Abstract
Diplocraterion, a U-shaped burrow attributed to infaunal invertebrates, is normally a shallow-marine trace fossil and not part of a continental vertebrate ichnoassemblage. Hence, the Glen Rose Formation (Aptian–Albian) of Texas (USA) presents an opportunity to study Diplocraterion associated with a world-class dinosaur tracksite. Most Diplocraterion are in a bioclastic wackestone–packstone bed just above the Taylor Tracklayer, a significant dinosaur track horizon. Diplocraterion are consistently sized, but with variable depths; most have protrusive spreiten and northeast–southwest trends. Smaller Arenicolites co-occur with Diplocraterion, and other trace fossils include Rhizocorallium and a large theropod trackway. Based on our analysis, a sea-level rise buried the Taylor Tracklayer, with a shallow-marine carbonate mud colonised by Diplocraterion and Arenicolites tracemakers. Protrusive Diplocraterion, eroded burrow tops, Rhizocorallium, and other criteria point towards firming and net erosion of the bed caused by a stillstand. The depositional environment of the Diplocraterion bed was possibly a subtidal lagoon that covered shoreward sediments impacted by large theropods. Burrow orientations suggest bidirectional currents consistent with trends of theropod trackways, implying each were controlled by a shoreline. The results of our study demonstrate how marine invertebrate and continental vertebrate trace fossils can be used together to define fine-scale changes in former carbonate shorelines.
1. Introduction
The Glen Rose Formation (Lower Cretaceous: Aptian–Albian) of east central Texas is ichnologically world famous for its abundant and exquisitely preserved theropod and sauropod tracks, particularly where the formation crops out in and near Dinosaur Valley State Park (Bird, Citation1985; Dattilo et al., Citation2014; Farlow, Citation1993, Citation2001; Farlow et al., Citation2012). In contrast, the invertebrate trace fossils of this marginal marine sequence of limestones and mudstones are less known; instead, researchers focused on its lithofacies and vertebrate trace fossils (Bird, Citation1985; Farlow, Citation1993; Farlow, Pittman, & Hawthorne, Citation1989; Jasinski, Citation2009; Kuban, Citation1989a, 1989b; Meyer & Pittman, Citation1994; Shuler, Citation1917).
Fortunately, a recent stratigraphic analysis of the Glen Rose Formation in and around Dinosaur Valley State Park provided a preliminary accounting of its invertebrate trace fossils and their stratigraphic positions (Dattilo et al., Citation2014). Of these trace fossils, potentially the most useful for interpreting depositional environments is Diplocraterion, a U-shaped, tubular, and vertically oriented burrow with spreiten. This ichnogenus, which has been reported from continental and marginal marine sediments ranging from the Cambrian through the Neogene, is also commonly applied to sedimentological analyses, particularly in marginal marine facies (Cornish, Citation1986; Fürsich, Citation1974a, Citation1975; Goldring, Citation1962, 1964; Mason & Christie, Citation1986; Oloriz & Rodriguez-Tovar, Citation2000; Rodríguez-Tovar & Pérez-Valera, Citation2013; Seilacher, Citation2007). In the Glen Rose Formation, a bioclastic wackestone–packstone bed-bearing abundant Diplocraterion, first described by Nagle (Citation1968), crops out at several places in and around Dinosaur Valley State Park. It is also located <.5 m above the Taylor Tracklayer, one of the best dinosaur track horizons in the Glen Rose Formation (Bird, Citation1985; Dattilo et al., Citation2014; Farlow et al., Citation2012; Kuban, Citation1989a, 1989b).
In our study, we confirmed that Diplocraterion and associated trace fossils (Arenicolites, Rhizocorallium) in this bed provide information pertinent to sedimentation rates and sea-level fluctuations during and just after formation of the Taylor Tracklayer. The Diplocraterion bed thus serves as an example of how marginal marine invertebrate trace fossils can be applied to better understand paleoenvironmental settings of nearshore continental vertebrate trace fossils.
2. Study area, stratigraphy
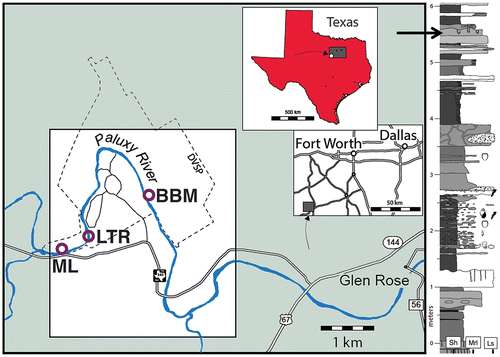
The Glen Rose Formation in the study area crops out mostly along the Paluxy River valley in and around Dinosaur Valley State Park, west of Glen Rose, Texas (USA) (Figure (a)). The formation varies from Aptian–Albian throughout its outcrop area, but is Albian in the study area (Dattilo et al., Citation2014; Farlow et al., Citation2012; Hawthorne, Citation1990; Nagle, Citation1968). Outcrops in the area of Dinosaur Valley State Park are from the Lower Member of the Glen Rose Formation (Dattilo et al., Citation2014). The Glen Rose Formation is best known for its abundant and well-preserved sauropod and theropod tracks, although its invertebrate trace fossils have also received some attention in a few recent studies (Dattilo et al., Citation2014; Farlow et al., Citation2012). Depositional environments of the Glen Rose Formation were supratidal-subtidal, with a mixture of carbonate–clastic systems that produced a varied sequence of limestones, marls, and shales (Dattilo et al., Citation2014; Hawthorne, Citation1990; Nagle, Citation1968). During the Albian, paleotemperatures of this region were considerably higher than today (White, González, Ludvigson, & Poulsen, Citation2001), and the paleolatitude was at about 25° (Ludvidson et al., Citation2004; Ufnar, Ludvigson, González, & Davis, Citation2005); hence, the overall paleoclimate was tropical–subtropical.
Figure 1. Locations and stratigraphic setting of the Diplocraterion Bed in the Glen Rose Formation. (a) Selected area of Dinosaur Valley State Park (denoted by dashed boundary) and Paluxy river outcrops within the context of Texas (USA), with LowT/Riverbend Cliff site (LTR), McFall Ledge (ML), and Buckeye Branch Mouth (BBM) outcrops indicated. (b) Glen Rose Formation stratigraphy in the study area, with Diplocraterion bed (arrow) indicated; stratigraphic profile based on relative amounts of shale (Sh), marly limestone (Mrl), and limestone (Ls).
Note: Figure adapted from Dattilo et al. (Citation2014); see the same for details on Glen Rose Formation stratigraphy.
Diplocraterion in the study area is concentrated in a laterally persistent and easily identified bioclastic wackestone–packstone bed (Figure (b)). As one of the better-cemented limestones in the 6-m-thick section exposed in the study area, it crops out prominently in streambed and riverbank exposures over more than 15 km2 in the study area. Thus, the physical appearance and trace fossils of this bed make it a useful biostratigraphic marker for mapping the lower member of the Glen Rose Formation in and around Dinosaur Valley State Park (Dattilo et al., Citation2014; Farlow et al., Citation2012; Hawthorne, Citation1990; Nagle, Citation1968). Other trace fossils co-occurring with Diplocraterion in this bed are Arenicolites, Rhizocorallium, and, at one locality, a large theropod trackway. The Diplocraterion bed is consistently less than 50 cm above one of the most significant dinosaur track horizons in the vicinity of Dinosaur Valley State Park, the Taylor Tracklayer. The Taylor Tracklayer contains numerous trackways of large theropods and has historical value for inspiring misguided evangelism around so-called man tracks amidst the dinosaur tracks (Farlow, Citation1993; Kuban, Citation1989a, 1989b).
Three localities with excellent exposures of the Diplocraterion bed were used for this study: the LowT/Riverbend Cliff, McFall Ledge, and Buckeye Branch Mouth sites (Figure (a)). The LowT/Riverbend Cliff and McFall Ledge sites provided bedding-plane views of the bed top. The Buckeye Branch Mouth site had continuous vertical expressions of the bed in a riverside outcrop, as well as large float blocks offering bedding-plane perspectives. Given sufficient specimens from these three localities, we characterised morphological and preservational variations of Diplocraterion in the area, as well as documented its co-occurrence with other trace fossils. This information lent to specific interpretations of paleoenvironmental conditions following formation of the Taylor Tracklayer.
3. Methods
The stratigraphic position of the Diplocraterion bed with relation to the Taylor Tracklayer was documented by two of us (BD and SH); Dattilo et al. (Citation2014) reported the main results of that survey. Two of us (AJM and MB) identified and measured burrows in the field at the designated three localities. One of us (JOF) had previously taken measurements of theropod trackway orientations in the Main Tracklayer, as well as for one trackway on top of the Diplocraterion bed at McFall Ledge.
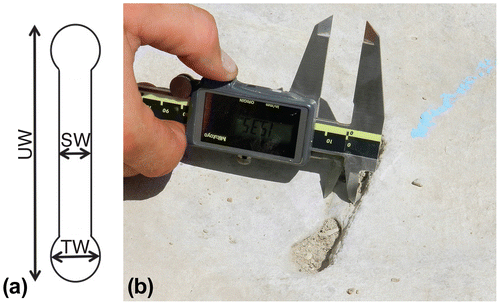
Size measurements of Diplocraterion and Arenicolites were taken with Mitutoyo digital calipers and only from in situ burrows. Measurements of Diplocraterion expressed on bedding planes were burrow U-width, burrow-tube width, and spreiten width; these were measured inside the minimum outlines of these features (Figure ). For vertical sections of Diplocraterion, burrow length (depth) was measured, along with burrow U-width and burrow-tube width. In instances where original tubes of burrows were not preserved or otherwise clearly defined, burrow-tube width was measured on distal ends of each burrow. A Silva compass was used to determine strikes of Diplocraterion tops, defined by linear trends of spreite on bedding planes. These measurements were later corrected for a magnetic declination of 4° in the study area. To avoid data duplication, each burrow was marked with blue chalk after taking measurements. Diplocraterion orientations were analysed and plotted in rose diagrams using PAST (PAleontological STatisics) software (Versions 3.04 and 2.17), which was originally developed by Hammer, Harper, and Ryan (Citation2001). All Diplocraterion and Arenicolites specimens were measured in the field to the nearest .1 mm, whereas the few specimens of Rhizocorallium reported from Buckeye Branch Mouth site were measured from photographs (with scale) to the nearest millimetre. Burrow measurements and orientations were entered in Excel spreadsheets and analysed using basic descriptive statistics, and data were summarised in histograms. A detailed description of the statistics applied to the Diplocraterion and dinosaur trackway orientations is in Appendix 1.
4. Diplocraterion and related trace fossils in the Glen Rose Formation
4.1. Ichnotaxonomic distinctions
The three invertebrate ichnogenera identified in the Diplocraterion bed are Diplocraterion, Arenicolites, and Rhizocorallium, which are all U-shaped burrows. However, each ichnogenus had distinctive morphological traits that enabled identification in the field. Ichnospecies were identified when possible, although detailed ichnotaxonomy was not the primary focus of our study.
Diplocraterion Torell, 1870, is a vertical, U-shaped tubular burrow oriented perpendicular to bedding and with spreiten (Fürsich, Citation1974a; Seilacher, Citation2007). Burrow openings may be wider, narrower, or the same width as the main part of the burrow. Spreiten can be either below the main burrow or above and inside the ‘U’ turn of the burrow. Spreiten below the main burrow indicate it is retrusive, representing where the tracemaker moved the burrow upward in response to increased sedimentation (Bromley, Citation1996; Goldring, Citation1962, 1964; Seilacher, Citation2007). In contrast, spreiten located above the main burrow are protrusive, indicating downward movement of the tracemaker, a behaviour likely triggered by erosion of the sediment surface above the burrow (Bromley, Citation1996; Goldring, Citation1962, 1964; Seilacher, Citation2007). Such vertical adjustments of burrow depth probably ensured optimum feeding depths for the burrow dweller or accommodated tracemaker growth (Bromley, Citation1996; Bromley & Hanken, Citation1991; Šimo & Olšavskỳ, Citation2007). Hence, Diplocraterion is considered a dwelling, feeding, and equilibrium trace in which its tracemakers attempted to maintain an ideal burrow depth in its host sediment.
Ichnospecies of Diplocraterion (e.g. D. parallelum, D. polyupsilon, D. habichi) are based on variations in U-width or tube width along the length of the burrow, or other such morphological details (Bromley, Citation1996). Individual specimens of Diplocraterion can also include both retrusive and protrusive spreiten from combinations of up-and-down movement, lending to the informal nickname ‘Diplocraterion yoyo’ (Goldring, Citation1962, 1964, 1971). However, ichnospecies names should not be applied to bedding-plane exposures of Diplocraterion, as these do not reveal sufficient information about their subsurface form.
Arenicolites Salter, 1866, lacks spreiten, but otherwise has the same salient traits of Diplocraterion: tubular, U-shaped, vertical, and oriented perpendicular to bedding. Accordingly, Diplocraterion is distinguished from Arenicolites in vertical section based on the presence or absence of a spreite, respectively. On bedding planes, Diplocraterion often differs from Arenicolites by having a central, indented linear zone connecting the two burrow openings. This feature is a result of sediment partially collapsing from the underlying spreite, or from different fill (i.e. active in the spreite vs. passive in the tube), and hence imparting differential compaction and weathering. Arenicolites intersecting bed tops normally lack this trait, but it also may have been imparted by sediment compacting between limbs of a U-burrow. Nonetheless, Arenicolites is still distinguishable as consistently paired and identical width holes on bedding-plane surfaces that are not joined by a central zone towards their tops.
Rhizocorallium Zenker, 1836, is virtually identical to Diplocraterion, with a U-shaped tube (burrow) and spreiten, but is oriented oblique or horizontal with respect to bedding (Knaust, Citation2013; Seilacher, Citation2007). Moreover, its spreiten are normally protrusive (Seilacher, Citation2007), although retrusive examples are known (Knaust, Citation2013). Rhizocorallium also may vary from Diplocraterion in its substrate setting, as this ichnogenus, and R. jenense specifically, is normally associated with firmgrounds (i.e. the Glossifungites ichnofacies), rather than soft-ground infaunal communities (Bromley, Citation1996; Knaust, Citation2013; Oloriz & Rodriguez-Tovar, Citation2000; Pazos et al., Citation2012). Furthermore, Diplocraterion and Arenicolites are usually interpreted as the dwelling burrows of infaunal suspension feeders, whereas Rhizocorallium can be regarded as either a suspension-feeding or deposit-feeding burrow (Dam, Citation1990; Rodríguez-Tovar & Pérez-Valera, Citation2008; Knaust, Citation2013).
Eubrontes Hitchcock, 1845, is an ichnogenus commonly applied to large tridactyl theropod tracks from the Early Jurassic through the Early Cretaceous (Lockley, Citation2009; Olsen et al., Citation1998). This ichnogenus was applied originally by Shuler (Citation1917) to the Glen Rose theropod tracks, and the ichnospecies most often assigned to large theropod tracks in the Glen Rose Formation is Eubrontes glenrosensis Shuler 1935, based on the type specimen in the bandstand of downtown Glen Rose, Texas (Adams et al., Citation2010; Farlow, Citation1993). In this study, we will use Shuler’s (Citation1917) naming of the Glen Rose theropod tracks, but without necessarily endorsing it.
In the study area and the examined bed, Diplocraterion and Arenicolites were expressed in both bedding-plane (cross-sectional) views at all three localities (LowT/Riverbend Cliff, McFall Ledge, and Buckeye Branch Mouth), but longitudinal (vertical) views of these trace fossils were only seen at Buckeye Branch Mouth. Moreover, Rhizocorallium was only observed in vertical section at Buckeye Branch Mouth, and Eubrontes occurs solely at McFall Ledge.
4.2. LowT/Riverbend Cliff site
Diplocraterion and Arenicolites are abundantly represented in bedding-plane exposures of the Diplocraterion bed at the LowT/Riverbend Cliff site (Figure ). This locality is also well known for its theropod trackways in the Taylor Tracklayer, which crops out in the main channel of the Paluxy River. However, it was submerged at the time of our study. The base of the Diplocraterion bed is only about 30 cm above the Taylor Tracklayer there, with the beds separated by a siltstone. The Diplocraterion bed crops out along east and west banks of the river, and all observed U-burrows were in top surfaces of the Diplocraterion bed. During our study, some surfaces were emergent, whereas others were 15–20 cm underwater. Owing to this logistical challenge, burrow widths, U-widths, and spreite widths, and burrow orientations were measured only on emergent bedding planes. However, we were able to measure a large sample of orientations on submerged examples of Diplocraterion by holding a compass just above the water level.
Figure 3. Diplocraterion at the LowT/Riverbend Cliff site. (a) Oblique view of bedding-plane exposure, showing abundance and distribution of burrows. Scale = 20 cm long. (b) Overhead view of bedding plane with relative density of burrows, with both Diplocraterion (Di) and Arenicolites (Ar). Scale = 20 cm long. (c) Burrows on bedding plane in various preservational states, including Arenicolites (Ar) and Diplocraterion (Di). Scale = 15 cm long. (d) Close-up of Ar, Di; note pelleted exterior of Diplocraterion. Scale in centimetres.
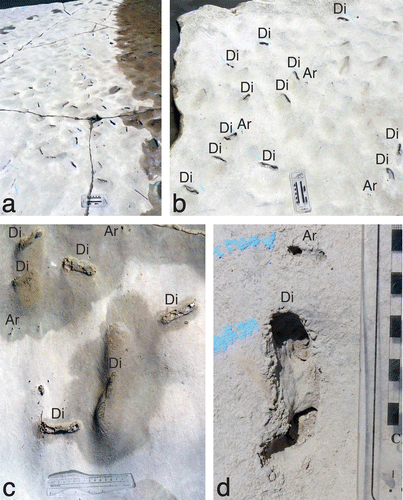
At this site, most specimens of Diplocraterion were evident as negative-relief endichnia, with paired open tubes connected by a straight, thinner zone of shallowly collapsed spreiten. Burrows showed relatively little variation in size parameters, with mean tube widths of 12.5 ± 1.7 mm, U-widths of 61.3 ± 9.6 mm, and spreiten widths of 10.7 ± 1.8 mm (n = 92; Figure (a)–(c), Table (a)). The smallest measured specimen had a tube width of 9.0 mm and U-width of 43.2 mm, whereas the largest was nearly twice as big, with a tube width of 17.4 mm and U-burrow of 84.5 mm. The mean U-width:tube width ratio for measured burrows was 5.0 (n = 92). In this same sample, 89% of burrows had straight outlines, whereas 11% were curved. Moreover, 15% (14 of 92) of Diplocraterion lacked open tubes and were only evident as shallow linear concavities. These likely represented the lowermost part of the original U-burrow, discussed later. A few burrows had 10–20 mm wide and 5–10 mm tall pelletal rims along their outer edges (Figure (d)), but most edges were smooth and nearly flush with the bedding plane.
Figure 4. Histograms and rose diagram of quantitative data for Diplocraterion at LowT/Riverbend Cliff site. (a) U-burrow widths; (b) burrow-tube widths; (c) spreiten widths; (d) orientations.
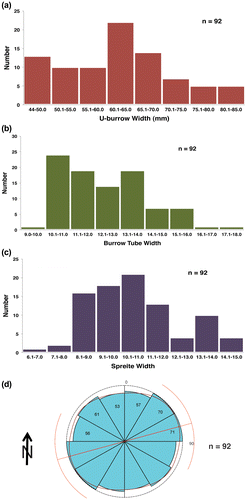
Table 1. Descriptive statistics of (a) Diplocraterion, (b) Arenicolites, and (c) Diplocraterion orientations at LowT/Riverbend site.
Arenicolites was more variable than Diplocraterion in its size parameters, with a mean tube width of 4.3 ± 1.6 mm and U-widths of 19.3 ± 4.8 mm (n = 46; Table (b)). Size ranges of Diplocraterion and Arenicolites were entirely separate from one another, as the biggest Arenicolites (8.0 mm tube width and 31.4 mm U-width) was less than the smallest Diplocraterion. A few specimens of Arenicolites had a central collapsed zone connecting the tubes, but these were poorly defined compared to those in Diplocraterion. Most Arenicolites were evident as proximally paired and equally sized holes with no deformation evident in the area between limbs.
Burrow orientations for Diplocraterion, taken from both emergent and submerged surfaces, could be broadly categorised as 54% northeast–southwest (NE–SW) quadrant and 46% northwest–southeast (NW–SE) (n = 368; Table (c)). The largest grouping of orientations is NE–SW (31–60°) at 20.1% of the total, and the second largest is NE–E to SW–W (61–90°) at 18.5% of the total (Figure (d)).
Although we did not attempt a thorough spatial analysis of distances between burrows on the bedding plane (e.g. nearest-neighbour analysis, sensu Pemberton & Frey, Citation1984), no Diplocraterion and Arenicolites were observed overlapping or otherwise cutting across other burrows. In one sample of the bedding plane for burrow density, we found 14 burrows/m2. Ends of separate burrows were as close as 2–3 cm from one another in places, but otherwise separated by more than a burrow width.
4.3. McFall Ledge Site
The Diplocraterion bed at the McFall Ledge site has a limited outcrop area compared to the LowT/Riverbend Cliff site. Accordingly, Diplocraterion and Arenicolites were less abundant in bedding-plane exposures there (Figure ). The bed crops out about 3 m above the river level on its southern bank and is the stratigraphically highest bed of the Glen Rose Formation there, with the Taylor Tracklayer about 30 cm below it. All observed U-shaped burrows were on top surfaces of the bed. However, the bed at this site differs from all other known exposures by having a large theropod trackway (Eubrontes isp.) on its upper surface, which also intersects several Diplocraterion.
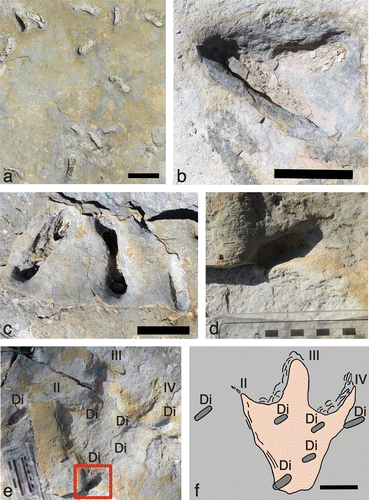
Figure 5. Diplocraterion at the McFall Ledge site. (a) Overhead view of bedding-plane with relative density and distribution of Diplocraterion, also in various preservational states. Scale = 10 cm. (b) Overlapping Diplocraterion. Scale = 5 cm. (c) Closely associated and similarly aligned Diplocraterion, but ranging from nearly complete (left and centre) to only the bottommost portion of the original ‘U’ (right). Scale = 5 cm. (d) Lower part of ‘U’ from Diplocraterion intersected by proximal left margin of theropod track (Eubrontes isp.). (e) Diplocraterion (Di) associated with right theropod track (Eubrontes isp.), with Diplocraterion depicted in (d) outlined and digits II-IV (II-IV) on track indicated. Scale (left) in centimetres. (e) Map of Diplocraterion (Di) intersected by, within, and near theropod track; pressure-release structures on left sides of digits III and IV. Scale = 10 cm.
Nearly all specimens of Diplocraterion were evident as negative-relief endichnia and paired open tubes, although tubes were absent from a few specimens. Spreiten were expressed as shallow, linear depressions between paired tubes. Despite a smaller sample size, burrow size parameters were nearly identical to those noted at the LowT/Riverbend Cliff site, with a mean tube width of 12.6 ± 1.5 mm, U-width of 60.9 ± 8.9 mm, and spreiten width of 11.2 ± 1.4 mm (n = 28; Figure (a)–(c); Table (a)). The smallest measured specimen had a tube width of 8.7 mm and U-width of 40.4 mm; the largest tube width was 15.8 mm and widest U-burrow of 74.8 mm, which were close to minimum–maximum values observed at LowT/Riverbend Cliff. The mean U-width:tube width ratio for measured specimens was also 5.0 (n = 28). About 79% of burrows (22 of 28) had straight outlines on bedding planes, whereas 21% had spreite curving between burrow limbs. Approximately 36% of Diplocraterion (10 of 28) lacked open tubes and only showed a shallow linear concavity.
Figure 6. Histograms and rose diagram of quantitative data for Diplocraterion at McFall Ledge site. (a) U-burrow widths; (b) burrow-tube widths; (c) spreiten widths; (d) orientations.
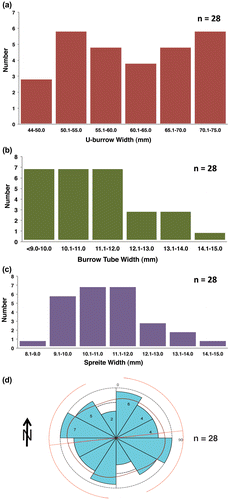
Table 2. Descriptive statistics of (a) Diplocraterion, (b) Arenicolites, and (c) Diplocraterion orientations at McFall Ledge site.
Owing to its comparatively limited outcrop area, Arenicolites was not nearly as abundant at McFall Ledge as at the LowT/Riverbend site, with only four specimens observed. The largest had a U-width of 22.7 mm and tube width of 6.0 mm, the smallest had a U-width of 15.6 mm and tube width of 4.4 mm, and the other two were between these (Table (b)). Size ranges of Arenicolites and Diplocraterion were again separate, as the largest Arenicolites (burrow width 6.0 mm and U-width 22.7 mm) was less than the smallest Diplocraterion (8.7 mm and 40.4 mm, respectively).
Burrow orientations for Diplocraterion differed slightly from those measured at LowT/Riverbend Cliff site, with 50% NE–SW and 50% NW–SE (n = 28; Table (c); Figure (d)). However, the number of measurements at this locality was more than an order of magnitude smaller than at the LowT/Riverbend Cliff site; hence, this sample may not be useful for comparison.
As at the LowT/Riverbend site, we did not apply spatial analysis of burrows. However, nearly all burrows were separate from one another, in most instances spaced more than a burrow U-width apart. The only exceptions to this generality were two instances of interconnecting Diplocraterion, which shared a burrow tube (Figure (b)) and a grouping of three closely spaced burrows (Figure (c)). One sample of the bedding plane had a burrow density more than twice that of the LowT/Riverbend site, at 31 burrows/m2. However, the smaller exposure at this site means any relating of its burrow density to that of the LowT/Riverbend site must be applied cautiously.
This was the only exposure of the Diplocraterion bed that also had dinosaur tracks as part of its ichnoassemblage. These tracks were represented by one theropod trackway (Eubrontes isp.) on the top surface of the bed. The trackway originally had four tracks in succession (left-right–left-right), although the third in the sequence was stolen, leaving a gap in the trackway. The remaining tracks are tridactyl, preserved as natural depressions (negative-relief epichnia) and are 40–41 cm long and 28 cm wide. Trackway width is relatively narrow: 40 cm, or about 1.5 times track width. Pace from the first to second track is 124 cm, and stride – taken from the second and fourth track (both right feet) – is 253 cm. The trackway orientation is 131° or to the southeast. A large bipedal theropod, such as an allosaurid, is the most likely tracemaker for these tracks (Dattilo et al., Citation2014; Farlow, Citation1993; Farlow et al., Citation2012). Like most dinosaur tracks in the Glen Rose Formation, these are easily distinguishable as vertebrate trace fossils. Nonetheless, other than narrow (sharp) clawmarks, these tracks mostly lack anatomical details, such as digital pads and skin impressions.
The most important track in this sequence is the second, a right footprint that overlaps with the surface expression of five Diplocraterion, with another nearby on the same bedding plane (Figure (d)–(f)). In contrast, the other two tracks lacked any Diplocraterion within or along their margins. For the track that co-occurs with Diplocraterion, two burrows are intersected by the track margin and three are entirely within the track. One of the intersected specimens straddles the lower left edge of the track – at the proximal end of digit II – whereas the other Diplocraterion is to the right, along the distal end of digit IV. The burrow on the lower left side of the track is 20–25 mm deep, with a U-width of 54 mm and burrow width of 18 mm wide; its original tubes are absent. The part of the burrow inside the track margin is a few millimetres shallower than outside of the track (Figure (d)). The intersected burrow on the upper right side of the track is slightly shallower (10–15 mm deep), but otherwise similarly sized, with a U-width of 51 mm and burrow width of 18 mm. This burrow also lacks defined tube openings.
Of the other Diplocraterion specimens located inside the track, one is at the distal intersection of digits II and III (left), another is at the intersection of digits III and IV (right), and a third is towards the right proximal (‘heel’) part of the track. All three burrows are shallower than the intersected burrows (5–10 mm deep) and otherwise smaller. These burrows are nearly identical in size: the upper left, upper right, and lower right burrows have U-widths of 34, 32, and 28 mm, and burrow widths of 13, 13, and 15 mm, respectively. The U-width: burrow width ratios differ noticeably from that calculated for Diplocraterion outside of the track (5.0), with values of 2.6, 2.5, and 1.9. None of the three burrows has open tubes, and instead are preserved as shallow, oval depressions. Interestingly, all five burrows associated with the track have similar orientations, ranging from 4 to 17° (N–NE), vs. the 131° orientation of the trackway.
Another Diplocraterion is about 9 cm to the left of the distal end of digit II. It is about 20–25 mm deep, has a U-width of 62 mm, a tube width of 15 mm. Similar to the five Diplocraterion specimens directly associated with the theropod track, burrow tubes are not evident, and its orientation is nearly north–south (2°). However, its U-width: burrow width ratio is 4.1 and thus closer to the norm of 5.0 for other Diplocraterion at this locality. All of the described traits of this and other burrows in and intersecting this theropod track imply that they are eroded lower parts of originally more complete Diplocraterion and perhaps were compressed by the theropod, significant points discussed more later.
4.4. Buckeye Branch Mouth site
The Diplocraterion bed at the Buckeye Branch Mouth site is the only outcrop we examined with a significant number of vertical (longitudinal) expressions of Diplocraterion. Hence, we could assess variations in burrow depths and expression of spreiten, i.e. whether these were protrusive, retrusive, or a combination of the two. The bed crops out above the river level and on the eastern bank at this site (Figure (a)). Based on 20 measurements taken along a 63-m-long exposure parallel to the Paluxy River, Diplocraterion bed thickness ranged from 17 to 31 cm, with an average of about 22 cm (Table ). The Taylor Tracklayer is about 30 cm below the Diplocraterion bed here, but notably thinner than at the LowT/Riverbend and McFall Ledge sites and is only exposed in vertical sections of the outcrop. The river bank also held large float blocks bearing Diplocraterion and Arenicolites that could be connected directly to the outcrop. These blocks provided bedding-plane expressions of their trace fossils and showed they were more densely populated here than at any other locality known in the field area (Figure (b) and (c)). For example, in one block, we calculated a density of about 450 burrows/m2. Arenicolites was not observed in vertical sections here, but was identified by its smaller, paired burrow openings on float-block bedding planes (Figure (c)). Furthermore, this site was the only one in which we observed examples of Rhizocorallium, although these trace fossils were rare compared to Diplocraterion, discussed later.
Figure 7. Diplocraterion at the Buckeye Branch Mouth site. (a) Outcrop view of Diplocraterion bed, with bed top indicated by dashed line. (b) Top bedding plane on float block with high density of Arenicolites and Diplocraterion, evident as open burrows and collapsed spreiten sandal and foot = 10 cm wide. (c) Close-up of Arenicolites (Ar) and Diplocraterion (Di) on bedding plane, with pelletal rim surrounding two Diplocraterion (right); scale = 5 cm. (d) Diplocraterion parallelum in longitudinal section, with open burrow tube and protrusive spreiten; scale = 5 cm. (e) Closely spaced Diplocraterion with differing preservation, with one specimen having only the basal part of its spreiten and ‘U’ burrow (left) and another with a more complete expression of its overall form and identifiable as D. parallelum with protrusive spreiten (right); scale = 5 cm. (f) Intersecting Diplocraterion, with one burrow (left) cross-cutting another (right); scale = 5 cm. (g) Multiple specimens of horizontally to obliquely oriented Rhizocorallium (Rh), with one specimen cross-cutting another (below) and one with open burrow tubes (above); scale = 5 cm.
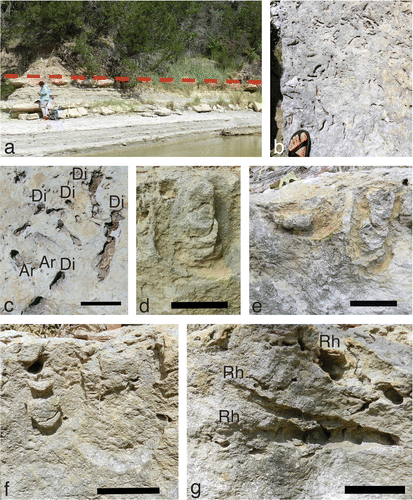
Table 3. (a) In situ thicknesses of Diplocraterion bed at Buckeye Branch Mouth, taken at 3-m intervals along 63-m length of outcrop. Measurements rounded to nearest .5 cm. (b) Descriptive statistics of bed measurements.
Specimens of Diplocraterion in longitudinal section were preserved as negative-relief endichnia with paired open tubes visible on bed tops, but also with either one or both limbs of their U-shaped tubes outlined (Figure (d)–(f)). A few Diplocraterion on bed tops of float blocks had raised sediment rims surrounding the burrows, similar to those seen at the LowT/Riverbend and McFall Ledge sites, but were better defined (Figure (c)). For in situ Diplocraterion, half of the vertical expressions of Diplocraterion had only one limb, and half had both (n = 92). Spreiten were clearly defined between each limb on most burrows. However, some specimens preserved only the lowermost bend of the original U-burrow and spreiten (Figure (e)). Diplocraterion also commonly cross-cut on another in vertical section (Figure (f)), affirming the high density of burrows noted on float-block bedding planes and implying multiple generations of burrowing.
Mean tube width of measured specimens was 11.5 ± 2.1 mm (n = 90), mean U-width was 58.3 ± 14.6 mm (n = 49), and mean burrow depth was 64.3 ± 21.6 mm (n = 92; Figure , Table ). The smallest tube width and U-width were 2.9 and 31.9 mm, respectively, which overlaps with the size range of Arenicolites at the other two localities. The largest Diplocraterion tube width and U-width were 15.4 mm and 91.8 mm, respectively; this tube width was nearly the same as that of the largest Diplocraterion at McFall Ledge site (15.8 mm), and the U-width was slightly greater than the largest specimen at LowT/Riverbend (84.5 mm). In terms of vertical dimensions, the shallowest measured specimen was 20.4 mm and the deepest was 137.5 mm; the former was represented only by the bottommost bend of spreiten from a former burrow. Diplocraterion depths accordingly ranged from 9% (20.4 mm) to 62% (137.5 mm) of average bed thickness (22 cm), and none penetrated the entire bed. The U-width: tube width ratio for measured specimens was 5.1, nearly the same as the 5.0 ratio derived from Diplocraterion at the LowT/Riverbend and McFall Ledge sites. In those Diplocraterion where burrow position relative to spreiten could be discerned, 83% were protrusive (burrow tube above the spreiten), 13% were retrusive (burrow tube below the spreiten), and 4% had spreiten both above and below the burrow tube (n = 54). The two specimens observed with both protrusive and retrusive spreiten had nearly identical depths (63.3 and 64.6 mm).
Figure 8. Histograms of quantitative data for Diplocraterion at Buckeye Branch Mouth site. (a) U-burrow widths; (b) burrow-tube widths; (c) U-burrow depths.
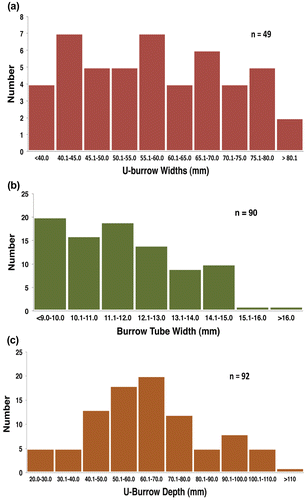
Table 4. Descriptive statistics of Diplocraterion at Buckeye Branch Mouth site.
Vertical expressions of these Diplocraterion also allowed for ichnotaxonomic designations, as summarised by Bromley (Citation1996, figure 9.9). Nearly every specimen observed was D. parallelum, in which the ‘U’ of the burrow has an almost uniform width throughout its length, and spreiten are joined directly to the inner part of the burrow tube. However, a few specimens had the ‘U’ part of the burrow slightly widened at their distal (deepest) ends and thus were more akin to D. helmerseni. Several other specimens also apparently had nested spreiten, in which spreiten are wider with depth; if so, these could be designated as D. polyupsilon. Nonetheless, numerous instances of Diplocraterion cross-cutting one another complicated our identification of ichnospecies, so we concluded that D. parallelum should be treated as the default ichnospecies for this bed.
The few specimens of Rhizocorallium detected at Buckeye Branch Mouth were limited to the upper 10 cm of the bed and were horizontal or inclined at 20–30° with respect to bedding. All spreiten were protrusive (Figure (g)), and open tubes were visible in some specimens, although we did not observe scratches on burrow interiors. These trace fossils were evident in longitudinal and cross-sectional views, as long as 154 mm and 59–83 mm wide (Table ). At least one Rhizocorallium observed in the outcrop cut across a Diplocraterion and hence was formed afterwards. Although specimens of Rhizocorallium were uncommon, they co-occurred with Diplocraterion and were comparably sized. Indeed, some near-vertically inclined and protrusive D. parallelum suggest transitions between Diplocraterion and Rhizocorallium (sensu Knaust, Citation2013). Owing to small sample size and lack of details in individual burrows, we could not determine ichnospecies for Rhizocorallium, such as R. jenese or R. commune. Nevertheless, an absence of faecal pellets in Rhizocorallium spreiten and tubes is typical of R. jenese and rules out R. commune as an ichnospecies (Knaust, Citation2013). Moreover, R. jenese is more commonly associated with firmgrounds, i.e. the Glossifungites ichnofacies.
Table 5. Rhizocorallium measurements from the Glen Rose Formation at Buckeye Branch. ‘L or C’ refers to longitudinal or cross-section, respectively. Measurements in millimetres and taken from photographs with included scale.
5. Analysis of results
Our results show that Diplocraterion specimens in the Diplocraterion bed of the Glen Rose Formation are remarkably consistent in size and other traits at the three examined localities. This morphological consistency is relevant to paleoenvironmental interpretations of this bed, as it probably reflects similar ecological conditions throughout the study area for the Diplocraterion tracemaker. By pooling data and qualitative traits from the three examined localities, we were able to create a Diplocraterion archetype for the study area. This idealised specimen would have a U-width of about 60 mm, a burrow-tube width of 12 mm, a burrow depth of 65 mm, protrusive spreiten, and would be oriented northeast–southwest (Figure ). Variations of this archetype – such as differing burrow-tube widths, spreite, absence or presence of burrow tubes, and burrow depths – are explainable through different biological and sedimentological factors that affected the Diplocraterion tracemakers’ behaviours and preservation of their traces afterwards.
Figure 9. Diplocraterion ‘archetype’ based on averages from descriptive statistics of measured specimens from three sites (LowT/Riverbend Cliff, McFall Ledge, Buckeye Branch Mouth) in the Diplocraterion bed, Glen Rose Formation. Scale = 1 cm.
The co-occurrence and similarly proportioned Arenicolites and Rhizocorallium in the same bed as Diplocraterion suggests that all three ichnogenera may have been made by similar tracemakers, with variations depending on tracemaker growth stages and substrate types. Tracemakers of Diplocraterion, Arenicolites, and Rhizocorallium in the bioclastic wackestone–packstone bed were most likely infaunal marine invertebrates and may have been the same species. For the two most abundant U-shaped burrows (Diplocraterion and Arenicolites), suspension-feeding invertebrates would have been the most likely tracemakers, such as polychaetes or decapod crustaceans (Bromley, Citation1996; Fürsich, Citation1974a; Gingras, Dashtgard, MacEachern, & Pemberton, Citation2008; Rodríguez-Tovar & Pérez-Valera, Citation2013; Seilacher, Citation2007; Šimo & Olšavskỳ, Citation2007). For example, burrows made by the modern amphipod Corophium volutator can resemble either Diplocraterion or Arenicolites (Dashtgard, Gingras, & Pemberton, Citation2008; Gingras et al., Citation2008). In modern lagoonal carbonate sediments of the Bahamas, upogebiid shrimp, such as Upogebia vasquezi, also make U-shaped burrows (Curran & Martin, Citation2003; Martin, Citation1999). Although we did not observe scratches or similar bioglyphs on burrow walls, which would correspond to arthropod legs, a few burrow tops retained pelletal exteriors (Figures (d) and (c)), a trait of some marine decapod burrows (Curran, Citation2007; Curran & Martin, Citation2003; Martin, Citation2013).
Bromley and Hanken (Citation1991) noted growth stages in Diplocraterion and other trace fossils, meaning the size gap between Arenicolites and Diplocraterion could either indicate two different species of tracemakers or different growth stages of the same species. The nearly identical U-width:burrow width ratio for both ichnogenera (~5.0), however, could be used to support either hypothesis. For instance, this ratio may reflect an architectural adaptation in which a U-shaped burrow optimised suspension feeding in two different species. Alternatively, the same species might have made both types of burrows, but the smaller growth stage of that species was incapable of adjusting its burrow up and down in response to sedimentation and erosion, respectively. Consequently, spreiten would be absent from such burrows. Oloriz and Rodriguez-Tovar (Citation2000) discerned growth stages in Diplocraterion from the Upper Jurassic of Spain; Rodríguez-Tovar and Pérez-Valera (Citation2013) further proposed that Middle Triassic Diplocraterion in Spain demonstrated connections between tracemaker growth stages and ecological conditions. In terms of settlement timing, though, we did not see any Arenicolites cross-cut Diplocraterion or vice versa. Hence, we could not tell whether their respective tracemakers preceded one another, or whether they overlapped in their residence time in the substrate.
The same tracemakers of Arenicolites and Diplocraterion also may have been responsible for Rhizocorallium in the Diplocraterion bed. Rhizocorallium is normally associated with polychaete tracemakers, but also has been attributed to decapod crustaceans, especially in Mesozoic examples (Knaust, Citation2013; and references therein). Specimens of Rhizocorallium at Buckeye Branch Mouth support this premise, bearing lengths and U-burrow widths in the same range as Diplocraterion depths and U-burrow widths, respectively. Given greater sediment firmness, the Rhizocorallium tracemakers would have behaved differently in making these burrows vs. those of Diplocraterion or Arenicolites. However, we do not regard these burrows as deposit-feeding traces, as opposed to suspension-feeding traces of Arenicolites and Diplocraterion (Dam, Citation1990; Knaust, Citation2013). Open burrow tubes in the Buckeye Branch Mouth Rhizocorallium imply these were dwelling burrows, which is more consistent with suspension feeding and would have been more likely in a firmground (Bromley, Citation1996; Knaust, Citation2013; Oloriz & Rodriguez-Tovar, Citation2000; Pazos et al., Citation2012).
Local variations in substrate consistency likely affected tracemaker colonisation, burrow orientation, and burrow morphology at the three examined localities. For example, moderate numbers of burrowers and nearly contemporaneous populations would have occupied softgrounds, such as at the LowT/Riverbend Cliff and McFall Ledge sites, whereas firmgrounds would have had separate generations in the same place over greater amounts of time (Frey & Seilacher, Citation1980; MacEachern, Pemberton, Gingras, & Bann, Citation2007; MacEachern, Pemberton, Gingras, Bann, & Dafoe, Citation2007; Pemberton & Frey, Citation1985). Only one locality, Buckeye Branch Mouth, showed densely packed and numerous overlapping burrows in the Diplocraterion bed, whereas the other two localities collectively had a single example of burrows cutting across one another (McFall Ledge). As a result, a spatial analysis of burrows, such as a nearest-neighbour analysis (sensu Pemberton & Frey, Citation1984), seemed unnecessary. Our preliminary assessment is that the Diplocraterion bed was not subject to long-term (e.g. thousands of years) colonisation. For instance, in modern firmgrounds, the elapsed time between the original ecosystem, burial, compression, exhumation, and exposure of a sedimentary bed to firmground tracemakers can take place in just a few hundred years (Morris & Rollins, Citation1977; Pemberton & Frey, Citation1985). Based on burrow abundance, size-frequency distributions that resemble populations, and qualitative features, the Diplocraterion bed at the LowT/Riverbend and McFall Ledge sites may represent only a few colonisation events by Arenicolites and Diplocraterion tracemakers. Regardless, this bed certainly had significant local differences in how it was used (or reused) by benthic infauna over time.
Variations in Diplocraterion spreiten, burrow depths, and preservation of lowermost portions of U-burrows all indicate that the top surface of the Diplocraterion bed was partially eroded in places, while also providing estimates of the minimal amount of that erosion. Spreiten at Buckeye Branch Mouth are mostly protrusive (83%), which is consistent with the majority of Diplocraterion tracemakers adjusting their burrows downward in response to erosion (Bromley, Citation1996; Goldring, Citation1962, 1964; Seilacher, Citation2007; Šimo & Olšavskỳ, Citation2007). Based on variations in burrow depth for Diplocraterion with protrusive spreiten at Buckeye Branch, approximately the top 5–12 cm of the bed was eroded. This estimate is based on the following: a maximum depth of about 14 cm for one specimen; most specimens having depths of about 6–7 cm; and some as little as 2–3 cm. These data also correspond with variable thickness measured for the Diplocraterion bed at Buckeye Branch Mouth, which ranged from 17 to 31 cm, a difference of 14 cm. Lastly, eroded Diplocraterion, preserved only as oval-shaped depressions on bedding planes, represent the bottom parts of U-burrows. These burrow remnants were at all three localities, and at McFall Ledge, a few co-occur with a theropod track (Eubrontes isp.) on the top surface of the bed. Preservation of these lowermost portions of burrows also indicates that the bed underwent partial erosion. The theropod trackway at McFall Ledge further suggests that the bed had already been eroded and perhaps was subaerially exposed when this dinosaur walked across it.
As mentioned previously, sediments composing the Diplocraterion bed at the Buckeye Branch Mouth site were probably colonised by multiple generations of infauna, and in a substrate that changed from a softground to a firmground. This would have signalled a shift from a Skolithos ichnofacies to a Glossifungites ichnofacies (MacEachern, Pemberton, Gingras, & Bann, Citation2007; MacEachern, Pemberton, Gingras, Bann, & Dafoe, Citation2007). This supposition is supported by interconnected ichnological and sedimentological evidence. First, a dominance of protrusive Diplocraterion, varying depths of Diplocraterion, eroded burrow tops, crowding and cross-cutting burrows, and open-tubed (i.e. suspension feeding) forms of Rhizocorallium, all imply a progressive firming and erosion of this bed (Knaust, Citation2013; Knaust & Costamagna, Citation2012). Furthermore, all observed Rhizocorallium were restricted to the uppermost 10 cm of the Diplocraterion bed, and in one instance cut across a Diplocraterion. This indicated that Rhizocorallium likely represents a final colonisation phase of infauna in these sediments. Additionally, the common preservational mode of Arenicolites, Diplocraterion, and Rhizocorallium as well-defined open tubes suggests that sediments were firm enough to retain burrow shapes. However, an absence of scratch marks on burrow walls, borings, encrusting organisms, pyritic or phosphatic mineralisation along the bed top, or other aspects of hardgrounds (Bromley, Citation1996; Frey & Seilacher, Citation1980; Knaust, Citation2013; Seilacher, Citation2007) shows that the firmground did not segue into a hardground.
Diplocraterion burrow orientations at the Riverbend/LowT and McFall Ledge sites also help to clarify the paleoenvironmental setting for the Diplocraterion bed. These orientations show a weakly defined northeast–southwest preference, but with a secondary grouping of northwest–southeast orientations. These groupings suggest that bidirectional currents may have had an influence on suspension-feeding Diplocraterion tracemakers. Crustaceans are known to align their burrows with currents (Hohenegger & Pervesler, Citation1985), and Diplocraterion and Rhizocorallium are well documented as burrows in which their tracemakers likely oriented with currents to facilitate suspension feeding (Fürsich, Citation1975; Knaust, Citation2013; Oloriz & Rodriguez-Tovar, Citation2000).
Interestingly, these burrow orientations also align with northeast–southwest bidirectional trends of theropod trackways in the Taylor Tracklayer, as reported previously by Farlow et al. (Citation2012) (Figure ; see Appendix 1 for details). The most parsimonious explanation for this coincidence is that both trace fossil assemblages were controlled by a northeast–southwest trending shoreline. In such a scenario, longshore currents would have influenced burrow orientations, whereas wave activity perpendicular to the shoreline could be assumed for a foreshore environment. Because the Diplocraterion bed overlies the Taylor Tracklayer, currents passing over a buried intertidal–supratidal zone would have prompted the tracemakers to orient the burrows; accordingly, the shoreline would have moved upslope. Nonetheless, this ‘new’ shoreline still could have been parallel to the one that influenced theropod behaviour during formation of the Taylor Tracklayer.
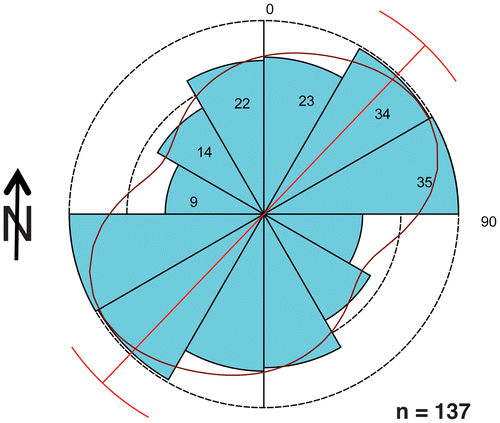
Figure 10. Dinosaur (theropod) track orientations in the Taylor Tracklayer, <1 m below the Diplocraterion bed and in the same study area. Data were degraded (combined) to reflect bimodally opposed directions of trackways; see Appendix 1 for further explanation.
A more specific explanation for similar orientations of theropod trackways and Diplocraterion burrows, but with each ichnoassemblage separated by time, is that of a linear lagoon (longer than wide) with tidal exchange. Ebb and flood tides would have carried bountiful detritus for infaunal suspension feeders, which would have oriented their burrows more or less parallel to these flow directions (Oloriz & Rodriguez-Tovar, Citation2000). Moreover, in a linear lagoon, tidal currents would have flowed parallel to its shoreline, but also may have varied in directions. An alternative explanation to a lagoon is an open tidal flat with an extensive shoreline, but with a shoreline that would have been consistent enough to produce the same preferred orientations observed in the burrows. Nonetheless, in either case, currents likely had preferred directions, which would have affected behaviours of benthic infaunal suspension feeders in those environments.
Given all of this ichnological, sedimentological, and stratigraphic information, the overall picture of Diplocraterion and associated trace fossils in the bed is that of a quiet-water, shallow subtidal (nearshore) environment that later underwent some dewatering (firming) and partial erosion. An analogous modern environment would be a low-energy lagoon composed of carbonate mud and sand, microtidal regime, and hosting an actively burrowing infauna. Curran and Martin (Citation2003) described such an environment from the Bahamas; in this example, upogebiid shrimp produce doubled and intersecting U-shaped burrows, which these shrimp develop on stabilised surfaces of callianassid-shrimp burrow mounds (Curran & Martin, Citation2003; see also Knaust, Curran, & Dronov, Citation2012; Martin, Citation1999, Citation2006). Although coastal carbonate facies are often characterised as ‘rapid-cementing’ when compared to clastic facies, rates of cementation are sufficiently variable in shallow-marine environments that carbonate firmgrounds do not necessarily become hardgrounds in a geological instant (Flügel, Citation2010; Moore & Wade, Citation2013). As a result, the Diplocraterion bed could have started as a softground, then transitioned to a firmground while still hosting infaunal invertebrates, but also may have remained a firmground while buried or later exhumed. Although a detailed look at vadose cementation, paleohydrology, and diagenesis of Glen Rose strata was well beyond the scope of our study, previous researchers have examined these facets (Ludvigson et al., Citation2004; Ufnar et al., Citation2005) and are worthy topics for work following our ichnological diagnoses.
The theropod trackway at the McFall Ledge site very likely was made well after the Diplocraterion and Arenicolites tracemakers were dead and otherwise no longer inhabiting a shallow subtidal environment. Because one track literally impacted several Diplocraterion and these represented the eroded lower parts of the burrows, Diplocraterion and Arenicolites burrows were occupied, abandoned, eroded, and possibly exposed subaerially before a theropod tromped on them. Additionally, the theropod tracks have clear, definite outlines, including one with sufficient quality to inspire its human-enabled theft. Nonetheless, we also acknowledge that this large theropod might have waded in shallow water while stepping on abandoned, eroded invertebrate burrows in carbonate firmgrounds. The absence of additional dinosaur tracks at this and the other two localities implies either a paleoenvironmental factor excluding their presence – such as water – or unfavourable conditions for preserving tracks.
With regard to the Taylor Tracklayer lying under the Diplocraterion bed, it has been interpreted as a supratidal to intertidal deposit, perhaps on the landward margin of a lagoon (Dattilo et al., Citation2014; Farlow et al., Citation2012). Interestingly, some theropod tracks in the Taylor Tracklayer have metatarsal impressions, implying that their trackmakers sank into soft muds that were either emergent or under shallow water (Kuban, Citation1989a, 1989b). Nonetheless, Diplocraterion, Arenicolites, and other trace fossils of suspension-feeding infauna are absent from the Taylor Tracklayer, although Arenicolites is exceedingly common in the Main Tracklayer (Dattilo et al., Citation2014). This likely means that the sedimentary environment for the Taylor Tracklayer bed was not submerged deeply enough to allow colonisation and occupation by suspension-feeding organisms and thus was indeed intertidal–supratidal. Accordingly, the Taylor Tracklayer would have best fit the Brontopodus ichnofacies (sensu Lockley, Hunt, & Meyer, Citation1994; Meyer & Pittman, Citation1994), which is particularly applicable to dinosaur tracksites associated with coastal carbonate facies.
Because the Diplocraterion bed is less than 50 cm stratigraphically above the Taylor Tracklayer, and given no evidence of a major time gap between the two beds, its diagnosis allows for fine-scale resolution of sea-level fluctuations that occurred during and just after these tracks were formed. Assuming that the Taylor Tracklayer is a supratidal–intertidal deposit formed on the landward margin of a lagoon, and that abundant U-shaped burrows (e.g. Arenicolites and Diplocraterion) reflect suspension feeding in submerged environments, the Diplocraterion bed represents a higher sea level and is probably subtidal in origin. In short, these burrows were formed in relatively deeper water than the dinosaur tracks. However, following a stillstand and/or drop in sea level that exposed the formerly submerged (or buried) bed, the bed top would have undergone firming and erosion, while also allowing dinosaurs to step on it. Consequently, the ichnofacies transitions within this <1 m thick interval – from the top of the Taylor Tracklayer to the top of the Diplocraterion bed – would have been Brontopodus, Skolithos, and Glossifungites, with the last of these overlapping a Brontopodus ichnofacies. Although only one theropod trackway is known from the Diplocraterion bed at the McFall Ledge site, it nonetheless gives important insights on how their respective marine infaunal invertebrate and continental vertebrate tracemakers were separated by both environments and time.
6. Significance of study
The Glen Rose Formation in the vicinity of Dinosaur Valley State Park is considered a world-class dinosaur tracksite (Bird, Citation1985; Dattilo et al., Citation2014; Farlow, Citation1993; Farlow et al., Citation1989, Citation2012; Jasinski, Citation2009; Kuban, Citation1989a, 1989b; Meyer & Pittman, Citation1994), yet our understanding of the environmental conditions that led to the formation and preservation of those tracks is still a work in progress. Our study of the Diplocraterion bed overlying one of the most important track-bearing horizons in the area – the Taylor Tracklayer – thus considerably advances our understanding of these processes. Most importantly, our study demonstrates how traces of shallow-marine invertebrate infauna can be connected to the behaviours of continental vertebrates. Through examining the smaller and often overlooked invertebrate trace fossils associated with these tracksites, we clarified much about changes in sedimentary environments associated with the tracksite, factors that could not have been gleaned from the lithofacies and dinosaur tracks alone.
For example, theropod trackways in the Taylor Tracklayer are mostly oriented northeast–southwest, which may reflect the orientation of the paleoshoreline. Sea level then raised enough to bury the Taylor Tracklayer, having deposited fine-grained carbonate sediments that allowed for colonisation by shallow-marine infaunal invertebrates. A significant proportion of the tracemakers oriented their burrows in northeast–southwest directions, again perhaps indicating the original shoreline. Colonisation was followed by firming of the sediments and erosion associated with a sea-level stillstand. A slight drop in sea level then enabled at least one large theropod to stroll through this formerly subtidal environment, leaving its tracks less than a metre above those of its dinosaurian predecessors.
Similar co-occurrences of dinosaur tracks with intertidal and formerly subtidal carbonates containing U-shaped burrows (e.g. Diplocraterion and Rhizocorallium) are in the Middle Jurassic Sundance Formation of Wyoming (Kvale et al., Citation2001), the Lower Cretaceous Dakota Sandstone of Colorado (Wright, Citation2004), and Lower Cretaceous strata in Patagonia, Argentina (Pazos et al., Citation2012). Although a detailed comparison between the Glen Rose Formation ichnoassemblages and these other sites is beyond the scope of our study, we hope future researchers will further unite the study of shallow-marine invertebrate burrows and dinosaur tracks made near or on them.
Acknowledgements
We appreciate the invitation of Huirye Dermican (MTA) and the organising committee to participate in the International Ichnofabric Workshop 2013 in Çanakkale, Turkey, where AJM presented the preliminary results of this research. We are doubly grateful to Dr Dermican for proposing and editing this volume, which further documents the important international research shared at the workshop. Following AJM’s presentation at the workshop, Luis Buatois, H.A. (Al) Curran, A.A. (Tony) Ekdale, Gabriela Mángano, Andrew (Andy) K. Rindsberg, and Alfred Uchman provided him with helpful feedback, some of which was incorporated into this manuscript. The manuscript was improved considerably by the aid of reviews by Dirk Knaust and an anonymous reviewer, and we thank Dr Erdin Bozkurt, the editor-in-chief of Geodinamica Acta, for his expert assistance in the post-review process. We are very thankful for the support of the National Geographic Society, which awarded Research Grant 205883 to JOF and funded field research for all of the authors during the summer of 2012. AJM thanks the Department of Environmental Sciences, Emory University for providing travel support to the study area. Ray Gildner, Peter Falkingham, Tara Joyce, April Knox, Dawn Stager, and Jim Whitcraft provided logistical and moral support during the field research. AJM is always thankful for the support of his wife, Ruth Schowalter, who did not do field work with him in Texas, but instead travelled with him to Çanakkale. The latter resulted in a superior culinary experience that fuelled their bodies and spirits for quite a while afterwards.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Adams, T. L., Strganac, C., Polcyn, M. J., & Jacobs, L. L. (2010). High resolution three-dimensional laser-scanning of the type specimen of Eubrontes (?) glenrosensis Shuler 1935, from the Comanchean (Lower Cretaceous) of Texas: Implications for digital archiving and preservation. Palaeontologia Electronica, 13(3), http://palaeo-electronica.org/2010_3/226/index.html
- Berens, P. (2009). CircStat: A MATLAB toolbox for circular statistics. Journal of Statistical Software, 31, 1–21.
- Bird, R. T. (1985). Bones for Barnum Brown: Adventures of a dinosaur hunter (225 p). Fort Worth, TX: Texas Christian University Press.
- Borradaile, G. (2003). Statistics of earth science data (355 p). Berlin: Springer. 10.1007/978-3-662-05223-5
- Bromley, R. G. (1996). Trace fossils (2nd ed., 361 p). London: Chapman & Hall.10.1007/978-1-4899-2875-7
- Bromley, R. G., & Hanken, N. M. (1991). The growth vector in trace fossils: Examples from the lower Cambrian of Norway. Ichnos, 1, 261–276.10.1080/10420949109386361
- Cornish, F. G. (1986). The trace-fossil Diplocraterion: Evidence of animal–sediment interactions in Cambrian tidal deposits. PALAIOS, 1, 478–491.10.2307/3514630
- Curran, H. A. (2007). Ichnofacies, ichnocoenoses, and ichnofabrics of quaternary shallow-marine to dunal tropical carbonates: A model and implications. In W. M. Miller III (Ed.), Trace fossils (pp. 232–247). Amsterdam: Elsevier.10.1016/B978-044452949-7/50140-6
- Curran, H. A., & Martin, A. J. (2003). Complex decapod burrows and ecological relationships in modern and pleistocene intertidal carbonate environments, San Salvador Island, Bahamas. Palaeogeography, Palaeoclimatology, Palaeoecology, 192, 229–245.10.1016/S0031-0182(02)00687-9
- Dam, G. (1990). Palaeoenvironmental significance of trace fossils from the shallow marine Lower Jurassic Neill Klinter Formation, East Greenland. Palaeogeography, Palaeoclimatology, Palaeoecology, 79, 221–248.10.1016/0031-0182(90)90019-4
- Dashtgard, S. E., Gingras, M. K., & Pemberton, S. G. (2008). Grain-size controls on the occurrence of bioturbation. Palaeogeography, Palaeoclimatology, Palaeoecology, 257, 224–243.10.1016/j.palaeo.2007.10.024
- Dattilo, B., Howald, S., Bonem, R., Farlow, J. O., Martin, A. J., O’Brien, M., … Joyce, T. (2014). Stratigraphy of the Paluxy river tracksites in and around Dinosaur Valley State Park, Lower Cretaceous Glen Rose Formation, Somervell County, Texas. New Mexico Museum of Natural History Bulletin, 62, 307–338.
- Farlow, J. O. (1993). The dinosaurs of Dinosaur Valley State Park (32 p). Austin, TX: Texas Parks and Wildlife Press.
- Farlow, J. O. (2001). Acrocanthosaurus and the maker of Comanchean large-theropod footprints. In D. H. Tanke & K. Carpenter (Eds.), Mesozoic vertebrate life (pp. 408–427). Bloomington: Indiana University Press.
- Farlow, J. O., O’Brien, M., Kuban, G. J., Dattilo, B. F., Bates, K. T., Falkingham, P. L., … Whitcraft, J. (2012). Dinosaur tracksites of the Paluxy river valley (Glen Rose Formation, Lower Cretaceous), Dinosaur Valley State Park, Somervell County, Texas. Actas de V Jornadas Internacionales sobre Paleontología de Dinosaurios y su Entorno (pp. 1–30). Burgos, Spain, Salas de los Infantes.
- Farlow, J. O., Pittman, J. G., & Hawthorne, J. M. (1989). Brontopodus birdi, Lower Cretaceous sauropod footprints from the U.S. Gulf Coastal Plain. In D. D. Gillette & M. G. Lockley (Eds.), Dinosaur tracks and traces (pp. 371–394). Cambridge: Cambridge University Press.
- Flügel, E. (2010). Microfacies of carbonate rocks: Analysis, interpretation and application (984 p). Berlin: Springer.10.1007/978-3-642-03796-2
- Frey, R. W., & Seilacher, A. (1980). Uniformity in marine invertebrate ichnology. Lethaia, 13, 183–207.10.1111/let.1980.13.issue-3
- Fürsich, F. T. (1974a). On Diplocraterion Torell 1870 and the significance of morphological features in vertical, spreiten-bearing U-shaped trace fossils. Journal of Paleontology, 48, 952–962.
- Fürsich, F. T. (1974b). Ichnogenus Rhizocorallium. Paläontologische Zeitschrift, 48, 16–28.10.1007/BF02986987
- Fürsich, F. T. (1975). Trace fossils as environmental indicators in the Corallian of England and Normandy. Lethaia, 8, 151–172.10.1111/let.1975.8.issue-2
- Gingras, M. K., Dashtgard, S. E., MacEachern, J. A., & Pemberton, S. G. (2008). Biology of shallow marine ichnology: A modern perspective. Aquatic Biology, 2, 255–268.
- Goldring, R. (1962). The trace fossils of the baggy beds (upper Devonian) of North Devon, England. Paläontologische Zeitschrift, 36, 232–251.10.1007/BF02986976
- Goldring, R. (1964). Trace fossils and the sedimentary surface in shallow water marine sediments. Developments in Sedimentology, 1, 136–143.10.1016/S0070-4571(08)70478-3
- Goldring, R. (1971). Shallow-water sedimentation as illustrated in the upper Devonian baggy beds. Memoirs of the Geological Society of London No. 5 (80 p). London: Burlington House.
- Hammer, Ø., Harper, D. A. T., & Ryan, P. D. (2001). Past: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica, 4, 1–9. Retrieved from http://palaeo-electronica.org/2001_1/past/issue1_01.htm
- Hawthorne, J. M. (1990). Dinosaur track-bearing strata of the Lampasas Cut Plain and Edwards Plateau, Texas. Baylor Geological Studies Bulletin, 49, 1–47.
- Hohenegger, J., & Pervesler, P. (1985). Orientation of crustacean burrows. Lethaia, 18, 323–339.10.1111/let.1985.18.issue-4
- Jasinski, L. E. (2009). Dinosaur highway: A history of Dinosaur Valley State Park (212 p). Fort Worth: Texas Christian University Press.
- Knaust, D. (2013). The ichnogenus Rhizocorallium: Classification, trace makers, palaeoenvironments and evolution. Earth Science Reviews, 126, 1–47.10.1016/j.earscirev.2013.04.007
- Knaust, D., & Costamagna, L. C. (2012). Ichnology and sedimentology of the triassic carbonates of north-west Sardinia, Italy. Sedimentology, 59, 1190–1207.10.1111/sed.2012.59.issue-4
- Knaust, D., Curran, H. A., & Dronov, A. V. (2012). Developments in sedimentology. In D. Knaust & R. G. Bromley (Eds.), Trace fossils as indicators of sedimentary environments (pp. 7-5-750). Amsterdam: Elsevier.
- Kuban, G. J. (1989a). Elongate dinosaur tracks. In D. D. Gillette & M. G. Lockley (Eds.), Dinosaur tracks and traces (pp. 57–72). Cambridge: Cambridge University Press.
- Kuban, G. J. (1989b). Color distinctions and other curious features of dinosaur tracks near Glen Rose, Texas. In D. D. Gillette & M. G. Lockley (Eds.), Dinosaur tracks and traces (pp. 427–440). Cambridge: Cambridge University Press.
- Kvale, E. P., Johnson, A. D., Mickelson, D. L., Keller, K., Furer, L. C., & Archer, A. W. (2001). Middle jurassic (bajocian and bathonian) dinosaur megatracksites, Bighorn Basin, Wyoming, U.S.A. PALAIOS, 16, 233–254.10.1669/0883-1351(2001)016<0233:MJBABD>2.0.CO;2
- Levitin, D. J., Russell, G. S. (1999). Circular data, Rao’s spacing test for. Encyclopedia of statistical sciences (Vol. 3, pp. 87–89). New York, NY: Wiley.
- Lockley, M. G. (2009). New perspective on morphological variation in tridactyl footprints: Clues to widespread convergence in developmental dynamics. Geological Quarterly, 53, 415–432.
- Lockley, M. G., Hunt, A. P., & Meyer, C. A. (1994). The ichnofacies concept in vertebrate ichnology. In S. K. Donovan (Ed.), The paleobiology of trace fossils (pp. 241–268). New York, NY: Wiley.
- Ludvigson, G. A., Ufnar, D. F., González, L. A., Witzke, B. J., Brenner, R. L., & Davis, J. (2004). Terrestrial paleoclimatology of the mid-Cretaceous greenhouse I: Cross-calibration of pedogenic siderite and calcite d18O proxies at the Hadley cell boundary. Geological Society of America, Abstracts with Programs, 36, 305.
- MacEachern, J. A., Pemberton, S. G., Gingras, M. K., & Bann, K. L. (2007). The ichnofacies paradigm: A fifty-year perspective. In W. M. Miller III (Ed.), Trace fossils (pp. 52–77). Amsterdam: Elsevier.10.1016/B978-044452949-7/50130-3
- MacEachern, J. A., Pemberton, S. G., Gingras, M. K., Bann, K. L., & Dafoe, L. T. (2007). Uses of trace fossils in genetic stratigraphy. In W. M. Miller III (Ed.), Trace fossils (pp. 110–134). Amsterdam: Elsevier.10.1016/B978-044452949-7/50133-9
- Martin, A. J. (1999). Fossil upogebiid burrows and their geologic significance: Grotto Beach Formation (Pleistocene), San Salvador, Bahamas. In H. A. Curran & J. E. Mylroie (Eds.), Proceedings of the 9th symposium on the geology of the Bahamas and other carbonate regions (pp. 81–92). San Salvador: Gerace Research Centre.
- Martin, A. J. (2006). Trace fossils of San Salvador (80 p). San Salvador: Gerace Research Centre.
- Martin, A. J. (2013). Life traces of the Georgia coast: Revealing the unseen lives of plants and animals (692 p). Bloomington: Indiana University Press.
- Mason, T. R., & Christie, A. D. M. (1986). Palaeoevironmental significance of ichnogenus Diplocraterion Torell from the Permian Vryheid formation of the Karoo Supergroup, South Africa. Palaeogeography, Palaeoclimatology, Palaeoecology, 52, 249–265.10.1016/0031-0182(86)90050-7
- Meyer, C. A., & Pittman, J. G. (1994). A comparison between the Brontopodus ichnofacies of Portugal, Switzerland, and Texas. Gaia, 10, 125–133.
- Moore, C. H., & Wade, W. J. (2013). Carbonate reservoirs: Porosity and diagenesis in a sequence stratigraphic framework (392 p). Amsterdam: Elsevier.
- Morris, R. W., & Rollins, H. B. (1977). Observations on intertidal organism associations on St. Catherines Island, Georgia. I. General description and paleoecological implications. Bulletin of the American Museum of Natural History, 159, 87–128.
- Nagle, J. S. (1968). Glen Rose cycles and facies, Paluxy River Valley, Somervell County, Texas. Bureau of Economic Geology Geological Circular, 68, 1–25.
- Oloriz, F., & Rodriguez-Tovar, F. J. (2000). Diplocraterion: A useful marker for sequence stratigraphy and correlation in the Kimmeridgian, Jurassic (Prebetic Zone, Betic Cordillera, southern Spain). PALAIOS, 15, 546–552.10.1669/0883-1351(2000)015<0546:DAUMFS>2.0.CO;2
- Olsen, P. E., Smith, J. B., & McDonald, C. A. (1998). The material of the type species of the classic theropod footprint genera Eubrontes, Anchisauripus, and Grallator (Early Jurassic), Hartford and Deerfield Basins, Connecticut and Massachusetts, U.S.A.). Journal of Vertebrate Paleontology, 18, 586–601.
- Pazos, P. J., Lazo, D. G., Tunik, M. A., Marsicano, C. A., Fernández, D. E., & Aguirre-Urreta, M. B. (2012). Paleoenvironmental framework of dinosaur tracksites and other ichnofossils in early Cretaceous mixed siliciclastic-carbonate deposits in the Neuquén Basin, northern Patagonia (Argentina). Gondwana Research, 22, 1125–1140.10.1016/j.gr.2012.02.003
- Pemberton, S. G., & Frey, R. W. (1984). Quantitative methods in ichnology spatial distribution among populations. Lethaia, 17, 33–49.10.1111/let.1984.17.issue-1
- Pemberton, S. G., & Frey, R. W. (1985). The Glossifungites ichnofacies: modern examples from the Georgia coast, U.S.A. In H. A. Curran (Ed.), Biogenic structures: Their use in interpreting depositional environments (Vol. 35, pp. 237–259). Tulsa, OK: Society of Economic Paleontologists and Mineralogists Special Publication.
- Rodriguez-Tovar, F. J., & Perez-Valera, F. (2008). Trace fossil rhizocorallium from the middle Triassic of the Betic Cordillera, southern Spain: Characterization and environmental implications. PALAIOS, 23, 78–86.10.2110/palo.2007.p07-007r
- Rodríguez-Tovar, F. J., & Pérez-Valera, F. (2013). Variations in population structure of Diplocraterion parallelum: Hydrodynamic influence, food availability, or nursery settlement? Palaeogeography, Palaeoclimatology, Palaeoecology, 369, 501–509.10.1016/j.palaeo.2012.11.022
- Seilacher, A. (2007). Trace fossil analysis (272 p). Berlin: Springer.
- Shuler, E. W. (1917). Dinosaur tracks in the Glen Rose limestone near Glen Rose, Texas. American Journal of Science, 44, 294–298.10.2475/ajs.s4-44.262.294
- Šimo, V., & Olšavskỳ, M. (2007). Diplocraterion parallelum Torell 1870 and other trace fossils from the lower Triassic succession of the Drienok Nappe in the Western Carpathians, Slovokia. Bulletin of Geosciences [Czech Geological Survey], 82, 165–173.
- Ufnar, D. F., Ludvigson, G. A., González, L. A., & Davis, J. (2005). Mid-Cretaceous evaporation rates estimated from pedogenic carbonate isotopic values in the Glen Rose Formation, Texas. Geological Society of America, Abstracts with Programs, 37, 305.
- White, T., González, L. A., Ludvigson, G. A., & Poulsen, C. (2001). Middle Cretaceous greenhouse hydrologic cycle of North America. Geology, 29, 363–366.10.1130/0091-7613(2001)029<0363:MCGHCO>2.0.CO;2
- Wright, J. L. (2004). Walking with dinosaurs: Walking with dinosaurs (and other extinct animals) along Colorado’s Front Range: A field trip to Paleozoic and Mesozoic terrestrial localities. GSA Field Guides, 5, 219–234.
Appendix 1
Explanation of Diplocraterion and dinosaur trackway orientation statistics
None of the orientation data sets for Diplocraterion show a Von Mises distribution, which is the circular analogue of a Gaussian (‘normal’) distribution (Borradaile, Citation2003), meaning they are neither uniform nor unimodal. Yet the dinosaur trackways from the Taylor Tracklayer, when treated as axial orientations, are close to such a distribution. This suggests that all distributions are multimodal, but that the secondary mode of the dinosaur trackways without direction is relatively small. However, when assuming these as directions, they are more or less bimodal. Because the distributions are non-Von Mises, their means have little importance, other than the axial (non-directional) dinosaur trackway trends. For this reason, Rayleigh’s test for significance of mean direction (Berens, Citation2009) does not provide any further insights, as it assumes a Von Mises distribution and thus fails to detect non-uniformity. However, Rao’s spacing test (Levitin & Russell, Citation1999) does not depend on a Von Mises distribution; thus, it is generally reliable, given enough observations.
In terms of the Diplocraterion orientation data reported here, the low number of observations for the McFall Ledge site makes this the least ‘significant’ of all the distributions. It is definitely multi-modal, but the low number of observations (n = 28) makes any display of more than six bin sizes (in this instance, 30° intervals) irrelevant. Rao’s spacing test (Levitin & Russell, Citation1999) suggests a weak non-uniform distribution of the data. In contrast, the Low T/Riverbend Cliff site, with its large number of observations (n = 368), strengthens its statistical meaning: under both Rao’s and Von Mises tests, it is definitely non-uniform, pointing towards a preferred alignment of northeast–southwest for Diplocraterion.
For the dinosaur track orientations – whether consisting of single isolated tracks or series of many tracks (trackways) – it is interesting to note that the two modes of dinosaur track directions are opposite one other, which renders these as more apparently uniform. Since the two modes are at 180° to one another, treating them as axial orientations greatly improves the error on their mean direction. This suggests that the mode for the dinosaur track orientations is far narrower than that of the Diplocraterion burrow orientations.
Hence, a statistical comparison between the distribution of Diplocraterion directions with the axial version of the distribution of dinosaur trackway directions is challenging, as the dinosaur track data had to be degraded. Because the distribution of the Diplocraterion orientation data is so broad and non-uniform, a comparison of means, using a Watson–Williams test (Berens, Citation2009), results in a relatively low probability (.0029817) that the two distributions (Diplocraterion and dinosaur tracks) have the same means. Although other statistical tests should detect ‘equal’ distributions, a visual inspection and differences in peak narrowness – i.e. the dinosaur trackways are much more precisely aligned than Diplocraterion – show that the distributions are not identical anyway. For this reason, other statistical tests should return a low probability that the two distributions are identical. In short, we are left pointing out that both distributions of the Diplocraterion and dinosaur track orientations have a primary mode in the northeast–southwest direction, but it is difficult to precisely test this as a correlation.