Callianassidae) from western Kyushu, Japan. Marine Biology, 144, 723–734. doi:10.1007/s00227-003-1237-y
- Shyu, J. B. H., Sieh, K., Chen, Y.-G., & Liu, C.-S. (2005). Neotectonic architecture of Taiwan and its implications for future large earthquakes. Journal of Geophysical Research: Solid Earth, 110, B08402. doi:10.1029/2004JB003251
- Stamhuis, E. J., Reede-Dekker, T., van Etten, Y., de Wiljes, J. J., & Videler, J. J. (1996). Behaviour and time allocation of the burrowing shrimp Callianassa subterranea (Decapoda, Thalassinidea). Journal of Experimental Marine Biology and Ecology, 204, 225–239. doi:10.1016/0022-0981(96)02587-7
- Suppe, J. (1984). Kinematics of arc-continent collision, flipping of subduction, and back-arc spreading near Taiwan. Memoir of the Geological Society of China, 6, 21–33.
- Suter, P. (1977). The biology of two species of Engaeus (Decapoda: Parastacidae) in Tasmania. II. Life history and larval development, with particular reference to E. cisternarius. Marine and Freshwater Research, 28, 85–93. doi:10.1071/MF9770085 10.1071/MF9770085
- Swinbanks, D. D., & Luternauer, J. L. (1987). Burrow distribution of Thalassinidean Shrimp on a Fraser Delta Tidal Flat, British Columbia. Journal of Paleontology, 61, 315–332. doi:10.2307/1305325
- Tamaki, A., Ikebe, K., Muramatsu, K., & Ingole, B. (1992). Utilizatiqn of adult burrows by juveniles of the ghost shrimp, Calllanassa poalica Ortmann: Evidence from resin casts of burrows. Researches on Crustacea, 21, 113–120.
- Tamaki, A., Mandal, S., Agata, Y., Aoki, I., Suzuki, T., Kanehara, H., Aoshima, T., Fukuda, Y., Tsukamoto, H., & Yanagi, T. (2010). Complex vertical migration of larvae of the ghost shrimp, Nihonotrypaea harmandi, in inner shelf waters of western Kyushu, Japan. Estuarine, Coastal and Shelf Science, 86, 125–136. doi:10.1016/j.ecss.2009.11.005 10.1016/j.ecss.2009.11.005
- Tamaki, A., Saitoh, Y., Itoh, J.-I., Hongo, Y., Sen-ju, S.-S., Takeuchi, S., & Ohashi, S. (2013). Morphological character changes through decapodid-stage larva and juveniles in the ghost shrimp Nihonotrypaea harmandi from western Kyushu, Japan: Clues for inferring pre- and post-settlement states and processes. Journal of Experimental Marine Biology and Ecology, 443, 90–113. doi:10.1016/j.jembe.2013.02.038 10.1016/j.jembe.2013.02.038
- Teng, L. S., Wang, Y., Tang, C. H., Huang, T. C., Yu, M. S., & Ke, A. (1991). Tectonic aspects of the Paleogene depositional basin of northern Taiwan. Proceedings of the Geological Society of China, 34, 313–316.
- Ter, P. C., & Buckeridge, J. S. J. S. (2012). Ophiomorpha beaumarisensis isp. nov., a trace fossil from the late Neogene Beaumaris Sandstone is the burrow of a thalassinidean lobster. Proceedings of the Royal Society of Victoria, 124, 223–231.
- Uchman, A. (2009). The Ophiomorpha rudis ichnosubfacies of the Nereites ichnofacies: Characteristics and constraints. Palaeogeography, Palaeoclimatology, Palaeoecology, 276, 107–119. doi:10.1016/j.palaeo.2009.03.003 10.1016/j.palaeo.2009.03.003
- Verde, M., & Martínez, S. (2004). A new ichnogenus for crustacean trace fossils from the upper Miocene Camacho formation of Uruguay. Palaeontology, 47, 39–49. doi:10.1111/j.0031-0239.2004.00346.x
- Wang, Y. (1953). Geology of the Chinkuashih and Chiufen districts, Taipei hsien, Taiwan. Acta geologica Taiwanica, 5, 47–67.
- Weber, K. A., Spanbauer, T. L., Wacey, D., Kilburn, M. R., Loope, D. B., & Kettler, R. M. (2012). Biosignatures link microorganisms to iron mineralization in a paleoaquifer. Geology, 40, 747–750. doi:10.1130/g33062.1
- Yen, T. P., & Chen, P. Y. (1953). Explanatory text of the geologic map of Taiwan, Tatunshan Sheet (9 pp). Taipei:Geological Survey of Taiwan.
- Yu, N. T. (1997). Sequence stratigraphy of the middle to upper Miocene strata, northern Taiwan: A preliminary study. Proceedings of the Geological Society of China, 40, 685–707.
- Yu, N. T., & Teng, L. S. (1999). Depositional environments of the Taliao and Shihti Formations, northern Taiwan. Bulletin of the Central Geological Survey, Taiwan, 12, 99–132.
- Yu, N. T., & Teng, S. L. (1996). Facies characteristics and depositional cycles of middle and upper Miocene strata of the Western Foothills, northern Taiwan. Ti-Chih, 15, 29–60.